Value-Added Utilization of Wheat Straw: From Cellulose and Cellulose Nanofiber to All-Cellulose Nanocomposite Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of CNF
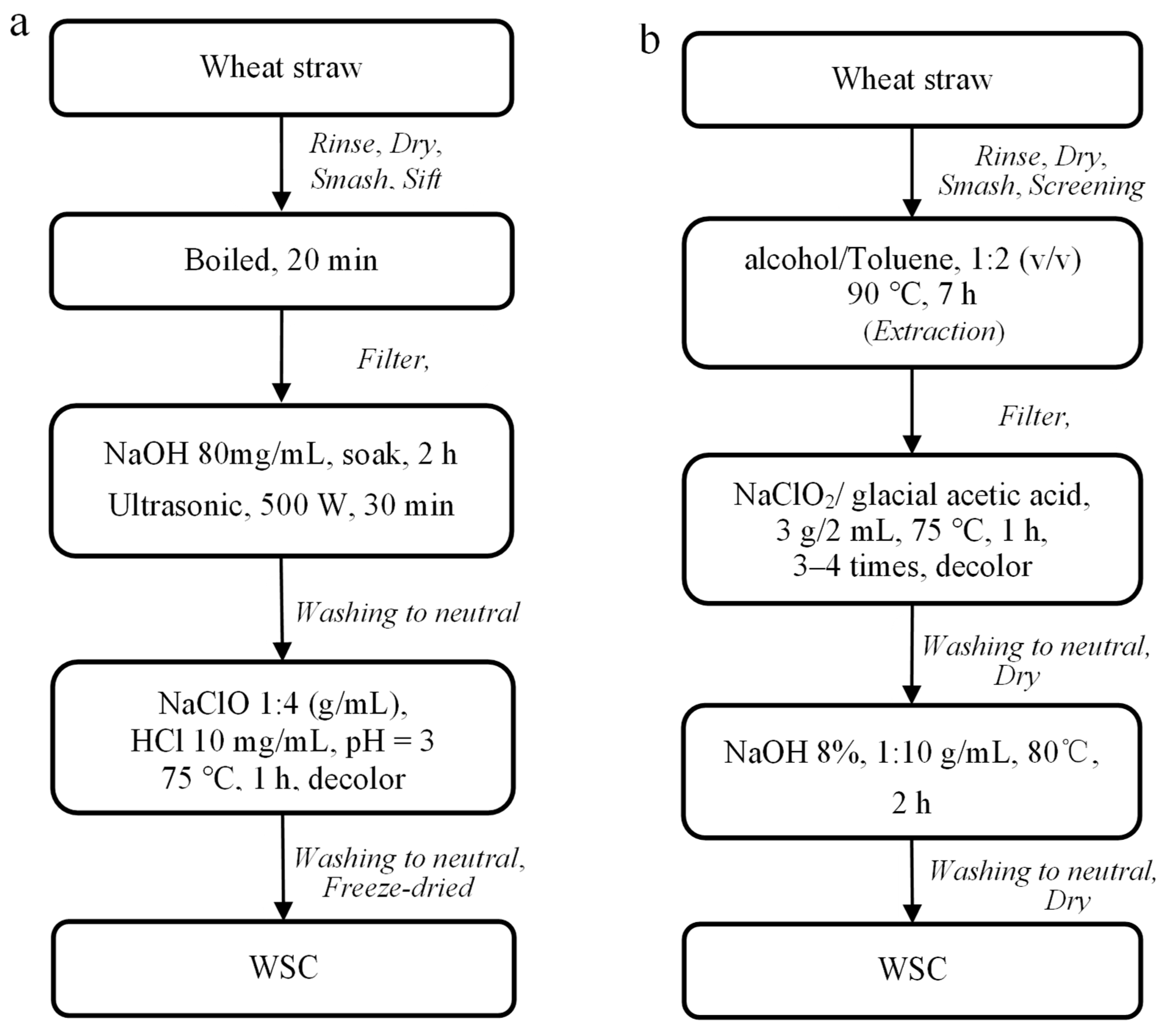
2.2.2. Preparation of WSC
2.2.3. Fabrication of All-Cellulose Nanocomposites
2.3. Instrumental Characterizations
2.3.1. CNF and Film Morphology
2.3.2. Crystal Structure
2.3.3. Thermal Stability
2.3.4. Mechanical Properties
3. Results and Discussion
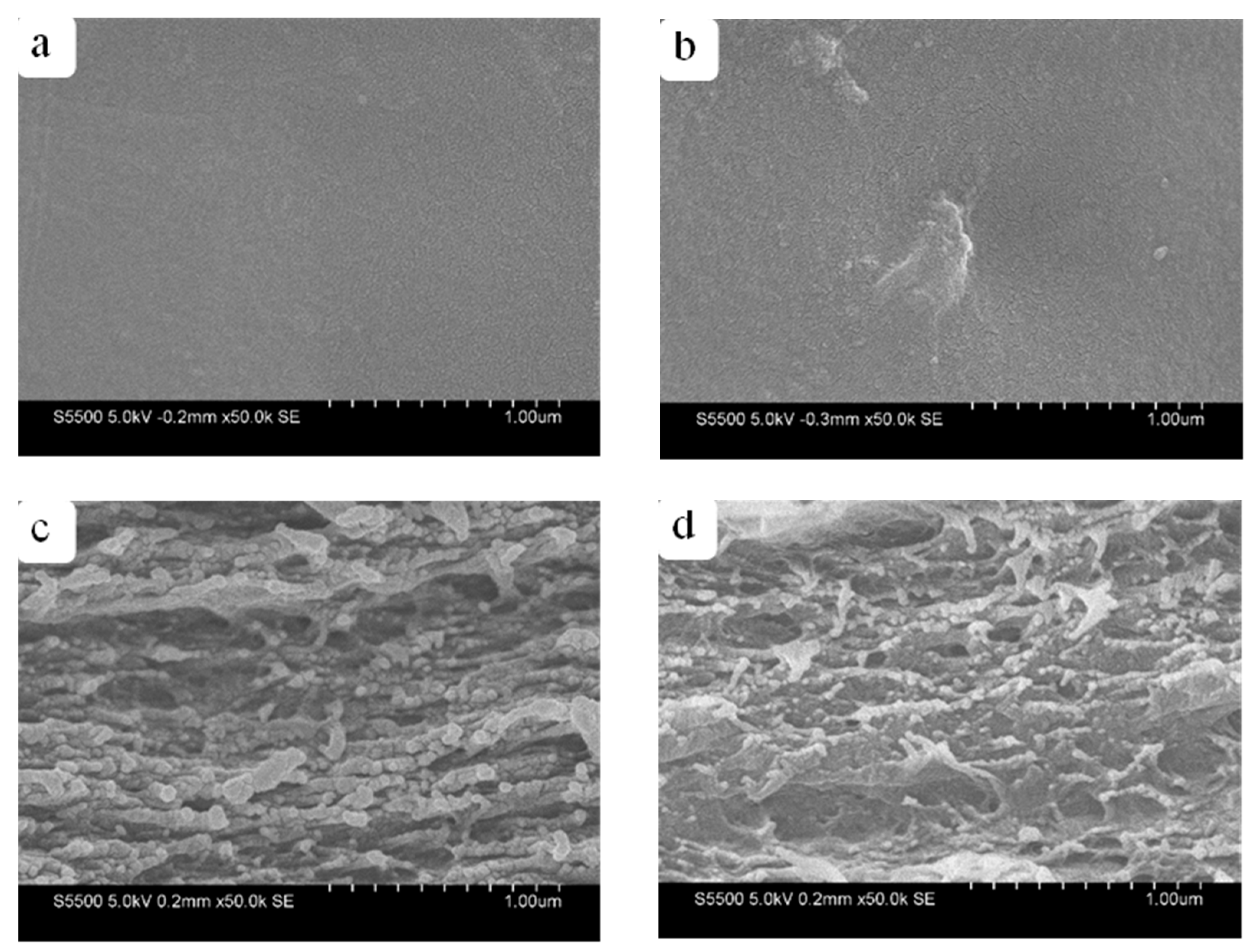
3.1. Morphology of CNFs, RWSC, and All-Cellulose Nanocomposites
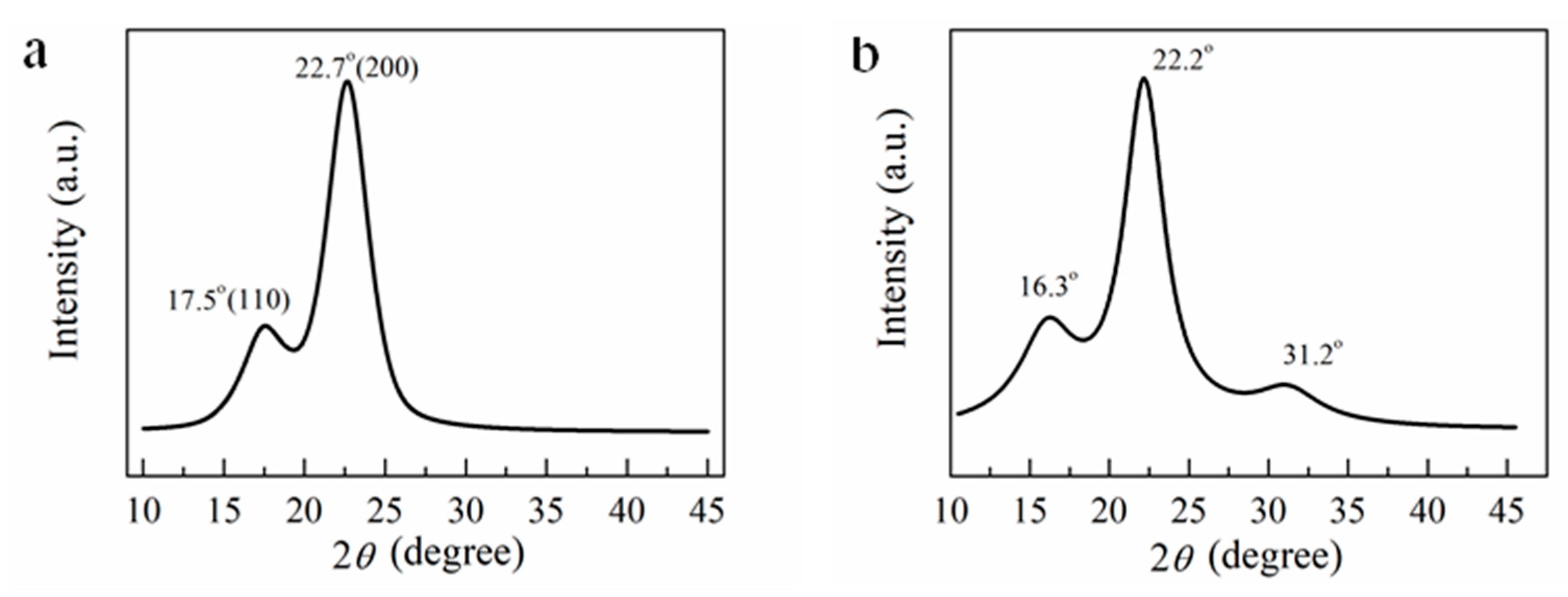
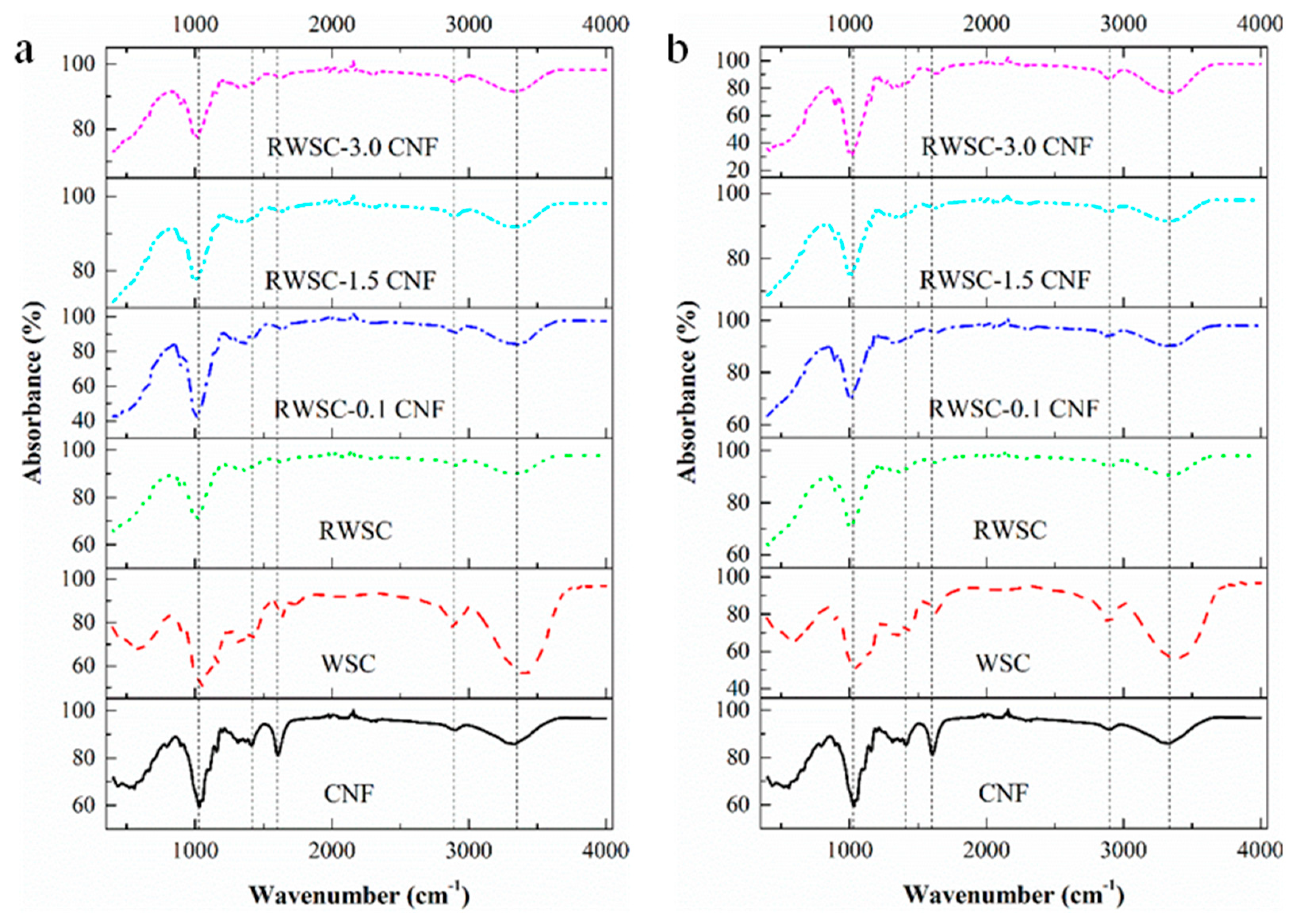
3.2. Structural Characteristics of CNF, RWSC and All−Cellulose Nanocomposites
3.3. Thermal Performance Analysis
3.4. Mechanical Properties of the All-Cellulose Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Statistical Bulletin of National Economic and Social Development of the People’s Republic of China in 2020 (Agriculture). 2021. Available online: http://www.stats.gov.cn/tjsj/zxfb/202102/t20210227_1814154.html (accessed on 28 February 2021).
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Conversion of biomass to biofuels and life cycle assessment: A review. Environ. Chem. Lett. 2021, 19, 4075–4118. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and application of granular activated carbon from biomass waste materials for water treatment: A review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Y.; Ma, C.; Gu, J. Research progress in the application of cellulose and its derivatives. Mater. Rep. 2016, 30, 75–82. [Google Scholar]
- Hon, D.N.-S. Cellulose: A random walk along its historical path. Cellulose 1994, 1, 1–25. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Buranov, A.U.; Mazza, G. Lignin in straw of herbaceous crops. Ind. Crops Prod. 2008, 28, 237–259. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.; Quick, A.; Taha, M.; Mignard, N.; Becquart, F.; Ayoub, A. Hemicellulose extraction and characterization for applications in paper coatings and adhesives. Ind. Crops Prod. 2017, 107, 370–377. [Google Scholar] [CrossRef]
