Pilot Scale Application of a Ceramic Membrane Bioreactor for Treating High-Salinity Oil Production Wastewater
Abstract
:1. Introduction
2. Materials and Methods
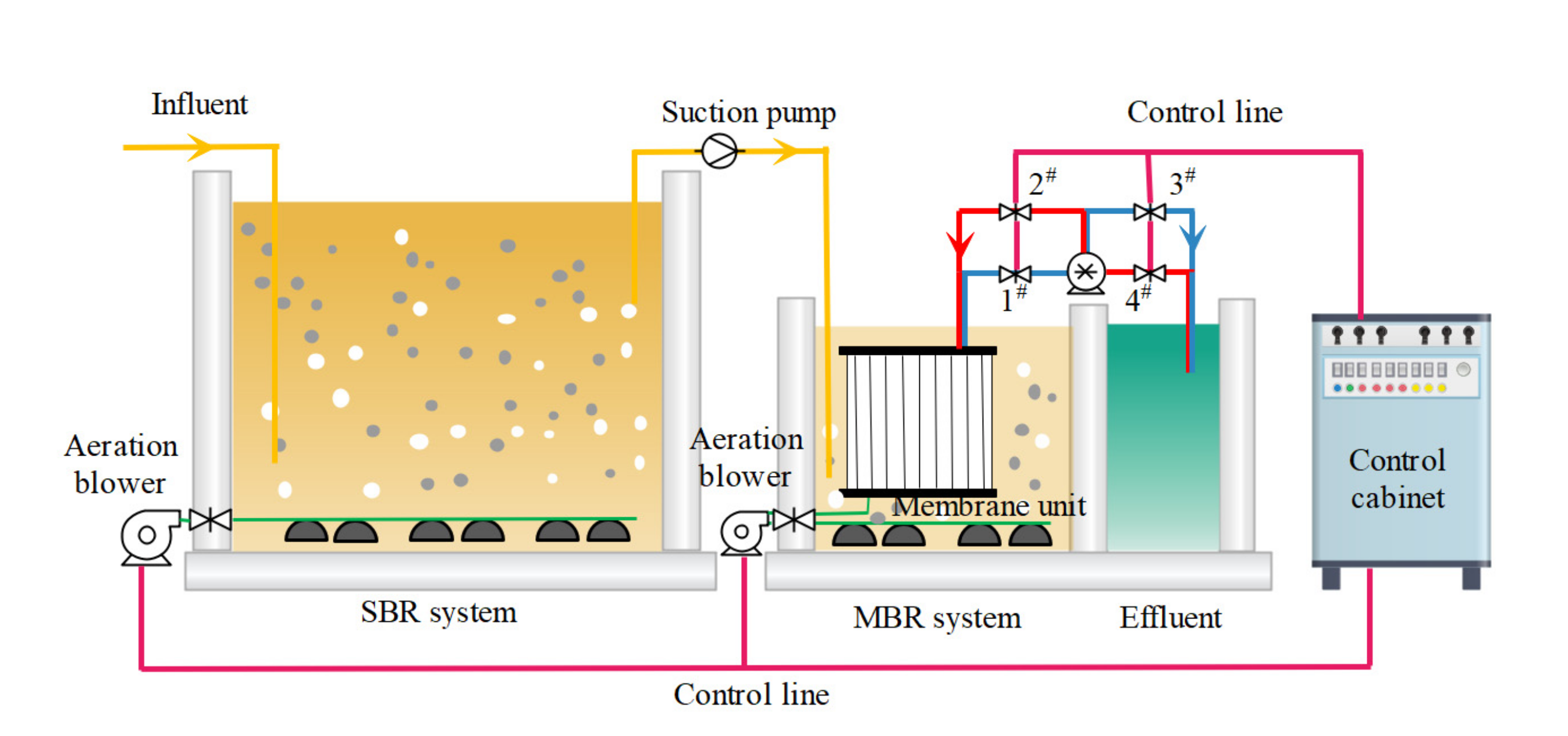
2.1. C−MBR Pilot Plant
2.2. Wastewater Composition
2.3. Reactor Operation Process
2.4. Analysis Method
2.4.1. Water Quality Analysis
2.4.2. Microbial Diversity Analysis
2.4.3. Data Analysis
3. Results and Discussion
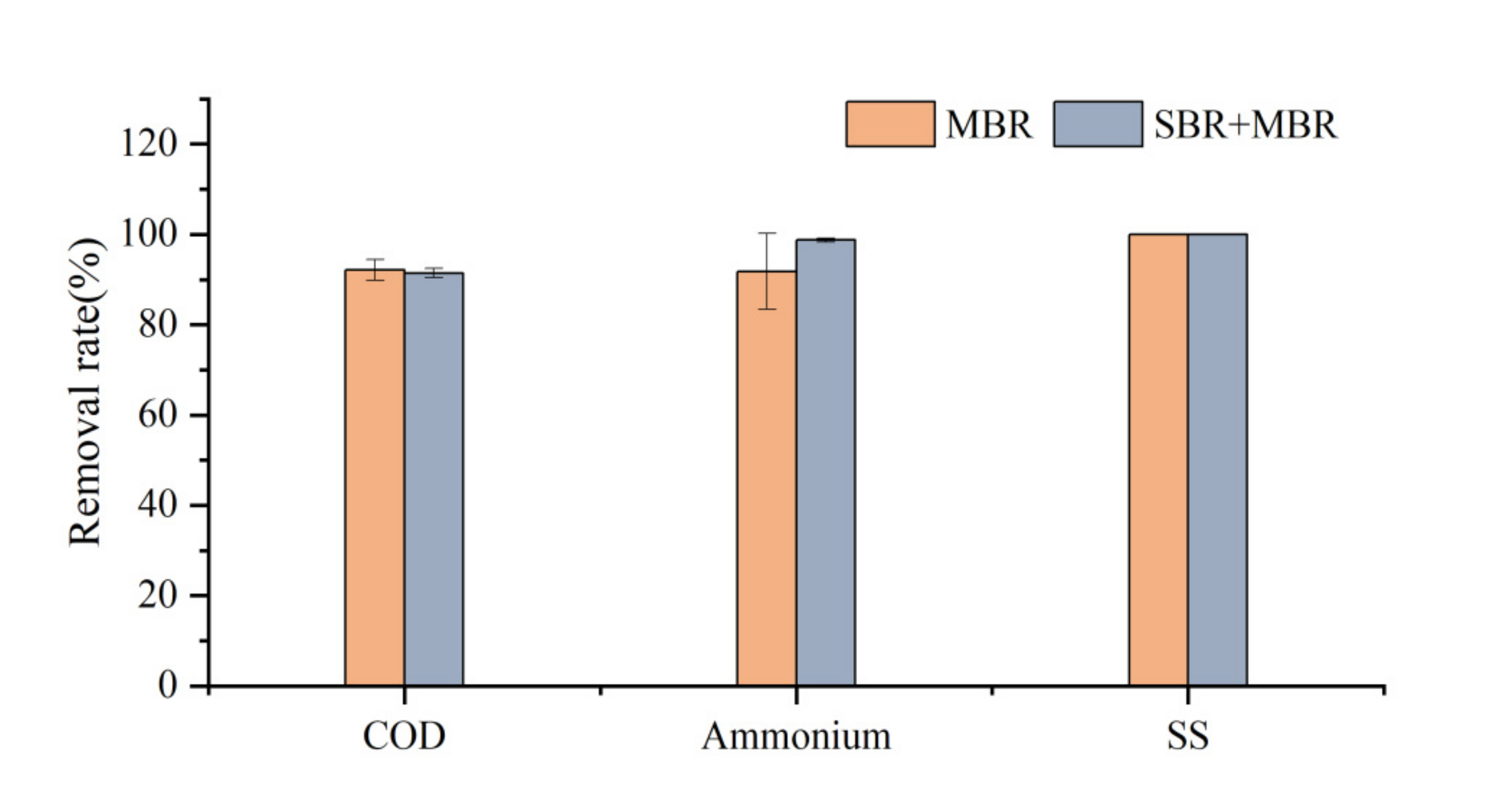
3.1. System Processing Performance
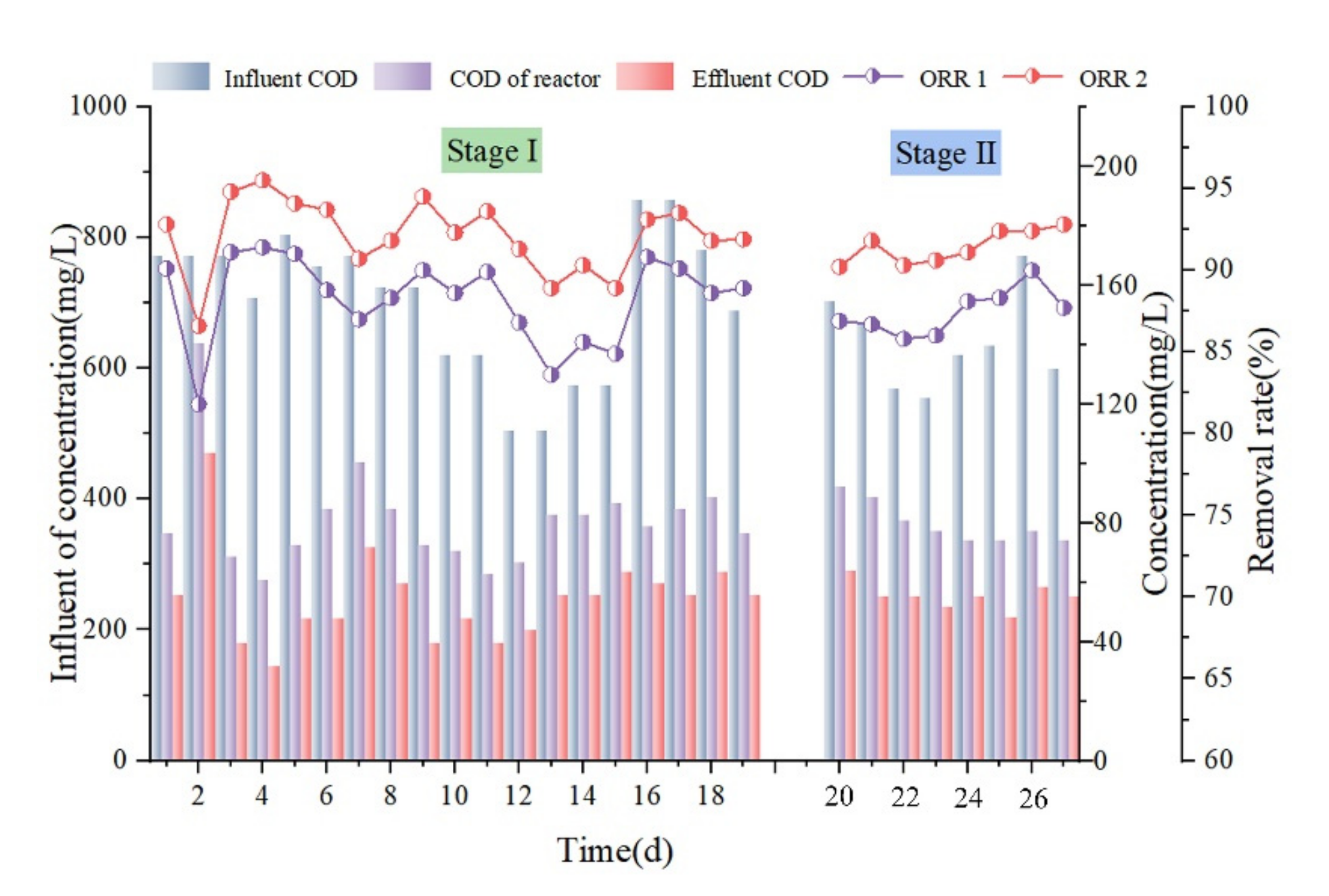
3.1.1. Chemical Oxygen Demand (COD) Removal
3.1.2. NH4+−N Removal
3.2. Microbial Diversity in the Reaction System
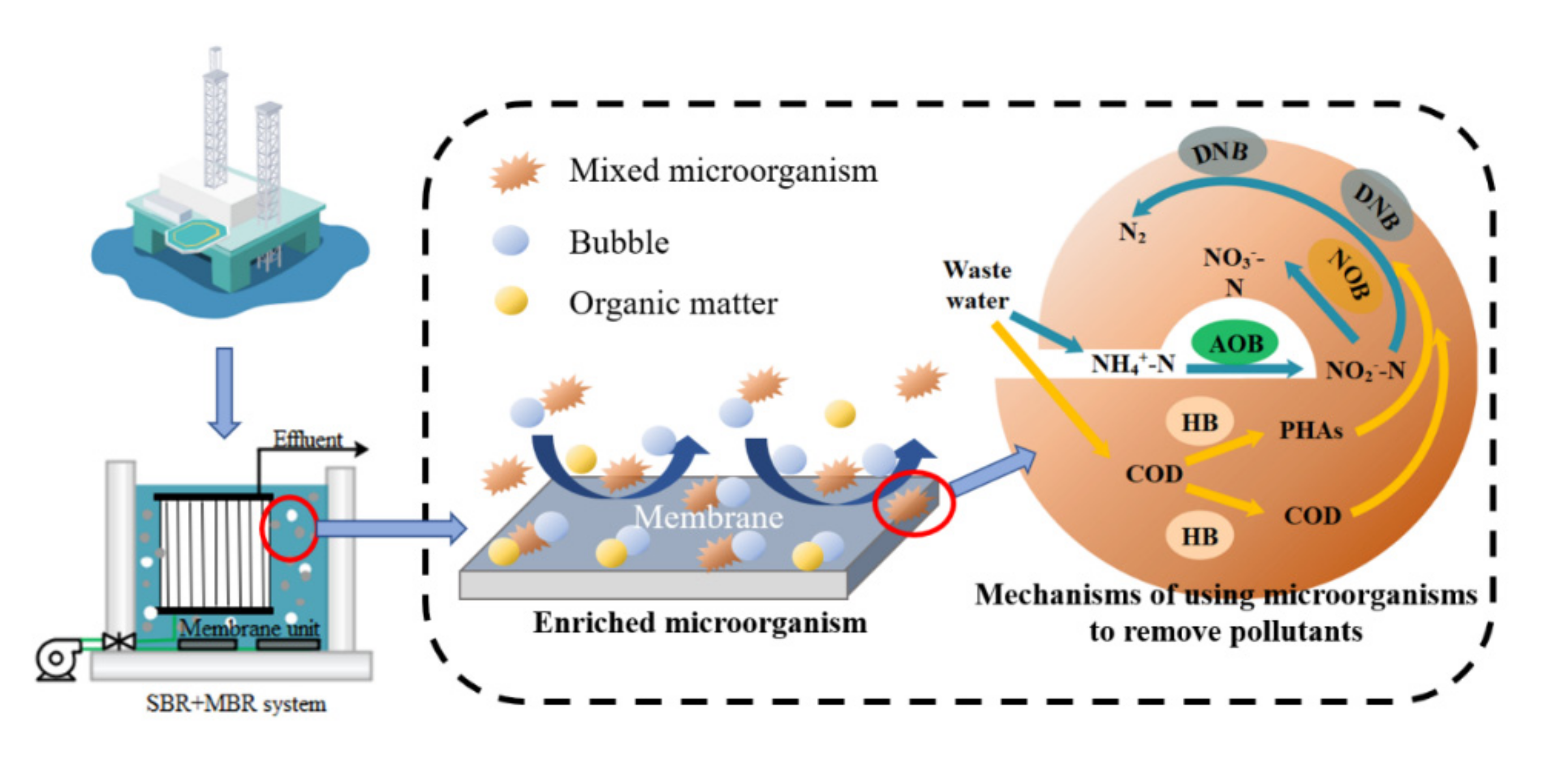
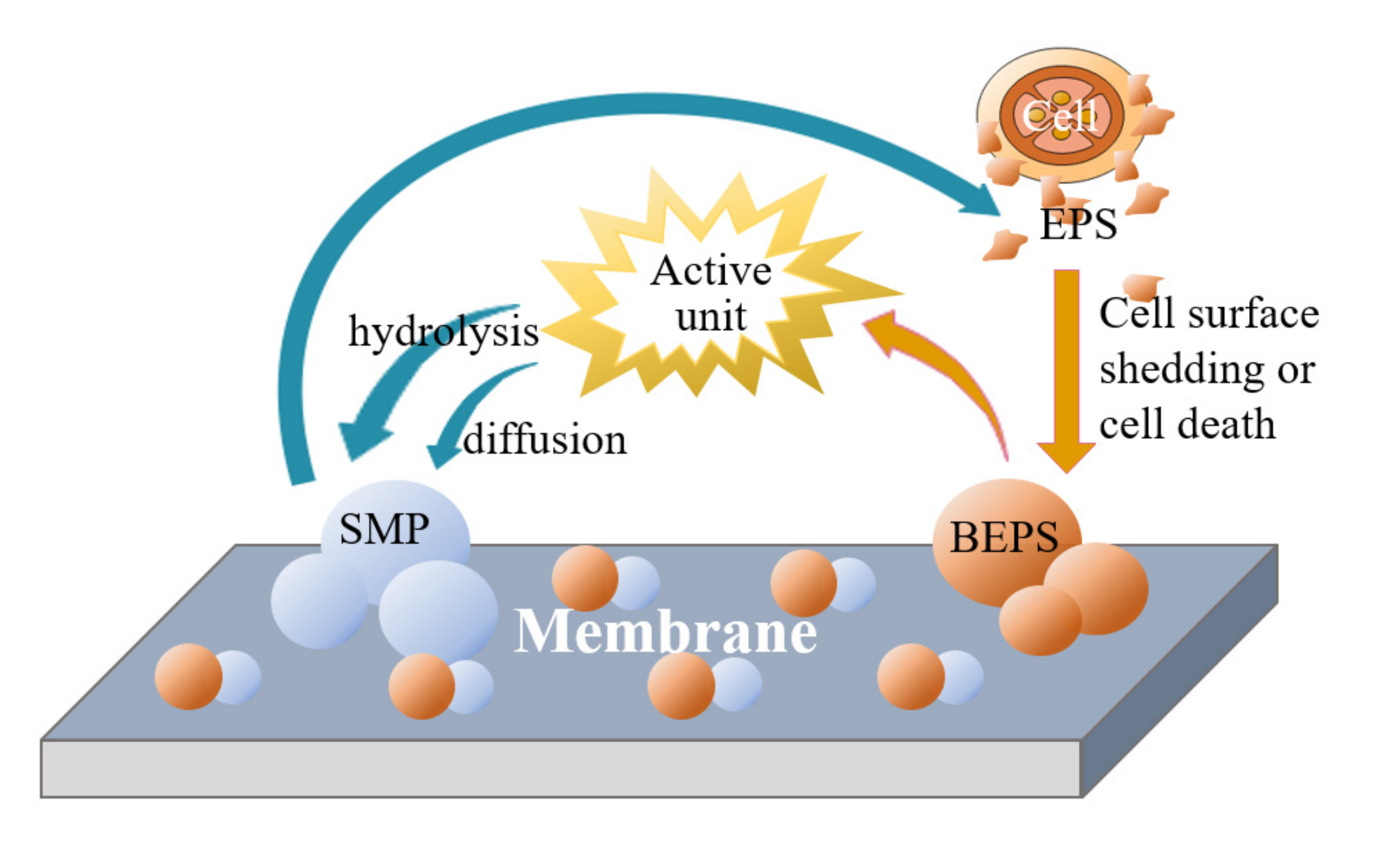
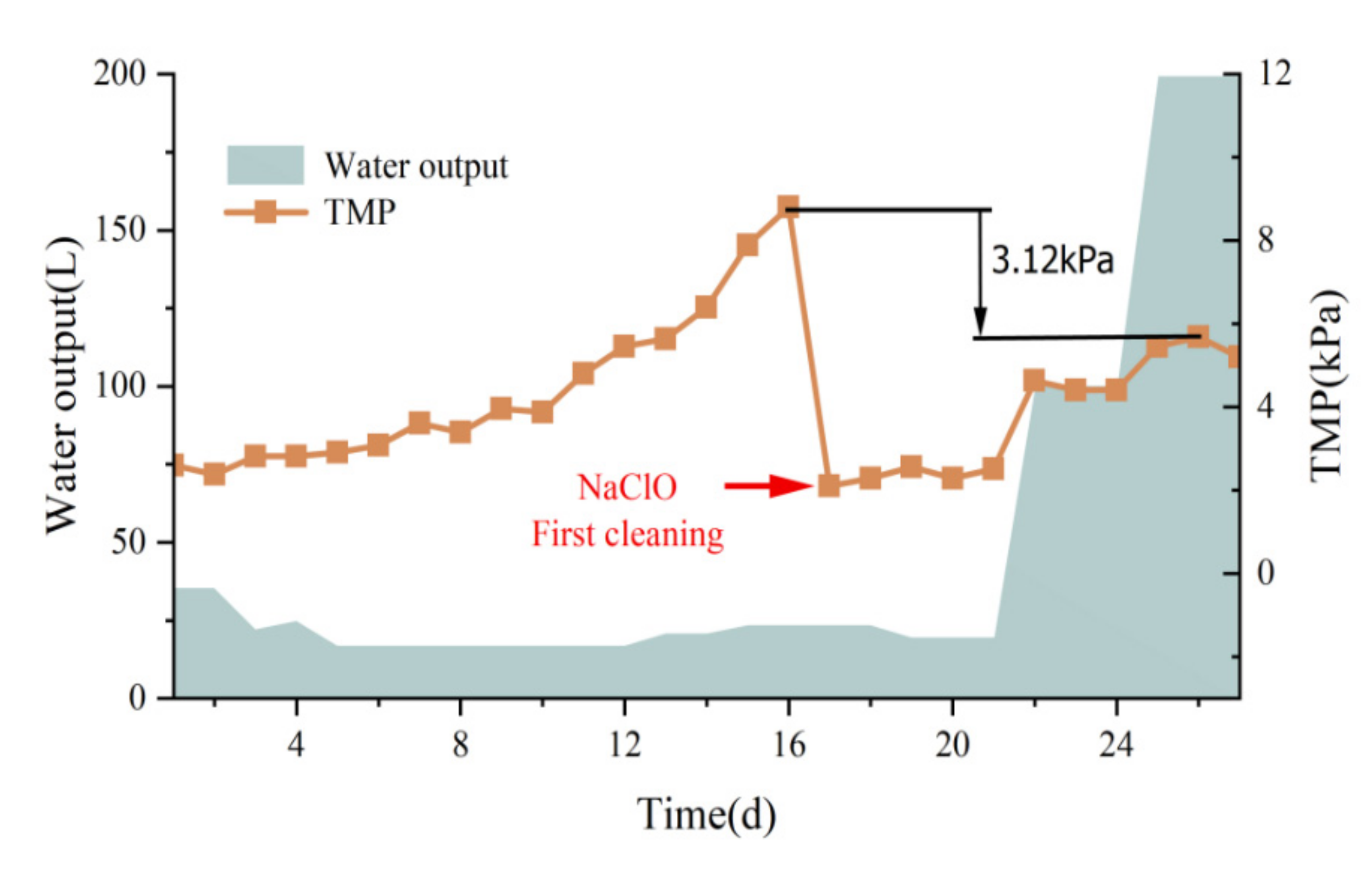
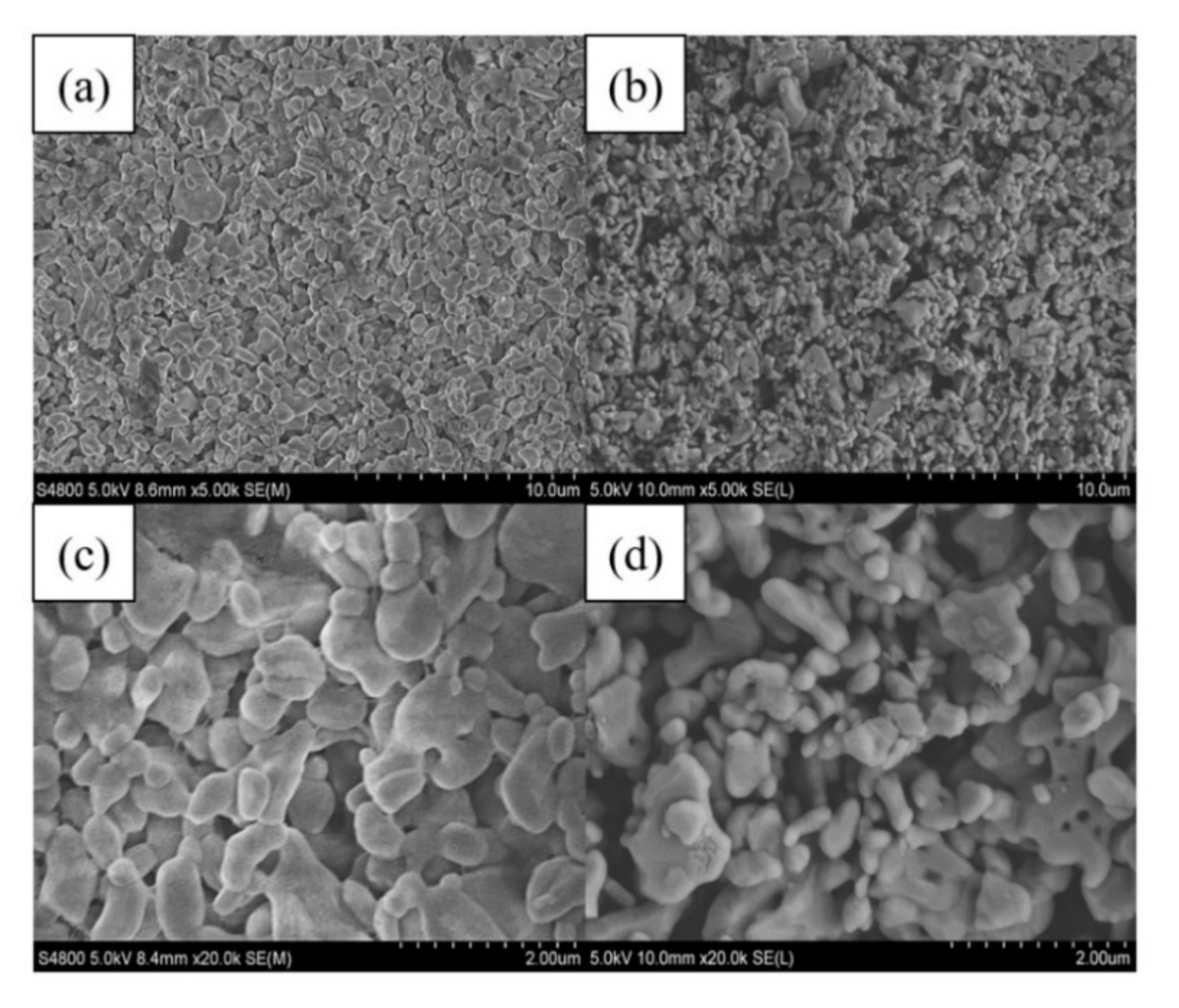
3.3. Membrane Contamination
3.4. Economic Analysis
3.4.1. Capital Expenditure (CAPEX)
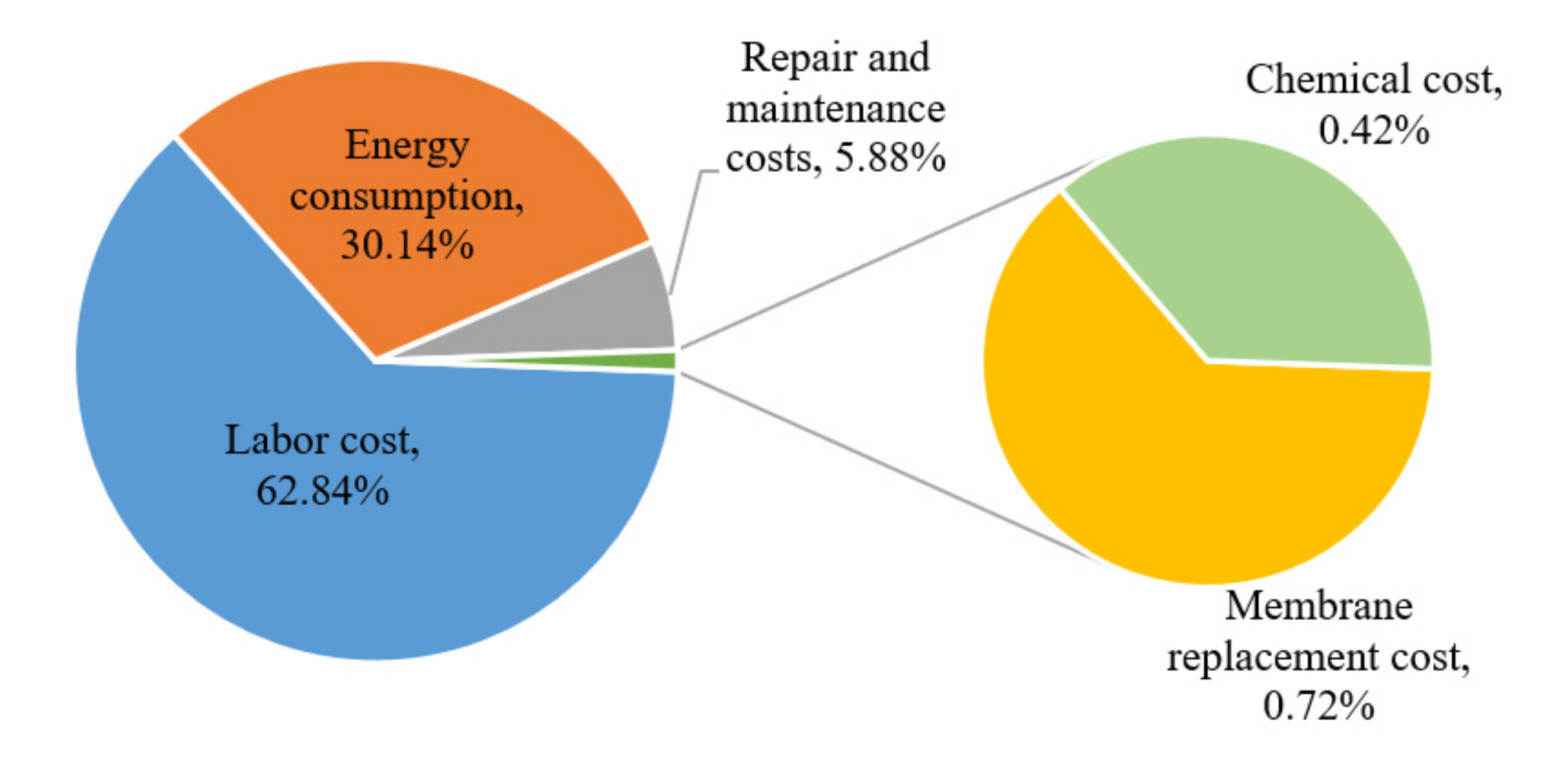
3.4.2. Operating Expenses (OPEX)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakke, T.; Klungsøyr, J.; Sanni, S. Environmental impacts of produced water and drilling waste discharges from the Norwegian offshore petroleum industry. Mar. Environ. Res. 2013, 92, 154–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappello, S.; Volta, A.; Santisi, S.; Morici, C.; Mancini, G.; Quatrini, P.; Genovese, M.; Yakimov, M.M.; Torregrossa, M. Oil-degrading bacteria from a membrane bioreactor (BF-MBR) system for treatment of saline oily waste: Isolation, identification and characterization of the biotechnological potential. Int. Biodeterior. Biodegrad. 2016, 110, 235–244. [Google Scholar] [CrossRef]
- Beyer, J.; Goksøyr, A.; Hjermann, D.O.; Klungsøyr, J. Environmental effects of offshore produced water discharges: A review focused on the Norwegian continental shelf. Mar. Environ. Res. 2020, 162, 105155. [Google Scholar] [CrossRef] [PubMed]
- Lofthus, S.; Almås, I.K.; Evans, P.; Pelz, O.; Brakstad, O.G. Biodegradation in seawater of PAH and alkylphenols from produced water of a North Sea platform. Chemosphere 2018, 206, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Brakstad, O.G.; Altin, D.; Davies, E.J.; Aas, M.; Nordtug, T. Interaction between microalgae, marine snow and anionic polyacrylamide APAM at marine conditions. Sci. Total Environ. 2020, 705, 135950. [Google Scholar] [CrossRef]
- Bagheri, M.; Roshandel, R.; Shayegan, J. Optimal selection of an integrated produced water treatment system in the upstream of oil industry. Process Saf. Environ. Prot. 2018, 117, 67–81. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Li, Y.; Xu, H.; Pan, Z.; Dai, P.; Wang, H.; Yang, Q. A review of treatment technologies for produced water in offshore oil and gas fields. Sci. Total Environ. 2021, 775, 145485. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Agrawal, S.; Nawaz, T.; Pan, S.; Selvaratnam, T. A review of algae-based produced water treatment for biomass and biofuel production. Water 2020, 12, 2351. [Google Scholar] [CrossRef]
- Zolghadr, E.; Firouzjaei, M.D.; Amouzandeh, G.; LeClair, P.; Elliott, M. The role of membrane-based technologies in environmental treatment and reuse of produced water. Front. Environ. Sci. 2021, 9, 71. [Google Scholar] [CrossRef]
