Studying the Effect of Shortening Carbon Nanotubes via Ball Milling on Cellulose Acetate Nanocomposite Membranes for Desalination Applications
Abstract
:1. Introduction
2. Materials and Methods
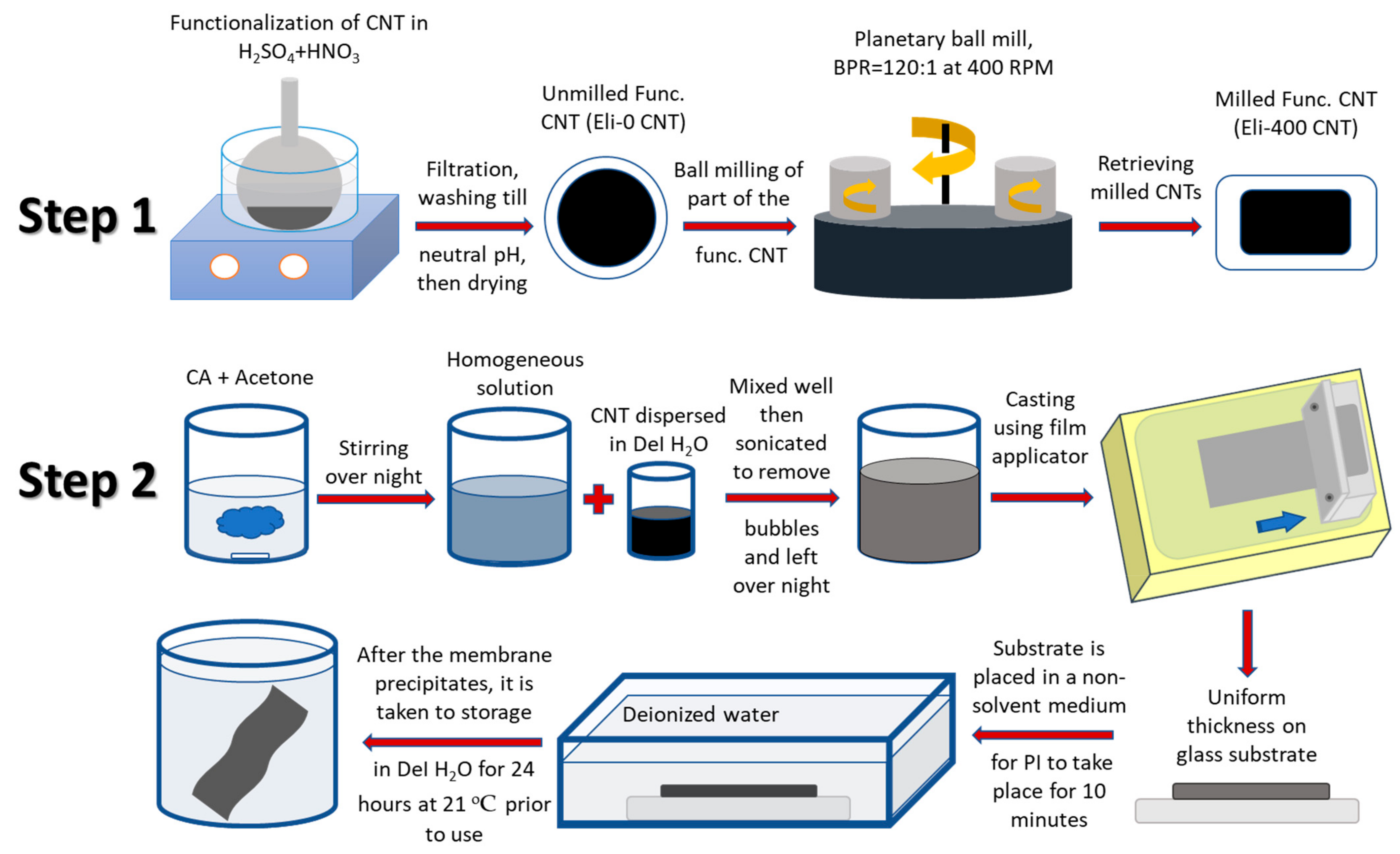
2.1. CNT Functionalization, Shortening, and Characterization
2.2. Membrane Preparation and Characterization
2.3. Separation Analysis
3. Results
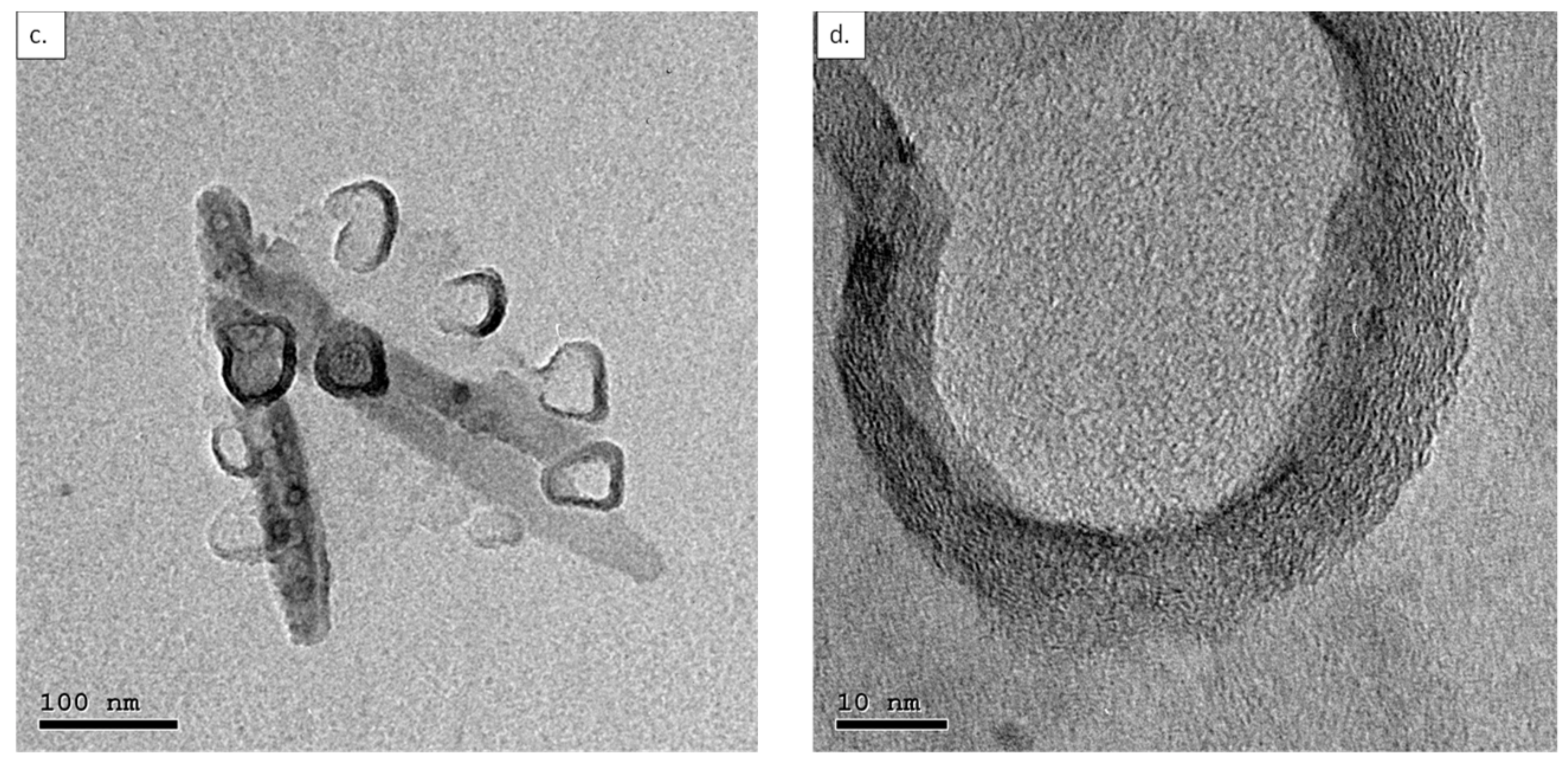
3.1. Characterization of Shortened CNTs
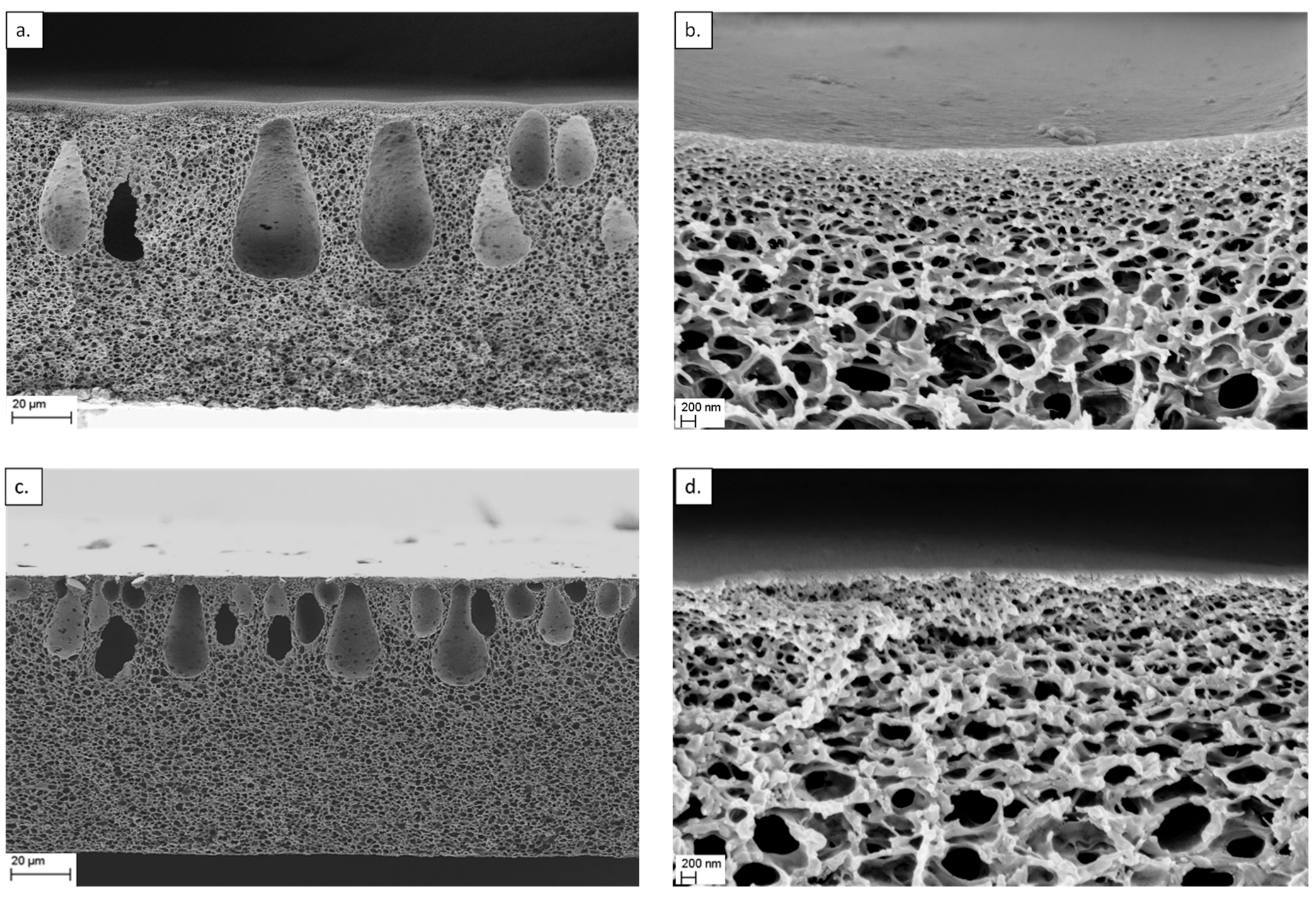
3.2. Characterization of the Nanocomposite Membranes
3.3. Permeation and Salt Retention Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berenguel, F.; Lara Galera, A.; Muñoz-Medina, M. Requirements for the construction of new desalination plants into a framework of sustainability. Sustainability 2020, 12, 5124. [Google Scholar] [CrossRef]
- The IDA Water Security Handbook. 2020–2021. Available online: https://www.globalwaterintel.com/products-and-services/market-research-reports/ida-water-security-handbook (accessed on 19 January 2021).
- World Water Day 2020: Desalination technologies Provide Safe and Sustainable Drinking Water. Available online: https://cordis.europa.eu/article/id/415568-world-water-day-2020-desalination-technologies-provide-safe-and-sustainable-drinking-water (accessed on 17 January 2021).
- Sola, I.; