Recent Progress of SAPO-34 Zeolite Membranes for CO2 Separation: A Review
Abstract
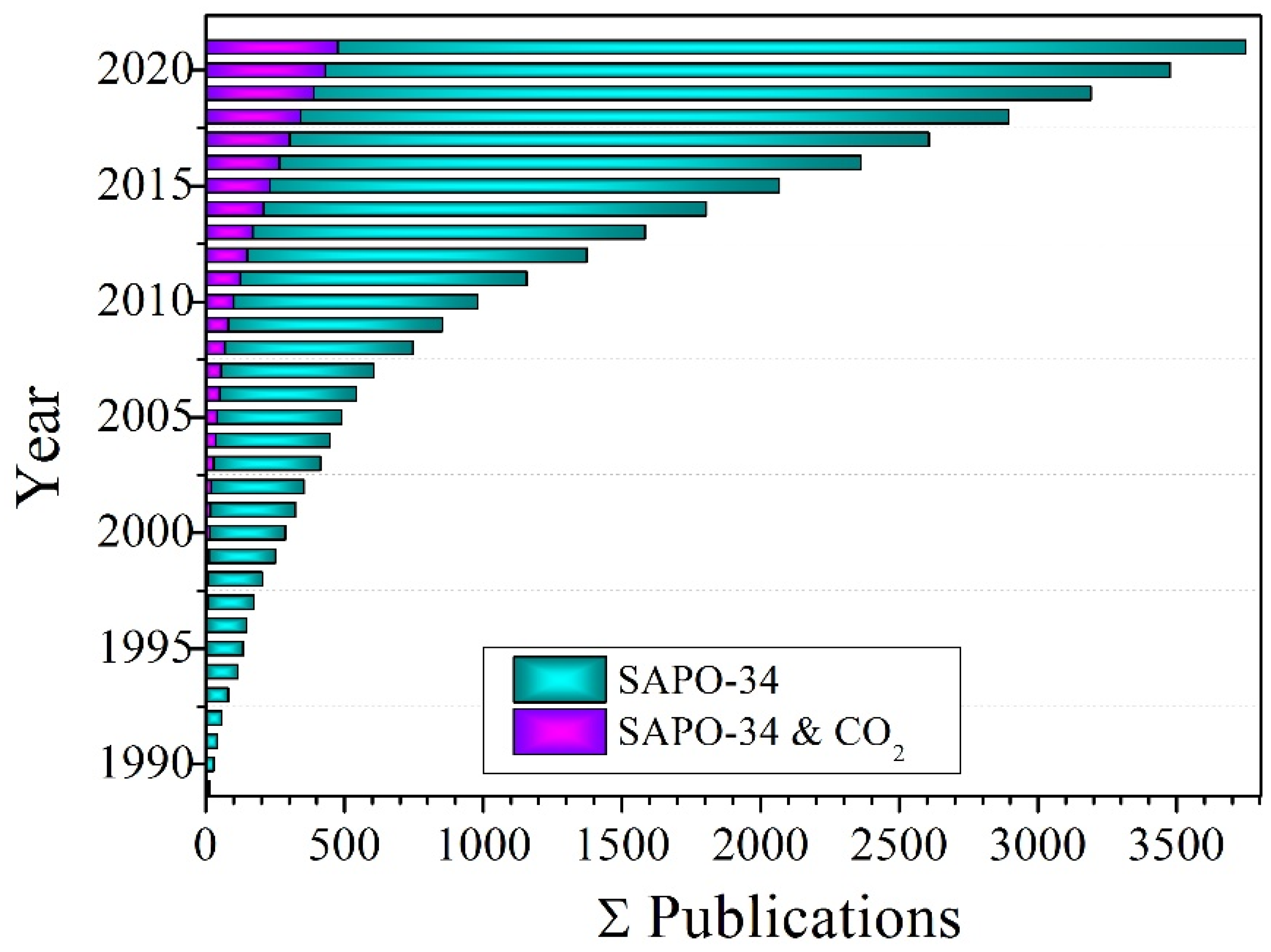
:1. Introduction
2. SAPO-34 Membranes for CO2 Separation
2.1. SAPO-34-Based Mixed Matrix Membranes
2.1.1. SAPO-34 MMMs
2.1.2. SAPO-34 Functionalized MMMs
2.1.3. SAPO-34 MMMs and Operating Conditions
2.2. Pure SAPO-34 Membranes
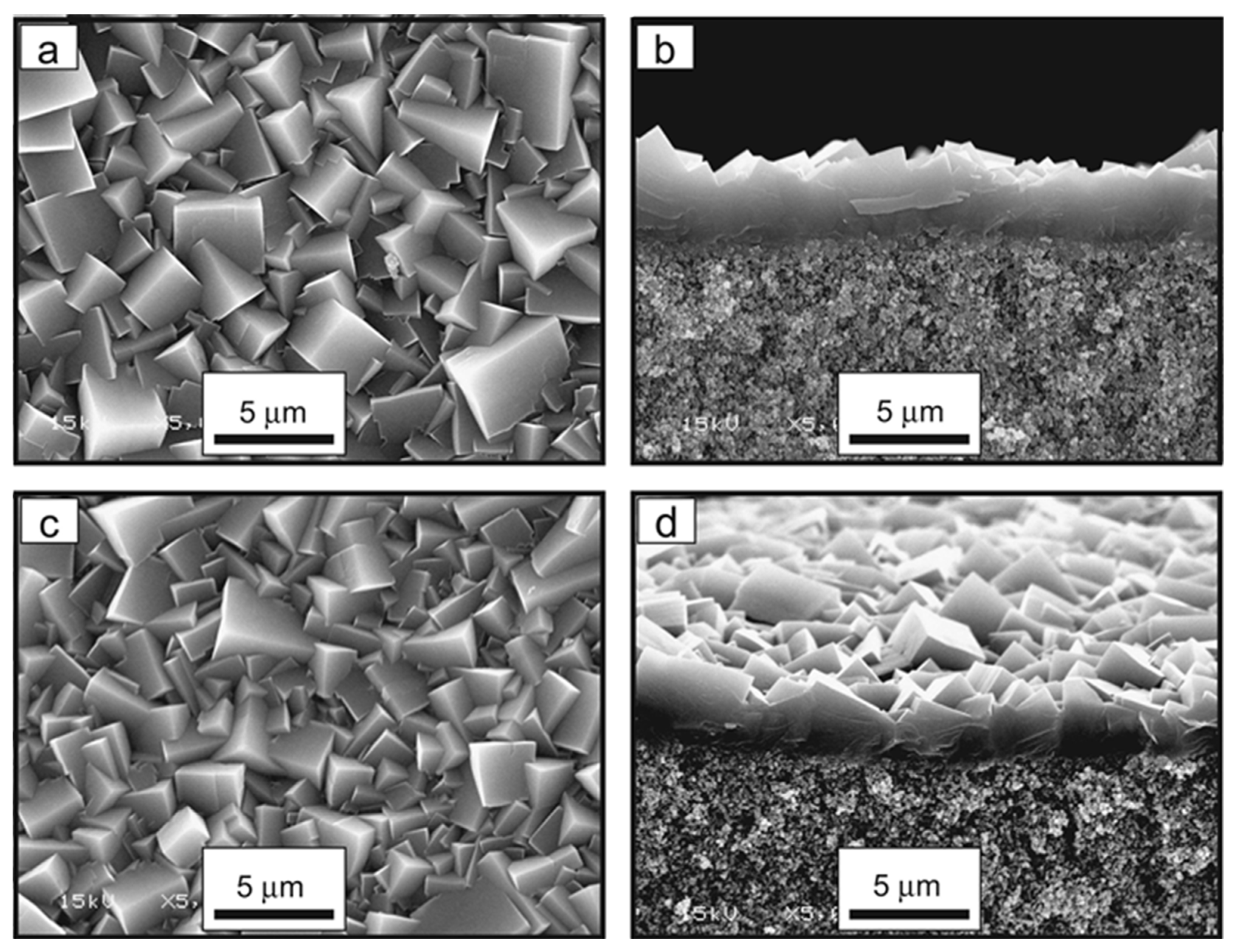
2.2.1. SAPO-34/Alumina Membranes
2.2.2. SAPO-34/Stainless Steel Membranes
2.2.3. SAPO-34/Silica Membranes
2.2.4. Scale-Up and Industrial Approach of Pure SAPO-34 Membranes
3. Summary and Outcome
- Rigid pore membranes and ion-exchange membranes must be developed to increase the solubility and rejection of particular gases.
- More studies should be focus on the transport mechanism of MMMs and pure SAPO-34 membranes.
- Regarding techno-economic analysis, the economics of each separation method must be evaluated in terms of factors such as cost per kilogram of product and energy consumption per kilogram, with a view to encourage and explore the research in this direction.
