Separation of Mercury(II) from Industrial Wastewater through Polymer Inclusion Membranes with Calix[4]pyrrole Derivative
Abstract
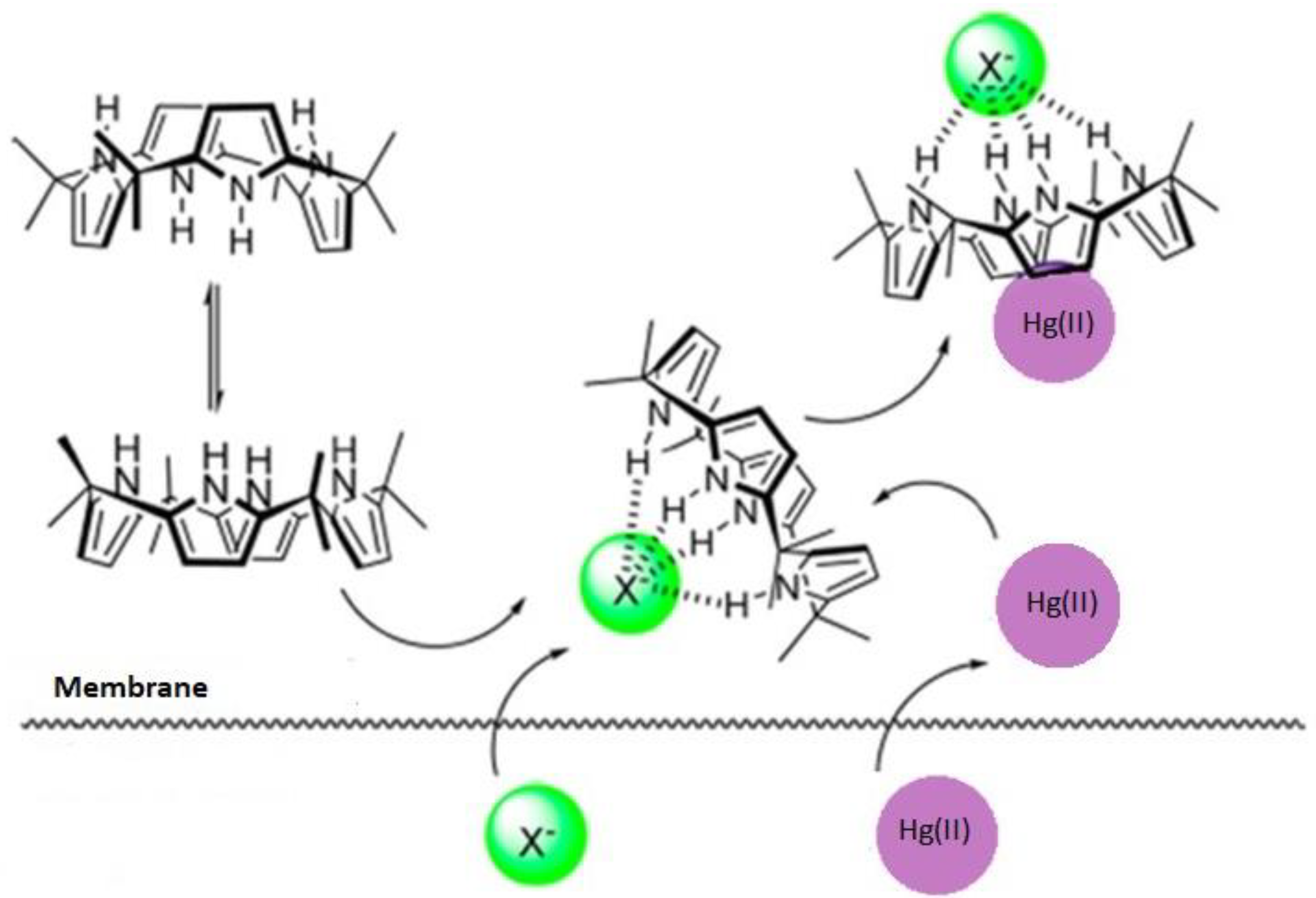
:1. Introduction
2. Materials and Methods
2.1. Reagents
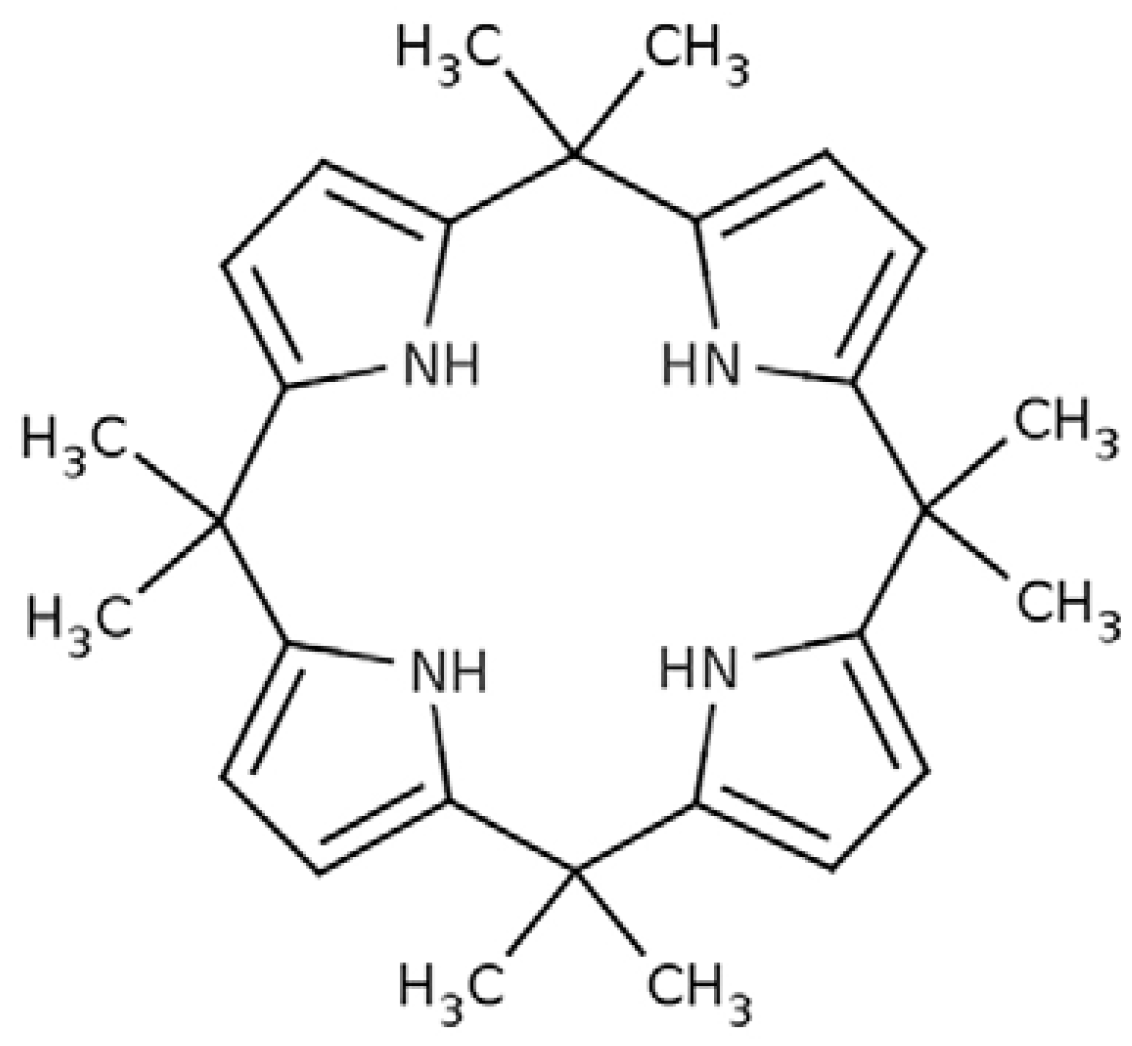
2.2. Synthesis of Meso-octamethylcalix[4]pyrrole
2.3. Preparation of Polymer Inclusion Membranes and Stability Test
2.4. Transport Studies
3. Results and Discussion
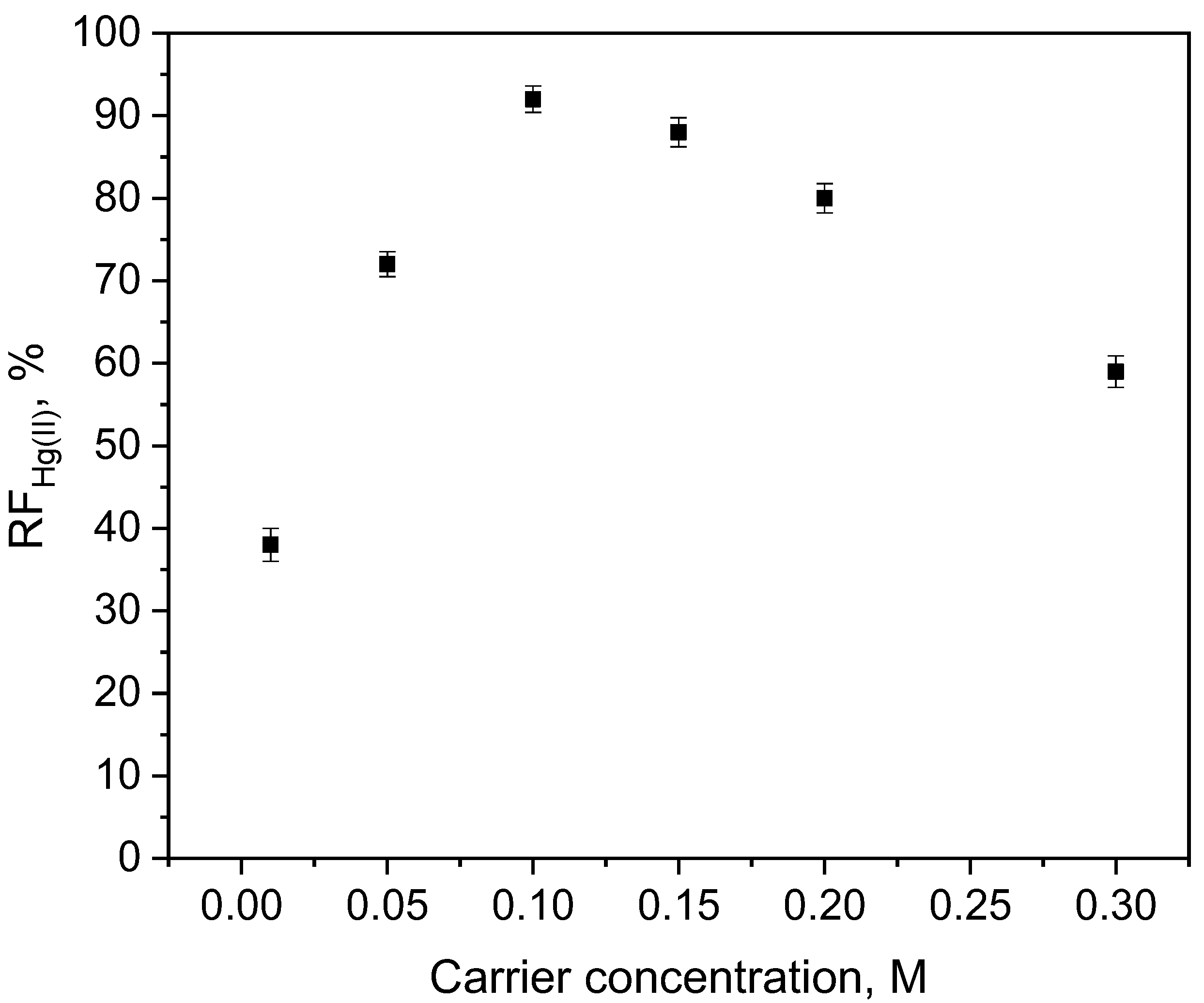
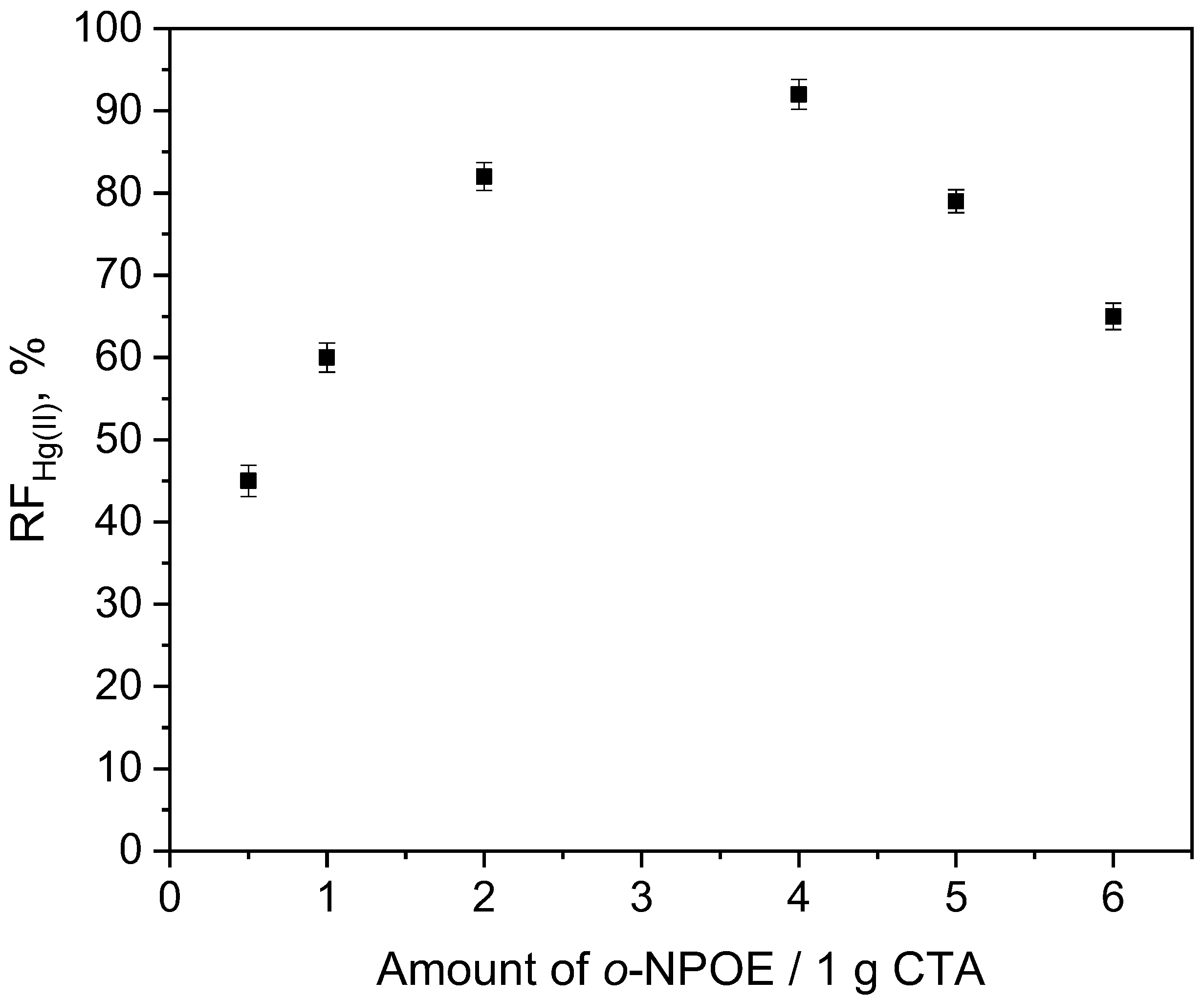
3.1. Effect of Membrane Composition
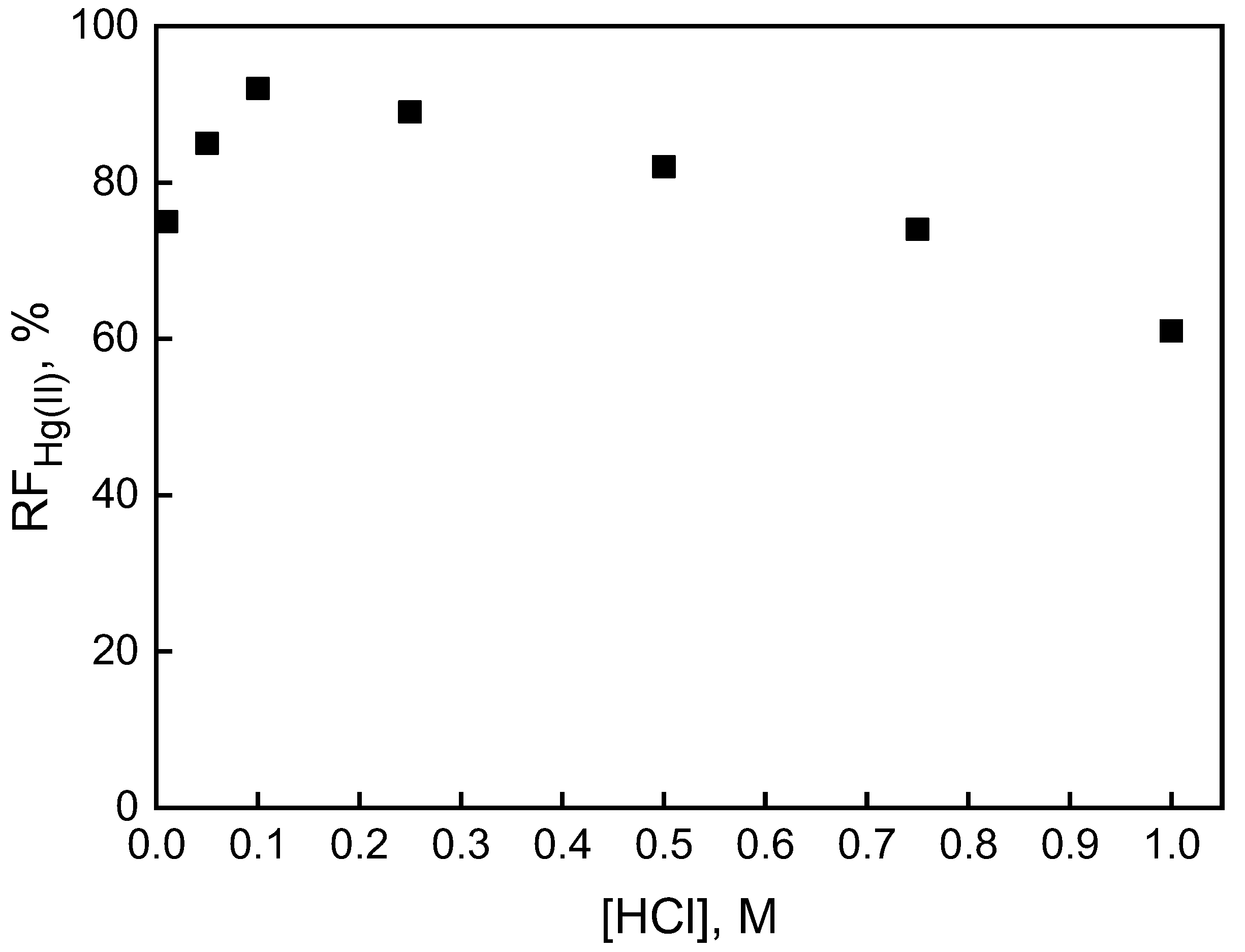
3.2. Modification of the Source Phase Acidity
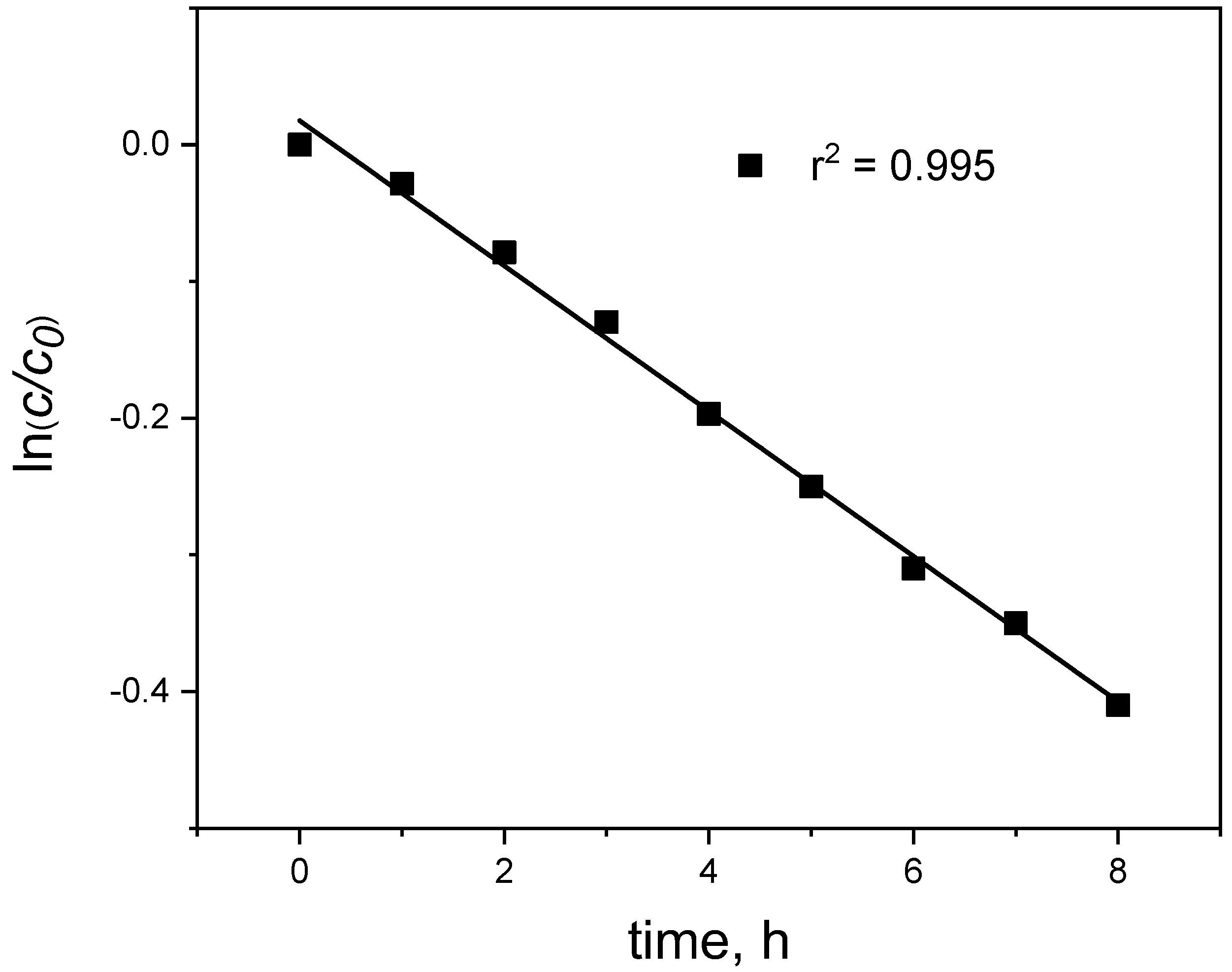
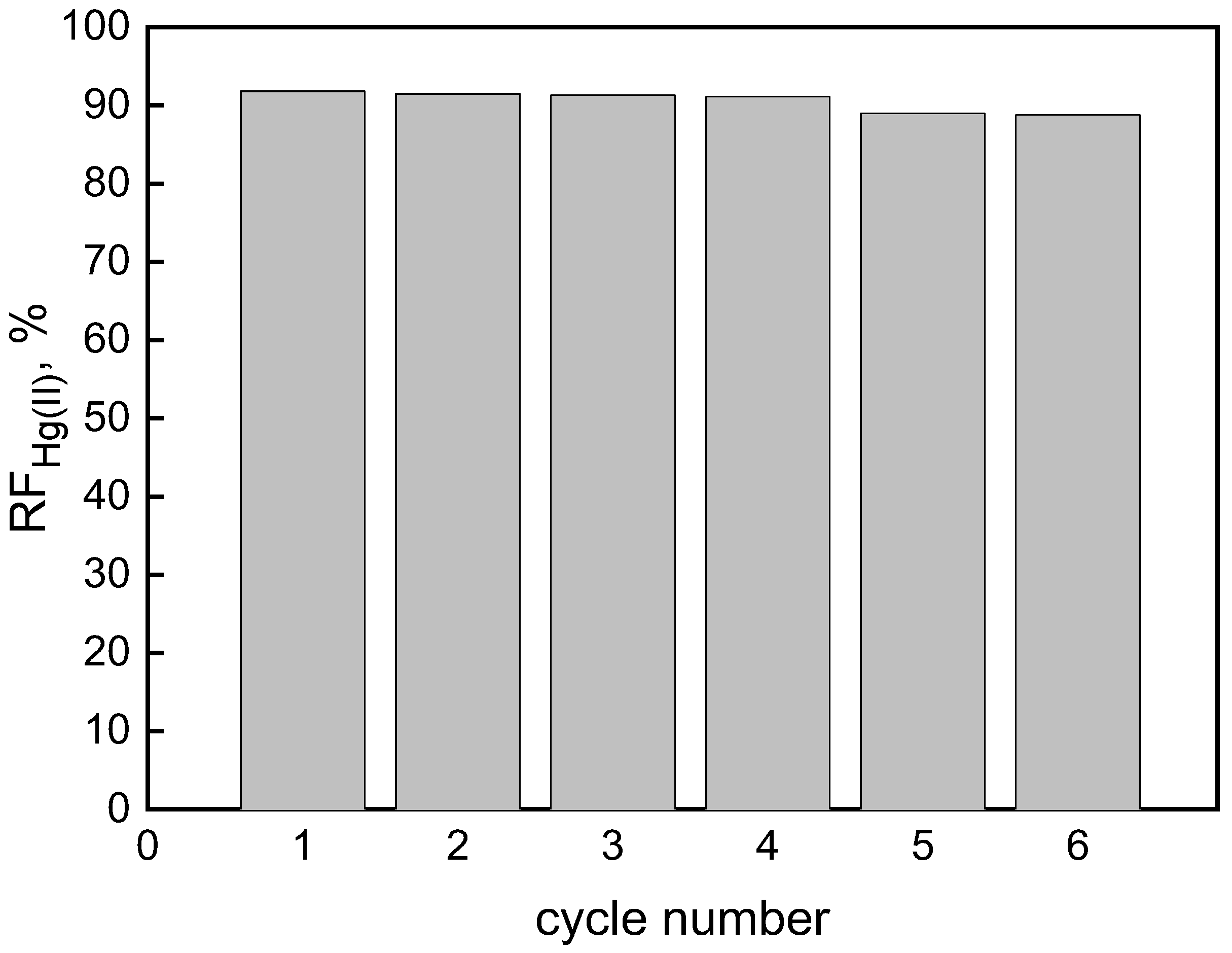
3.3. Membrane Reusability
3.4. Effect of the Type of Agents in the Receiving Phase
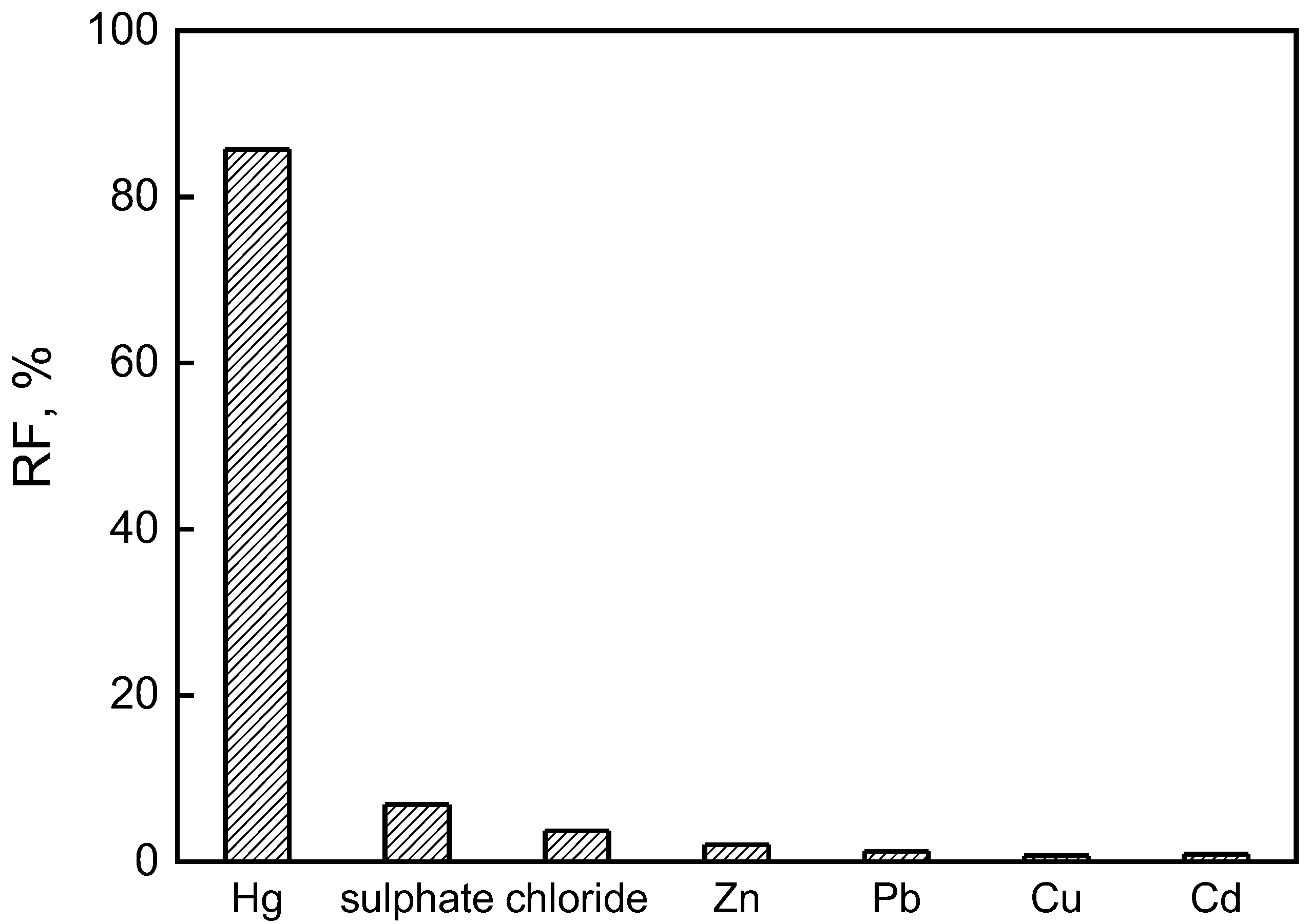
3.5. Removal of Hg(II) from Zinc Smelting Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Jang, Y.-C.; Yo Hong, Y.-S. Substance flow analysis of mercury from industrial and municipal wastewater treatment facilities. Int. J. Appl. Eng. Res. 2017, 12, 5332–5338. [Google Scholar]
- Abbas, K.; Znad, H.; Awual, R. A ligand anchored conjugate adsorbent for effective mercury(II) detection and removal from aqueous media. Chem. Eng. J. 2018, 334, 432–443. [Google Scholar] [CrossRef]
- Kabiri, S.; Diana, N.H.T.; Azari, S.; Losic, D. Graphene-diatom silica aerogels for efficient removal of mercury from water. ACS Appl. Mater. Interfaces 2015, 7, 11815–11823. [Google Scholar] [CrossRef] [PubMed]
- Gajec, M.; Krol, A.; Kukulska-Zajac, E. Determination of metals in selected elements of the environment in the context of applicable legal regulations. Nafta-Gaz 2019, 5, 283–292. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.; Abu-Dieyeh, M.; Khraisheh, M. Adsorptive removal of mercury from water by adsorbents derived from date pits. Sci. Rep. 2019, 9, 15327. [Google Scholar] [CrossRef] [Green Version]
- Hua, K.; Xu, X.; Luo, Z.; Fang, D.; Bao, R.; Yi, J. Effective removal of mercury ions in aqueous solutions: A review. Curr. Nanosci. 2020, 16, 363–375. [Google Scholar] [CrossRef]
- Albakri, M.A.; Saleh, T.A.; Mankour, Y.; Garrison, T.F.; Al Hamouz, O.C.S. Synthesis of a new thiophenol-thiophene polymer for the removal of mercury from wastewater and liquid hydrocarbons. J. Colloid Interface Sci. 2021, 582, 428–438. [Google Scholar] [CrossRef]
- Lecler, M.; Zimmermann, F.; Silvente, E.; Masson, A.; Morele, Y.; Remy, A.; Chollot, A. Improving the work environment in the fuorescent lamp recycling sector by optimizing mercury elimination. Waste Manag. 2018, 76, 250–260. [Google Scholar] [CrossRef]
- Zawierucha, I.; Kozlowski, C.; Malina, G. Immobilized materials for removal of toxic metal ions from surface/groundwaters and aqueous waste streams. Environ. Sci. Proc. Impacts 2016, 18, 429–444. [Google Scholar] [CrossRef]
- Zawierucha, I.; Nowik-Zajac, A.; Kozlowski, C. Application of Cr(VI) transport across the polymer inclusion membrane with calixresorcin4arene derivative as ion carrier. Sep. Sci. Technol. 2020, 55, 2204–2210. [Google Scholar] [CrossRef]
- Zawierucha, I.; Nowik-Zajac, A.; Kozlowski, C. Removal of Pb(II) ions using polymer inclusion membranes containing calix [4]resorcinarene derivative as ion carrier. Polymers 2019, 11, 2111. [Google Scholar] [CrossRef] [Green Version]
- Zawierucha, I.; Nowik-Zajac, A.; Malina, G. Selective removal of As(V) ions from acid mine drainage using polymer inclusion membranes. Minerals 2020, 10, 909. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Pospiech, B.; Kujawski, W. Ionic liquids as selective extractants and ion carriers of heavy metal ions from aqueous solutions utilized in extraction and membrane separation. Rev. Chem. Eng. 2015, 31, 179–191. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Maslowska, K.; Urbaniak, W. Removal of copper (II), zinc (II), cobalt (II), and nickel (II) ions by PIMs doped 2-Alkylimidazoles. Membranes 2022, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Pont, N.; Salvado, V.; Fontas, C. Selective transport and removal of Cd from chloride solutions by polymer inclusion membranes. J. Membr. Sci. 2008, 318, 340–345. [Google Scholar] [CrossRef]
- Asiri, A.M.; Petrosino, F.; Pugliese, V.; Khan, S.B.; Alamry, K.A.; Alfifi, S.Y.; Marwani, H.M.; Alotaibi, M.M.; Algieri, C.; Chakraborty, S. Synthesis and Characterization of Blended Cellulose Acetate Membranes. Polymers 2022, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- de Agreda, D.; Garcia-Diaz, I.; López, F.A.; Alguacil, F.J. Supported liquid membranes technologies in metals removal from liquid effluents. Rev. Metal. 2011, 47, 146–168. [Google Scholar]
- Mercader-Trejo, F.E.; Rodrıguez de San Miguel, E.; de Gyves, J. Mercury(II) removal using polymer inclusion membranes containing Cyanex 471X. J. Chem. Technol. Biotechnol. 2009, 84, 1323–1330. [Google Scholar] [CrossRef]
- Kallithrakas-Kontos, N.; Foteinis, S. Simple and low cost dithizone-functionalized polymer membranes as a tool for mercury preconcentration and monitoring in aqueous bodies. Preliminary results using X-ray absorption near-edge structure (XANES). Glob. Nest J. 2019, 21, 471–476. [Google Scholar]
- García-Beleño, J.; Rodríguez de San Miguel, E. Integration of response surface methodology (RSM) and principal component analysis (PCA) as an optimization tool for polymer inclusion membrane based-optodes designed for Hg(II), Cd(II), and Pb(II). Membranes 2021, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Turull, M.; Elias, G.; Fontàs, C.; Díez, S. Exploring new DGT samplers containing a polymer inclusion membrane for mercury monitoring. Environ. Sci. Pollut. Res. 2017, 24, 10919–10928. [Google Scholar] [CrossRef] [PubMed]
- Elias, G.; Marguí, E.; Díez, S.; Fontàs, C. Polymer inclusion membrane as an effective sorbent to facilitate mercury storage and detection by X-ray fluorescence in natural waters. Anal. Chem. 2018, 90, 4756−4763. [Google Scholar] [CrossRef] [PubMed]
- Elias, G.; Díez, S.; Fontàs, C. System for mercury preconcentration in natural waters based on a polimer inclusion membrane incorporating an ionic liquid. J. Hazard. Mater. 2019, 371, 316–322. [Google Scholar] [CrossRef]
- Nowik-Zajac, A.; Zawierucha, I.; Kozlowski, C. Selective transport of Ag(I) through a polymer inclusion membrane containing a calix[4]pyrrole derivative from nitrate aqueous solutions. Int. J. Mol. Sci. 2020, 21, 5348. [Google Scholar] [CrossRef] [PubMed]
- Nowik-Zajac, A.; Zawierucha, I.; Kozlowski, C. Selective removal of silver(I) using polymer inclusion membranes containing calixpyrroles. RSC Adv. 2019, 9, 31122–31132. [Google Scholar] [CrossRef] [Green Version]
- Gale, P.A.; Anzenbacher, P., Jr.; Sessler, J.L. Calixpyrroles II. Coord. Chem. Rev. 2001, 222, 57–102. [Google Scholar] [CrossRef]
- Lappano, R.; Rosano, C.; Pisano, A.; Santolla, M.F.; De Francesco, E.M.; De Marco, P.; Dolce, V.; Ponassi, M.; Felli, L.; Cafeo, G.; et al. A calixpyrrole derivative acts as an antagonist to GPER, a G-protein coupled receptor: Mechanisms and models. Dis. Model. Mech. 2015, 8, 1237–1246. [Google Scholar]
- Baeyer, A. Ueber ein Condensations product von Pyrrol mit Aceton. Ber. Dtsch. Chem. Ges. 1886, 19, 2184–2185. [Google Scholar] [CrossRef] [Green Version]
- Rothemund, P.; Gage, C.L. Concerning the structure of “Acetonepyrrole”. J. Am. Chem. Soc. 1995, 77, 3340–3342. [Google Scholar] [CrossRef]
- Alguacil, F.J.; López-Delgado, A.; Alonso, M.; Sastre, A.M. The phosphine oxides Cyanex 921 and Cyanex 923 as carriers for facilitated transport of chromium (VI)-chloride aqueous solutions. Chemosphere 2004, 57, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.; Figoli, A. Arsenic removal by liquid membranes. Membranes 2015, 5, 150–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, A.; Alpoguz, H.K.; Yilmaz, A. Application of Cr(VI) transport through the polymer inclusion membrane with a new synthesized calix[4]arene derivative. Ind. Eng. Chem. Res. 2013, 52, 5428–5436. [Google Scholar] [CrossRef]
- Konczyk, J.; Ciesielski, W. Calixresorcin[4]arene-Mediated Transport of Pb(II) Ions Through Polymer Inclusion Membrane. Membranes 2021, 11, 285. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (EPA). Method 8061A: Phthalate Esters by Gas Chromatography with Electron Capture Detection (GC/ECD); US Environmental Protection Agency: Cincinnati, OH, USA, 1996. [Google Scholar]
- Chen, X.; Xu, S.; Tan, T.; Lee, S.T.; Cheng, S.H.; Lee, F.W.F.; Xu, S.J.L.; Ho, K.C. Toxicity and estrogenic endocrine disrupting activity of phthalates and their mixtures. Int. J. Environ. Res. Public Health 2014, 11, 3156–3168. [Google Scholar] [CrossRef]
- Baczynska, M.; Regel-Rosocka, M.; Nowicki, M.; Wisniewski, M. Effect of the structure of polymer inclusion membranes on Zn(II) transport from chloride aqueous solutions. J. Appl. Polym. Sci. 2015, 42319–42330. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Bracco, G., Holst, B., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2013; Volume 51, pp. 3–34. [Google Scholar]
- Marr, R.; Cop, A. Liquid membrane technology—A survey of phenomena, mechanisms and models. Int. Chem. Eng. 1982, 22, 44–59. [Google Scholar]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Fu, J. Lead and zinc smelting wastewater treatment and reclamation by coagulation-flocculation-sedimentation, ultrafiltration and reverse osmosis technique. J. Energy Environ. Chem. Eng. 2021, 6, 94–105. [Google Scholar]
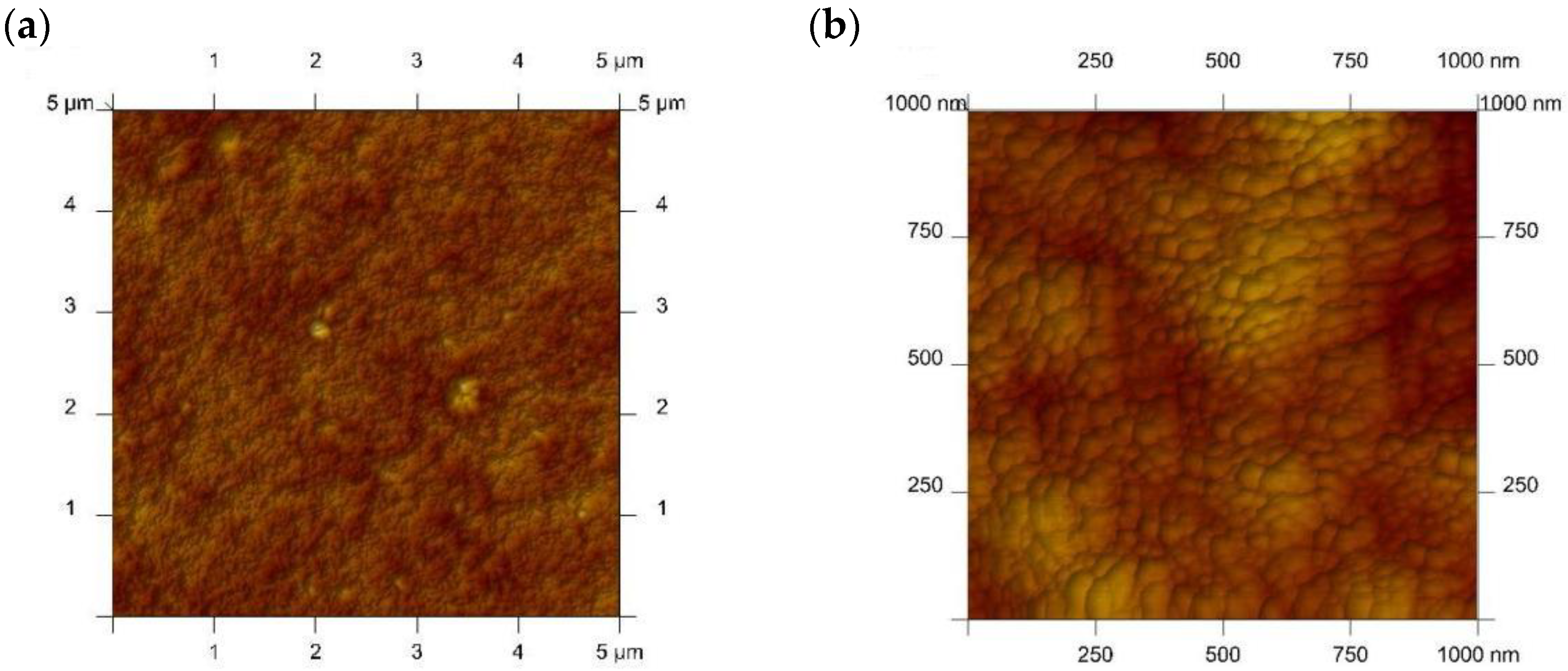
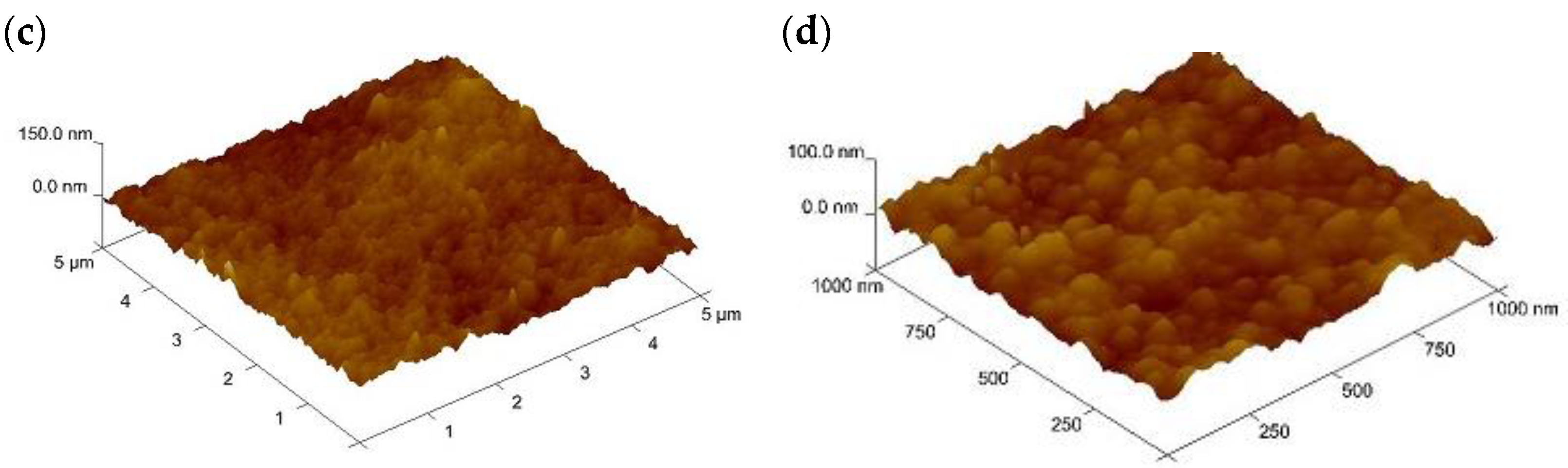
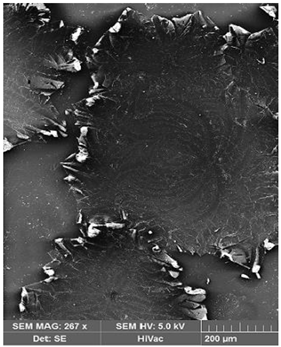
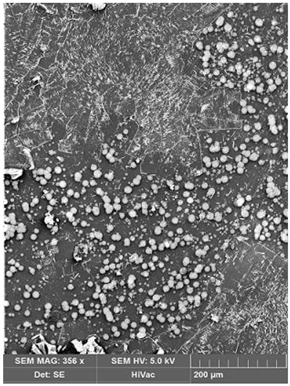
| Before Transport of Hg(II) Ions | After Transport of Hg(II) Ions |
|---|---|
 |  |
| Before Transport of Hg(II) Ions across the PIM | After Transport of Hg(II) Ions across the PIM |
|---|---|
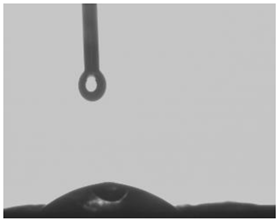 25.26° | 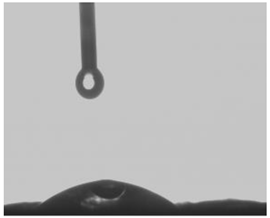 48.26° |
| Receiving Agent | Percentage of Hg(II) Transported into the Receiving Phase | Percentage of Hg(II) Remaining in the Receiving Phase |
|---|---|---|
| 0.1 M NaCl | 92 | 4 |
| 0.1 M KI distilled water | 67 54 | 14 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawierucha, I.; Nowik-Zajac, A.; Lagiewka, J.; Malina, G. Separation of Mercury(II) from Industrial Wastewater through Polymer Inclusion Membranes with Calix[4]pyrrole Derivative. Membranes 2022, 12, 492. https://doi.org/10.3390/membranes12050492
Zawierucha I, Nowik-Zajac A, Lagiewka J, Malina G. Separation of Mercury(II) from Industrial Wastewater through Polymer Inclusion Membranes with Calix[4]pyrrole Derivative. Membranes. 2022; 12(5):492. https://doi.org/10.3390/membranes12050492
Chicago/Turabian StyleZawierucha, Iwona, Anna Nowik-Zajac, Jakub Lagiewka, and Grzegorz Malina. 2022. "Separation of Mercury(II) from Industrial Wastewater through Polymer Inclusion Membranes with Calix[4]pyrrole Derivative" Membranes 12, no. 5: 492. https://doi.org/10.3390/membranes12050492
APA StyleZawierucha, I., Nowik-Zajac, A., Lagiewka, J., & Malina, G. (2022). Separation of Mercury(II) from Industrial Wastewater through Polymer Inclusion Membranes with Calix[4]pyrrole Derivative. Membranes, 12(5), 492. https://doi.org/10.3390/membranes12050492










