A State-of-Art on the Development of Nafion-Based Membrane for Performance Improvement in Direct Methanol Fuel Cells
Abstract
:1. Introduction
2. Functional Requirements
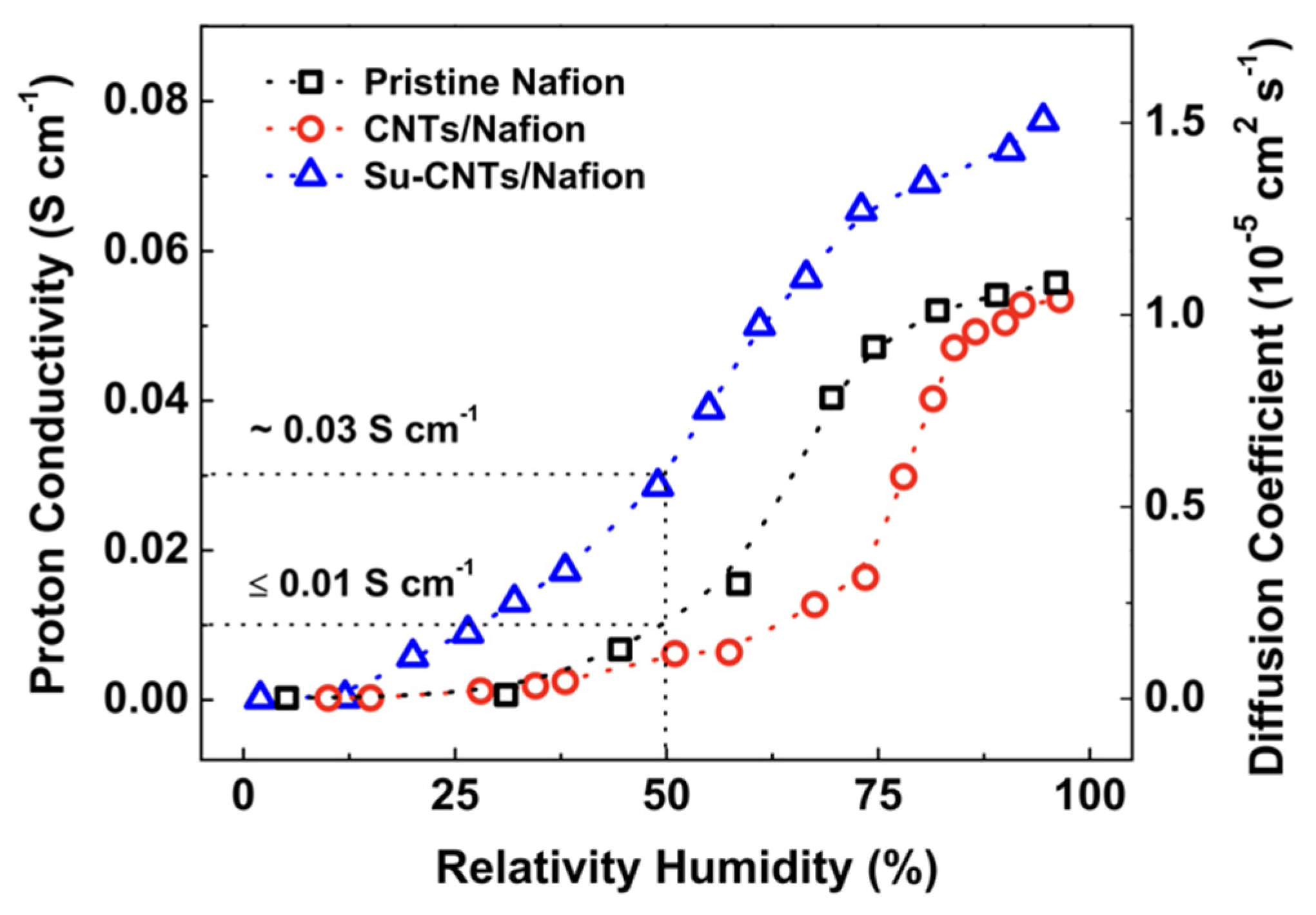
2.1. High Proton Conductivity
2.2. Low Methanol Permeability
2.3. High Electrical Resistivity
2.4. Suitable Chemical Stability
2.5. Suitable Mechanical Stability
3. Proton Transport Mechanism in Nafion
4. Preparation Methods
4.1. Blending
4.2. In Situ Sol-Gel
4.3. Multilayer Method
5. Modification Strategies
5.1. Polymeric Blend and Composite Membranes
5.2. Adding Inorganic Filler
5.3. Adding Ionic Liquid
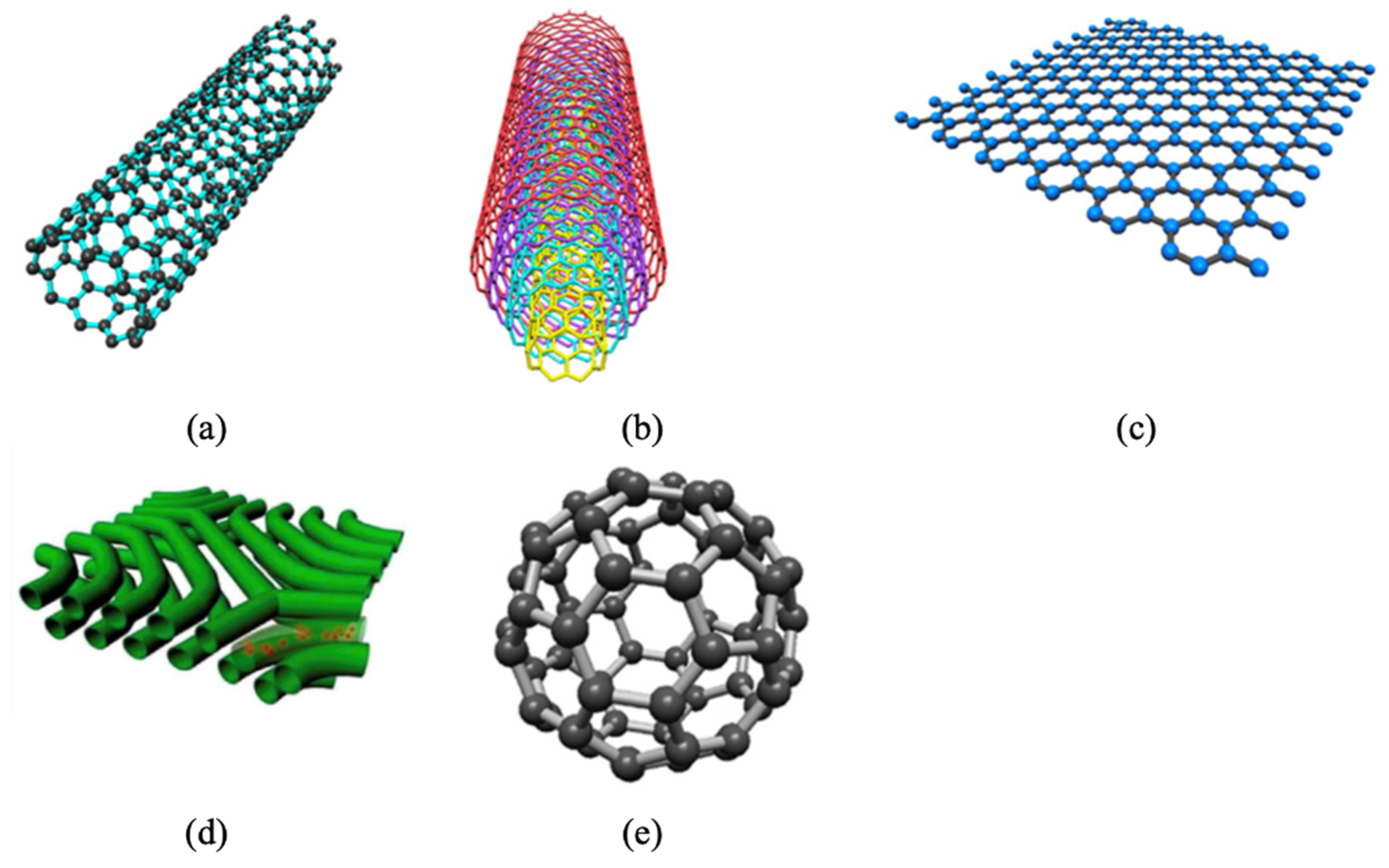
5.4. Incorporating Carbon Nanomaterials
6. Other Structural Modifications
7. Challenges and Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Cao, J.; Zhang, Y.; Liu, J.; Chen, J.; Li, M.; Wang, W.; Liu, X. Overcoming undesired fuel crossover: Goals of methanol-resistant modification of polymer electrolyte membranes. Renew. Sustain. Energy Rev. 2020, 138, 110660. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Bakangura, E.; Wu, L.; Ge, L.; Yang, Z.; Xu, T. Mixed matrix proton exchange membranes for fuel cells: State of the art and perspectives. Prog. Polym. Sci. 2016, 57, 103–152. [Google Scholar] [CrossRef]
- Elmer, T.; Worall, M.; Wu, S.; Riffat, S.B. Fuel cell technology for domestic built environment applications: State of-the-art review. Renew. Sustain. Energy Rev. 2015, 42, 913–931. [Google Scholar] [CrossRef]
- Mallick, R.K.; Thombre, S.B.; Shrivastava, N.K. Vapor feed direct methanol fuel cells (DMFCs): A review. Renew. Sustain. Energy Rev. 2016, 56, 51–74. [Google Scholar] [CrossRef]
- Velayutham, P.; Sahu, A.; Parthasarathy, S. A Nafion-Ceria Composite Membrane Electrolyte for Reduced Methanol Crossover in Direct Methanol Fuel Cells. Energies 2017, 10, 259. [Google Scholar] [CrossRef]
- Alias, M.S.; Kamarudin, S.K.; Zainoodin, A.M.; Masdar, M.S. Active direct methanol fuel cell: An overview. Int. J. Hydrog. Energy 2020, 45, 19620–19641. [Google Scholar] [CrossRef]
- Chia, M.Y.; Thiam, H.S.; Leong, L.K.; Koo, C.H.; Saw, L.H. Study on improvement of the selectivity of proton exchange membrane via incorporation of silicotungstic acid-doped silica into SPEEK. Int. J. Hydrog. Energy 2020, 45, 22315–22323. [Google Scholar] [CrossRef]
- Joghee, P.; Malik, J.N.; Pylypenko, S.; O’Hayre, R. A review on direct methanol fuel cells–In the perspective of energy and sustainability. MRS Energy Sustain. 2015, 2, E3. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Sheng, W.; Tao, W.; Li, Z. Direct methanol fuel cells system–A review of dual-role electrocatalysts for oxygen reduction and methanol oxidation. J. Mater. Sci. Technol. 2022, 114, 29–41. [Google Scholar] [CrossRef]
- Sapkota, P.; Boyer, C.; Dutta, R.; Cazorla, C.; Aguey-Zinsou, K.F. Planar polymer electrolyte membrane fuel cells: Powering portable devices from hydrogen. Sustain. Energy Fuels 2019, 4, 439–468. [Google Scholar] [CrossRef]
- Branco, C.M.; Sharma, S.; de Camargo Forte, M.M.; Steinberger-Wilckens, R. New approaches towards novel composite and multilayer membranes for intermediate temperature-polymer electrolyte fuel cells and direct methanol fuel cells. J. Power Sources 2016, 316, 139–159. [Google Scholar] [CrossRef] [Green Version]
- Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Potential carbon nanomaterials as additives for state-of-the-art Nafion electrolyte in proton-exchange membrane fuel cells: A concise review. RSC Adv. 2021, 11, 18351–18370. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Li, H.Y.; Lai, J.Y.; Liu, Y.L. Nanocomposite membranes of Nafion and Fe3O4-anchored and Nafion-functionalized multiwalled carbon nanotubes exhibiting high proton conductivity and low methanol permeability for direct methanol fuel cells. RSC Adv. 2013, 3, 12895. [Google Scholar] [CrossRef]
- Boutsika, L.G.; Enotiadis, A.; Nicotera, I.; Simari, C.; Charalambopoulou, G.; Giannelis, E.P.; Steriotis, T. Nafion® nanocomposite membranes with enhanced properties at high temperature and low humidity environments. Int. J. Hydrog. Energy 2016, 41, 22406–22414. [Google Scholar] [CrossRef] [Green Version]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrog. Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Prykhodko, Y.; Fatyeyeva, K.; Hespel, L.; Marais, S. Progress in hybrid composite Nafion®-based membranes for proton exchange fuel cell application. Chem. Eng. J. 2021, 409, 127329. [Google Scholar] [CrossRef]
- Hooshyari, K.; Horri, B.A.; Abdoli, H.; Vostakola, M.F.; Kakavand, P.; Salarizadeh, P. A Review of Recent Developments and Advanced Applications of High-Temperature Polymer Electrolyte Membranes for PEM Fuel Cells. Energies 2021, 14, 5440. [Google Scholar] [CrossRef]
- Sun, X.; Simonsen, S.C.; Norby, T.; Chatzitakis, A. Composite Membranes for High Temperature PEM Fuel Cells and Electrolysers: A Critical Review. Membranes 2019, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Raja Rafidah, R.S.; Rashmi, W.; Khalid, M.; Wong, W.Y.; Priyanka, J. Recent Progress in the Development of Aromatic Polymer-Based Proton Exchange Membranes for Fuel Cell Applications. Polymers 2020, 12, 1061. [Google Scholar]
- Li, J.; Bu, F.; Ru, C.; Jiang, H.; Duan, Y.; Sun, Y.; Pu, X.; Shang, L.; Li, X.; Zhao, C. Enhancing the selectivity of Nafion membrane by incorporating a novel functional skeleton molecule to improve the performance of direct methanol fuel cells. J. Mater. Chem. A 2020, 8, 196–206. [Google Scholar] [CrossRef]
- Feng, K.; Tang, B.; Wu, P. Sulfonated graphene oxide–silica for highly selective Nafion-based proton exchange membranes. J. Mater. Chem. A 2014, 2, 16083–16092. [Google Scholar] [CrossRef]
- Romano, S.M. Application of Nanofibres in Polymer Composite Membranes for Direct Methanol Fuel Cells. Ph.D. Thesis, Universidad Politécnica de Valencia, Valencia, Spain, 2015. [Google Scholar]
- Ahmad, H.; Kamarudin, S.K.; Hasran, U.A.; Daud, W.R.W. Overview of hybrid membranes for direct-methanol fuel-cell applications. Int. J. Hydrog. Energy 2010, 35, 2160–2175. [Google Scholar] [CrossRef]
- Pattanapisutkun, N. Silaned-Graphene oxide-Mordenite/Nafion Composite Membrane for Direct Methanol Fuel Cell. Master’s Thesis, Kasetsart University, Bangkok, Thailand, 2017. [Google Scholar]
- Wan, N. High performance direct methanol fuel cell with thin electrolyte membrane. J. Power Sources 2017, 354, 167–171. [Google Scholar] [CrossRef]
- Lue, S.J.; Pai, Y.L.; Shih, C.M.; Wu, M.C.; Lai, S.M. Novel bilayer well-aligned Nafion/graphene oxide composite membranes prepared using spin coating method for direct liquid fuel cells. J. Membr. Sci. 2015, 493, 212–223. [Google Scholar] [CrossRef]
- Xie, M.; Chu, T.; Wang, T.; Wan, K.; Yang, D.; Li, B.; Ming, P.; Zhang, C. Preparation, Performance and Challenges of Catalyst Layer for Proton Exchange Membrane Fuel Cell. Membranes 2021, 11, 879. [Google Scholar] [CrossRef]
- Xing, Y.; Li, H.; Avgouropoulos, G. Research Progress of Proton Exchange Membrane Failure and Mitigation Strategies. Materials 2021, 14, 2591. [Google Scholar] [CrossRef]
- Zatoń, M.; Rozière, J.; Jones, D.J. Current understanding of chemical degradation mechanisms of perfluorosulfonic acid membranes and their mitigation strategies: A review. Sustain. Energy Fuels 2017, 1, 409–438. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Hariprasad, R.; Ramakrishnan, S.; Kim, A.R.; Yoo, D.J. Potential Bifunctional Filler (CeO2 –ACNTs) for Nafion Matrix toward Extended Electrochemical Power Density and Durability in Proton-Exchange Membrane Fuel Cells Operating at Reduced Relative Humidity. ACS Sustain. Chem. Eng. 2019, 7, 12847–12857. [Google Scholar] [CrossRef]
- Sood, R.; Cavaliere, S.; Jones, D.J.; Rozière, J. Electrospun nanofibre composite polymer electrolyte fuel cell and electrolysis membranes. Nano Energy 2016, 26, 729–745. [Google Scholar] [CrossRef] [Green Version]
- Rambabu, G.; Bhat, S.D.; Figueiredo, F.M.L. Carbon Nanocomposite Membrane Electrolytes for Direct Methanol Fuel Cells-A Concise Review. Nanomaterials 2019, 9, 1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitzel, J.; Arena, F.; Walter, T.; Stefener, M.; Hempelmann, R. Direct Methanol Fuel Cell—Alternative Materials and Catalyst Preparation. Z. Phys. Chem. 2013, 227, 497–540. [Google Scholar] [CrossRef]
- Liu, L.; Chen, W.; Li, Y. An overview of the proton conductivity of nafion membranes through a statistical analysis. J. Membr. Sci. 2016, 504, 1–9. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrog. Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Telfah, A.; Majer, G.; Kreuer, K.D.; Schuster, M.; Maier, J. Formation and mobility of protonic charge carriers in methyl sulfonic acid–water mixtures: A model for sulfonic acid based ionomers at low degree of hydration. Solid State Ion. 2010, 181, 461–465. [Google Scholar] [CrossRef]
- Esmaeili, N.; Gray, E.M.; Webb, C.J. Non-Fluorinated Polymer Composite Proton Exchange Membranes for Fuel Cell Applications—A Review. Chemphysche: Eur. J. Chem. Phys. Phys. Chem. 2019, 20, 2016–2053. [Google Scholar] [CrossRef] [Green Version]
- Ru, C.; Gu, Y.; Duan, Y.; Na, H.; Zhao, C. Nafion based semi-interpenetrating polymer network membranes from a cross-linkable SPAEK and a fluorinated epoxy resin for DMFCs. Electrochim. Acta 2019, 324, 134873. [Google Scholar] [CrossRef]
- Bonis, C.; D’Epifanio, A.; Mecheri, B.; Licoccia, S.; Tavares, A. 10. Solid polymer proton conducting electrolytes for fuel cells. In Functional Materials: For Energy, Sustainable Development and Biomedical Sciences; Leclerc, M., Gauvin, R., Eds.; De Gruyter: Berlin, Germany; München, Germany; Boston, MA, USA, 2014; pp. 207–240. [Google Scholar]
- Dutta, K.; Das, S.; Kundu, P.P. Partially sulfonated polyaniline induced high ion-exchange capacity and selectivity of Nafion membrane for application in direct methanol fuel cells. J. Membr. Sci. 2015, 473, 94–101. [Google Scholar] [CrossRef]
- Li, Y.; He, G.; Wang, S.; Yu, S.; Pan, F.; Wu, H.; Jiang, Z. Recent advances in the fabrication of advanced composite membranes. J. Mater. Chem. A 2013, 1, 10058. [Google Scholar] [CrossRef]
- Moon, S.Y.; Bae, J.S.; Jeon, E.; Park, J.W. Organic sol-gel synthesis: Solution-processable microporous organic networks. Angew. Chem. 2010, 49, 9504–9508. [Google Scholar] [CrossRef]
- Harun, N.A.M.; Shaari, N.; Nik Zaiman, N.F.H. A review of alternative polymer electrolyte membrane for fuel cell application based on sulfonated poly(ether ether ketone). Int. J. Energy Res. 2021, 45, 19671–19708. [Google Scholar] [CrossRef]
- Zheng, J.; He, Q.; Liu, C.; Yuan, T.; Zhang, S.; Yang, H. Nafion-microporous organic polymer networks composite membranes. J. Membr. Sci. 2014, 476, 571–579. [Google Scholar] [CrossRef]
- Xue, Y.; Chan, S. Layer-by-layer self-assembly of CHI/PVS–Nafion composite membrane for reduced methanol crossover and enhanced DMFC performance. Int. J. Hydrog. Energy 2015, 40, 1877–1885. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Zhang, Q.; Qin, G. Assembly of an unbalanced charged polyampholyte onto Nafion® to produce high-performance composite membranes. Chem. Commun. 2012, 48, 12201–12203. [Google Scholar] [CrossRef]
- Ru, C.; Gu, Y.; Duan, Y.; Zhao, C.; Na, H. Enhancement in proton conductivity and methanol resistance of Nafion membrane induced by blending sulfonated poly(arylene ether ketones) for direct methanol fuel cells. J. Membr. Sci. 2019, 573, 439–447. [Google Scholar] [CrossRef]
- Tellez-Cruz, M.M.; Escorihuela, J.; Solorza-Feria, O.; Compañ, V. Proton Exchange Membrane Fuel Cells (PEMFCs): Advances and Challenges. Polymers 2021, 13, 3064. [Google Scholar] [CrossRef]
- Gonzalez-Ausejo, J.; Cabedo, L.; Gámez-Pérez, J.; Mollá, S.; Giménez, E.; Compañ, V. Modification of Nafion Membranes with Polyaniline to Reduce Methanol Permeability. J. Electrochem. Soc. 2015, 162, E325–E333. [Google Scholar] [CrossRef] [Green Version]
- Munar, A.; Suarez, K.; Solorza, O.; Berezina, N.P.; Compañ, V. Performance of Hydrogen Fuel Cell MEAs Based on Perfluorinated Nanocomposite Membranes Modified by Polyaniline. J. Electrochem. Soc. 2010, 157, B1186–B1194. [Google Scholar] [CrossRef]
- Escudero-Cid, R.; Montiel, M.; Sotomayor, L.; Loureiro, B.; Fatás, E.; Ocón, P. Evaluation of polyaniline-Nafion ® composite membranes for direct methanol fuel cells durability tests. Int. J. Hydrog. Energy 2015, 40, 8182–8192. [Google Scholar] [CrossRef]
- Cho, K.Y.; Park, J.K.; Jung, H.Y. The Effect of Solvent Casting Condition on the Electrochemical Properties of Nafion/Polyvinylidene Fluoride Copolymer Blends for Direct Methanol Fuel Cell. Chem. Eng. Commun. 2015, 202, 593–599. [Google Scholar] [CrossRef]
- Li, Y.; Hui, J.; Kawchuk, J.; O’Brien, A.; Jiang, Z.; Hoorfar, M. Composite Membranes of PVDF Nanofibers Impregnated with Nafion for Increased Fuel Concentrations in Direct Methanol Fuel Cells. Fuel Cells 2019, 19, 43–50. [Google Scholar] [CrossRef]
- Kumar, P.; Dutta, K.; Das, S.; Kundu, P.P. Membrane prepared by incorporation of crosslinked sulfonated polystyrene in the blend of PVdF-co-HFP/Nafion: A preliminary evaluation for application in DMFC. Appl. Energy 2014, 123, 66–74. [Google Scholar] [CrossRef]
- Kumar, P.; Kundu, P.P. Coating and lamination of Nafion117 with partially sulfonated PVdF for low methanol crossover in DMFC applications. Electrochim. Acta 2015, 173, 124–130. [Google Scholar] [CrossRef]
- Mondal, S.; Soam, S.; Kundu, P.P. Reduction of methanol crossover and improved electrical efficiency in direct methanol fuel cell by the formation of a thin layer on Nafion 117 membrane: Effect of dip-coating of a blend of sulphonated PVdF-co-HFP and PBI. J. Membr. Sci. 2015, 474, 140–147. [Google Scholar] [CrossRef]
- Yusoff, Y.N.; Shaari, N. An overview on the development of nanofiber-based as polymer electrolyte membrane and electrocatalyst in fuel cell application. Int. J. Energy Res. 2021, 45, 18441–18472. [Google Scholar] [CrossRef]
- Shabanpanah, S.; Omrani, A.; Mansour Lakouraj, M. Fabrication and characterization of PVA/NNSA/GLA/nano-silica proton conducting composite membranes for DMFC applications. Des. Monomers Polym. 2019, 22, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.L.; Wang, S.H. Nafion/poly(vinyl alcohol) nano-fiber composite and Nafion/poly(vinyl alcohol) blend membranes for direct methanol fuel cells. J. Membr. Sci. 2014, 452, 253–262. [Google Scholar] [CrossRef]
- Zizhou, R.E.; Çay, A.; Akçakoca Kumbasar, E.P.; Çolpan, C.Ö. Production of poly(vinyl alcohol)/Nafion® nanofibers and their stability assessment for the use in direct methanol fuel cells. J. Ind. Text. 2019, 50, 773–793. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Nassr, A.A.; Kashyout, A.B.; Hassan, E.A. Novel sulfonated poly(glycidyl methacrylate) grafted Nafion membranes for fuel cell applications. Polym. Bull. 2017, 74, 5195–5220. [Google Scholar] [CrossRef]
- Arslanova, A.A.; Sanginova, E.A.; Dobrovol’skii, Y.A. New Composite Proton-Conducting Membranes Based on Nafion and Cross-Linked Sulfonated Polystyrene. Russ. J. Electrochem. 2018, 54, 318–323. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Liu, C.; Xing, W.; Sun, J. Self-Healing Proton-Exchange Membranes Composed of Nafion-Poly(vinyl alcohol) Complexes for Durable Direct Methanol Fuel Cells. Adv. Mater. 2018, 30, e1707146. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, K.; Cai, W.; Ma, L.; Xu, G.; Xu, S.; Cheng, H. An in-situ nano-scale swelling-filling strategy to improve overall performance of Nafion membrane for direct methanol fuel cell application. J. Power Sources 2016, 332, 37–41. [Google Scholar] [CrossRef]
- Xu, G.; Li, J.; Ma, L.; Xiong, J.; Mansoor, M.; Cai, W.; Cheng, H. Performance dependence of swelling-filling treated Nafion membrane on nano-structure of macromolecular filler. J. Membr. Sci. 2017, 534, 68–72. [Google Scholar] [CrossRef]
- Cai, W.; Fan, K.; Li, J.; Ma, L.; Xu, G.; Xu, S.; Ma, L.; Cheng, H. A bi-functional polymeric nano-sieve Nafion composite membrane: Improved performance for direct methanol fuel cell applications. Int. J. Hydrog. Energy 2016, 41, 17102–17111. [Google Scholar] [CrossRef]
- Sigwadi, R.; Mokrani, T.; Dhlamini, S.; Msomi, P.F. Nafion® reinforced with polyacrylonitrile/ ZrO2 nanofibers for direct methanol fuel cell application. J. Appl. Polym. Sci. 2020, 138, 49978. [Google Scholar] [CrossRef]
- Ben Jadi, S.; El Guerraf, A.; Bazzaoui, E.A.; Wang, R.; Martins, J.I.; Bazzaoui, M. Synthesis, characterization, and transport properties of Nafion-polypyrrole membrane for direct methanol fuel cell (DMFC) application. J. Solid State Electrochem. 2019, 23, 2423–2433. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Sahoo, M.; Perez-Page, M.; Baylis, S.R.; Patel, F.; Holmes, S.M. Improving the performance of direct methanol fuel cells by implementing multilayer membranes blended with cellulose nanocrystals. Int. J. Hydrog. Energy 2019, 44, 30409–30419. [Google Scholar] [CrossRef]
- Ying, Y.P.; Kamarudin, S.K.; Masdar, M.S. Silica-related membranes in fuel cell applications: An overview. Int. J. Hydrog. Energy 2018, 43, 16068–16084. [Google Scholar] [CrossRef]
- Su, D.Y.; Ma, J.; Pu, H.K. The Research of Nafion/PTFE/Inorganic Composite Membrane Used in Direct Methanol Fuel Cell. Adv. Mater. Res. 2014, 881–883, 927–930. [Google Scholar] [CrossRef]
- Alvarez, A.; Guzmán, C.; Carbone, A.; Saccà, A.; Gatto, I.; Passalacqua, E.; Nava, R.; Ornelas, R.; Ledesma-García, J.; Arriaga, L.G. Influence of silica morphology in composite Nafion membranes properties. Int. J. Hydrog. Energy 2011, 36, 14725–14733. [Google Scholar] [CrossRef]
- Mazinani, V.; Tabaian, S.H.; Rezaei, M.; Mallahi, M.; Mohammadijoo, M.; Omidvar, H. Synthesis and Characterization of Nafion-ZrOH-CaO Hybrid Membrane for Proton Exchange Membrane Fuel Cell. J. Fuel Cell Sci. Technol. 2014, 11, 031004. [Google Scholar] [CrossRef]
- Ozden, A.; Ercelik, M.; Ozdemir, Y.; Devrim, Y.; Colpan, C.O. Enhancement of direct methanol fuel cell performance through the inclusion of zirconium phosphate. Int. J. Hydrog. Energy 2017, 42, 21501–21517. [Google Scholar] [CrossRef]
- Sigwadi, R.; Dhlamini, M.S.; Mokrani, T.; Nemavhola, F.; Nonjola, P.F.; Msomi, P.F. The proton conductivity and mechanical properties of Nafion®/ ZrP nanocomposite membrane. Heliyon 2019, 5, e02240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigwadi, R.; Dhlamini, S.; Mokrani, T.; Nemavhola, F. Wettability and mechanical strength of modified Nafion nanocomposite membrane for fuel cell. Dig. J. Nanomater. Biostructures 2017, 12, 1137–1148. [Google Scholar]
- Sigwadi, R.; Nemavhola, F.; Dhlamini, S.; Mokrani, T. Mechanical Strength of Nafion®/ZrO2 Nano-Composite Membrane. Int. J. Manuf. Mater. Mech. Eng. 2018, 8, 54–65. [Google Scholar] [CrossRef]
- Sigwadi, R.; Mokrani, T.; Dhlamini, M.S.; Nonjola, P.; Msomi, P.F. Nafion®/ sulfated zirconia oxide-nanocomposite membrane: The effects of ammonia sulfate on fuel permeability. J. Polym. Res. 2019, 26, 108. [Google Scholar] [CrossRef]
- Sigwadi, R.; Mokrani, T.; Msomi, P.; Nemavhola, F. The Effect of Sulfated Zirconia and Zirconium Phosphate Nanocomposite Membranes on Fuel Cell Efficiency. Polymers 2022, 14, 263. [Google Scholar] [CrossRef]
- Prapainainar, P.; Du, Z.; Theampetch, A.; Prapainainar, C.; Kongkachuichay, P.; Holmes, S.M. Properties and DMFC performance of nafion/mordenite composite membrane fabricated by solution-casting method with different solvent ratio. Energy 2019, 190, 116451. [Google Scholar] [CrossRef]
- Sun, X.; Yang, C.; Xia, Z.; Qi, F.; Sun, H.; Sun, G. Molecular sieve as an effective barrier for methanol crossover in direct methanol fuel cells. Int. J. Hydrog. Energy 2020, 45, 8994–9003. [Google Scholar] [CrossRef]
- Prapainainar, P.; Du, Z.; Kongkachuichay, P.; Holmes, S.M.; Prapainainar, C. Mordenite/Nafion and analcime/Nafion composite membranes prepared by spray method for improved direct methanol fuel cell performance. Appl. Surf. Sci. 2017, 421, 24–41. [Google Scholar] [CrossRef] [Green Version]
- Prapainainar, P.; Pattanapisutkun, N.; Prapainainar, C.; Kongkachuichay, P. Incorporating graphene oxide to improve the performance of Nafion-mordenite composite membranes for a direct methanol fuel cell. Int. J. Hydrog. Energy 2018, 44, 362–378. [Google Scholar] [CrossRef]
- Cui, Y.; Baker, A.P.; Xu, X.; Xiang, Y.; Wang, L.; Lavorgna, M.; Wu, J. Enhancement of Nafion based membranes for direct methanol fuel cell applications through the inclusion of ammonium-X zeolite fillers. J. Power Sources 2015, 294, 369–376. [Google Scholar] [CrossRef]
- Ercelik, M.; Ozden, A.; Devrim, Y.; Colpan, C.O. Investigation of Nafion based composite membranes on the performance of DMFCs. Int. J. Hydrog. Energy 2016, 42, 2658–2668. [Google Scholar] [CrossRef]
- Wang, M.; Liu, G.; Cui, X.; Feng, Y.; Zhang, H.; Wang, G.; Zhong, S.; Luo, Y. Self-crosslinked organic-inorganic nanocomposite membranes with good methanol barrier for direct methanol fuel cell applications. Solid State Ion. 2018, 315, 71–76. [Google Scholar] [CrossRef]
- Yang, C.W.; Chen, C.C.; Chen, K.H.; Cheng, S. Effect of pore-directing agents in SBA-15 nanoparticles on the performance of Nafion®/SBA-15n composite membranes for DMFC. J. Membr. Sci. 2017, 526, 106–117. [Google Scholar] [CrossRef]
- Thiam, H.S.; Daud, W.R.W.; Kamarudin, S.K.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S.; Majlan, E.H. Nafion/Pd–SiO2 nanofiber composite membranes for direct methanol fuel cell applications. Int. J. Hydrog. Energy 2013, 38, 9474–9483. [Google Scholar] [CrossRef]
- Thiam, H.S.; Chai, M.Y.; Cheah, Q.R.; Koo, C.C.H.; Lai, S.O.; Chong, K.C. Proton conductivity and methanol permeability of Nafion-SiO2/SiWA composite membranes. In Proceedings of the AIP Conference Proceedings, Perak, Malaysia, 10–13 January 2017; p. 020007. [Google Scholar]
- Feng, K.; Tang, B.; Wu, P. A “H2O donating/methanol accepting” platform for preparation of highly selective Nafion-based proton exchange membranes. J. Mater. Chem. A 2015, 3, 18546–18556. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhuang, X.; Cheng, B.; Wang, W.; Kang, W.; Shi, L.; Li, H. Modification of Nafion membrane with biofunctional SiO2 nanofiber for proton exchange membrane fuel cells. J. Power Sources 2017, 340, 201–209. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Feng, X.; Liu, Y.; Kang, W.; Xu, X.; Zhuang, X.; Cheng, B. Novel proton-conductive nanochannel membranes with modified SiO2 nanospheres for direct methanol fuel cells. J. Solid State Electrochem. 2018, 22, 3475–3484. [Google Scholar] [CrossRef]
- Cozzi, D.; Bonis, C.; D’Epifanio, A.; Mecheri, B.; Tavares, A.C.; Licoccia, S. Organically functionalized titanium oxide/Nafion composite proton exchange membranes for fuel cells applications. J. Power Sources 2014, 248, 1127–1132. [Google Scholar] [CrossRef]
- Allodi, V.; Brutti, S.; Giarola, M.; Sgambetterra, M.; Navarra, M.A.; Panero, S.; Mariotto, G. Structural and spectroscopic characterization of a nanosized sulfated TiO2 filler and of nanocomposite Nafion membranes. Polymers 2016, 8, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scipioni, R.; Gazzoli, D.; Teocoli, F.; Palumbo, O.; Paolone, A.; Ibris, N.; Brutti, S.; Navarra, M.A. Preparation and Characterization of Nanocomposite Polymer Membranes Containing Functionalized SnO2 Additives. Membranes 2014, 4, 123–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liu, C.T.; Zhao, L.; Wang, Z.B. Highly Durable Direct Methanol Fuel Cell with Double-Layered Catalyst Cathode. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Advani, S.G.; Prasad, A.K. Self-Hydrating Pt/CeO2-Nafion Composite Membrane for Improved Durability and Performance. ECS Electrochem. Lett. 2014, 3, F30–F32. [Google Scholar] [CrossRef]
- Weissbach, T.; Peckham, T.J.; Holdcroft, S. CeO2, ZrO2 and YSZ as mitigating additives against degradation of proton exchange membranes by free radicals. J. Membr. Sci. 2016, 498, 94–104. [Google Scholar] [CrossRef]
- Toikkanen, O.; Nisula, M.; Pohjalainen, E.; Hietala, S.; Havansi, H.; Ruotsalainen, J.; Halttunen, S.; Karppinen, M.; Kallio, T. Al2O3 coating grown on Nafion membranes by atomic layer deposition. J. Membr. Sci. 2015, 495, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Liu, Y.; Wu, J.; Zhang, F.; Baker, A.P.; Lavorgna, M.; Wu, Q.; Tang, Q.; Lu, J.; Xiao, Z.; et al. Porous silicon-aluminium oxide particles functionalized with acid moieties: An innovative filler for enhanced Nafion-based membranes of direct methanol fuel cell. J. Power Sources 2018, 403, 118–126. [Google Scholar] [CrossRef]
- Hamid, N.S.A.; Kamarudin, S.K.; Karim, N.A. Potential of Nafion /eggshell composite membrane for application in direct methanol fuel cell. Int. J. Energy Res. 2020, 45, 2245–2264. [Google Scholar] [CrossRef]
- Felice, C.; Ye, S.; Qu, D. Nafion−Montmorillonite Nanocomposite Membrane for the Effective Reduction of Fuel Crossover. Ind. Eng. Chem. Res. 2010, 49, 1514–1519. [Google Scholar] [CrossRef]
- Azimi, M.; Peighambardoust, S.J. Methanol Crossover and Selectivity of Nafion/Heteropolyacid/Montmorillonite Nanocomposite Proton Exchange Membranes for DMFC Applications. Iran. J. Chem. Eng. 2017, 14, 65–81. [Google Scholar]
- Hernández-Fernández, F.J.; de los Ríos, A.P.; Mateo-Ramírez, F.; Juarez, M.D.; Lozano-Blanco, L.J.; Godínez, C. New application of polymer inclusion membrane based on ionic liquids as proton exchange membrane in microbial fuel cell. Sep. Purif. Technol. 2015, 160, 51–58. [Google Scholar] [CrossRef]
- Rynkowska, E.; Fatyeyeva, K.; Kujawski, W. Application of polymer-based membranes containing ionic liquids in membrane separation processes: A critical review. Rev. Chem. Eng. 2017, 34, 341–363. [Google Scholar] [CrossRef]
- Goh, J.T.E.; Abdul Rahim, A.R.; Masdar, M.S.; Shyuan, L.K. Enhanced Performance of Polymer Electrolyte Membranes via Modification with Ionic Liquids for Fuel Cell Applications. Membranes 2021, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.S.; Nikazar, M.; Molla-Abbasi, P.; Hasani-Sadrabadi, M.M. Nafion®/histidine functionalized carbon nanotube: High-performance fuel cell membranes. Int. J. Hydrog. Energy 2013, 38, 5894–5902. [Google Scholar] [CrossRef]
- Da Trindade, L.G.; Zanchet, L.; Martins, P.C.; Borba, K.M.N.; Santos, R.D.M.; Paiva, R.d.S.; Vermeersch, L.A.F.; Ticianelli, E.A.; de Souza, M.O.; Martini, E.M.A. The influence of ionic liquids cation on the properties of sulfonated poly (ether ether ketone)/polybenzimidazole blends applied in PEMFC. Polymer 2019, 179, 121723. [Google Scholar] [CrossRef]
- Hudson, R.; Adhikari, R.Y.; Tuominen, M.T.; Hanzu, I.; Wilkening, M.; Thayumanavan, S.; Katz, J.L. Evaluation of carboxylic, phosphonic, and sulfonic acid protogenic moieties on tunable poly(meta-phenylene oxide) ionomer scaffolds. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2209–2213. [Google Scholar] [CrossRef] [Green Version]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Neves, L.A.; Coelhoso, I.M.; Crespo, J.G. Methanol and gas crossover through modified Nafion membranes by incorporation of ionic liquid cations. J. Membr. Sci. 2010, 360, 363–370. [Google Scholar] [CrossRef]
- Yang, J.; Che, Q.; Zhou, L.; He, R.; Savinell, R.F. Studies of a high temperature proton exchange membrane based on incorporating an ionic liquid cation 1-butyl-3-methylimidazolium into a Nafion matrix. Electrochim. Acta 2011, 56, 5940–5946. [Google Scholar] [CrossRef]
- Su, D.S.; Centi, G. A perspective on carbon materials for future energy application. J. Energy Chem. 2013, 22, 151–173. [Google Scholar] [CrossRef]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the Development of Advanced Catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xin, L.; Xia, Y.; Dai, L.; Qu, K.; Huang, K.; Fan, Y.; Xu, Z. Advanced Nafion hybrid membranes with fast proton transport channels toward high-performance vanadium redox flow battery. J. Membr. Sci. 2021, 624, 119047. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Majedi, F.S.; Wu, S.; Bertsch, A.; Moaddel, H.; Renaud, P. Nafion/chitosan-wrapped CNT nanocomposite membrane for high-performance direct methanol fuel cells. RSC Adv. 2013, 3, 7337. [Google Scholar] [CrossRef]
- Molla-Abbasi, P.; Janghorban, K.; Asgari, M.S. A novel heteropolyacid-doped carbon nanotubes/Nafion nanocomposite membrane for high performance proton-exchange methanol fuel cell applications. Iran. Polym. J. 2018, 27, 77–86. [Google Scholar] [CrossRef]
- Yin, C.; Li, J.; Zhou, Y.; Zhang, H.; Fang, P.; He, C. Enhancement in Proton Conductivity and Thermal Stability in Nafion Membranes Induced by Incorporation of Sulfonated Carbon Nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 14026–14035. [Google Scholar] [CrossRef]
- Nicotera, I.; Simari, C.; Coppola, L.; Zygouri, P.; Gournis, D.; Brutti, S.; Minuto, F.D.; Aricò, A.S.; Sebastian, D.; Baglio, V. Sulfonated Graphene Oxide Platelets in Nafion Nanocomposite Membrane: Advantages for Application in Direct Methanol Fuel Cells. J. Phys. Chem. C 2014, 118, 24357–24368. [Google Scholar] [CrossRef]
- Hu, S.; Lozada-Hidalgo, M.; Wang, F.C.; Mishchenko, A.; Schedin, F.; Nair, R.R.; Hill, E.W.; Boukhvalov, D.W.; Katsnelson, M.I.; Dryfe, R.A.W.; et al. Proton transport through one-atom-thick crystals. Nature 2014, 516, 227–230. [Google Scholar] [CrossRef] [Green Version]
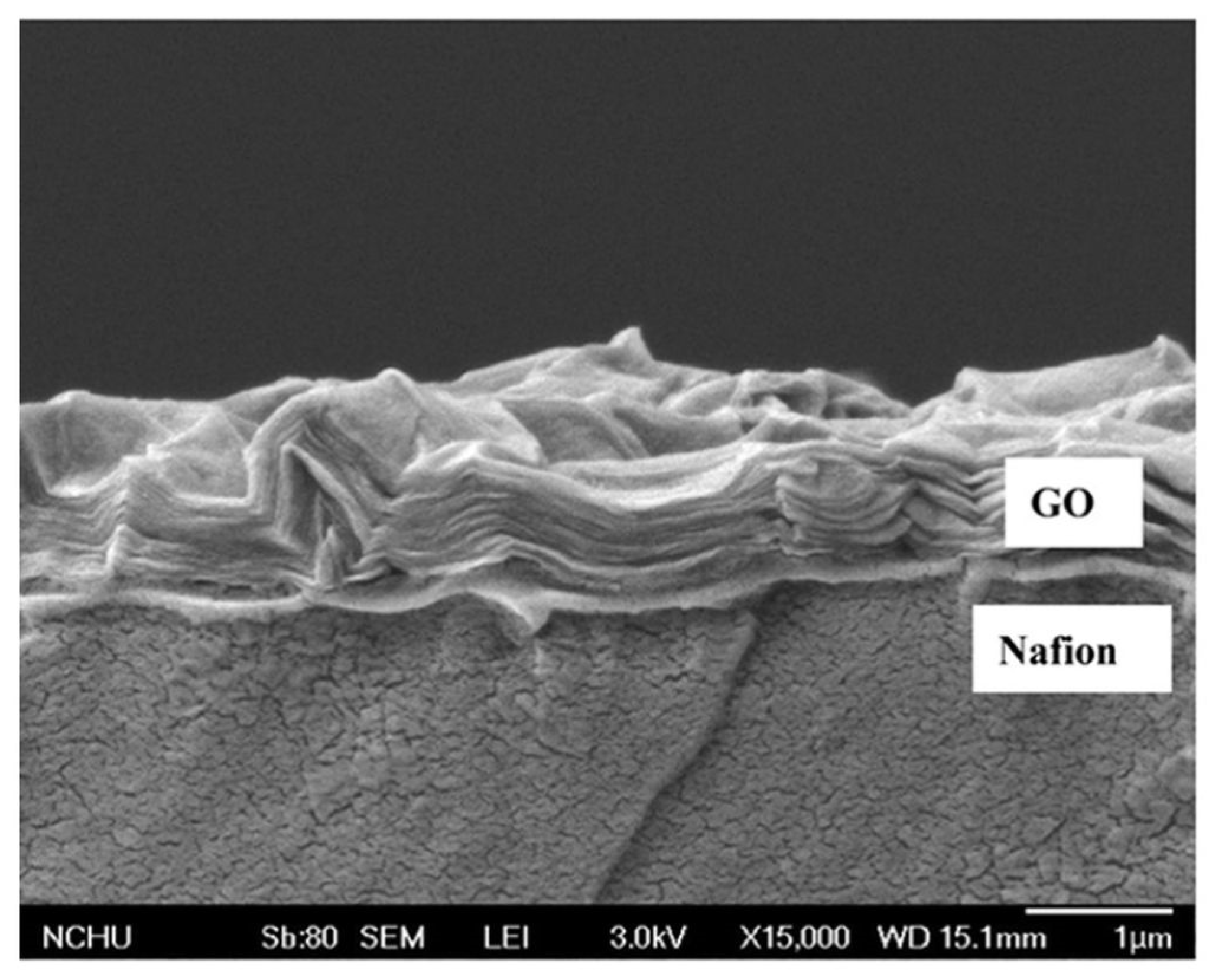
- Lin, C.W.; Lu, Y.S. Highly ordered graphene oxide paper laminated with a Nafion membrane for direct methanol fuel cells. J. Power Sources 2013, 237, 187–194. [Google Scholar] [CrossRef]
- Wang, L.S.; Lai, A.N.; Lin, C.X.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Orderly sandwich-shaped graphene oxide/Nafion composite membranes for direct methanol fuel cells. J. Membr. Sci. 2015, 492, 58–66. [Google Scholar] [CrossRef]
- Yan, X.H.; Wu, R.; Xu, J.B.; Luo, Z.; Zhao, T.S. A monolayer graphene—Nafion sandwich membrane for direct methanol fuel cells. J. Power Sources 2016, 311, 188–194. [Google Scholar] [CrossRef]
- Parthiban, V.; Akula, S.; Peera, S.G.; Islam, N.; Sahu, A.K. Proton Conducting Nafion-Sulfonated Graphene Hybrid Membranes for Direct Methanol Fuel Cells with Reduced Methanol Crossover. Energy Fuels 2015, 30, 725–734. [Google Scholar] [CrossRef]
- Tsai, L.D.; Chien, H.C.; Huang, W.H.; Huang, C.P.; Kang, C.Y.; Lin, J.N.; Chang, F.C. Novel Bilayer Composite Membrane for Passive Direct Methanol Fuel Cells with Pure Methanol. Int. J. Electrochem. Sci. 2013, 8, 9704–9713. [Google Scholar]
- Yuan, T.; Pu, L.; Huang, Q.; Zhang, H.; Li, X.; Yang, H. An effective methanol-blocking membrane modified with graphene oxide nanosheets for passive direct methanol fuel cells. Electrochim. Acta 2014, 117, 393–397. [Google Scholar] [CrossRef]
- Li, J.; Xu, G.; Cai, W.; Xiong, J.; Ma, L.; Yang, Z.; Huang, Y.; Cheng, H. Non-destructive modification on Nafion membrane via in-situ inserting of sheared graphene oxide for direct methanol fuel cell applications. Electrochim. Acta 2018, 282, 362–368. [Google Scholar] [CrossRef]