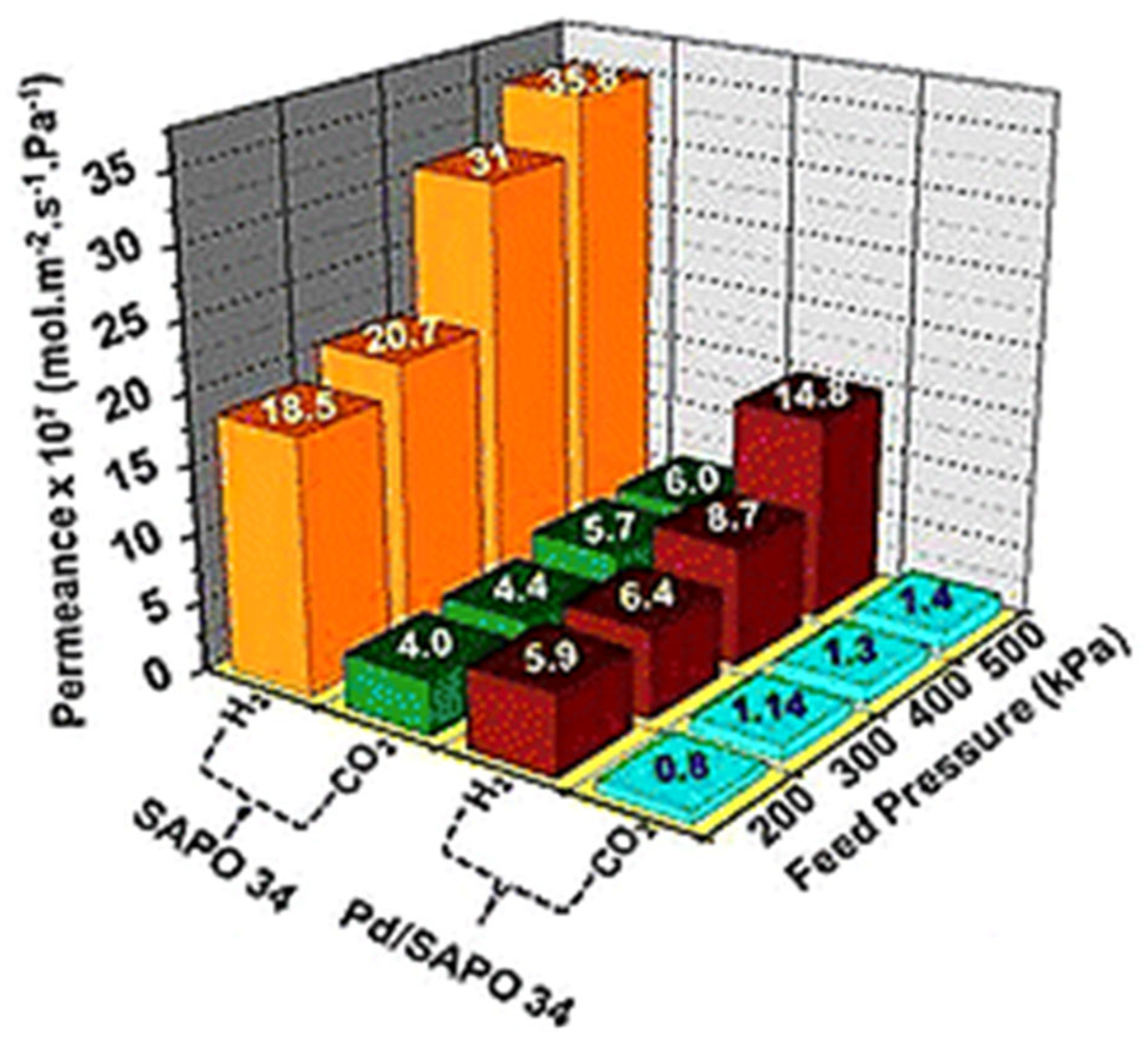
- In hydrogen separation using Pd-based membrane, SAPO-34 interlayer can play an important role as a diffusion barrier and substrate modifier over the support. SAPO-34 membranes were prepared on α-Al2O3 four-channel hollow fiber (4CHF) supported by secondary growth method. Moreover, the 4CHF supported SAPO-34 membranes could also provide high membrane packing density for membrane modules and cut fabrication costs, which is a promising candidate for practical applications. Therefore, more work is required to explore its application in Pd-based membranes by using vacuum-assisted seeding and secondary growth methods on the different substrates.
- Regarding He separation applications, SAPO-34 membranes are less explored in helium separation from He/CH4 since this is one the most attractive separations; due to size sieving and diffusivity difference, SAPO-34 membrane surpassed the Robeson limit upper bound, making these membranes appealing for the recovery of helium from natural gas.
- In natural gas purification, SAPO-34 is one of the best candidates due to its unique pore size and adsorption capabilities. However, it decomposes over the time (years) due to the presence of moisture in the raw natural gas. Therefore, it is recommended to improve its moisture resistance property by modifying its surface using hydrophobic barrier. The chemical modification strategy of SAPO-34 would strengthen the properties of the membranes. More studies of this type are required in SAPO-34 membrane research for boosting membrane steadiness and performances under high humidity conditions.
- Regarding defect-free membranes, thin SAPO-34 membranes over the supports have been produced through various synthetic strategies to reduce defects; however, it is extremely challenging to obtain defect-free membranes. Many studies have been attempted to heal these defects using post-treatments, but some methods are costly and some result in producing thicker membranes which ultimately lower the permeance. Therefore, more work is required to heal the defects of SAPO-34 membranes by carefully designing the post-synthetic modifications that should balance the resultant membranes’ cost and permeance.
- The demand of the scaling up of mixed matrix membranes is a hot topic. However, the commercialization of the SAPO-34-based MMMs is still in the early stage and requires more work to develop facile synthetic methods with cost effective techniques. Scaling up the Pure SAPO-34 membranes is a pivotal requirement for industrial commercialization. However, systematic optimization of the fabrication parameter is required to acquire the scaling-up procedure. Pure SAPO-34 thin membranes are produced on solid supports. However, they face many challenges when it comes to scaling-up their fabrication on longer tubular supports (more than 20 cm). In large-scale production, these membranes might require better approaches such as modified synthetic schemes and big autoclaves, etc. These approaches are reviewed in the above sections. The scalability of SAPO-34 membranes and mixed matrix membranes are still in the early stage and need more investigation and development in the scalability aspect before reaching the commercialization stage. Thus, more efforts are needed to produce longer SAPO-34 membranes before being implemented at an industrial scale. Hollow-fiber-based module systems could provide an alternative pathway owing to their large area to volume ratios.
- The high cost of the SAPO-34 zeolite membrane modules as compared with the polymeric membranes is one barrier to implementing these membranes in the industry for real practical applications. The cost in synthesizing SAPO-34 or zeolite membranes comes mainly from templates, Al/Si sources, and supports. A limited number of research studies have reported an alternative synthesis approach using low-priced alumina and/or template-free synthesis. The entire membrane cost consists of the zeolite filler and membrane support. As a result, research activities should consider choosing and fabricating less expensive membrane supports. Therefore, more studies are required to seek a cost-effective synthesis approach for SAPO-34 membranes to achieve cost-effective, scalability, and stability SAPO-34 membranes. In addition, improvement of SAPO-34 membrane toward industrial implementation at high operating conditions should be considered.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burns, T.D.; Pai, K.N.; Subraveti, S.G.; Collins, S.P.; Krykunov, M.; Rajendran, A.; Woo, T.K. Prediction of MOF Performance in Vacuum Swing Adsorption Systems for Postcombustion CO2 Capture Based on Integrated Molecular Simulations, Process Optimizations, and Machine Learning Models. Environ. Sci. Technol. 2020, 54, 4536–4544. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Iqbal, N.; Noor, T.; Zaman, N.; Asghar, A.; Abdelnaby, M.M.; Galadima, A.; Helal, A. Advanced strategies in Metal-Organic Frameworks for CO2 Capture and Separation. Chem. Rec. 2021. [Google Scholar] [CrossRef]
- Garba, M.D.; Usman, M.; Khan, S.; Shehzad, F.; Galadima, A.; Ehsan, M.F.; Ghanem, A.S.; Humayun, M. CO2 towards fuels: A review of catalytic conversion of carbon dioxide to hydrocarbons. J. Environ. Chem. Eng. 2021, 9, 104756. [Google Scholar] [CrossRef]
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R.M. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar] [CrossRef]
- Khan, S.; Khulief, Y.A.; Al-Shuhail, A.A. Effects of reservoir size and boundary conditions on pore-pressure buildup and fault reactivation during CO2 injection in deep geological reservoirs. Environ. Earth Sci. 2020, 79, 294. [Google Scholar] [CrossRef]
- Khan, S.; Khulief, Y.A.; Al-Shuhail, A.A. Numerical Modeling of the Geomechanical Behavior of Biyadh Reservoir Undergoing CO2 Injection. Int. J. Geomech. 2017, 17, 04017039. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sust. Energ. Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. New approach for biogas purification using cryogenic separation and distillation process for CO2 capture. Energy 2018, 156, 328–351. [Google Scholar] [CrossRef]
- Tengku Hassan, T.N.A.; Shariff, A.M.; Mohd Pauzi, M.M.i.; Khidzir, M.S.; Surmi, A. Insights on Cryogenic Distillation Technology for Simultaneous CO2 and H2S Removal for Sour Gas Fields. Molecules 2022, 27, 1424. [Google Scholar] [CrossRef]
- Breck, D.W.; Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; John Wiley & Sons: New York, NY, USA, 1973. [Google Scholar]
- Yaseen, M.; Humayun, M.; Khan, A.; Usman, M.; Ullah, H.; Tahir, A.A.; Ullah, H. Preparation, Functionalization, Modification, and Applications of Nanostructured Gold: A Critical Review. Energies 2021, 14, 1278. [Google Scholar] [CrossRef]
- Usman, M.; Humayun, M.; Shah, S.S.; Ullah, H.; Tahir, A.A.; Khan, A.; Ullah, H. Bismuth-Graphene Nanohybrids: Synthesis, Reaction Mechanisms, and Photocatalytic Applications—A Review. Energies 2021, 14, 2281. [Google Scholar] [CrossRef]
- Usman, M.; Humayun, M.; Garba, M.D.; Ullah, L.; Zeb, Z.; Helal, A.; Suliman, M.H.; Alfaifi, B.Y.; Iqbal, N.; Abdinejad, M.; et al. Electrochemical Reduction of CO2: A Review of Cobalt Based Catalysts for Carbon Dioxide Conversion to Fuels. Nanomaterials 2021, 11, 2029. [Google Scholar] [CrossRef] [PubMed]
- Israf Ud, D.; Qazi, N.; Mustapha, D.G.; Abdulrahman, I.A.; Mshari, A.A.; Muhammad, U. A Review of Preparation Methods for Heterogeneous Catalysts. Mini-Rev. Org. Chem. 2022, 19, 92–110. [Google Scholar] [CrossRef]
- Humayun, M.; Zada, A.; Li, Z.; Xie, M.; Zhang, X.; Qu, Y.; Raziq, F.; Jing, L. Enhanced visible-light activities of porous BiFeO3 by coupling with nanocrystalline TiO2 and mechanism. Appl. Catal. B 2016, 180, 219–226. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Tahir, A.A.; bin Mohd Yusoff, A.R.; Mat Teridi, M.A.; Nazeeruddin, M.K.; Luo, W. An Overview of the Recent Progress in Polymeric Carbon Nitride Based Photocatalysis. Chem. Rec. 2021. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Usman, M.; Habibi-Yangjeh, A.; Tahir, A.A.; Wang, C.; Luo, W. Perovskite-type lanthanum ferrite based photocatalysts: Preparation, properties, and applications. J. Energy Chem. 2022, 66, 314–338. [Google Scholar] [CrossRef]
- Helal, A.; Usman, M.; Arafat, M.E.; Abdelnaby, M.M. Allyl functionalized UiO-66 metal-organic framework as a catalyst for the synthesis of cyclic carbonates by CO2 cycloaddition. J. Ind. Eng. Chem. 2020, 89, 104–110. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N.; Nasir, H.; Sohail, M.; Zaman, N.; Usman, M. Nanocomposites of cobalt benzene tricarboxylic acid MOF with rGO: An efficient and robust electrocatalyst for oxygen evolution reaction (OER). Renew. Energy 2020, 156, 1040–1054. [Google Scholar] [CrossRef]
- Ullah, L.; Zhao, G.; Xu, Z.; He, H.; Usman, M.; Zhang, S. 12-Tungstophosphoric acid niched in Zr-based metal-organic framework: A stable and efficient catalyst for Friedel-Crafts acylation. Sci. China Chem. 2018, 61, 402–411. [Google Scholar] [CrossRef]
- Ullah, L.; Zhao, G.; Ma, J.-X.; Usman, M.; Khan, R.; Hedin, N. Pd-promoted heteropolyacid on mesoporous zirconia as a stable and bifunctional catalyst for oxidation of thiophenes. Fuel 2022, 310, 122462. [Google Scholar] [CrossRef]
- Usman, M.; Zhu, J.; Chuiyang, K.; Arslan, M.T.; Khan, A.; Galadima, A.; Muraza, O.; Khan, I.; Helal, A.; Al-Maythalony, B.A.; et al. Propene Adsorption-Chemisorption Behaviors on H-SAPO-34 Zeolite Catalysts at Different Temperatures. Catalysts 2019, 9, 919. [Google Scholar] [CrossRef] [Green Version]
- Arslan, M.T.; Qureshi, B.A.; Gilani, S.Z.A.; Cai, D.; Ma, Y.; Usman, M.; Chen, X.; Wang, Y.; Wei, F. Single-Step Conversion of H2-Deficient Syngas into High Yield of Tetramethylbenzene. ACS Catal. 2019, 9, 2203–2212. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Muhammad, U.; Wang, D.; Wang, Y. Effect of alkene co-feed on the MTO reactions over SAPO-34. Chem. Eng. J. 2017, 316, 187–195. [Google Scholar] [CrossRef]
- Ma, Y.; Cai, D.; Li, Y.; Wang, N.; Muhammad, U.; Carlsson, A.; Tang, D.; Qian, W.; Wang, Y.; Su, D.; et al. The influence of straight pore blockage on the selectivity of methanol to aromatics in nanosized Zn/ZSM-5: An atomic Cs-corrected STEM analysis study. RSC Adv. 2016, 6, 74797–74801. [Google Scholar] [CrossRef]
- Usman, M.; Li, D.; Razzaq, R.; Yaseen, M.; Li, C.; Zhang, S. Novel MoP/HY catalyst for the selective conversion of naphthalene to tetralin. J. Ind. Eng. Chem. 2015, 23, 21–26. [Google Scholar] [CrossRef]
- Wang, H.; Cao, Y.; Li, D.; Muhammad, U.; Li, C.; Li, Z.; Zhang, S. Catalytic hydrorefining of tar to liquid fuel over multi-metals (W-Mo-Ni) catalysts. J. Renew. Sustain. Energy 2013, 5, 053114. [Google Scholar] [CrossRef]
- Zhang, H.H.; Cao, Y.M.; Usman, M.; Li, L.J.; Li, C.S. Study on the Hydrotreating Catalysts Containing Phosphorus of Coal Tar to Clean Fuels. Adv. Mat. Res. 2012, 531, 263–267. [Google Scholar] [CrossRef]
- Kan, T.; Sun, X.; Wang, H.; Li, C.; Muhammad, U. Production of Gasoline and Diesel from Coal Tar via Its Catalytic Hydrogenation in Serial Fixed Beds. Energy Fuels 2012, 26, 3604–3611. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Usman, M.; Li, Z.; Han, L.; Li, C.; Zhang, S. Study on the hydrotreatment of C9 aromatics over supported multi-metal catalysts on γ-Al2O3. J. Renew. Sustain. Energy 2014, 6, 033132. [Google Scholar] [CrossRef]
- Lok, B.M.; Messina, C.A.; Patton, R.L.; Gajek, R.T.; Cannan, T.R.; Flanigen, E.M. Silicoaluminophosphate molecular sieves: Another new class of microporous crystalline inorganic solids. J. Am. Chem. Soc. 1984, 106, 6092–6093. [Google Scholar] [CrossRef]
- Song, S.; Gao, F.; Zhang, Y.; Li, X.; Zhou, M.; Wang, B.; Zhou, R. Preparation of SSZ-13 membranes with enhanced fluxes using asymmetric alumina supports for N2/CH4 and CO2/CH4 separations. Sep. Purif. Technol. 2019, 209, 946–954. [Google Scholar] [CrossRef]
- Liu, H.; Gao, X.; Wang, S.; Hong, Z.; Wang, X.; Gu, X. SSZ-13 zeolite membranes on four-channel α-Al2O3 hollow fibers for CO2 separation. Sep. Purif. Technol. 2021, 267, 118611. [Google Scholar] [CrossRef]
