Effects of Different Draw Solutions on Biogas Slurry Concentration in Forward Osmosis Membrane: Performance and Membrane Fouling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Materials
2.2. Experimental Protocol
2.3. Analytic Methods
3. Results and Discussion
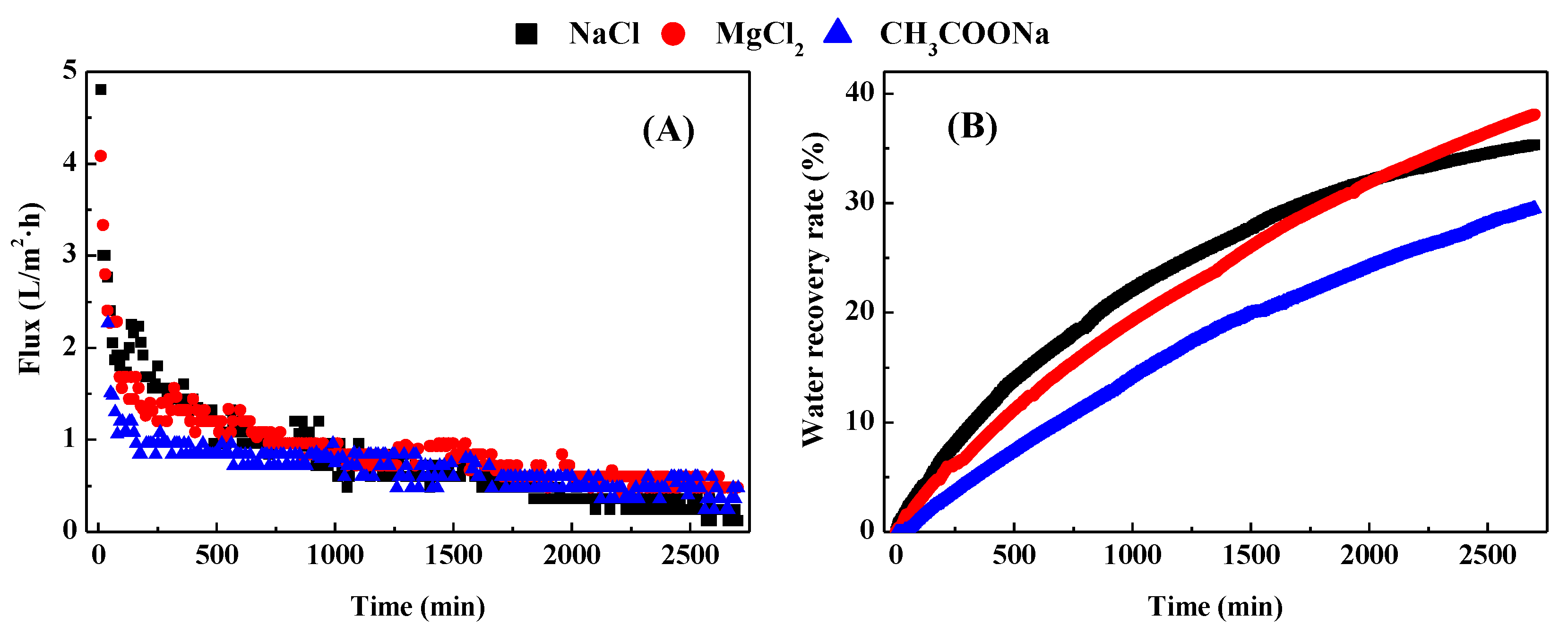
3.1. Water Flux and Water Recovery Rate
3.2. Basic Characteristics, Organic and Nutrient Enrichment
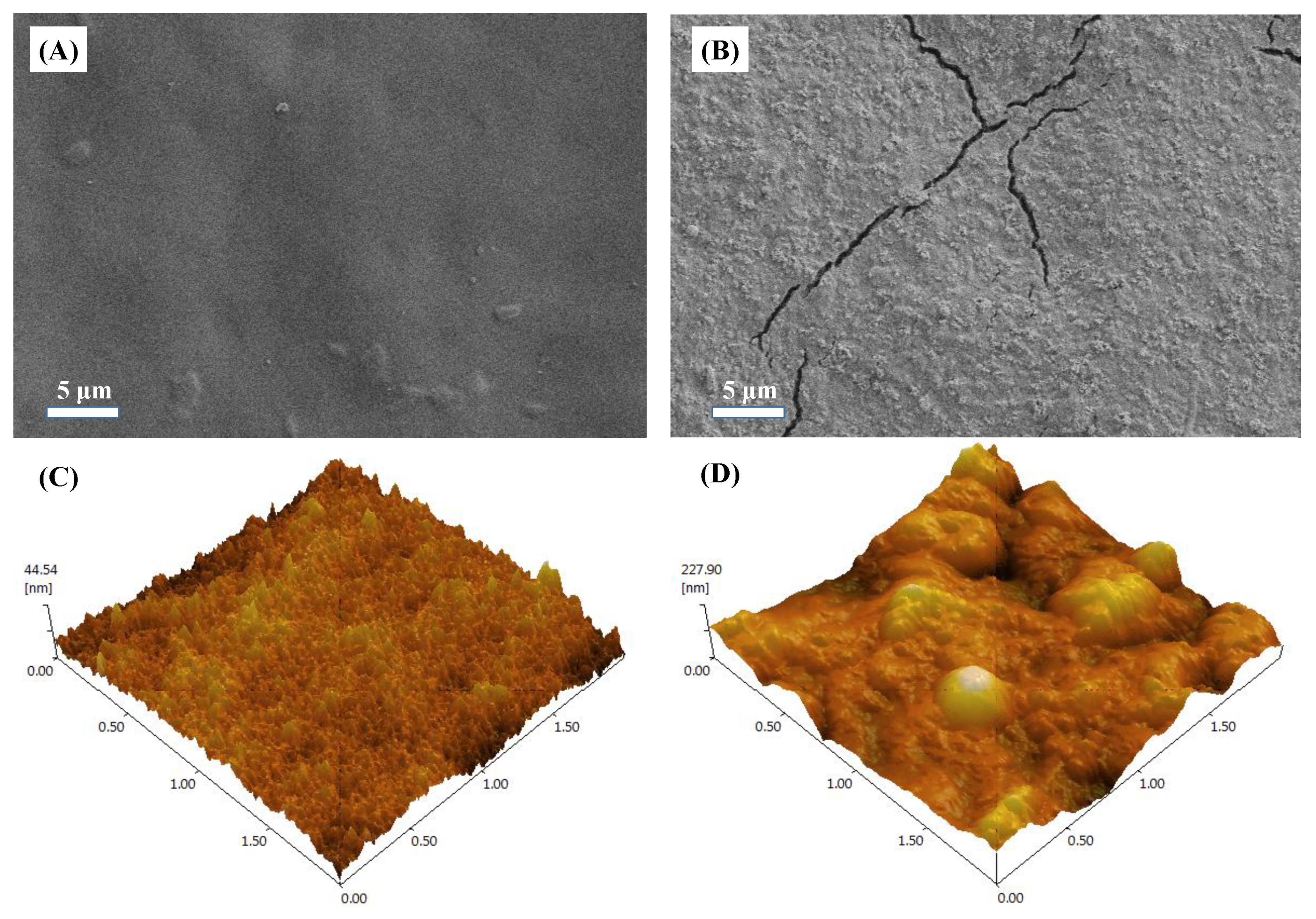
3.3. Membrane Surface Topography
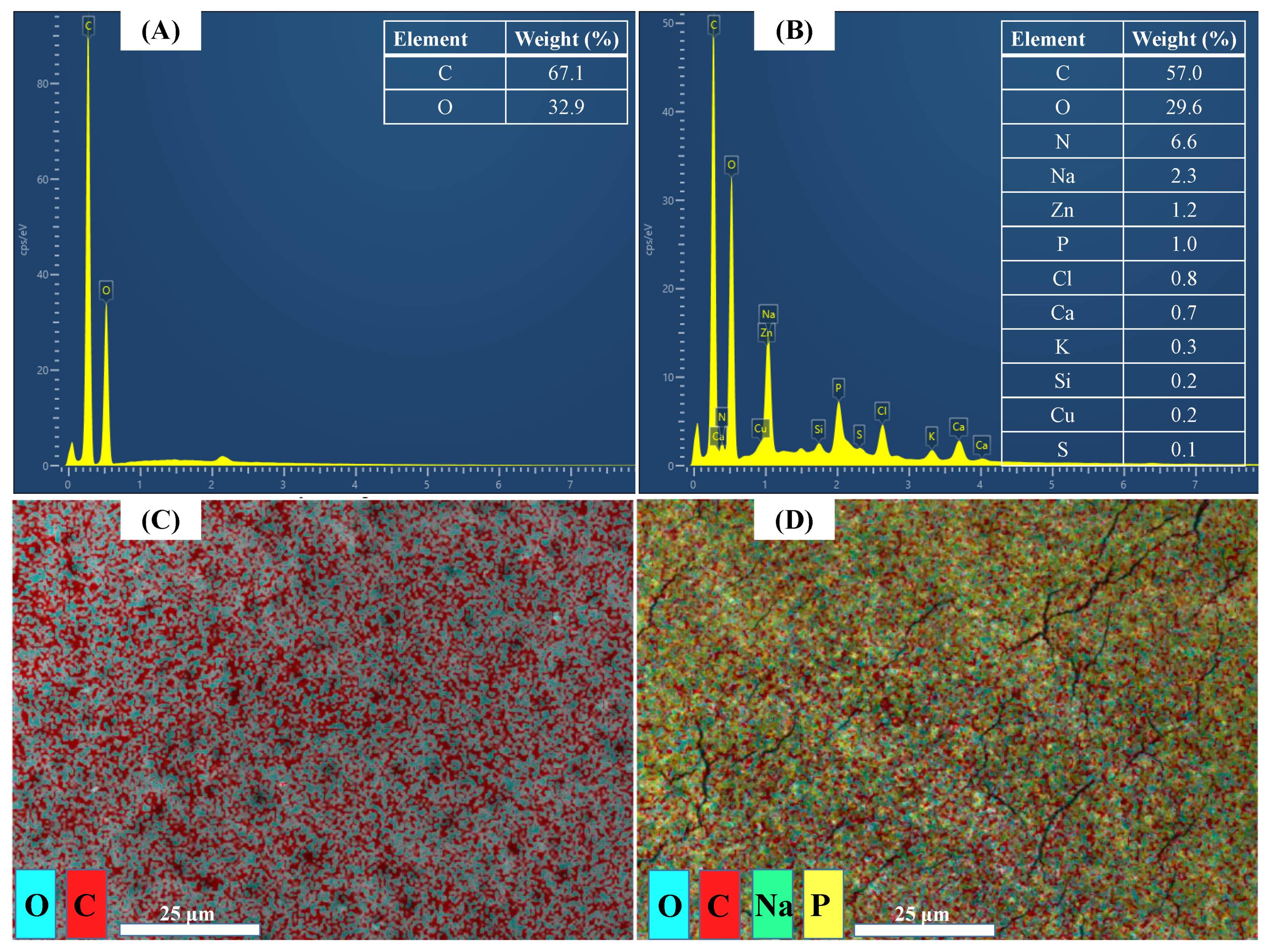
3.4. Membrane Surface Element Distribution
3.5. Membrane Surface Functional Groups
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamari, M.L.; Maleki, A.; Nazari, M.A.; Sadeghi, M.; Rosen, M.A.; Pourfayaz, F. Assessment of a biomass-based polygeneration plant for combined power, heat, bioethanol and biogas. Appl. Therm. Eng. 2021, 198, 117425. [Google Scholar] [CrossRef]
- Zaks, D.P.M.; Winchester, N.; Kucharik, C.J.; Barford, C.C.; Paltsev, S.; Reilly, J.M. Contribution of Anaerobic Digesters to Emissions Mitigation and Electricity Generation Under U.S. Climate Policy. Environ. Sci. Technol. 2011, 45, 6735–6742. [Google Scholar] [CrossRef] [PubMed]
- Gioelli, F.; Dinuccio, E.; Balsari, P. Residual biogas potential from the storage tanks of non-separated digestate and digested liquid fraction. Bioresour. Technol. 2011, 102, 10248–10251. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Jin, H.; Chang, Z. Distribution of Heavy Metal Contents and Chemical Fractions in Anaerobically Digested Manure Slurry. Appl. Biochem. Biotechnol. 2011, 164, 268–282. [Google Scholar] [CrossRef]
- Perazzolo, F.; Mattachini, G.; Riva, E.; Provolo, G. Nutrient Losses during Winter and Summer Storage of Separated and Unseparated Digested Cattle Slurry. J. Environ. Qual. 2017, 46, 879–888. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Li, G.; Luo, W.; Sun, Y. Manure digestate storage under different conditions: Chemical characteristics and contaminant residuals. Sci. Total Environ. 2018, 639, 19–25. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef] [Green Version]
- Akhiar, A.; Battimelli, A.; Torrijos, M.; Carrere, H. Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manag. 2017, 59, 118–128. [Google Scholar] [CrossRef]
- Xie, M.; Shon, H.K.; Gray, S.R.; Elimelech, M. Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future direction. Water Res. 2016, 89, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Masse, L.; Massé, D.I.; Pellerin, Y. The use of membranes for the treatment of manure: A critical literature review. Biosyst. Eng. 2007, 98, 371–380. [Google Scholar] [CrossRef]
- Tampio, E.; Marttinen, S.; Rintala, J. Liquid fertilizer products from anaerobic digestion of food waste: Mass, nutrient and energy balance of four digestate liquid treatment systems. J. Clean. Prod. 2016, 125, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Konieczny, K.; Kwiecińska, A.; Gworek, B. The recovery of water from slurry produced in high density livestock farming with the use of membrane processes. Sep. Purif. Technol. 2011, 80, 490–498. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Y.; Zhou, M.; Liu, M.; Yu, S.; Gao, C. Comparative study on the treatment of raw and biologically treated textile effluents through submerged nanofiltration. J. Hazard. Mater. 2015, 284, 121–129. [Google Scholar] [CrossRef]
- Lidén, A.; Persson, K.M. Feasibility Study of Advanced NOM-Reduction by Hollow Fiber Ultrafiltration and Nanofiltration at a Swedish Surface Water Treatment Plant. Water 2016, 8, 150. [Google Scholar] [CrossRef] [Green Version]
- Madaeni, S.S.; Eslamifard, M.R. Recycle unit wastewater treatment in petrochemical complex using reverse osmosis process. J. Hazard. Mater. 2010, 174, 404–409. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Guo, W.; Ngo, H.H.; Price, W.E.; Nghiem, L.D. Factors governing the pre-concentration of wastewater using forward osmosis for subsequent resource recovery. Sci. Total Environ. 2016, 566–567, 559–566. [Google Scholar] [CrossRef]
- Iskander, S.M.; Zou, S.; Brazil, B.; Novak, J.T.; He, Z. Energy consumption by forward osmosis treatment of landfill leachate for water recovery. Waste Manag. 2017, 63, 284–291. [Google Scholar] [CrossRef]
- Xue, W.; Yamamoto, K.; Tobino, T. Membrane fouling and long-term performance of seawater-driven forward osmosis for enrichment of nutrients in treated municipal wastewater. J. Membr. Sci. 2016, 499, 555–562. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Organic fouling of forward osmosis membranes: Fouling reversibility and cleaning without chemical reagents. J. Membr. Sci. 2010, 348, 337–345. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Xie, M.; Zhang, B.; Li, G.; Luo, W. Resource recovery from digested manure centrate: Comparison between conventional and aquaporin thin-film composite forward osmosis membranes. J. Membr. Sci. 2020, 593, 117436. [Google Scholar] [CrossRef]
- Zhang, J.; Lü, F.; Shao, L.; He, P. The use of biochar-amended composting to improve the humification and degradation of sewage sludge. Bioresour. Technol. 2014, 168, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.J.; Hai, F.I.; Guo, W.; Ngo, H.H.; Price, W.E.; Nghiem, L.D. Selection of forward osmosis draw solutes for subsequent integration with anaerobic treatment to facilitate resource recovery from wastewater. Bioresour. Technol. 2015, 191, 30–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancock, N.T.; Cath, T.Y. Solute Coupled Diffusion in Osmotically Driven Membrane Processes. Environ. Sci. Technol. 2009, 43, 6769–6775. [Google Scholar] [CrossRef]
- Luo, W.; Hai, F.I.; Price, W.E.; Elimelech, M.; Nghiem, L.D. Evaluating ionic organic draw solutes in osmotic membrane bioreactors for water reuse. J. Membr. Sci. 2016, 514, 636–645. [Google Scholar] [CrossRef] [Green Version]
- Achilli, A.; Cath, T.Y.; Marchand, E.A.; Childress, A.E. The forward osmosis membrane bioreactor: A low fouling alternative to MBR processes. Desalination 2009, 239, 10–21. [Google Scholar] [CrossRef]
- Qiu, G.; Ting, Y.-P. Osmotic membrane bioreactor for wastewater treatment and the effect of salt accumulation on system performance and microbial community dynamics. Bioresour. Technol. 2013, 150, 287–297. [Google Scholar] [CrossRef]
- Schneider, C.; Rajmohan, R.S.; Zarebska, A.; Tsapekos, P.; Hélix-Nielsen, C. Treating anaerobic effluents using forward osmosis for combined water purification and biogas production. Sci. Total Environ. 2019, 647, 1021–1030. [Google Scholar] [CrossRef]
- Ledda, C.; Schievano, A.; Salati, S.; Adani, F. Nitrogen and water recovery from animal slurries by a new integrated ultrafiltration, reverse osmosis and cold stripping process: A case study. Water Res. 2013, 47, 6157–6166. [Google Scholar] [CrossRef]
- Holloway, R.W.; Childress, A.E.; Dennett, K.E.; Cath, T.Y. Forward osmosis for concentration of anaerobic digester centrate. Water Res. 2007, 41, 4005–4014. [Google Scholar] [CrossRef] [PubMed]
- Irvine, G.J.; Rajesh, S.; Georgiadis, M.; Phillip, W.A. Ion Selective Permeation Through Cellulose Acetate Membranes in Forward Osmosis. Environ. Sci. Technol. 2013, 47, 13745–13753. [Google Scholar] [CrossRef] [PubMed]
- Masse, L.; Massé, D.I.; Pellerin, Y. The effect of pH on the separation of manure nutrients with reverse osmosis membranes. J. Membr. Sci. 2008, 325, 914–919. [Google Scholar] [CrossRef]
- Mondor, M.; Masse, L.; Ippersiel, D.; Lamarche, F.; Massé, D.I. Use of electrodialysis and reverse osmosis for the recovery and concentration of ammonia from swine manure. Bioresour. Technol. 2008, 99, 7363–7368. [Google Scholar] [CrossRef]