- Ammar, S.H.; Khadim, H.J.; Mohamed, A.I. Cultivation of nannochloropsis oculata and isochrysis galbana microalgae in produced water for bioremediation and biomass production. Environ. Technol. Innov. 2018, 10, 132–142. [Google Scholar] [CrossRef]
- Han, M.; Zhang, J.; Chu, W.; Chen, J.; Zhou, G. Research progress and prospects of marine oily wastewater treatment: A review. Water 2019, 11, 2517. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Jin, Y.; Zhang, W. Treatment of high-concentration wastewater from an oil and gas field via a paired sequencing batch and ceramic membrane reactor. Int. J. Environ. Res. Public Health 2020, 17, 1953. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.-Y.; Zhang, X.-B.; Li, J. Advanced treatment of oilfield production wastewater by an integration of coagulation/flotation, catalytic ozonation and biological processes. Environ. Technol. 2016, 37, 2536–2544. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, Y.; Jin, Y. Full-scale processing by anaerobic baffle reactor, sequencing batch reactor, and sand filter for treating high-salinity wastewater from offshore oil rigs. Processes 2018, 6, 256. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liu, F.; Wang, D.; Jin, Y. Impact of reactor configuration on treatment performance and microbial diversity in treating high-strength dyeing wastewater: Anaerobic flat-sheet ceramic membrane bioreactor versus upflow anaerobic sludge blanket reactor. Bioresour. Technol. 2018, 269, 269–275. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, B.; Bin, L. Research Progress in Biofilm-Membrane Bioreactor: A Critical Review. Ind. Eng. Chem. Res. 2017, 56, 6900–6909. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Wang, D.; Koga, Y.; Rouse, J.; Furukawa, K. Trace elements enhance biofilm formation in UASB reactor for solo simple molecule wastewater treatment. Bioresour. Technol. 2011, 102, 9296–9299. [Google Scholar] [CrossRef]
- Li, J.; Sun, S.; Yan, P.; Fang, L.; Yu, Y.; Xiang, Y.; Wang, D.; Gong, Y.; Gong, Y.; Zhang, Z. Microbial communities in the functional areas of a biofilm reactor with anaerobic–aerobic process for oily wastewater treatment. Bioresour. Technol. 2017, 238, 7–15. [Google Scholar] [CrossRef]
- Niwa, T.; Hatamoto, M.; Yamashita, T.; Noguchi, H.; Takase, O.; Kekre, K.A.; Ang, W.S.; Tao, G.; Seah, H.; Yamaguchi, T. Demonstration of a full-scale plant using an UASB followed by a ceramic MBR for the reclamation of industrial wastewater. Bioresour. Technol. 2016, 218, 1–8. [Google Scholar] [CrossRef]
- Zhu, J.; Lin, C.F.; Kao, J.C.M.; Yang, P.Y. Evaluation of potential integration of entrapped mixed microbial cell and membrane bioreactor processes for biological wastewater treatment/reuse. Clean Technol. Environ. Policy 2011, 13, 153–160. [Google Scholar] [CrossRef]
- Krzeminski, P.; Langhorst, W.; Schyns, P.; De Vente, D.; Broeck, R.V.D.; Smets, I.; Van Impe, J.; Van der Graaf, J.; Van Lier, J. The optimal MBR configuration: Hybrid versus stand-alone—Comparison between three full-scale MBRs treating municipal