- Kalipcilar, H.; Bowen, T.C.; Noble, R.D.; Falconer, J.L. Synthesis and Separation Performance of SSZ-13 Zeolite Membranes on Tubular Supports. Chem. Mater. 2002, 14, 3458–3464. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Natsui, M.; Ikeda, A. Gas Permeation Properties of High-Silica CHA-Type Zeolite Membrane. Membranes 2021, 11, 249. [Google Scholar] [CrossRef]
- Liang, L.; Zhu, M.; Chen, L.; Zhong, C.; Yang, Y.; Wu, T.; Wang, H.; Kumakiri, I.; Chen, X.; Kita, H. Single Gas Permeance Performance of High Silica SSZ-13 Zeolite Membranes. Membranes 2018, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Wang, C.; Dyballa, M.; Wu, G.; Guan, N.; Li, L.; Xie, Z.; Hunger, M. Understanding the Early Stages of the Methanol-to-Olefin Conversion on H-SAPO-34. ACS Catal. 2015, 5, 317–326. [Google Scholar] [CrossRef]
- Nishiyama, N.; Kawaguchi, M.; Hirota, Y.; Van Vu, D.; Egashira, Y.; Ueyama, K. Size control of SAPO-34 crystals and their catalyst lifetime in the methanol-to-olefin reaction. Appl. Catal. A-Gen. 2009, 362, 193–199. [Google Scholar] [CrossRef]
- Sun, Q.; Xie, Z.; Yu, J. The state-of-the-art synthetic strategies for SAPO-34 zeolite catalysts in methanol-to-olefin conversion. Natl. Sci. Rev. 2017, 5, 542–558. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zhang, L.; Zhou, Y.; Wang, H.; Wen, L.; Kianfar, E. Investigation of effective parameters on SAPO-34 nanocatalyst in the methanol-to-olefin conversion process: A review. Rev. Inorg. Chem. 2020, 40, 91–105. [Google Scholar] [CrossRef]
- Yang, G.; Han, J.; Huang, Y.; Chen, X.; Valtchev, V. Busting the efficiency of SAPO-34 catalysts for the methanol-to-olefin conversion by post-synthesis methods. Chin. J. Chem. Eng. 2020, 28, 2022–2027. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Cheng, C.K.; Bhuyar, P.; Atabani, A.E.; Pugazhendhi, A.; Chi, N.T.L.; Witoon, T.; Lim, J.W.; Juan, J.C. Effect of reaction conditions on the lifetime of SAPO-34 catalysts in methanol to olefins process—A review. Fuel 2021, 283, 118851. [Google Scholar] [CrossRef]
- Askari, S.; Bashardoust Siahmard, A.; Halladj, R.; Miar Alipour, S. Different techniques and their effective parameters in nano SAPO-34 synthesis: A review. Powder Technol. 2016, 301, 268–287. [Google Scholar] [CrossRef]
- Nasser, G.A.; Muraza, O.; Nishitoba, T.; Malaibari, Z.; Al-Shammari, T.K.; Yokoi, T. OSDA-free chabazite (CHA) zeolite synthesized in the presence of fluoride for selective methanol-to-olefins. Micrpor. Mesopor. Mater. 2019, 274, 277–285. [Google Scholar] [CrossRef]
- Nasser, G.A.; Muraza, O.; Nishitoba, T.; Malaibari, Z.; Yamani, Z.H.; Al-Shammari, T.K.; Yokoi, T. Microwave-Assisted Hydrothermal Synthesis of CHA Zeolite for Methanol-to-Olefins Reaction. Ind. Eng. Chem. Res. 2019, 58, 60–68. [Google Scholar] [CrossRef]
- Salih, H.A.; Muraza, O.; Abussaud, B.; Al-Shammari, T.K.; Yokoi, T. Catalytic Enhancement of SAPO-34 for Methanol Conversion to Light Olefins Using in Situ Metal Incorporation. Ind. Eng. Chem. Res. 2018, 57, 6639–6646. [Google Scholar] [CrossRef]
- Nasser, G.A.; Al-Qadri, A.A.; Jamil, A.K.; Bakare, I.A.; Sanhoob, M.A.; Muraza, O.; Yamani, Z.H.; Yokoi, T.; Saleem, Q.; Alsewdan, D. Conversion of Methanol to Olefins over Modified OSDA-Free CHA Zeolite Catalyst. Ind. Eng. Chem. Res. 2021, 60, 12189–12199. [Google Scholar] [CrossRef]
- Liang, J.; Li, H.; Zhao, S.; Guo, W.; Wang, R.; Ying, M. Characteristics and performance of SAPO-34 catalyst for methanol-to-olefin conversion. Appl. Catal. 1990, 64, 31–40. [Google Scholar] [CrossRef]
- Yu, L.; Nobandegani, M.S.; Hedlund, J. Industrially relevant CHA membranes for CO2/CH4 separation. J. Membr. Sci. 2022, 641, 119888. [Google Scholar] [CrossRef]
- Wang, B.; Huang, W.; Zhu, Y.; Zhou, R.; Xing, W. Ultra-permeable high-selective SAPO-34 membranes for efficient CO2 capture. J. Membr. Sci. 2022, 650, 120420. [Google Scholar] [CrossRef]
- Wang, B.; Wang, N.; Li, X.; Zhou, R.; Xing, W. Exfoliation of lamellar SAPO-34 zeolite to nanosheets and synthesis of thin SAPO-34 membranes by a nanosheet-seeded secondary growth approach. J. Membr. Sci. 2022, 645, 120177. [Google Scholar] [CrossRef]
- Ahmed, A.; Ishiguro, S.; Seshimo, M.; Subramanian, B.; Matsukata, M. Synthesis of SAPO-34 Membrane and Its Application to the Separation of Water/Acetic Acid Mixtures by Vapor Permeation. J. Chem. Eng. Jpn. 2022, 55, 97–104. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, S.; Hao, J.; Song, L.; Chong, M.; Cheng, D.-G.; Chen, F. Bifunctional catalysts composed of low silicon-content SAPO-34 nanosheets and In2O3/ZrO2 with improved performance for CO2 hydrogenation. Greenh. Gases Sci. Technol. 2022, 12, 305–320. [Google Scholar] [CrossRef]
- Usman, M.; Ghanem, A.S.; Ali Shah, S.N.; Garba, M.D.; Khan, M.Y.; Khan, S.; Humayun, M.; Khan, A.L. A review on SAPO-34 zeolite materials for CO2 capture and conversion. Chem. Rec. 2022, e202200039. [Google Scholar] [CrossRef]
- Xu, J.; Haw, K.-G.; Li, Z.; Pati, S.; Wang, Z.; Kawi, S. A mini-review on recent developments in SAPO-34 zeolite membranes and membrane reactors. React. Chem. Eng. 2021, 6, 52–66. [Google Scholar] [CrossRef]
- Rimaz, S.; Kosari, M.; Zarinejad, M.; Ramakrishna, S. A comprehensive review on sustainability-motivated applications of SAPO-34 molecular sieve. J. Mater. Sci. 2022, 57, 848–886. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastava, R.; Koh, J. Utilization of zeolites as CO2 capturing agents: Advances and future perspectives. J. CO2 Util. 2020, 41, 101251. [Google Scholar] [CrossRef]
- Perez, E.V.; Kalaw, G.J.D.; Ferraris, J.P.; Balkus, K.J.; Musselman, I.H. Amine-functionalized (Al) MIL-53/VTEC™ mixed-matrix membranes for H2/CO2 mixture separations at high pressure and high temperature. J. Membr. Sci. 2017, 530, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Mahmoudi, A.; Ghasemi, H. The potential of hydrogen hydrate as a future hydrogen storage medium. iScience 2021, 24, 101907. [Google Scholar] [CrossRef]
- Ackley, M.W. Medical oxygen concentrators: A review of progress in air separation technology. Adsorption 2019, 25, 1437–1474. [Google Scholar] [CrossRef]
- Marwat, M.A.; Humayun, M.; Afridi, M.W.; Zhang, H.; Abdul Karim, M.R.; Ashtar, M.; Usman, M.; Waqar, S.; Ullah, H.; Wang, C.; et al. Advanced Catalysts for Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2021, 4, 12007–12031. [Google Scholar] [CrossRef]
- Elrasheedy, A.; Nady, N.; Bassyouni, M.; El-Shazly, A. Metal Organic Framework Based Polymer Mixed Matrix Membranes: Review on Applications in Water Purification. Membranes 2019, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Shafiq, S.; Al-Maythalony, B.A.; Usman, M.; Ba-Shammakh, M.S.; Al-Shammari, A.A. ZIF-95 as a filler for enhanced gas separation performance of polysulfone membrane. RSC Adv. 2021, 11, 34319–34328. [Google Scholar] [CrossRef]
- Usman, M.; Ali, M.; Al-Maythalony, B.A.; Ghanem, A.S.; Saadi, O.W.; Ali, M.; Jafar Mazumder, M.A.; Abdel-Azeim, S.; Habib, M.A.; Yamani, Z.H.; et al. Highly Efficient Permeation and Separation of Gases with Metal–Organic Frameworks Confined in Polymeric Nanochannels. ACS Appl. Mater. Interfaces 2020, 12, 49992–50001. [Google Scholar] [CrossRef]
- Husna, A.; Hossain, I.; Jeong, I.; Kim, T.-H. Mixed Matrix Membranes for Efficient CO2 Separation Using an Engineered UiO-66 MOF in a Pebax Polymer. Polymers 2022, 14, 655. [Google Scholar] [CrossRef]
- Tara, N.; Shamair, Z.; Habib, N.; Craven, M.; Bilad, M.R.; Usman, M.; Tu, X.; Khan, A.L. Simultaneous increase in CO2 permeability and selectivity by BIT-72 and modified BIT-72 based mixed matrix membranes. Chem. Eng. Res. Des. 2022, 178, 136–147. [Google Scholar] [CrossRef]
- Xiong, S.; Li, L.; Dong, L.; Tang, J.; Yu, G.; Pan, C. Covalent-organic frameworks (COFs)-based membranes for CO2 separation. J. CO2 Util. 2020, 41, 101224. [Google Scholar] [CrossRef]
- Khan, N.A.; Humayun, M.; Usman, M.; Ghazi, Z.A.; Naeem, A.; Khan, A.; Khan, A.L.; Tahir, A.A.; Ullah, H. Structural Characteristics and Environmental Applications of Covalent Organic Frameworks. Energies 2021, 14, 2267. [Google Scholar] [CrossRef]
- Kang, Z.; Peng, Y.; Qian, Y.; Yuan, D.; Addicoat, M.A.; Heine, T.; Hu, Z.; Tee, L.; Guo, Z.; Zhao, D. Mixed Matrix Membranes (MMMs) Comprising Exfoliated 2D Covalent Organic Frameworks (COFs) for Efficient CO2 Separation. Chem. Mater. 2016, 28, 1277–1285. [Google Scholar] [CrossRef]
- Duan, K.; Wang, J.; Zhang, Y.; Liu, J. Covalent organic frameworks (COFs) functionalized mixed matrix membrane for effective CO2/N2 separation. J. Membr. Sci. 2019, 572, 588–595. [Google Scholar] [CrossRef]
- Wang, X.; Shi, X.; Wang, Y. In Situ Growth of Cationic Covalent Organic Frameworks (COFs) for Mixed Matrix Membranes with Enhanced Performances. Langmuir 2020, 36, 10970–10978. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Kiran, M.D.; Govindaraju, H.K.; Jayaraju, T.; Kumar, N. Review-Effect of Fillers on Mechanical Properties of Polymer Matrix Composites. Mater. Today Proc. 2018, 5, 22421–22424. [Google Scholar] [CrossRef]
- Cai, W.; Xie, J.; Luo, J.; Chen, X.; Wang, M.; Wang, Y.; Li, J. n-Octyltrichlorosilane Modified SAPO-34/PDMS Mixed Matrix Membranes for Propane/Nitrogen Mixture Separation. Separations 2022, 9, 64. [Google Scholar] [CrossRef]
- Santaniello, A.; Di Renzo, A.; Di Maio, F.; Belov, N.A.; Yampolskii, Y.P.; Golemme, G. Competing non ideal behaviour of SAPO-34 and Poly(hexafluoropropylene) in mixed matrix membranes. Micrpor. Mesopor. Mater. 2020, 303, 110241. [Google Scholar] [CrossRef]
- Peydayesh, M.; Asarehpour, S.; Mohammadi, T.; Bakhtiari, O. Preparation and characterization of SAPO-34—Matrimid® 5218 mixed matrix membranes for CO2/CH4 separation. Chem. Eng. Res. Des. 2013, 91, 1335–1342. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.S.; Kumakiri, I.; Tanaka, K.; Chen, X.S.; Kita, H. Preparation and Permeation Properties of PESU-Based Mixed Matrix Membranes with Nano-Sized CHA Zeolites. J. Chem. Eng. Jpn. 2019, 52, 514–520. [Google Scholar] [CrossRef]
- Carter, D.; Tezel, F.H.; Kruczek, B.; Kalipcilar, H. Investigation and comparison of mixed matrix membranes composed of polyimide matrimid with ZIF-8, silicalite, and SAPO-34. J. Membr. Sci. 2017, 544, 35–46. [Google Scholar] [CrossRef]
- Belhaj Messaoud, S.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Mixed matrix membranes using SAPO-34/polyetherimide for carbon dioxide/methane separation. Sep. Purif. Technol. 2015, 148, 38–48. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.Z.; Li, H.; Hua, K.S.; Deng, M.C. Poly(amide-6-b-ethylene oxide)/SAPO-34 mixed matrix membrane for CO2 separation. J. Energy Chem. 2014, 23, 227–234. [Google Scholar] [CrossRef]
- Junaidi, M.U.M.; Leo, C.P.; Ahmad, A.L.; Kamal, S.N.M.; Chew, T.L. Carbon dioxide separation using asymmetric polysulfone mixed matrix membranes incorporated with SAPO-34 zeolite. Fuel Process. Technol. 2014, 118, 125–132. [Google Scholar] [CrossRef]
- Junaidi, M.U.M.; Khoo, C.P.; Leo, C.P.; Ahmad, A.L. The effects of solvents on the modification of SAPO-34 zeolite using 3-aminopropyl trimethoxy silane for the preparation of asymmetric polysulfone mixed matrix membrane in the application of CO2 separation. Micrpor. Mesopor. Mater. 2014, 192, 52–59. [Google Scholar] [CrossRef]
- Cakal, U.; Yilmaz, L.; Kalipcilar, H. Effect of feed gas composition on the separation of CO2/CH4 mixtures by PES-SAPO 34-HMA mixed matrix membranes. J. Membr. Sci. 2012, 417, 45–51. [Google Scholar] [CrossRef]
- Oral, E.E.; Yilmaz, L.; Kalipcilar, H. Effect of Gas Permeation Temperature and Annealing Procedure on the Performance of Binary and Ternary Mixed Matrix Membranes of Polyethersulfone, SAPO-34, and 2-Hydroxy 5-Methyl Aniline. J. Appl. Polym. Sci. 2014, 131, 40679. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Mukhtar, H.; Mohshim, D.F.; Nasir, R.; Man, Z. Effect of different organic amino cations on SAPO-34 for PES/SAPO-34 mixed matrix membranes toward CO2/CH4separation. J. Appl. Polym. Sci. 2016, 133, 43387. [Google Scholar] [CrossRef]
- Nasir, R.; Ahmad, N.N.R.; Mukhtar, H.; Mohshim, D.F. Effect of ionic liquid inclusion and amino–functionalized SAPO-34 on the performance of mixed matrix membranes for CO2/CH4 separation. J. Environ. Chem. Eng 2018, 6, 2363–2368. [Google Scholar] [CrossRef]
- Junaidi, M.U.M.; Leo, C.P.; Ahmad, A.L.; Ahmad, N.A. Fluorocarbon functionalized SAPO-34 zeolite incorporated in asymmetric mixed matrix membranes for carbon dioxide separation in wet gases. Micrpor. Mesopor. Mater. 2015, 206, 23–33. [Google Scholar] [CrossRef]
- Nawar, A.; Ghaedi, H.; Ali, M.; Zhao, M.; Iqbal, N.; Khan, R. Recycling waste-derived marble powder for CO2 capture. Process Saf. Environ. Prot. 2019, 132, 214–225. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Leo, C.P.; Mohammad, A.W.; Ahmad, A.L. Modification of gas selective SAPO zeolites using imidazolium ionic liquid to develop polysulfone mixed matrix membrane for CO2 gas separation. Micrpor. Mesopor. Mater. 2017, 244, 21–30. [Google Scholar] [CrossRef]
- Mohshim, D.F.; Mukhtar, H.; Man, Z. The effect of incorporating ionic liquid into polyethersulfone-SAPO34 based mixed matrix membrane on CO2 gas separation performance. Sep. Purif. Technol. 2014, 135, 252–258. [Google Scholar] [CrossRef]
- Mohshim, D.F.; Mukhtar, H.; Dutta, B.K.; Man, Z. Predicting CO2 Permeation through an Enhanced Ionic Liquid Mixed Matrix Membrane (IL3M). Int. J. Chem. Eng. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sen, M.; Das, N. In situ carbon deposition in polyetherimide/SAPO-34 mixed matrix membrane for efficient CO2/CH4 separation. J. Appl. Polym. Sci. 2017, 134, 45508. [Google Scholar] [CrossRef]
- Sodeifian, G.; Raji, M.; Asghari, M.; Rezakazemi, M.; Dashti, A. Polyurethane-SAPO-34 mixed matrix membrane for CO2/CH4 and CO2/N2 separation. Chin. J. Chem. Eng. 2019, 27, 322–334. [Google Scholar] [CrossRef]
- Rabiee, H.; Meshkat Alsadat, S.; Soltanieh, M.; Mousavi, S.A.; Ghadimi, A. Gas permeation and sorption properties of poly(amide-12-b-ethyleneoxide)(Pebax1074)/SAPO-34 mixed matrix membrane for CO2/CH4 and CO2/N2 separation. J. Ind. Eng. Chem. 2015, 27, 223–239. [Google Scholar] [CrossRef]
- Lixiong, Z.; Mengdong, J.; Enze, M. Synthesis of SAPO-34/ceramic composite membranes. In Studies in Surface Science and Catalysis; Chon, H., Ihm, S.-K., Uh, Y.S., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 105, pp. 2211–2216. [Google Scholar]
- Poshusta, J.C.; Tuan, V.A.; Falconer, J.L.; Noble, R.D. Synthesis and permeation properties of SAPO-34 tubular membranes. Ind. Eng. Chem. Res 1998, 37, 3924–3929. [Google Scholar] [CrossRef]
- Poshusta, J.C.; Tuan, V.A.; Pape, E.A.; Noble, R.D.; Falconer, J.L. Separation of light gas mixtures using SAPO-34 membranes. AlChE J. 2000, 46, 779–789. [Google Scholar] [CrossRef]
- Zhou, R.F.; Ping, E.W.; Funke, H.H.; Falconer, J.L.; Noble, R.D. Improving SAPO-34 membrane synthesis. J. Membr. Sci. 2013, 444, 384–393. [Google Scholar] [CrossRef]
- Carreon, M.A.; Li, S.; Falconer, J.L.; Noble, R.D. Alumina-supported SAPO-34 membranes for CO2/CH4 separation. J. Am. Chem. Soc. 2008, 130, 5412–5413. [Google Scholar] [CrossRef]
- Li, S.; Fan, C.Q. High-Flux SAPO-34 Membrane for CO2/N2 Separation. Ind. Eng. Chem. Res. 2010, 49, 4399–4404. [Google Scholar] [CrossRef]
- Li, S.G.; Carreon, M.A.; Zhang, Y.F.; Funke, H.H.; Noble, R.D.; Falconer, J.L. Scale-up of SAPO-34 membranes for CO2/CH4 separation. J. Membr. Sci. 2010, 352, 7–13. [Google Scholar] [CrossRef]
- Chew, T.L.; Ahmad, A.L.; Bhatia, S. Microwave heating-synthesized zeolite membrane for CO2/CH4 separation. Desalination Water Treat. 2012, 47, 139–149. [Google Scholar] [CrossRef]
- Chew, T.L.; Yeong, Y.F.; Ho, C.D.; Ahmad, A.L. Ion-Exchanged Silicoaluminophosphate-34 Membrane for Efficient CO2/N2 Separation with Low CO2 Concentration in the Gas Mixture. Ind. Eng. Chem. Res. 2018, 58, 729–735. [Google Scholar] [CrossRef]
- Li, G.; Yang, J.H.; Wang, J.Q.; Xiao, W.; Zhou, L.; Zhang, Y.; Lu, J.M.; Yin, D.H. Thin carbon/SAPO-34 microporous composite membranes for gas separation. J. Membr. Sci. 2011, 374, 83–92. [Google Scholar] [CrossRef]
- Shi, H. Organic template-free synthesis of SAPO-34 molecular sieve membranes for CO2–CH4 separation. RSC Adv. 2015, 5, 38330–38333. [Google Scholar] [CrossRef]
- Liu, X.; Du, S.; Zhang, B. The seeded growth of dense and thin SAPO-34 membranes on porous α-Al2O3 substrates under microwave irradiation. Mater. Lett. 2013, 91, 195–197. [Google Scholar] [CrossRef]
- Das, J.K.; Das, N. Mercaptoundecanoic acid capped palladium nanoparticles in a SAPO 34 membrane: A solution for enhancement of H(2)/CO(2) separation efficiency. ACS Appl. Mater. Interfaces 2014, 6, 20717–20728. [Google Scholar] [CrossRef]
- Falconer, J.; Funke, H.; Noble, R.; Wu, T.; Diaz, M.; Zhou, R.F. Influence of propane on CO2/CH4 and N2/CH4 separations in CHA zeolite membranes. Abstr. Pap. Am. Chem. S 2015, 249, 201–209. [Google Scholar] [CrossRef]
- Bing, L.C.; Wang, G.J.; Wang, F.; Liu, X.F.; Zhang, B.Q. Preparation of a preferentially oriented SAPO-34 membrane by secondary growth under microwave irradiation. RSC Adv. 2016, 6, 56170–56173. [Google Scholar] [CrossRef]
- Kgaphola, K.; Sigalas, I.; Daramola, M.O. Synthesis and characterization of nanocomposite SAPO-34/ceramic membrane for post-combustion CO2 capture. Asia-Pac. J. Chem. Eng. 2017, 12, 894–904. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, Y.; Zhang, C.; Zhu, Z.; Gu, X. Diethylamine template-directed synthesis of hollow fiber supported SAPO-34 membranes. CIESC J. 2019, 70, 2316–2324. [Google Scholar] [CrossRef]
- Liu, B.; Tang, C.Y.; Li, X.W.; Wang, B.; Zhou, R.F. High-performance SAPO-34 membranes for CO2 separations from simulated flue gas. Micrpor. Mesopor. Mater. 2020, 292, 109712. [Google Scholar] [CrossRef]
- Mu, Y.B.; Chen, H.H.; Xiang, H.; Lan, L.; Shao, Y.; Fan, X.L.; Hardacre, C. Defects-healing of SAPO-34 membrane by post-synthesis modification using organosilica for selective CO2 separation. J. Membr. Sci. 2019, 575, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wu, T.; Yu, M.; Li, S.; Zhou, R.; Xing, W. Highly Ordered Nanochannels in a Nanosheet-Directed Thin Zeolite Nanofilm for Precise and Fast CO2 Separation. Small 2020, 16, e2002836. [Google Scholar] [CrossRef] [PubMed]
- Li, S.G.; Martinek, J.G.; Falconer, J.L.; Noble, R.D.; Gardner, T.Q. High-pressure CO2/CH4 separation using SAPO-34 membranes. Ind. Eng. Chem. Res 2005, 44, 3220–3228. [Google Scholar] [CrossRef]
- Tian, Y.Y.; Fan, L.L.; Wang, Z.Y.; Qiu, S.L.; Zhu, G.S. Synthesis of a SAPO-34 membrane on macroporous supports for high permeance separation of a CO2/CH4 mixture. J. Mater. Chem. 2009, 19, 7698–7703. [Google Scholar] [CrossRef]
- Li, S.G.; Falconer, J.L.; Noble, R.D. Improved SAPO-34 membranes for CO2/CH4 separations. Adv. Mater. 2006, 18, 2601–2603. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Amino-functionalized SAPO-34 membranes for CO2/CH4 and CO2/N2 separation. Langmuir 2011, 27, 2888–2894. [Google Scholar] [CrossRef]
- Makertihartha, I.G.B.N.; Kencana, K.S.; Dwiputra, T.R.; Khoiruddin, K.; Mukti, R.R.; Wenten, I.G. Silica supported SAPO-34 membranes for CO2/N2 separation. Micrpor. Mesopor. Mater. 2020, 298, 110068. [Google Scholar] [CrossRef]
- Ping, E.W.; Zhou, R.F.; Funke, H.H.; Falconer, J.L.; Noble, R.D. Seeded-gel synthesis of SAPO-34 single channel and monolith membranes, for CO2/CH4 separations. J. Membr. Sci. 2012, 415, 770–775. [Google Scholar] [CrossRef]
- Mirfendereski, S.M. RETRACTED: Development of a multi-step hybrid method to synthesize highly-permeable and well-oriented SAPO-34 membranes for CO2 removal applications. Chem. Eng. Sci. 2019, 208, 115157. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhang, C.; Jiang, J.; Gu, X. Fabrication of high-flux SAPO-34 membrane on α-Al2O3 four-channel hollow fibers for CO2 capture from CH 4. J. CO2 Util. 2017, 18, 30–40. [Google Scholar] [CrossRef]
- Rehman, R.U.; Song, Q.N.; Peng, L.; Wu, Z.Q.; Gu, X.H. A facile coating to intact SAPO-34 membranes for wet CO2/CH4 mixture separation. Chem. Eng. Res. Des. 2020, 153, 37–48. [Google Scholar] [CrossRef]
- Rehman, R.U.; Song, Q.N.; Peng, L.; Chen, Y.; Gu, X.H. Hydrophobic modification of SAPO-34 membranes for improvement of stability under wet condition. Chin. J. Chem. Eng. 2019, 27, 2397–2406. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Pati, S.; Chen, T.; Deng, Y.; Dewangan, N.; Meng, L.; Lin, J.Y.S.; Kawi, S. High H2 permeable SAPO-34 hollow fiber membrane for high temperature propane dehydrogenation application. AlChE J. 2020, 66, e16278. [Google Scholar] [CrossRef]

| Filler | Substrate | CO2 Permeance | CO2/CH4 Selectivity | CO2/N2 Selectivity | Ref. |
|---|---|---|---|---|---|
| Neat | Matrimid 5218 | 4.4 Barrer | 34 | - | [77] |
| SAPO-34 2 wt% | Matrimid 5218 | 4.5 Barrer | 41.98 | - | [77] |
| SAPO-34 5 wt% | Matrimid 5218 | 4.6 Barrer | 44.24 | - | [77] |
| SAPO-34 10 wt% | Matrimid 5218 | 5.3 Barrer | 50.82 | - | [77] |
| SAPO-34 15 wt% | Matrimid 5218 | 5.9 Barrer | 58.14 | - | [77] |
| SAPO-34 20 wt% | Matrimid 5218 | 6.9 Barrer | 66.99 | - | [77] |
| Neat | Polyethersulfone (PESU) | 6.7 Barrer | 37.8 | - | [78] |
| SAPO-34 NP 20 wt% | Polyethersulfone (PESU) | 8.2 Barrer | 42.6 | - | [78] |
| SAPO-34 NP 30 wt% | Polyethersulfone (PESU) | 8.9 Barrer | 48.3 | - | [78] |
| Neat | Matrimid 5218 | 9.5 ± 1.07 GPU | 29.81 | 13.63 | [79] |
| SAPO-34 10 wt% uncalcined | Matrimid 5218 | 7.63 ± 0.81 GPU | 31.79 | 26.31 | [79] |
| SAPO-34 10 wt% calcined | Matrimid 5218 | 12.5 ± 1.3 GPU | 9.32 | 10.50 | [79] |
| Neat | Polyetherimide | 6 × 10−10 mol/(m2 s Pa) | 0.02 | - | [80] |
| SAPO-34 5 wt% | Polyetherimide | 4.4 × 10−10 mol/(m2 s Pa) | 60 | - | [80] |
| SAPO-34 10 wt% | Polyetherimide | 6 × 10−10 mol/(m2 s Pa) | 8 | - | [80] |
| Neat | Pebax 1657 | 100 Barrer | 16.7 | 53.8 | [81] |
| SAPO-34 23 wt% | Pebax 1657 | 134 Barrer | 21.7 | 55.2 | [81] |
| SAPO-34 33 wt% | Pebax 1657 | 252 Barrer | 17 | 55 | [81] |
| SAPO-34 50 wt% | Pebax 1657 | 339 Barrer | 16.8 | 53.2 | [81] |
| Neat | Polysulfone (Asymmetric) | 22.0 ± 3.42 GPU | 17.3 | 16.5 | [82] |
| SAPO-34 5 wt% | Polysulfone (Asymmetric) | 205.9 ± 7.26 GPU | 22.5 | 21.4 | [82] |
| SAPO-34 10 wt% | Polysulfone (Asymmetric) | 314.0 ± 4.65 GPU | 28.2 | 26.1 | [82] |
| SAPO-34 20 wt% | Polysulfone (Asymmetric) | 281.18 ± 6.92 GPU | 10.9 | 10.7 | [82] |
| SAPO-34 30 wt% | Polysulfone (Asymmetric) | 232. ± 3.21 GPU | 3 | 2.9 | [82] |
| Neat | Polysulfone (Asymmetric) | 105 GPU | 15 | 13 | [83] |
| SAPO-34 10 wt% | Polysulfone (Asymmetric) | 459 GPU | 27 | 21 | [83] |
| SAPO-34E 10 wt% | Polysulfone (Asymmetric) | 706 GPU | 31 | 28 | [83] |
| SAPO-34I 10 wt% | Polysulfone (Asymmetric) | 775 GPU | 28 | 22 | [83] |
| Neat | Polyhexafluoropropylene (PHFP) | 290 Barrer | 14.1 | - | [76] |
| SAPO-34 NP 24.6 v% | Polyhexafluoropropylene (PHFP) | 468 Barrer | 15.8 | - | [76] |
| SAPO-34 NP 36 v% | Polyhexafluoropropylene (PHFP) | 437 Barrer | 17.5 | - | [76] |
| Neat | PES | 4.45 Barrer | 33.2 | - | [84] |
| HMA 10% | PES | 0.8 Barrer | 32.3 | - | [84] |
| SAPO-34 20 wt% | PES | 5.7 Barrer | 37 | - | [84] |
| SAPO-34 20 wt% + HMA 10% | PES | 1.3 Barrer | 44.7 | - | [84] |
| HMA 4% | PES | 5.1 Barrer | 39.3 | - | [85] |
| SAPO-34 20 wt% | PES | 13.8 Barrer | 32.7 | - | [85] |
| SAPO-34 20 wt% + HMA 4% | PES | 7.8 Barrer | 41.6 | - | [85] |
| SAPO-34 | PES | 18 GPU | 1.2 | - | [86] |
| SAPO-34 20 wt% | PES | 30 GPU | 1.3 | - | [86] |
| SAPO-34 20 wt% m-EDA | PES | 10.0 GPU | 12.14 | - | [86] |
| SAPO-34 20 wt% | PES | 50 GPU | 2.5 | - | [87] |
| SAPO-34 20 wt%/IL | PES | 0.03 GPU | 4.9 | - | [87] |
| SAPO-34 20 wt% m-EDA/IL | PES | 0.09 GPU | 26.5 | - | [87] |
| SAPO-34 20 wt% m-HA/IL | PES | 0.045 GPU | 37.2 | - | [87] |
| Neat | Polysulfone (PSf) | 21.3 ± 2.8 GPU | 17.2 | - | [88] |
| SAPO-34 10 wt% | Polysulfone (PSf) | 317.0 ± 3.5 GPU | 27.9 | - | [88] |
| SAPO-34 20 wt% | Polysulfone (PSf) | 283.0 ± 2.2 GPU | 10.8 | - | [88] |
| SAPO-34 10 wt% + 0.5 wt%HFDS | Polysulfone (PSf) | 310.4 ± 1.7 GPU | 30.4 | - | [88] |
| SAPO-34 10 wt% + 1 wt%HFDS | Polysulfone (PSf) | 278.8 ± 2.1 GPU | 38.9 | - | [88] |
| SAPO-34 10 wt% + 1.5 wt%HFDS | Polysulfone (PSf) | 259.7 ± 4.2 GPU | 37.3 | - | [88] |
| SAPO-34 20 wt% + 0.5 wt%HFDS | Polysulfone (PSf) | 332.1 ± 5.5 GPU | 11.9 | - | [88] |
| SAPO-34 20 wt% + 1 wt%HFDS | Polysulfone (PSf) | 293.7 ± 4.9 GPU | 27.5 | - | [88] |
| SAPO-34 20 wt% + 1.5 wt%HFDS | Polysulfone (PSf) | 306.8 ± 5.2 GPU | 24.8 | - | [88] |
| SAPO-34 5 wt% | Polysulfone (PSf) | 6.1 GPU | 4.9 | 5.1 | [90] |
| SAPO-34 5 wt%/IL(0.2 M) | Polysulfone (PSf) | 24.89 GPU | 35.06 | 40.15 | [90] |
| Neat | Polysulfone (PSf) | 5.60 ± 0.75 GPU | 3.24 | 6.15 | [91] |
| SAPO-34 5 wt% | Polysulfone (PSf) | 6.53 ± 1.22 GPU | 3.47 | 5.67 | [91] |