- Jia, W.; Tang, B.; Wu, P. Carbon dots with multi-functional groups and the application in proton exchange membranes. Electrochim. Acta 2018, 260, 92–100. [Google Scholar] [CrossRef]
- Saga, S.; Matsumoto, H.; Saito, K.; Minagawa, M.; Tanioka, A. Polyelectrolyte membranes based on hydrocarbon polymer containing fullerene. J. Power Sources 2008, 176, 16–22. [Google Scholar] [CrossRef]
- Rambabu, G.; Nagaraju, N.; Bhat, S.D. Functionalized fullerene embedded in Nafion matrix: A modified composite membrane electrolyte for direct methanol fuel cells. Chem. Eng. J. 2016, 306, 43–52. [Google Scholar] [CrossRef]
- Lee, J.S.; Hwang, I.T.; Jung, C.H.; Choi, J.H. Surface modification of Nafion membranes by ion implantation to reduce methanol crossover in direct methanol fuel cells. RSC Adv. 2013, 6, 62467–62470. [Google Scholar] [CrossRef]
- Wang, M.; Liu, G.; Tian, Z.; Shao, Y.; Wang, L.; Ye, F.; Tran, M.X.; Yun, Y.; Lee, J.K. Microstructure-modified proton exchange membranes for high-performance direct methanol fuel cells. Energy Convers. Manag. 2017, 148, 753–758. [Google Scholar] [CrossRef]
- Ling, X.; Bonn, M.; Parekh, S.H.; Domke, K.F. Nanoscale Distribution of Sulfonic Acid Groups Determines Structure and Binding of Water in Nafion Membranes. Angew. Chem. 2016, 55, 4011–4015. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.S.; Rashmi, K.R.; Manjunatha, D.V.; Jayarama, A.; Prabhu, S.; Pinto, R. Pore size tuning of Nafion membranes by UV irradiation for enhanced proton conductivity for fuel cell applications. Int. J. Hydrog. Energy 2019, 44, 23762–23774. [Google Scholar] [CrossRef]
- Li, L.; Su, L.; Zhang, Y. Enhanced performance of supercritical CO2 treated Nafion 212 membranes for direct methanol fuel cells. Int. J. Hydrog. Energy 2012, 37, 4439–4447. [Google Scholar] [CrossRef]
- Hasanabadi, N.; Ghaffarian, S.R.; Hasani-Sadrabadi, M.M. Nafion-based magnetically aligned nanocomposite proton exchange membranes for direct methanol fuel cells. Solid State Ion. 2013, 232, 58–67. [Google Scholar] [CrossRef]
- Feng, K.; Tang, B.; Wu, P. Selective growth of MoS2 for proton exchange membranes with extremely high selectivity. ACS Appl. Mater. Interfaces 2013, 5, 13042–13049. [Google Scholar] [CrossRef]
- Parthiban, V.; Sahu, A.K. Performance enhancement of direct methanol fuel cells using a methanol barrier boron nitride–Nafion hybrid membrane. New J. Chem. 2020, 44, 7338–7349. [Google Scholar] [CrossRef]
- Tian, A.H.; Hou, Z.S.; Sun, X.F.; Shen, D.H. Sodium dodecyl sulfate/Pd-modified Nafion membrane for direct methanol fuel cells applications. In Proceedings of the 2013 International Conference on Materials for Renewable Energy and Environment (ICMREE), Chengdu, China, 19–21 August 2013. [Google Scholar]
- Iwai, Y.; Ikemoto, S.; Haramaki, K.; Hattori, R.; Yonezawa, S. Influence of ligands of palladium complexes on palladium/Nafion composite membranes for direct methanol fuel cells by supercritical CO2 impregnation method. J. Supercrit. Fluids 2014, 94, 48–58. [Google Scholar] [CrossRef]
- Rao, S.; Xiu, R.; Si, J.; Lu, S.; Yang, M.; Xiang, Y. In situ synthesis of nanocomposite membranes: Comprehensive improvement strategy for direct methanol fuel cells. ChemSusChem 2014, 7, 822–828. [Google Scholar] [CrossRef]
- Abouzari-lotf, E.; Nasef, M.M.; Ghassemi, H.; Zakeri, M.; Ahmad, A.; Yadollah, A. Improved methanol barrier property of Nafion hybrid membrane by incorporating nanofibrous interlayer self-immobilized with high level of phosphotungstic acid. ACS Appl. Mater. Interfaces 2015, 7, 1944–8244. [Google Scholar] [CrossRef]
- Wang, S.H.; Lin, H.L. Poly (vinylidene fluoride-co-hexafluoropropylene)/polybenzimidazole blend nanofiber supported Nafion membranes for direct methanol fuel cells. J. Power Sources 2014, 257, 254–263. [Google Scholar] [CrossRef]
- Jiang, G.P.; Zhang, J.; Qiao, J.L.; Jiang, Y.M.; Zarrin, H.; Chen, Z.; Hong, F. Bacterial nanocellulose/Nafion composite membranes for low temperature polymer electrolyte fuel cells. J. Power Sources 2015, 273, 697–706. [Google Scholar] [CrossRef]
- Shen, L.; Sun, Z.; Chu, Y.; Zou, J.; Deshusses, M.A. Novel sulfonated Nafion®-based composite membranes with pillararene as selective artificial proton channels for application in direct methanol fuel cells. Int. J. Hydrog. Energy 2015, 40, 13071–13079. [Google Scholar] [CrossRef]
- Yao, J.; Xu, G.; Zhao, Z.; Guo, J.; Li, S.; Cai, W.; Zhang, S. An enhanced proton conductivity and reduced methanol permeability composite membrane prepared by sulfonated covalent organic nanosheets/Nafion. Int. J. Hydrog. Energy 2019, 44, 24985–24996. [Google Scholar] [CrossRef]
- Guo, X.; Fan, Y.; Xu, J.; Wang, L.; Zheng, J. Amino-MIL-53(Al)-nanosheets@Nafion composite membranes with improved proton/methanol selectivity for passive direct methanol fuel cells. Ind. Eng. Chem. Res. 2020, 59, 14825–14833. [Google Scholar] [CrossRef]
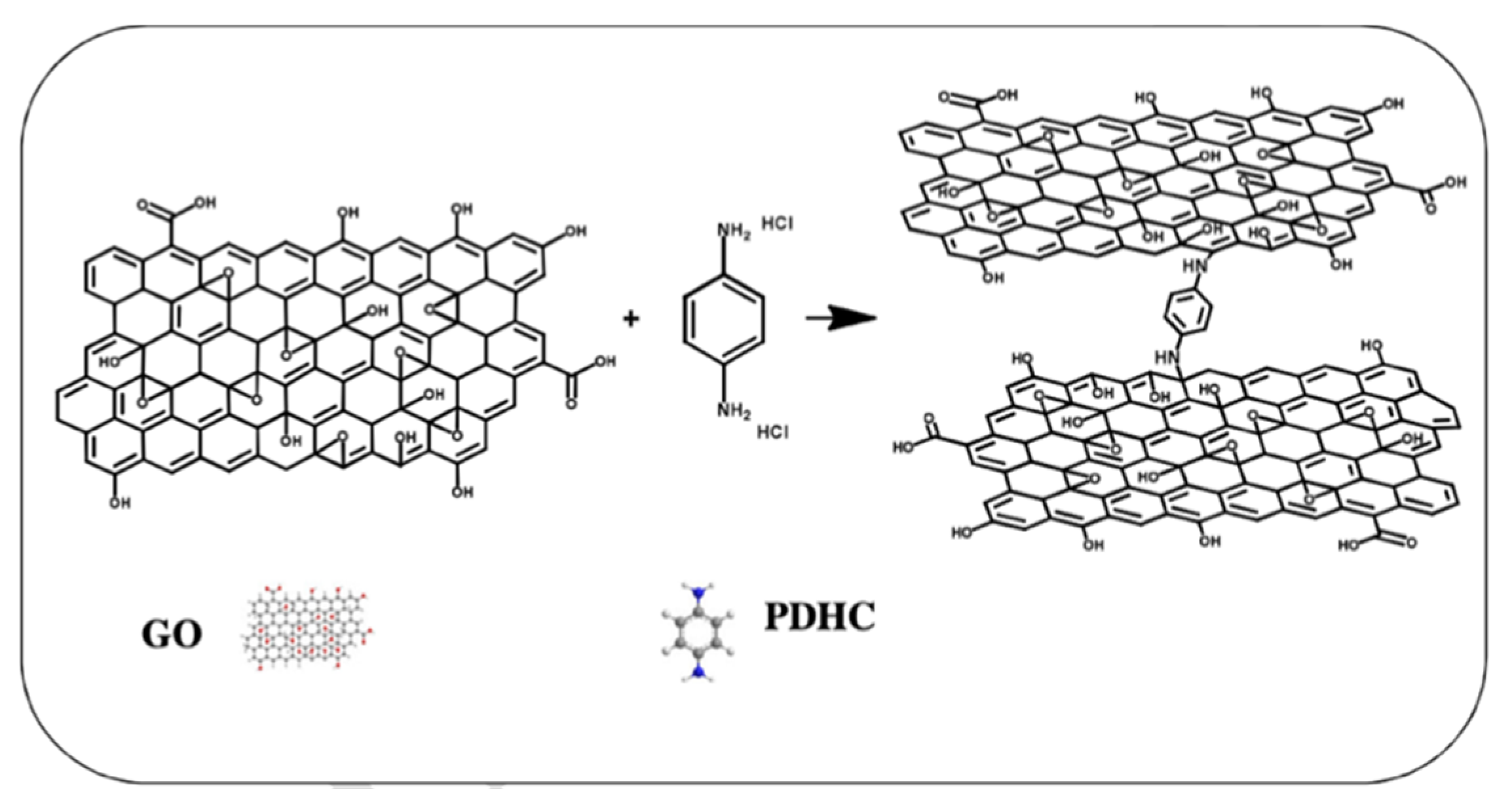
- He, G.; He, X.; Wang, X.; Chang, C.; Zhao, J.; Li, Z.; Wu, H.; Jiang, Z. A highly proton-conducting, methanol-blocking Nafion composite membrane enabled by surface-coating crosslinked sulfonated graphene oxide. Chem. Commun. 2016, 52, 2173–2176. [Google Scholar] [CrossRef] [Green Version]
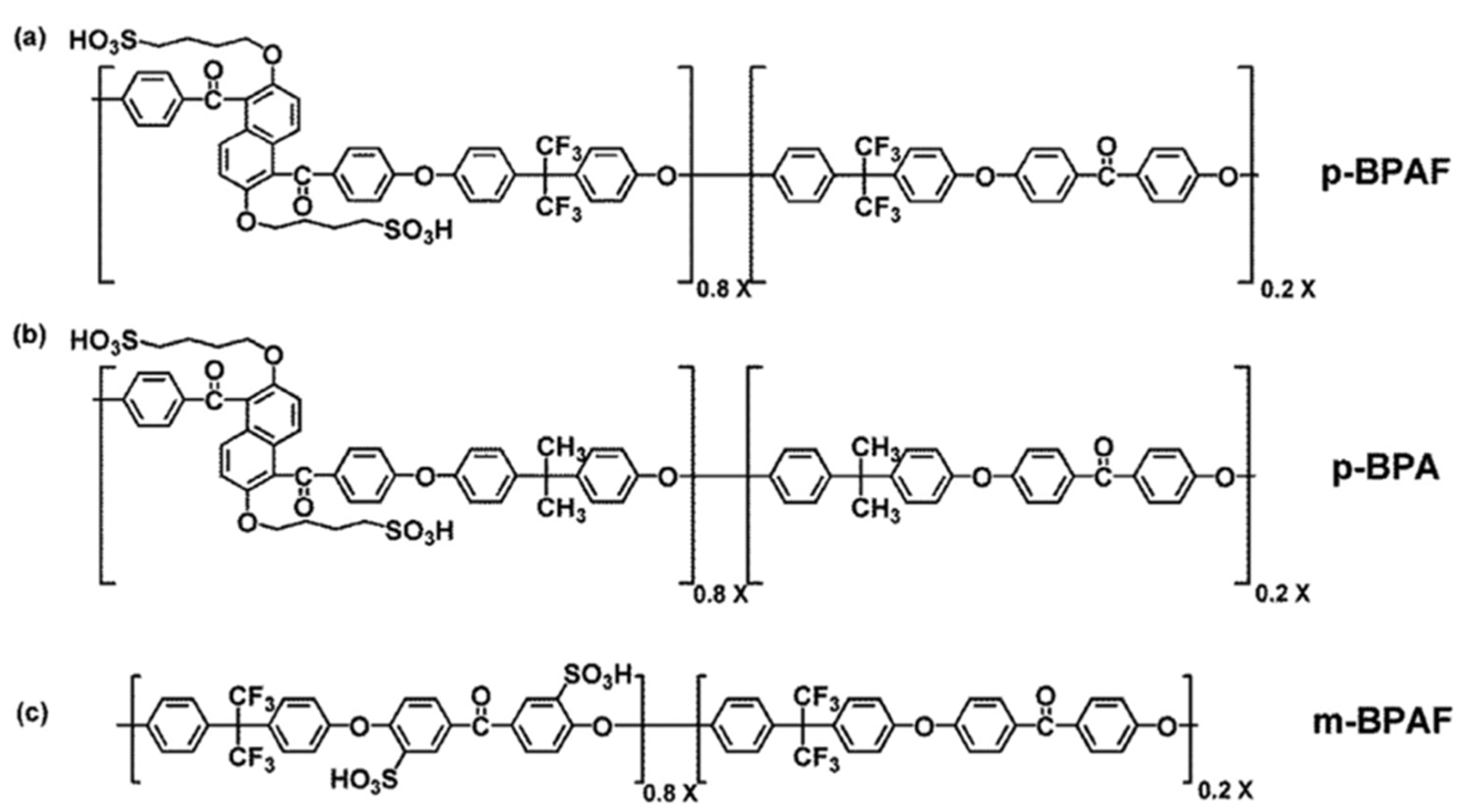
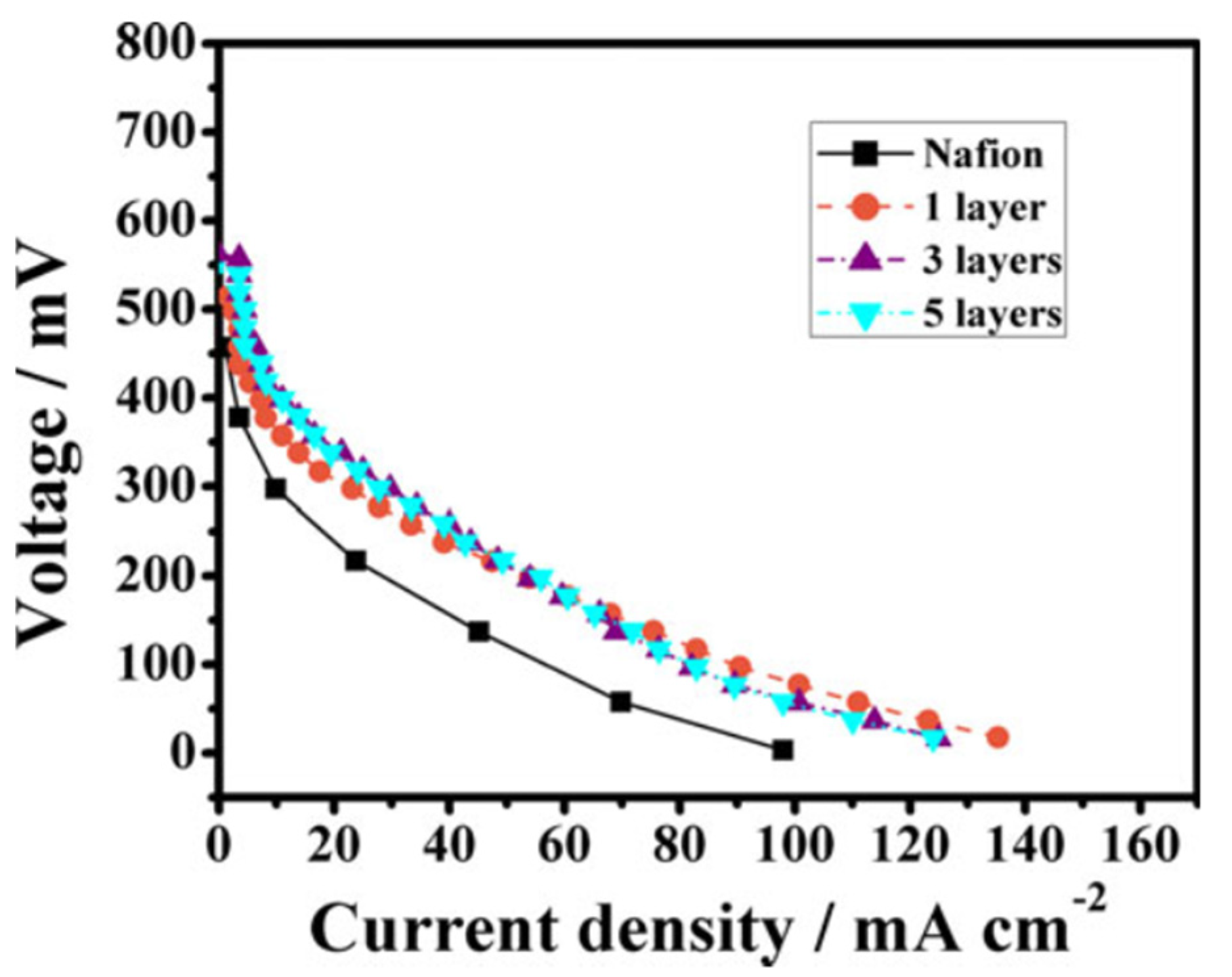
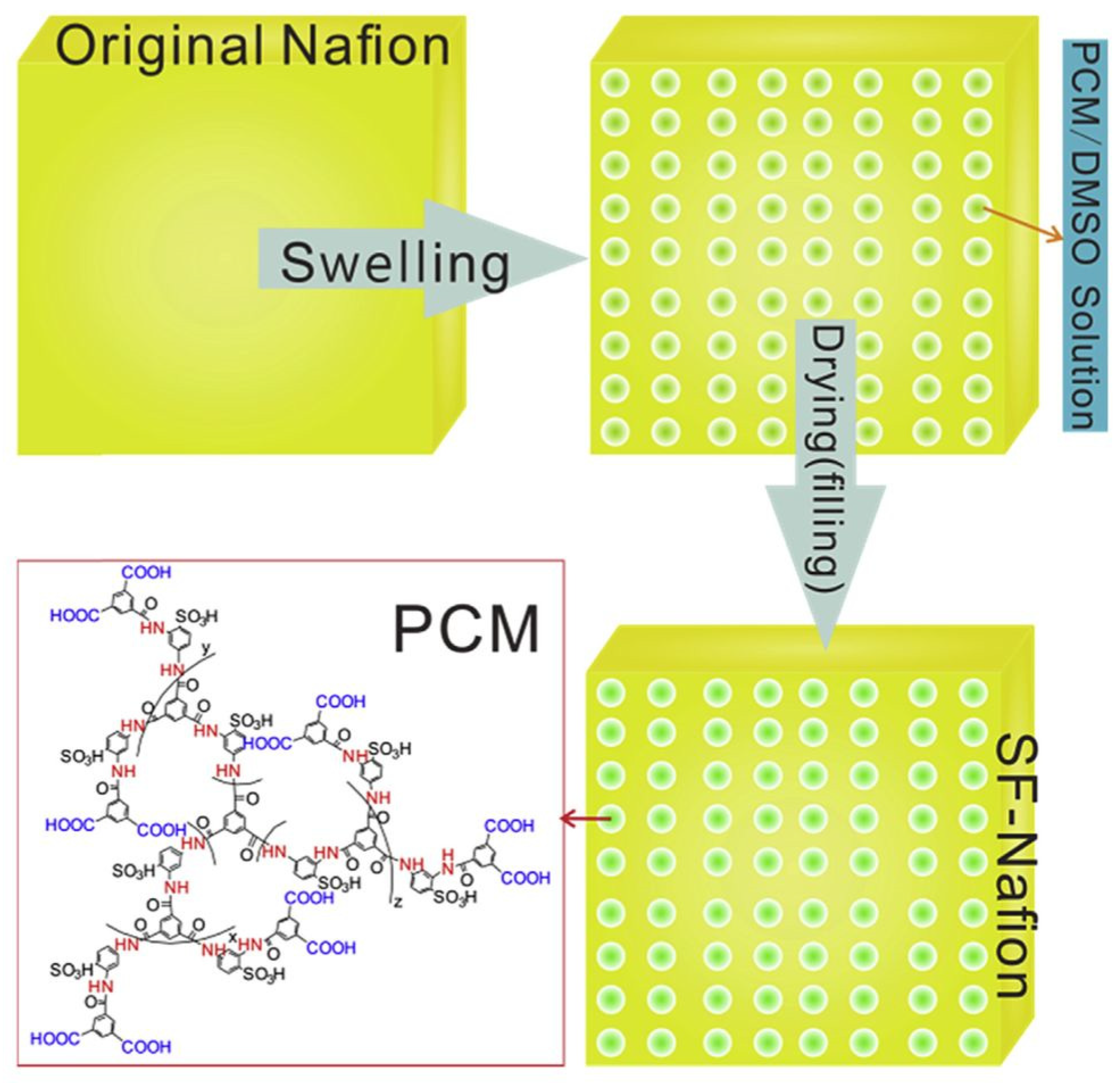
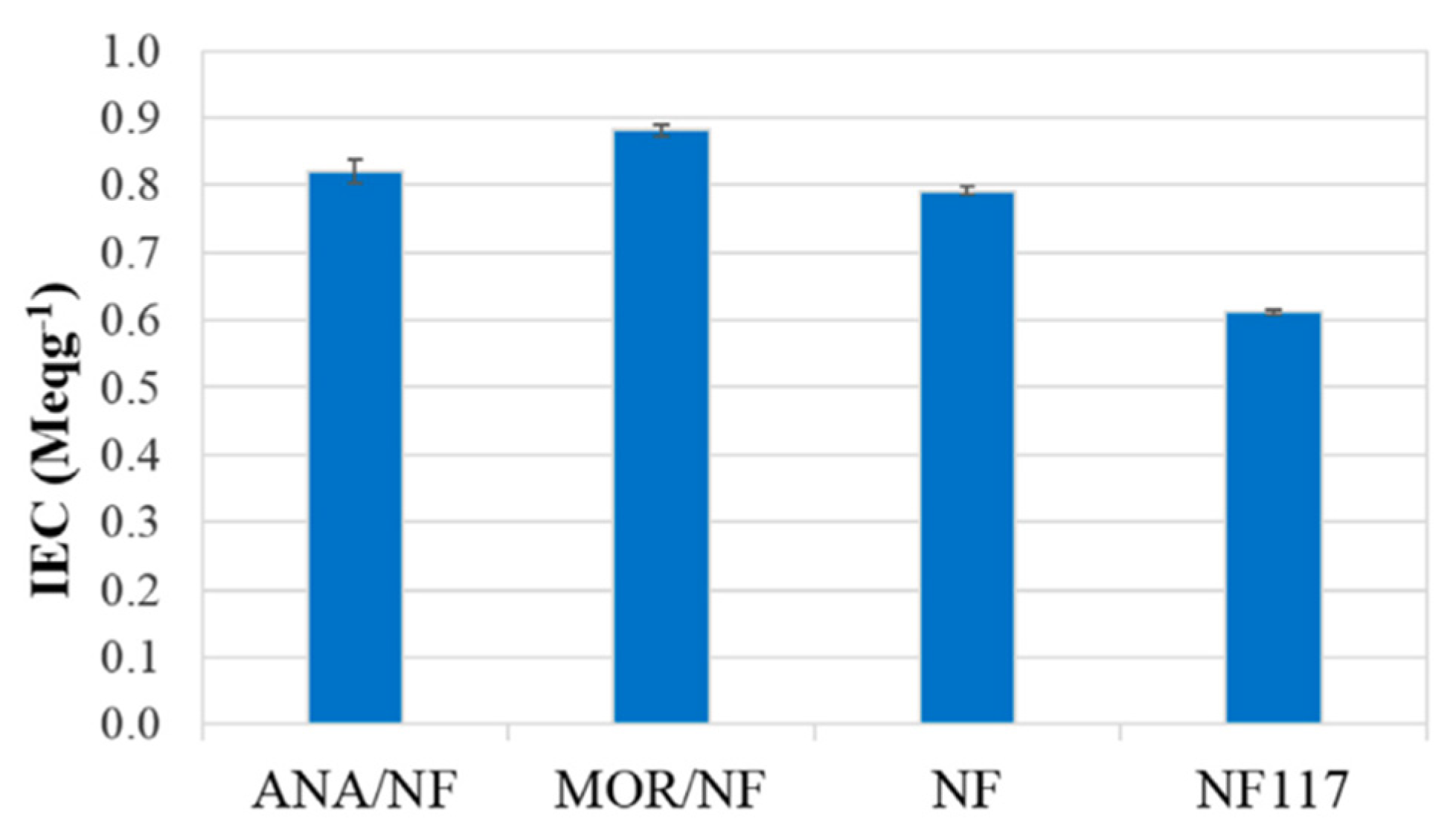
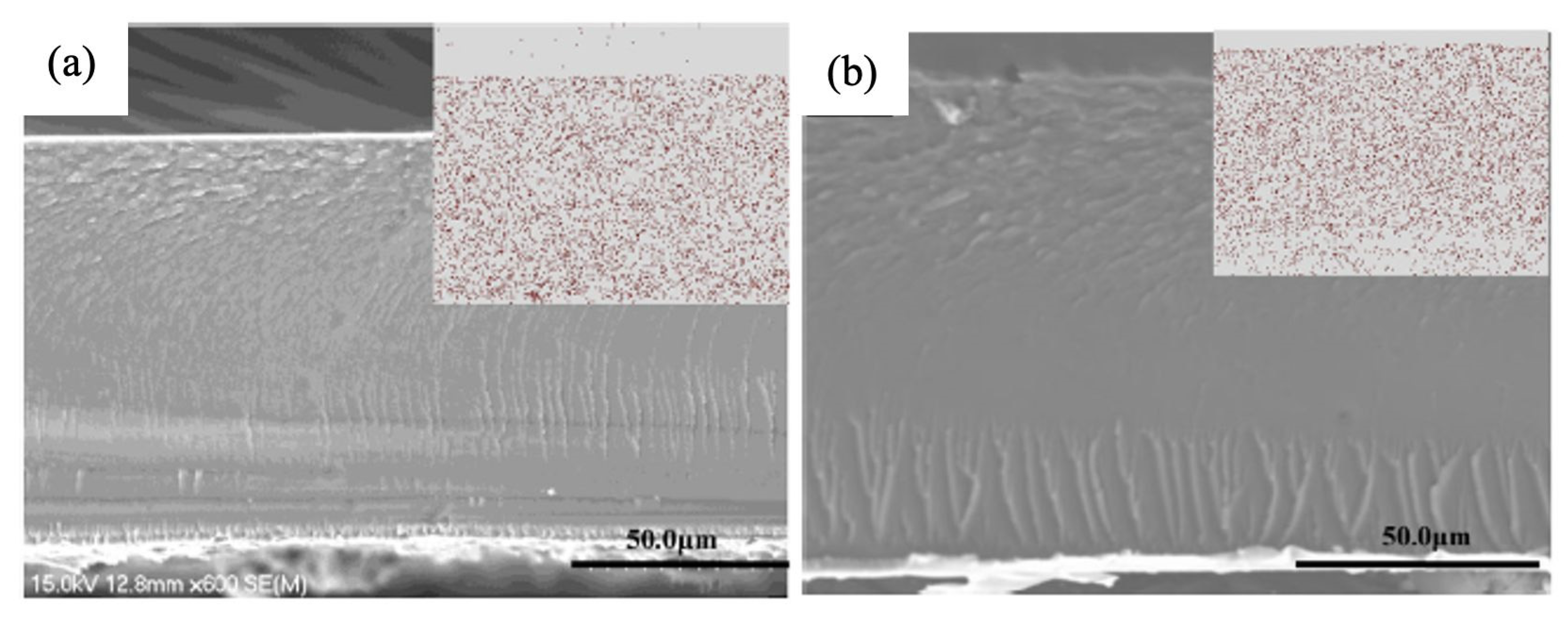

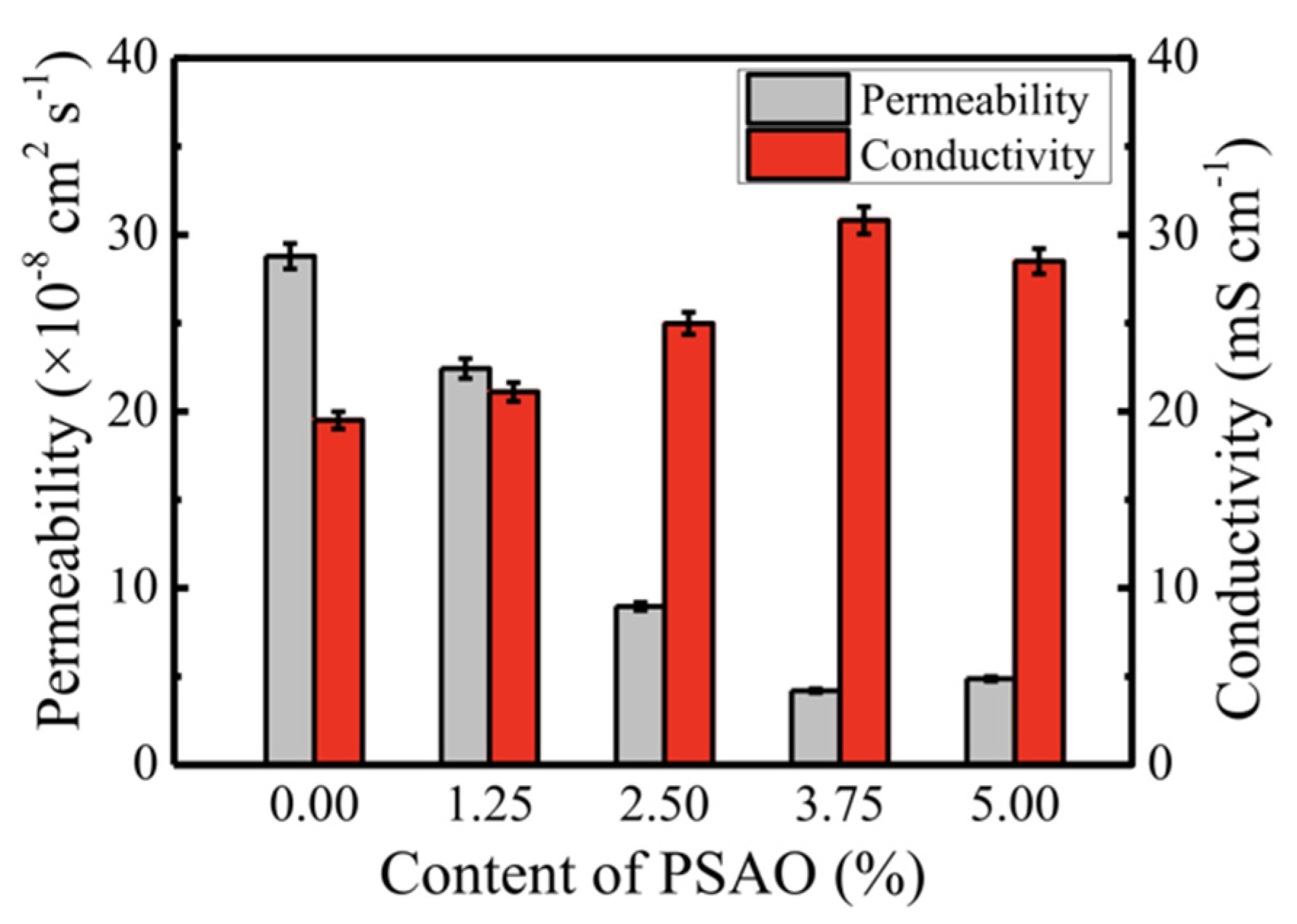
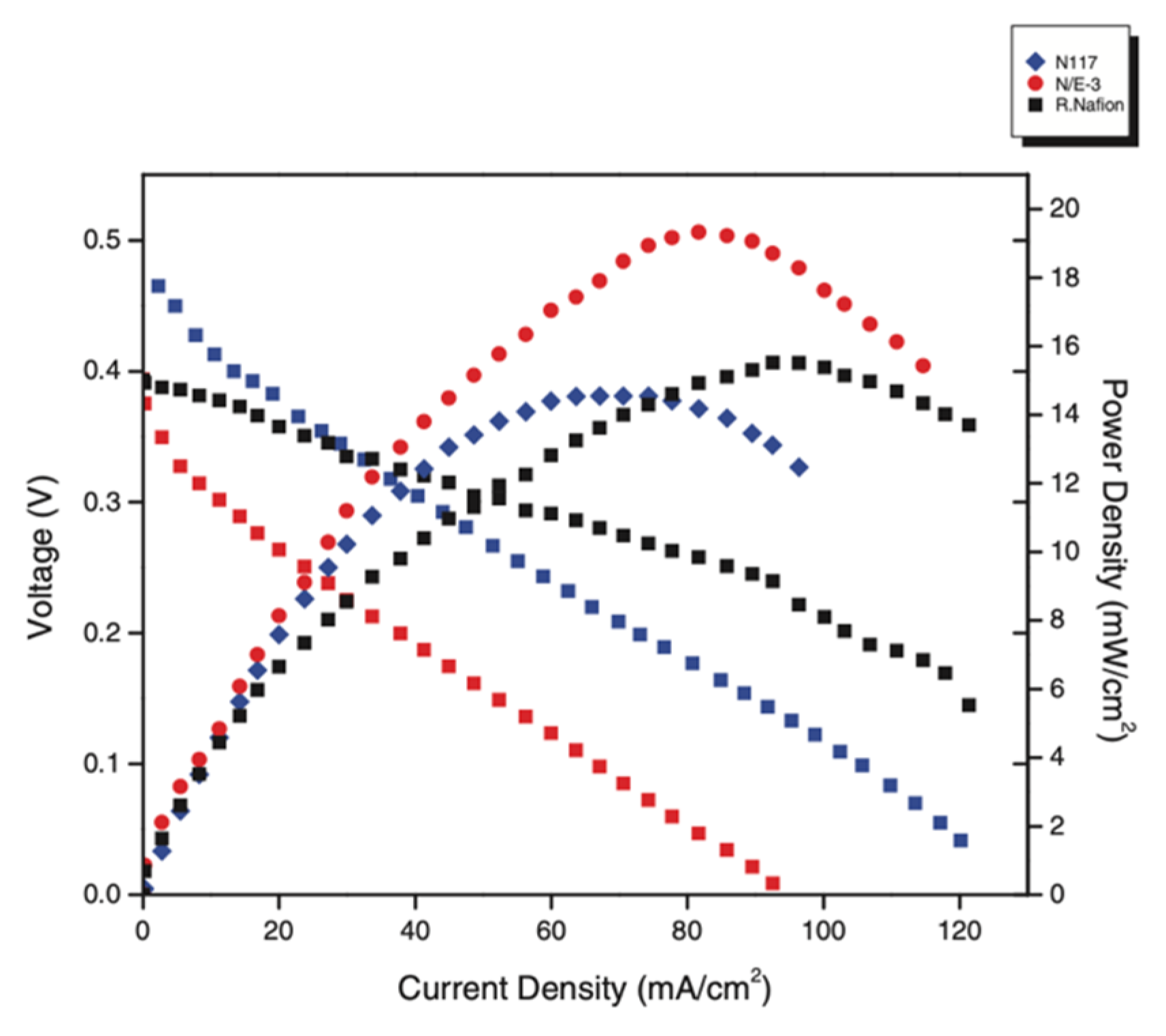
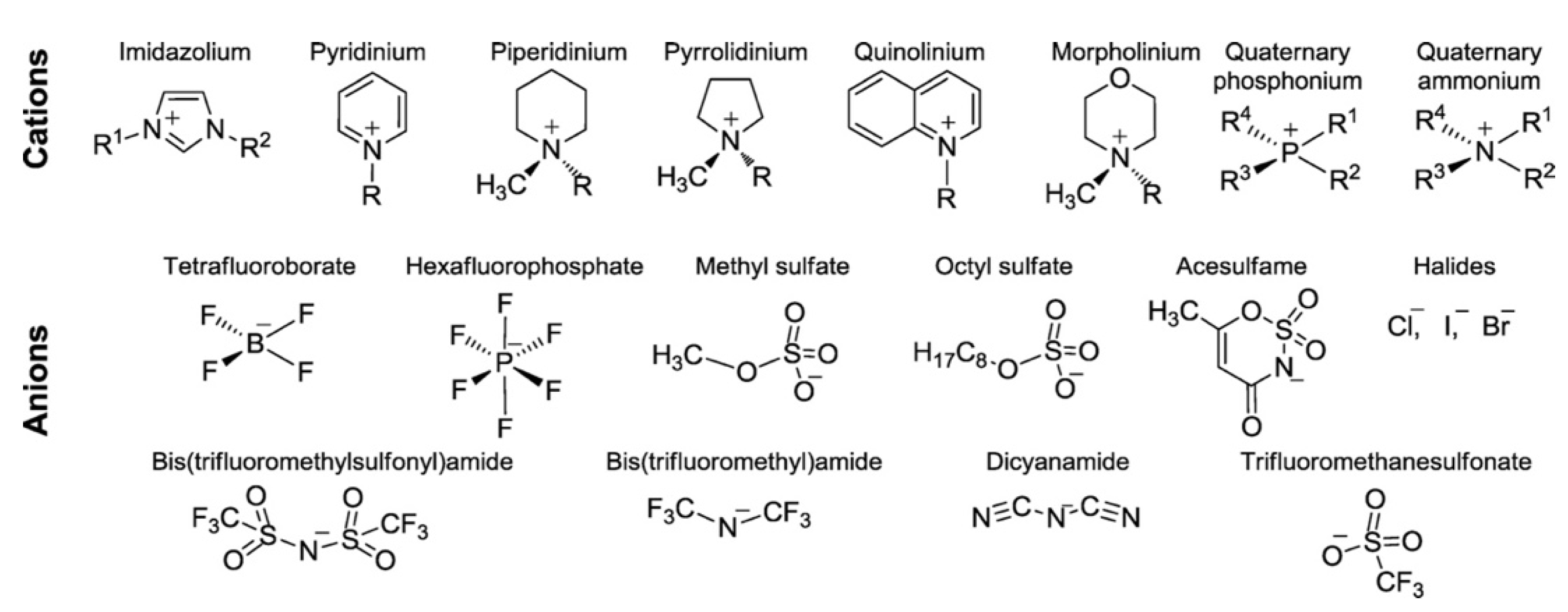

| Modified Nafion Membrane | Filler Content (wt%) | Test Condition Temperature (°C) | Proton Conductivity (mS/cm) | Methanol Permeability (cm2/s) | Reference |
|---|---|---|---|---|---|
| SDF-PAEK@Nafion | 15 | 80 | ↑ (197) | ↓ (2.03 × 10−6) | [48] |
| Nafion/PAni | - | 90 | ↓ (10.66) | ↓ (7.71 × 10−7) | [52] |
| Nafion/SPAni | 30 | 20 | ↓ (7.21) | ↓ (9.12 × 10−8) | [41] |
| Nafion/PVdF | - | 70 | ↓ (0.59) | ↓ (11.7 × 10−7) | [54] |
| PVdF-co-HFP/Nafion | 20 | 20 | ↑ (31.6) | ↑ (1.76 × 10−6) | [55] |
| SPVdF-co-HFP/PBI-coated Nafion | - | - | ↓ (15.1) | ↓ (4.92 × 10−7) | [56] |
| Nafion/PVA-fiber | 5 | 70 | ↓ (14) | ↓ (3.47 × 10−6) | [60] |
| 10 | ↓ (11) | ↓ (2.83 × 10−6) | |||
| Nafion/poly (vinyl alcohol) blend | 5 | 70 | ↓ (9) | ↓ (4.11 × 10−6) | [60] |
| 10 | ↓ (4.8) | ↓ (3.22 × 10−6) | |||
| CBA/Nafion-PVA | - | 80 | ↓ (90) | ↓ (6.79 × 10−7) | [64] |
| SF-Nafion | - | Room temperature | ↑ (130) | ↓ | [65] |
| NH-Nafion | - | 80 | ↑ (247) | ↓ (4.75 × 10−7) | [66] |
| BFPS-Nafion | - | 80 | ↓ (310) | ↓ | [67] |
| Nafion-PPy | - | - | ↓ (49.4) | ↓ (2.38 × 10−8) | [69] |
| Nafion/CNC | 1.5 | 50, 60, 70 | ↓ | ↓ | [70] |
| Modified Nafion Membrane | Filler Content (wt%) | Test Condition Temperature (°C) | Proton Conductivity (mS/cm) | Methanol Permeability (cm2/s) | Reference |
|---|---|---|---|---|---|
| Nafion-CaO-ZrOH | - | - | ↑ (510) | ↓ (0.08 × 10−6) | [74] |
| Nafion/ZrP | 2.5 | 60 | ↑ (41) | ↓ (0) | [76] |
| Nafion/ZrP | 2.5 | 25, 50, 60, 70, 80 | ↑ | - | [75] |
| Nafion/S-ZrO2(NH3SO4) | 30 | 20 | ↓ (7.21) | ↓ (1.5 × 10−7) | [79] |
| Nafion/S-ZrO2 | 5 | 25 | ↓ (78.9) | ↓ (0) | [80] |
| Nafion/S-GO-MOR | 5 | 80 | ↑ (86.45) | ↓ | [84] |
| NH4-X/Nafion | 5 | 20, 40, 60, 80 | ↑ | ↓ | [85] |
| Nafion/SiO2 | 2.5 | 30 | ↓ (115) | - | [86] |
| Nafion/TiO2 | 2.5 | 30 | ↓ (130) | - | [86] |
| Nafion/Pd-SiO2 | 3 | 25 | ↑ (129.2) | ↓ (8.36 × 10−7) | [89] |
| SiO2@sPS + Nafion | 1 | 25 | ↑ | ↓ (2.31 × 10−8) | [91] |
| PVdF/Nafion/SiO2–NH2 | - | 80 | ↑ (210) | ↓ (5.2 × 10−7) | [93] |
| Nafion_TiO2-RSO3H | 10 | 140 | ↑ (80) | ↓ (0.75 × 10−7) | [94] |
| Nafion/CsPW/MMT | - | ↑ (3.71) | ↓ (1.651 × 10−6) | [104] |
| Ionic Liquid in Nafion-Based Membrane | Methanol Crossover (cm2/s) |
|---|---|
| Tetramethylammonium chloride, TMA+ Cl− | 4.21 × 10−8 |
| Phenyltrimethylammonium chloride, TMPA+ Cl− | 5.16 × 10−8 |
| n-Dodecyltrimethylammonium chloride, DTA+ Cl− | 3.89 × 10−8 |
| Hexadecyltrimethylammonium bromide, CTA+ Br− | 2.59 × 10−8 |
| 1-Butyl-3-methylimidazolium bis(trifluoromethanesulfonimide), BMIM+ Tf2N− | 1.56 × 10−8 |
| 1-Octyl-3-methylimidazolium bis(trifluoromethanesulfonimide), OMIM+ Tf2N− | 1.21 × 10−8 |
| Methyl-tricaprylylammonium dicyanamide, ALIQUAT+ DCA− | 4.05 × 10−9 |
| Filler Type | Membrane | Filler Content | Performance | Reference |
|---|---|---|---|---|
| Inorganic material | Nafion/sulfonated γ-Fe2O3 | ≤1.0 wt% |
| [137] |
| MoS2/Nafion composite membrane MoS2+Nafion blending membrane | ≤0.5 wt% |
| [138] | |
| Nafion–h-BN | 0.75 wt% |
| [139] | |
| Organic material | Sodium dodecyl sulfate/Palladium (SDS/Pd)-modified Nafion | - |
| [140] |
| Metal | Palladium/Nafion | ≤3 wt% |
| [141] |
| Solid acid | CsPW-Nafion | ≤10 wt% |
| [142] |
| PWA and recast Nafion | - |
| [143] | |
| Polymer | Nafion/PVFP-BI | - |
| [144] |
| CHI/PVS-Nafion | - |
| [46] | |
| BC/Nafion | B: N = 1: 7 |
| [145] | |
| Metal organic framework | Sulfonated pillar [5] arene/Nafion | ≤10 wt% |
| [146] |
| Nafion-SCONs | ≤0.6 wt% |
| [147] | |
| Amino-MIL-53(Al)-Nanosheets@Nafion (AMA@Nafion) | ≤2.0 wt% |
| [148] | |
| Carbon nanomaterials | Nafion/MWCNT-MNP-Nafion | ≤0.1 wt% |
| [14] |
| Nafion/GO@PDASA | 20 layers |
| [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, W.W.; Thiam, H.S.; Pang, Y.L.; Chong, K.C.; Lai, S.O. A State-of-Art on the Development of Nafion-Based Membrane for Performance Improvement in Direct Methanol Fuel Cells. Membranes 2022, 12, 506. https://doi.org/10.3390/membranes12050506
Ng WW, Thiam HS, Pang YL, Chong KC, Lai SO. A State-of-Art on the Development of Nafion-Based Membrane for Performance Improvement in Direct Methanol Fuel Cells. Membranes. 2022; 12(5):506. https://doi.org/10.3390/membranes12050506
Chicago/Turabian StyleNg, Wei Wuen, Hui San Thiam, Yean Ling Pang, Kok Chung Chong, and Soon Onn Lai. 2022. "A State-of-Art on the Development of Nafion-Based Membrane for Performance Improvement in Direct Methanol Fuel Cells" Membranes 12, no. 5: 506. https://doi.org/10.3390/membranes12050506
APA StyleNg, W. W., Thiam, H. S., Pang, Y. L., Chong, K. C., & Lai, S. O. (2022). A State-of-Art on the Development of Nafion-Based Membrane for Performance Improvement in Direct Methanol Fuel Cells. Membranes, 12(5), 506. https://doi.org/10.3390/membranes12050506