- Sun, R.C.; Tomkinson, J.; Ma, P.L.; Liang, S.F. Comparative study of hemicelluloses from rice straw by alkali and hydrogen peroxide treatments. Carbohydr. Polym. 2000, 42, 111–122. [Google Scholar] [CrossRef]
- Jin, A.X.; Ren, J.L.; Peng, F.; Xu, F.; Zhou, G.Y.; Sun, R.C.; Kennedy, J.F. Comparative characterization of degraded and non-degradative hemicelluloses from barley straw and maize stems: Composition, structure, and thermal properties. Carbohydr. Polym. 2009, 78, 609–619. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Zhao, X.; Han, Z. Comparative study on the method of extracting straw cellulose. Chin. Agric. Sci. Bull. 2013, 29, 130–134. [Google Scholar]
- Miao, X.; Lin, J.; Tian, F.; Li, X.; Bian, F.; Wang, J. Cellulose nanofibrils extracted from the byproduct of cotton plant. Carbohydr. Polym. 2016, 136, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Pham, K.A.; Breedveld, V. Rheological characterization and modeling of cellulose nanocrystal and TEMPO-oxidized cellulose nanofibril suspensions. Cellulose 2020, 27, 3741–3757. [Google Scholar] [CrossRef]
- Dong, X.M.; Revol, J.-F.; Gray, D.G. Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 1998, 5, 19–32. [Google Scholar] [CrossRef]
- Huq, T.; Salmieri, S.; Khan, A.; Khan, R.A.; Le Tien, C.; Riedl, B.; Fraschini, C.; Bouchard, J.; Uribe-Calderon, J.; Kamal, M.R.; et al. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr. Polym. 2012, 90, 1757–1763. [Google Scholar] [CrossRef]
- Yang, Q.; Saito, T.; Berglund, L.A.; Isogai, A. Cellulose nanofibrils improve the properties of all-cellulose composites by the nano-reinforcement mechanism and nanofibril-induced crystallization. Nanoscale 2015, 7, 17957–17963. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Song, Y.S. Viscoelastic characteristics of all cellulose suspension and nanocomposite. Carbohydr. Polym. 2016, 151, 119–129. [Google Scholar] [CrossRef]
- Lomelí-Ramírez, M.G.; Valdez-Fausto, E.M.; Rentería-Urquiza, M.; Jiménez-Amezcua, R.M.; Hernández, J.A.; Torres-Rendon, J.G.; Enriquez, S.G. Study of green nanocomposites based on corn starch and cellulose nanofibrils from Agave tequilana Weber. Carbohydr. Polym. 2018, 201, 9–19. [Google Scholar] [CrossRef]
- Wu, T.; Cai, B.; Wang, J.; Zhang, C.; Shi, Z.; Yang, Q.; Hu, G.H.; Xiong, C. TEMPO-oxidized cellulose nanofibril/layered double hydroxide nanocomposite films with improved hydrophobicity, flame retardancy and mechanical properties. Compos. Sci. Technol. 2019, 171, 111–117. [Google Scholar] [CrossRef]
- Jayanth Babu, K.; Sasya, M.; Nesakumarl, N.; Shankar, P.; Gumpu, M.B.; Ramachandra, B.L.; Kulandaisamy, A.J.; Rayappan, J.B.B. Non-enzymatic detection of glucose in fruits using TiO2–Mn3O4 hybrid nano interface. Appl. Nanosci. 2017, 7, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Nishino, T.; Matsuda, I.; Hirao, K. All-cellulose composite. Marcromolecules 2004, 37, 7683–7687. [Google Scholar] [CrossRef]
- Zhao, J.; He, X.; Wang, Y.; Zhang, W.; Zhang, X.; Zhang, X.; Deng, Y.; Lu, C. Reinforcement of all-cellulose nanocomposite films using native cellulose nanofibrils. Carbohydr. Polym. 2014, 104, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, Q.; Zhang, X.; Ren, S.; Lei, T.; Li, W.; Xu, G.; Zhang, Q. Nanocellulose films with combined cellulose nanofibers and nanocrystals: Tailored thermal, optical and mechanical properties. Cellulose 2018, 25, 1103–1115. [Google Scholar] [CrossRef]
- Ghaderi, M.; Mousavi, M.; Yousefi, H.; Labbafi, M. All-cellulose nanocomposite film made from bagasse cellulose nanofibers for food packing application. Carbohydr. Polym. 2014, 104, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Alexander Bismarck, A.A. Self-reinforced cellulose nanocomposites. Cellulose 2010, 17, 779–791. [Google Scholar]
- Hai, L.V.; Kim, H.C.; Kafy, A.; Zhai, L.; Kim, J.W.; Kim, J. Green all-cellulose nanocomposites made with cellulose nanofibers reinforced in dissolved cellulose matrix without heat treatment. Cellulose 2017, 24, 3301–3311. [Google Scholar] [CrossRef]
- Samyn, P.; Barhoum, A.; Öhlund, T.; Dufresne, A. Review: Nanoparticles and nanostructured materials in papermaking. J. Mater. Sci. 2019, 53, 146–184. [Google Scholar] [CrossRef]
- Oksman, K.; Aitomaki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Compos. Part A Appl. Sci. Manuf. 2016, 83, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Sun, H.; Shao, X.; Ma, Z. Effect of Incorporation Nanocrystalline Corn Straw Cellulose and Polyethylene Glycol on Properties of Biodegradable Films. J. Food Sci. 2016, 81, E2529–E2537. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, L.; Li, M.C.; Wu, Q.; Zhou, D. Cellulose Nanocrystals (CNCs) from Corn Stalk: Activation Energy Analysis. Materials 2017, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Bian, H.; Tu, P.; Chen, J.Y. Fabrication of all-cellulose nanocomposites from corn stalk. J. Sci. Food Agric. 2020, 100, 4390–4399. [Google Scholar] [CrossRef] [PubMed]
- Ruland, W. X-ray determination of crystallinity and diffuse disorder scattering. Acta Crystallogr. 1961, 14, 1180–1185. [Google Scholar] [CrossRef]
- Hindeleh, A.M.; Johnson, D.J. Crystallinity and crystallite size measurement in cellulose fibers: 2. Viscose rayon. Polymer 1974, 15, 697–705. [Google Scholar] [CrossRef]
- Jing, A.J.; Zhang, A.; Wu, Z. Thermal analysis of polymer fibers in handbook of thermal analysis and calorimetry. In Applications to Polymer and Plastics; Cheng, S.Z.D., Gallagher, P.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 3, pp. 409–482. [Google Scholar]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.P.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulose performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.Y.; Sun, N.; Stoner, B.; Jiang, X.Y.; Lu, X.M.; Rogers, R.D. Rapid dissolution of lignocellulosic biomass in ionic liquids using temperatures above the glass transition of lignin. Green Chem. 2011, 13, 2038–2047. [Google Scholar] [CrossRef]
- Zhai, L.; Mun, S.; Gao, X.; Kim, J.W.; Kim, J. Cellulose electroactive paper fabricated by facile solvent exchange pretreatment and its physical and electromechanical properties. Cellulose 2015, 22, 927–933. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Ioelovich, M. Cellulose-Nanostructured Natural Polymer; Lambert Academic Publishing: Saarbrücken, Germany, 2014. [Google Scholar]
- Kargarzadeh, H.; Ahmad, I.; Thomas, S.; Dufresne, A. Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley-VCH Publishing: Weinheim, Germany, 2017. [Google Scholar]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues–wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Timell, T.E.; Wimmer, R. Crystalline Cellulose and Derivatives. In Springer Series in Wood Science; Springer: New York, NY, USA, 2008; p. 103. [Google Scholar]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Kale, R.D.; Bansal, P.S.; Gorade, V.G. Extraction of microcrystalline cellulose from cotton sliver and its comparison with commercial microcrystalline cellulose. J. Polym. Environ. 2017, 26, 355–364. [Google Scholar] [CrossRef]
- Aulin, C.; Ahola, S.; Josefsson, P.; Nishino, T.; Hirose, Y.; Österberg, M.; Wågberg, L. Nanoscale Cellulose Films with Different Crystallinities and Mesostructures-Their Surface Properties and Interaction with Water. Langmuir 2009, 25, 7675–7685. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Park, H.S.; Yoon, K.J.; Hauser, P.J. Enhancing the durability of linen-like properties of low temperature mercerized cotton. Text. Res. J. 2004, 74, 146–154. [Google Scholar]
- Liu, Y.P.; Hu, H. X-ray diffraction study of bamboo fibers treated with NaOH. Fibers Polym. 2008, 9, 735–739. [Google Scholar] [CrossRef]
- Liu, R.; Yu, H.; Huang, Y. Structure and morphology of cellulose in wheat straw. Cellulose 2005, 12, 25–34. [Google Scholar] [CrossRef]
- Senthil, M.K.T.; Rajini, N.; Obi Reddy, K.; Varada Rajulu, A.; Siengchin, S.; Ayrilmis, N. All-cellulose composite films with cellulose matrix and Napier grass cellulose fibril fillers. Int. J. Biol. Macromol. 2018, 112, 1310–1315. [Google Scholar] [CrossRef]
- Shi, W.; Li, S.; Jia, J.; Zhao, Y. Highly Efficient Conversion of Cellulose to Bio-Oil in Hot-Compressed Water with Ultrasonic Pretreatment. Ind. Eng. Chem. Res. 2013, 52, 586–593. [Google Scholar] [CrossRef]
- Pullawan, T.; Wilkinson, A.N.; Eichhorn, S.J. Discrimination of matrix–fibre interactions in all-cellulose nanocomposites. Compos. Sci. Technol. 2010, 70, 2325–2330. [Google Scholar] [CrossRef] [Green Version]
- Lourdin, D.; Peixinho, J.; Joël, B.; Bernard, C.; Eric, L.; Benoît, D. Concentration driven cocrystallisation and percolation in all-cellulose nanocomposites. Cellulose 2016, 23, 529–543. [Google Scholar] [CrossRef]
- Kondo, T. A Fourier transform infra-red spectroscopic analysis of the character of hydrogen bonds in amorphous cellulose. Polymer 1996, 37, 393–399. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Liang, C.Y.; Marchessault, R.H. Infrared spectra of crystalline polysaccharides. I. Hydrogen bonds in native celluloses. J. Polym. Sci. 1959, XXXVII, 385–395. [Google Scholar] [CrossRef]
- Gwon, J.G.; Lee, S.Y.; Doh, G.H.; Kim, J.H. Characterization of chemically modified wood fibers using FTIR spectroscopy for biocomposites. J. Appl. Polym. Sci. 2010, 116, 3212–3219. [Google Scholar] [CrossRef]
- Yue, Y. A Comparative Study of Cellulose I and II Fibers and Nanocrystals. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2011. [Google Scholar]
- Colom, X.; Carrillo, F. Crystallinity changes in lyocell and viscose-type fibers by caustic treatment. Eur. Polym. J. 2002, 38, 2225–2230. [Google Scholar] [CrossRef]
- Liang, C.Y.; Marchessault, R.H. Infrared Spectra of Crystalline Polysaccharides. II. Native Celluloses in the Region from 640 to 1700 cm−1. J. Polym. Sci. 1959, XXXIX, 269–278. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’connor, R.T. Relation of Certain Infrared Bands to Cellulose Crystallinity and Crystal Lattice Type. Part I. Spectra of Lattice Types I, II, III and of Amorphous Cellulose. J. Appl. Polym. Sci. 1964, 8, 1311–1324. [Google Scholar] [CrossRef]
- Li, Q.; Renneckar, S. Supramolecular Structure Characterization of Molecularly Thin Cellulose I Nanoparticles. Biomacromolecules 2011, 12, 650–659. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Lee, S.-W.; Kim, J. Effect of heat treatment on the structure, piezoelectricity and actuation behavior of a cellulose electroactive-paper actuator. Acta Mater. 2008, 56, 1868–1875. [Google Scholar] [CrossRef]
- Qi, H.; Cai, J.; Zhang, L. Properties of films composed of cellulose nanowhiskers and a cellulose matrix regenerated from alkali/urea solution. Biomacromolecules 2009, 10, 1597–1602. [Google Scholar] [CrossRef]

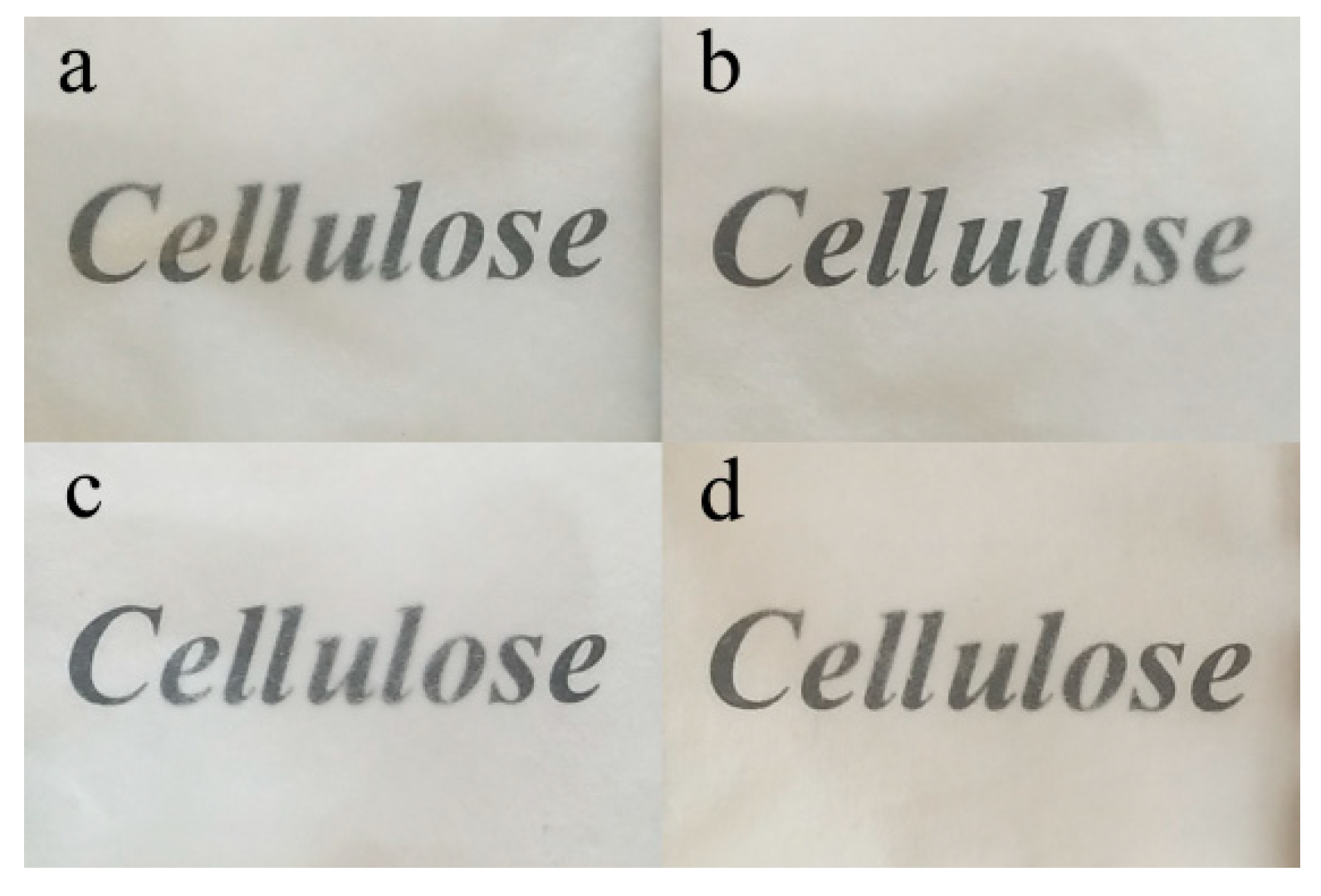




| Extraction Method | WSC | RWSC | All-Cellulose Nanocomposite | ||
|---|---|---|---|---|---|
| 0.1% | 1.5% | 3.0% | |||
| Prepared by AM | 68.7 | 55.5 | 47.9 | 42.9 | 46.9 |
| Prepared by EM | 50.7 | 44.3 | 40.8 | 58.9 | 50.9 |
| WSC | Strain Rate (mm/min) | CNFs (%) | Yield Strength (MPa) | Tensile Strength (MPa) | Young’s Modulus (GPa) |
|---|---|---|---|---|---|
| Prepared by AM | 0.05 | 0 | 6.5 ± 2.1 | 29.1 ± 6.6 | 0.32 ± 0.07 |
| 0.1 | 11.0 ± 2.1 | 46.1 ± 15.0 | 0.55 ± 0.08 | ||
| 1.5 | 21.3 ± 2.1 | 37.3 ± 4.2 | 1.07 ± 0.14 | ||
| 3.0 | 15.4 ± 2.1 | 53.6 ± 13.0 | 0.77 ± 0.18 | ||
| 5 | 0 | 22.6 ± 4.5 | 39.0 ± 6.3 | 1.13 ± 0.23 | |
| 0.1 | 26.6 ± 0.14 | 40.5 ± 7.9 | 1.41 ± 0.07 | ||
| 1.5 | 29.2 ± 0.19 | 39.7 ± 0.01 | 1.40 ± 0.07 | ||
| 3.0 | 22.6 ± 0.08 | 41.4 ± 0.1 | 1.13 ± 0.00 | ||
| Prepared by EM | 0.05 | 0 | 11.1 ± 2.1 | 38.0 ± 1.8 | 0.56 ± 0.10 |
| 0.1 | 13.1 ± 2.1 | 41.5 ± 12.0 | 0.66 ± 0.13 | ||
| 1.5 | 19 ± 2.1 | 56.0 ± 3.8 | 0.95 ± 0.24 | ||
| 3.0 | 12.3 ± 2.1 | 65.1 ± 2.2 | 0.61 ± 0.27 | ||
| 5 | 0 | 24.0 ± 4.2 | 42.0 ± 4.3 | 1.20 ± 0.21 | |
| 0.1 | 24.1 ± 2.4 | 50.1 ± 1.4 | 1.21 ± 0.12 | ||
| 1.5 | 20.8 ± 1.3 | 52.9 ± 2.9 | 1.04 ± 0.06 | ||
| 3.0 | 18.5 ± 3.7 | 59.6 ± 9.1 | 0.93 ± 0.18 |
| WSC | Strain Rate (mm/min) | CNF Content | 0.0% | 0.1% | 1.5% | 3.0% |
|---|---|---|---|---|---|---|
| Prepared by AM | 0.05 | 3.0% | 20 ** | 5 | 7 | |
| 1.5% | 13 * | 2 | ||||
| 0.1% | 15 ** | |||||
| 0.0% | ||||||
| 5 | 3.0% | 5 | 7 | 4 | ||
| 1.5% | 2 | 4 | ||||
| 0.1% | 2 | |||||
| 0.0% | ||||||
| Prepared by EM | 0.05 | 3.0% | 28 * | 19 | 11 | |
| 1.5% | 17 | 9 | ||||
| 0.1% | 8 | |||||
| 0.0% | ||||||
| 5 | 3.0% | 17 ** | 19 ** | 24 ** | ||
| 1.5% | 7 | 5 | ||||
| 0.1% | 2 | |||||
| 0.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, H.; Yang, Y.; Tu, P.; Chen, J.Y. Value-Added Utilization of Wheat Straw: From Cellulose and Cellulose Nanofiber to All-Cellulose Nanocomposite Film. Membranes 2022, 12, 475. https://doi.org/10.3390/membranes12050475
Bian H, Yang Y, Tu P, Chen JY. Value-Added Utilization of Wheat Straw: From Cellulose and Cellulose Nanofiber to All-Cellulose Nanocomposite Film. Membranes. 2022; 12(5):475. https://doi.org/10.3390/membranes12050475
Chicago/Turabian StyleBian, Hongxia, Yanyan Yang, Peng Tu, and Jonathan Y. Chen. 2022. "Value-Added Utilization of Wheat Straw: From Cellulose and Cellulose Nanofiber to All-Cellulose Nanocomposite Film" Membranes 12, no. 5: 475. https://doi.org/10.3390/membranes12050475
APA StyleBian, H., Yang, Y., Tu, P., & Chen, J. Y. (2022). Value-Added Utilization of Wheat Straw: From Cellulose and Cellulose Nanofiber to All-Cellulose Nanocomposite Film. Membranes, 12(5), 475. https://doi.org/10.3390/membranes12050475