| SAPO-34 5 wt%/IL(0.4 M) | Polysulfone (PSf) | 4.82 ± 1.28 GPU | 4.86 | 8.04 | [91] |
| SAPO-34 5 wt%/IL(0.6 M) | Polysulfone (PSf) | 7.24 ± 1.78 GPU | 20.35 | 18.82 | [91] |
| SAPO-34 20 wt% | Polyethersulfone (PES) | 85.7 GPU | 20.67 | - | [92] |
| SAPO-34 20 wt% + IL 5 wt% | Polysulfone (PSf) | 230.8 GPU | - | 46.20 | [92] |
| SAPO-34 20 wt% + IL 10 wt% | Polysulfone (PSf) | 255.69 GPU | - | 58.83 | [92] |
| SAPO-34 20 wt% + IL 15 wt% | Polysulfone (PSf) | 279.2 GPU | - | 60.62 | [92] |
| SAPO-34 20 wt% + IL 20 wt% | Polysulfone (PSf) | 300.0 GPU | - | 62.58 | [92] |
| Neat | Polyetherimide | 3.8 × 10−10 mol/(m2 s Pa) | - | 2.23 | [94] |
| SAPO-34 10 wt% | Polyetherimide | 2.8 × 10−8 mol/(m2 s Pa) | - | 2.54 | [94] |
| SAPO-34 25 wt% + Carbonization | Polyetherimide | 8.42 × 10−8 mol/(m2 s Pa) | - | 6.47 | [94] |
| SAPO-34 40 wt% | Polyetherimide | 9.1 × 10−7 mol/(m2 s Pa) | - | 5.05 | [94] |
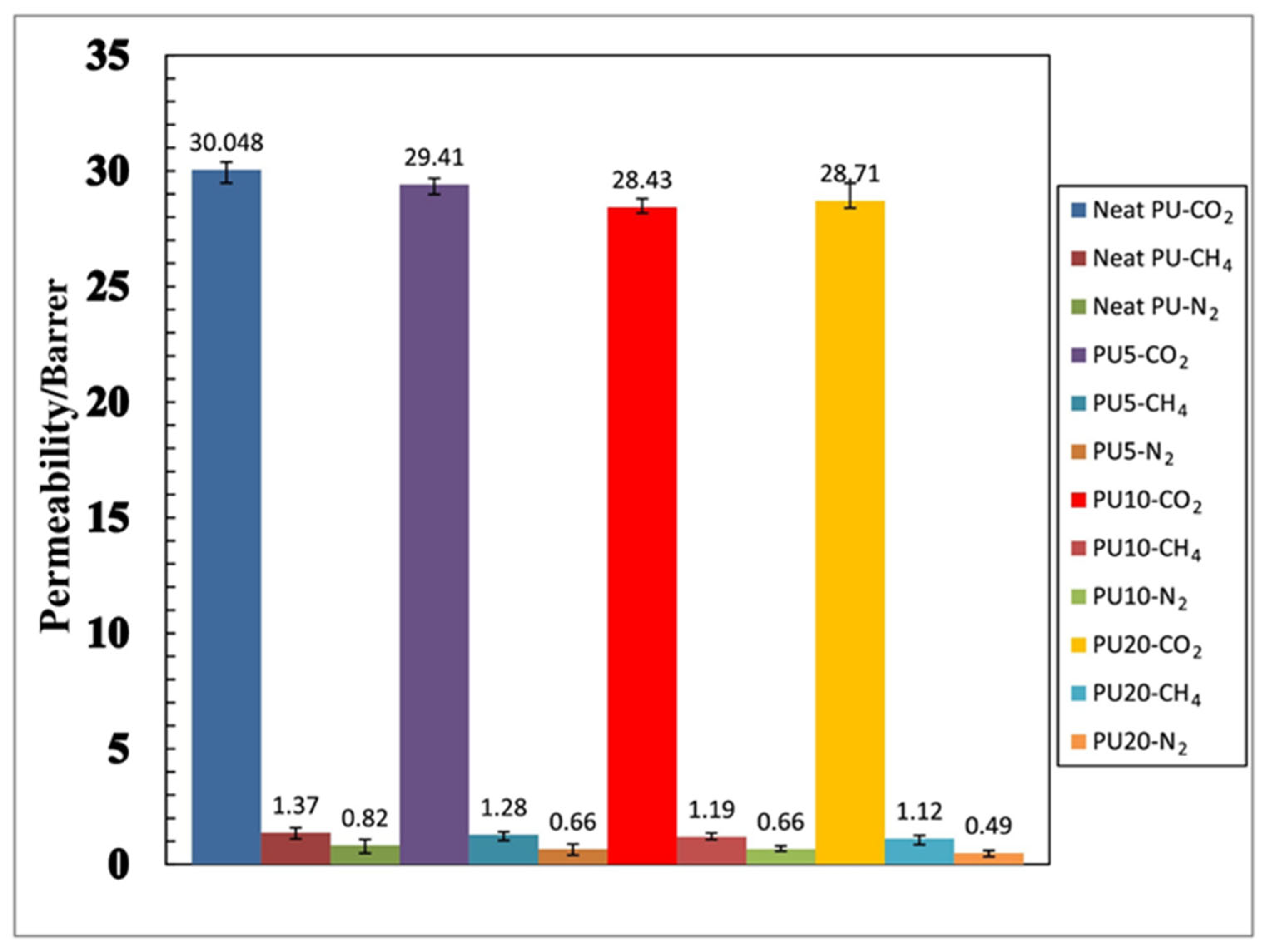
| Neat | Polyurethane | 30.05 Barrer | 21.93 | 36.64 | [89] |
| SAPO-34 NP 5 wt% | Polyurethane | 29.41 Barrer | 22.97 | 44.56 | [89] |
| SAPO-34 NP 10 wt% | Polyurethane | 28.43 Barrer | 23.89 | 54.67 | [89] |
| SAPO-34 NP 20 wt% | Polyurethane | 28.71 Barrer | 25.63 | 58.59 | [89] |
| Neat | Pebax 1074 | 120 Barrer | 17.5 | 60.3 | [95] |
| SAPO-34 5 wt% | Pebax 1074 | 123 Barrer | 18.5 | 61 | [95] |
| SAPO-34 10 wt% | Pebax 1074 | 130 Barrer | 22 | 62.5 | [95] |
| SAPO-34 20 wt% | Pebax 1074 | 152 Barrer | 29 | 68 | [95] |
| SAPO-34 30 wt% | Pebax 1074 | 156 Barrer | 35 | 69 | [95] |
| Filler | Substrate | CO2 Permeance | CO2/CH4 Selectivity | CO2/N2 Selectivity | Ref. |
|---|---|---|---|---|---|
| SAPO-34 | Stainless steel | 2 × 10−7 mol/(m2 s Pa) | 270 | - | [116] |
| SAPO-34 (M1) | Stainless steel | 1.1 × 10−7 mol/(m2 s Pa) | 27 | - | [117] |
| SAPO-34 (M2) | Stainless steel | 1.4 × 10−7 mol/(m2 s Pa) | 54 | - | [117] |
| SAPO-34 (M3) | Stainless steel | 1.4 × 10−7 mol/(m2 s Pa) | 87 | - | [117] |
| SAPO-34 (M3) | Stainless steel | 4.9 × 10−8 mol/(m2 s Pa) | 55 | - | [117] |
| SAPO-34 | Stainless steel tube | 1.2 × 10−7 mol/(m2 s Pa) | 170 | - | [118] |
| SAPO-34 | Stainless steel | 2.52 × 10−6 mol/(m2 s Pa) | 9.30 | - | [119] |
| SAPO-34 (nonfunctionalized) | Stainless steel | 4.6 × 10−7 mol/(m2 s Pa) | 159 | 29 | [119] |
| SAPO-34 (0.15 mmol of HA) | Stainless steel | 3.7 × 10−7 mol/(m2 s Pa) | 238 | 36 | [119] |
| SAPO-34 (0.15 mmol of OA) | Stainless steel | 1.9 × 10−7 mol/(m2 s Pa) | 229 | 30 | [119] |
| SAPO-34 (0.15 mmol of ED) | Stainless steel | 5 × 10−7 mol/(m2 s Pa) | 245 | 39 | [119] |
| SAPO-34 | α-Al2O3 disk | 6.40 × 10−8 mol/(m2 s Pa) | - | 4.16 | [96] |
| SAPO-34 | α-Al2O3 disk | 29.9 × 10−8 mol/(m2 s Pa) | - | 11.17 | [119] |
| SAPO-34 | α-Al2O3 tubes | 2.4 × 10−8 mol/(m2 s Pa) | 19 | 5.7 | [97] |
| SAPO-34 | α-Al2O3 tubes | 15.5 × 10−8 mol/(m2 s Pa) | 20 | 7.1 | [98] |
| SAPO-34 | α-Al2O3 | 1.2×10−6 mol/(m2 s Pa) | 70 | - | |
| SAPO-34 | α-Al2O3 | 1.8 × 10−6 mol/(m2 s Pa) | 171 | - | [100] |
| SAPO-34 | α-Al2O3 | 1.2 × 10−6 mol/(m2 s Pa) | - | 32 | [101] |
| SAPO-34 | α-Al2O3 | 0.45 × 10−6 mol/(m2 s Pa) | - | 9.5 | [101] |
| SAPO-34 | α-Al2O3 | 0.7 × 10−7 mol/(m2 s Pa) | - | 10 | [101] |
| Ba-SAPO-34 | α-Al2O3 tubes | 37.6 × 10−8 mol/(m2 s Pa) | 103 | - | [102] |
| Ba-SAPO-34 | α-Al2O3 tubes | 17 × 10−8 mol/(m2 s Pa) | 36 | - | [102] |
| SAPO-34 | α-Al2O3 disk | 17.5 × 10−7 mol/(m2 s Pa) | - | 78 | [104] |
| SAPO-34 | α-Al2O3 disk | 8.1 × 10−7 mol/(m2 s Pa) | - | 25.1 | [104] |
| Thin carbon/SAPO-34 | α-Al2O3 tubes | 8.7 × 10−8 mol/(m2 s Pa) | 87 | - | [105] |
| SAPO-34 | seven-channel monolith Al2O3 | 3.7 × 10−7 mol/(m2 s Pa) | 21 | - | [121] |
| SAPO-34 | seven-channel monolith Al2O3 | 6.3 × 10−7 mol/(m2 s Pa) | 44 | - | [121] |
| SAPO-34 | seven-channel monolith Al2O3 | 6.3 × 10−7 mol/(m2 s Pa) | 56 | - | [121] |
| SAPO-34 | α-Al2O3 disk | 1.63 × 10−6 mol/(m2 s Pa) | 258 | - | [106] |
| SAPO-34 | α-Al2O3 disk | 1.72 × 10−6 mol/(m2 s Pa) | 213 | - | [106] |
| SAPO-34 | α-Al2O3 disk | 1.26 × 10−6 | 95 | - | [107] |
| SAPO-34 | α-Al2O3 tubes | 1.5 × 10−6 mol/(m2 s Pa) | 100 | - | [109] |
| SAPO-34 | α-Al2O3 tubes | 5 × 10−7 mol/(m2 s Pa) | 100 | - | [109] |
| SAPO-34 | α-Al2O3 | 1.57 × 10−6 mol/(m2 s Pa) | 109 | - | [110] |
| SAPO-34 | α-Al2O3 tubes | 2.44 × 10−7 mol/(m2 s Pa) | - | 7.9 | [111] |
| SAPO-34 | α-Al2O3 | 6.2 × 10−7 mol/(m2 s Pa) | 89 | - | [112] |
| SAPO-34 | α-Al2O3 tubes | 1.82 × 10−6 mol/(m2 s Pa) | - | 32.9 | [113] |
| SAPO-34 | α-Al2O3 tubes | 4.67 × 10−7 mol/(m2 s Pa) | - | 10.3 | [113] |
| SAPO-34 | α-Al2O3 tubes (untreated) | 2.5 × 10−7 mol/(m2 s Pa) | 61 | - | [114] |
| SAPO-34 | α-Al2O3 tubes (treated) | 2.4 × 10−7 mol/(m2 s Pa) | 158 | - | [114] |
| SAPO-34 | α-Al2O3 tubes | 27 ± 1.41 × 10−7 mol/(m2 s Pa) | 146 ± 5.6 | - | [122] |
| SAPO-34 | α-Al2O3 tubes | 1.2 × 10−5 mol/(m2 s Pa) | 135 | 41 | [115] |
| SAPO-34 | α-Al2O3/(4CHF) | 1.18 × 10−6 mol/(m2 s Pa) | 160 | - | [123] |
| SAPO-34 | α-Al2O3/(4CHF) | 2.3 × 10−7 mol/(m2 s Pa) | 53 | [124] | |
| SAPO-34/PDMS | α-Al2O3/(4CHF) | 1.18 × 10−7 mol/(m2 s Pa) | 68 | - | [124] |
| SAPO-34/PDMS | α-Al2O3/(4CHF) | 4.2 × 10−7 mol/(m2 s Pa) | 86 | - | [124] |
| SAPO-34 | α-Al2O3/(4CHF) | 1.7 × 10−8 mol/(m2 s Pa) | 0.9 | - | [125] |
| SAPO-34/PDMS | α-Al2O3/(4CHF) | 1.18 × 10−6 mol/(m2 s Pa) | 160 | - | [125] |
| SAPO-34 | Silica tubes | 2.01 × 10−6 mol/(m2 s Pa) | - | 53 | [120] |
| SAPO-34 | Silica tubes | 2.01 × 10−6 mol/(m2 s Pa) | - | 2.08 | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, M. Recent Progress of SAPO-34 Zeolite Membranes for CO2 Separation: A Review. Membranes 2022, 12, 507. https://doi.org/10.3390/membranes12050507
Usman M. Recent Progress of SAPO-34 Zeolite Membranes for CO2 Separation: A Review. Membranes. 2022; 12(5):507. https://doi.org/10.3390/membranes12050507
Chicago/Turabian StyleUsman, Muhammad. 2022. "Recent Progress of SAPO-34 Zeolite Membranes for CO2 Separation: A Review" Membranes 12, no. 5: 507. https://doi.org/10.3390/membranes12050507
APA StyleUsman, M. (2022). Recent Progress of SAPO-34 Zeolite Membranes for CO2 Separation: A Review. Membranes, 12(5), 507. https://doi.org/10.3390/membranes12050507