- Linares, R.V.; Li, Z.; Abu-Ghdaib, M.; Wei, C.-H.; Amy, G.; Vrouwenvelder, J.S. Water harvesting from municipal wastewater via osmotic gradient: An evaluation of process performance. J. Membr. Sci. 2013, 447, 50–56. [Google Scholar] [CrossRef]
- Xue, W.; Tobino, T.; Nakajima, F.; Yamamoto, K. Seawater-driven forward osmosis for enriching nitrogen and phosphorous in treated municipal wastewater: Effect of membrane properties and feed solution chemistry. Water Res. 2015, 69, 120–130. [Google Scholar] [CrossRef]
- Kedwell, K.C.; Quist-Jensen, C.A.; Giannakakis, G.; Christensen, M.L. Forward osmosis with high-performing TFC membranes for concentration of digester centrate prior to phosphorus recovery. Sep. Purif. Technol. 2018, 197, 449–456. [Google Scholar] [CrossRef]
- Wu, Z.; Zou, S.; Zhang, B.; Wang, L.; He, Z. Forward osmosis promoted in-situ formation of struvite with simultaneous water recovery from digested swine wastewater. Chem. Eng. J. 2018, 342, 274–280. [Google Scholar] [CrossRef]
- Chen, R.; Nie, Y.; Hu, Y.; Miao, R.; Utashiro, T.; Li, Q.; Xu, M.; Li, Y.-Y. Fouling behaviour of soluble microbial products and extracellular polymeric substances in a submerged anaerobic membrane bioreactor treating low-strength wastewater at room temperature. J. Membr. Sci. 2017, 531, 1–9. [Google Scholar] [CrossRef]
- Basri, H.; Ismail, A.F.; Aziz, M. Microstructure and anti-adhesion properties of PES/TAP/Ag hybrid ultrafiltration membrane. Desalination 2012, 287, 71–77. [Google Scholar] [CrossRef]
- Ivnitsky, H.; Katz, I.; Minz, D.; Volvovic, G.; Shimoni, E.; Kesselman, E.; Semiat, R.; Dosoretz, C.G. Bacterial community composition and structure of biofilms developing on nanofiltration membranes applied to wastewater treatment. Water Res. 2007, 41, 3924–3935. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; de O. Manes, C.-L.; Aubry, C.; Gutierrez, L.; Croue, J.P. Kinetic Study of Seawater Reverse Osmosis Membrane Fouling. Environ. Sci. Technol. 2013, 47, 10884–10894. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Arhatari, B.; Gray, S.R.; Xie, M. Seeing is believing: Insights from synchrotron infrared mapping for membrane fouling in osmotic membrane bioreactors. Water Res. 2018, 137, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Teng, J.; Liao, B.-Q.; Li, R.; Lin, H. Molecular insights into the impacts of iron(III) ions on membrane fouling by alginate. Chemosphere 2020, 242, 125232. [Google Scholar] [CrossRef]
- Soler-Cabezas, J.L.; Mendoza-Roca, J.A.; Vincent-Vela, M.C.; Luján-Facundo, M.J.; Pastor-Alcañiz, L. Simultaneous concentration of nutrients from anaerobically digested sludge centrate and pre-treatment of industrial effluents by forward osmosis. Sep. Purif. Technol. 2018, 193, 289–296. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xie, X.; Yin, R.; Dong, Q.; Wei, Q.; Zhang, B. Effects of Different Draw Solutions on Biogas Slurry Concentration in Forward Osmosis Membrane: Performance and Membrane Fouling. Membranes 2022, 12, 476. https://doi.org/10.3390/membranes12050476
Li Y, Xie X, Yin R, Dong Q, Wei Q, Zhang B. Effects of Different Draw Solutions on Biogas Slurry Concentration in Forward Osmosis Membrane: Performance and Membrane Fouling. Membranes. 2022; 12(5):476. https://doi.org/10.3390/membranes12050476
Chicago/Turabian StyleLi, Yun, Xiaomin Xie, Rongxiu Yin, Qingzhao Dong, Quanquan Wei, and Bangxi Zhang. 2022. "Effects of Different Draw Solutions on Biogas Slurry Concentration in Forward Osmosis Membrane: Performance and Membrane Fouling" Membranes 12, no. 5: 476. https://doi.org/10.3390/membranes12050476
APA StyleLi, Y., Xie, X., Yin, R., Dong, Q., Wei, Q., & Zhang, B. (2022). Effects of Different Draw Solutions on Biogas Slurry Concentration in Forward Osmosis Membrane: Performance and Membrane Fouling. Membranes, 12(5), 476. https://doi.org/10.3390/membranes12050476