wastewater. Desalination 2012, 284, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Yadu, A.; Sahariah, B.; Anandkumar, J. Influence of COD/ammonia ratio on simultaneous removal of NH4+−N and COD in surface water using moving bed batch reactor. J. Water Process Eng. 2018, 22, 66–72. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, G.; Li, J.; Chen, S.-Y.; Li, Y.; Li, W.-T.; Li, A.-M. Response of performance and ammonia oxidizing bacteria community to high salinity stress in membrane bioreactor with elevated ammonia loading. Bioresour. Technol. 2016, 216, 714–721. [Google Scholar] [CrossRef]
- Du, X.; Shi, Y.; Jegatheesan, V.; Haq, I.U. A review on the mechanism, impacts and control methods of membrane fouling in MBR system. Membranes 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhang, H.; Zhang, K.; Qian, Y.; Yuan, X.; Ji, B.; Han, W. Membrane fouling mitigation in different biofilm membrane bioreactors with pre-anoxic tanks for treating mariculture wastewater. Sci. Total Environ. 2020, 724, 138311. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Bo, R.; Jin, Y.; Zhang, W. Membrane fouling and cleaning in anaerobic flat-sheet ceramic membrane bioreactor for sewage treatment. J. Environ. Eng. 2021, 147, 04021041. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.; Tang, B.; Ding, J.; Zheng, Y.; Zhang, Z. Membrane fouling mechanism of biofilm-membrane bioreactor (BF-MBR): Pore blocking model and membrane cleaning. Bioresour. Technol. 2018, 250, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Koh, Y.K.K.; Ng, H.Y. Membrane fouling mitigation by NaClO-assisted backwash in anaerobic ceramic membrane bioreactors for the treatment of domestic wastewater. Bioresour. Technol. 2018, 268, 622–632. [Google Scholar] [CrossRef]
- Wang, H.; Ma, D.; Shi, W.; Yang, Z.; Cai, Y.; Gao, B. Formation of disinfection by-products during sodium hypochlorite cleaning of fouled membranes from membrane bioreactors. Front. Environ. Sci. Eng. 2021, 15, 102. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ngo, H.H.; Guo, W. Pilot scale study on a new membrane bioreactor hybrid system in municipal wastewater treatment. Bioresour. Technol. 2013, 141, 8–12. [Google Scholar] [CrossRef]
- Iglesias, R.; Simón, P.; Moragas, L.; Arce, A.; Rodriguez-Roda, I. Cost comparison of full-scale water reclamation technologies with an emphasis on membrane bioreactors. Water Sci. Technol. 2017, 75, 2562–2570. [Google Scholar] [CrossRef]
- Yang, X.; López-Grimau, V.; Vilaseca, M.; Crespi, M. Treatment of textile wastewater by CAS, MBR, and MBBR: A comparative study from technical, economic, and environmental perspectives. Water 2020, 12, 1306. [Google Scholar] [CrossRef]

| Parameter | Unit | Influent |
|---|---|---|
| Temperature | °C | 38–50 |
| pH | - | 7.1–8.2 |
| Total salinity | g/L | 27.4–31.8 |
| SS | mg/L | 140–610 |
| COD | mg/L | 100–1479 |
| BOD5 | mg/L | 36.1–650 |
| NH4+−N | mg/L | 11.3–40.2 |
| TN | mg/L | 9–13 |
| TP | mg/L | 7–12 |
| Oil | mg/L | 11.5–15 |
| COD | NH4+−N | ORR | NRR | |
|---|---|---|---|---|
| NH4+−N | −0.014 | |||
| ORR | 0.406 * | −0.214 | ||
| NRR | −0.398 * | 0.248 | −0.107 | |
| C/N ratio | 0.357 | −0.880 ** | 0.336 | −0.286 |
| Sample | Genus | Contain (%) | Sample | Genus | Contain (%) |
|---|---|---|---|---|---|
| A0 | Unclassified-Bacteria | 16.68 | B | Marinobacterium | 19.01 |
| Unclassified-Bacteroidetes | 8.04 | Marinobacter | 17.61 | ||
| Unclassified-Planctomycetaceae | 7.44 | Unclassified-Rhodobacteraceae | 16.2 | ||
| Nitrospira | 1.22 | Pseudidiomarina | 14.08 | ||
| Gp10 | 0.68 | Thiomicrospira | 11.27 | ||
| Unclassified-Rhodobacteraceae | 0.18 | Meyerozyma | 9.59 | ||
| Nitrosomonas | 0.17 | Nitrosomonas | 7.04 | ||
| Mycobacterium | 0.02 | Mycobacterium | 2.11 | ||
| Unclassified-Rhodospirillaceae | 0.02 | Unclassified-Rhodospirillaceae | 2.11 |
| Project | Quantity | Unit | Unit Price (USD) | Total Price (USD) | Remark | |
|---|---|---|---|---|---|---|
| Construction cost | Raw pool, SBR pool, MBR pool, chemical cleaning medicine tank, water outlet pool | 1 | Item | / | 56,600 | The tank is built with a reinforced concrete structure, with a thickness of about 300 mm |
| Subtotal | / | / | / | 56,600 | ||
| Non-engineering costs | PLC control system | 1 | Set | / | 157,000 | The total power of the PLC control cabinet is 3.5 kw |
| Lift pump | 4 | Tower | 239 | 960 | 2 use 2 backup; the power is 0.25 kw | |
| High-precision electromagnetic flowmeter | 2 | Set | 157.50 | 315 | ||
| Ceramic membrane module | 148 | Piece | 78.60 | 11,700 | 148 pieces per group (including interface, silicone tube, etc.) | |
| Microfiltration membrane self-priming pump | 2 | Tower | 158.50 | 317 | 1 use and 1 backup; the power is 0.45 kw | |
| Cleaning system | 1 | Set | / | 3160 | With CIP pump, the power of the pump is 2.2 kw | |
| Aeration system | 2 | Set | 315 | 630 | Including pipeline valve | |
| Aeration blower | 4 | Tower | 395 | 1580 | 2 use 2 backup; the power is 0.55 kw | |
| Shipping fee | 1 | Item | / | 2390 | ||
| Commissioning fee | 1 | Item | / | 3440 | ||
| Subtotal | / | / | / | 181,492 | ||
| Pipe network cost | Pipe network | 1 | Set | / | 9500 | Including pipes, fittings, etc. |
| Wire and cable | 1 | Set | / | 1100 | ||
| Hardware parts | 1 | Set | / | 745 | ||
| Plumbing installation fee | 1 | Item | / | 2280 | ||
| Subtotal | / | / | / | 13,625 | ||
| Total | / | / | / | 251,717 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Jin, Y. Pilot Scale Application of a Ceramic Membrane Bioreactor for Treating High-Salinity Oil Production Wastewater. Membranes 2022, 12, 473. https://doi.org/10.3390/membranes12050473
Sun R, Jin Y. Pilot Scale Application of a Ceramic Membrane Bioreactor for Treating High-Salinity Oil Production Wastewater. Membranes. 2022; 12(5):473. https://doi.org/10.3390/membranes12050473
Chicago/Turabian StyleSun, Ronglin, and Yue Jin. 2022. "Pilot Scale Application of a Ceramic Membrane Bioreactor for Treating High-Salinity Oil Production Wastewater" Membranes 12, no. 5: 473. https://doi.org/10.3390/membranes12050473
APA StyleSun, R., & Jin, Y. (2022). Pilot Scale Application of a Ceramic Membrane Bioreactor for Treating High-Salinity Oil Production Wastewater. Membranes, 12(5), 473. https://doi.org/10.3390/membranes12050473





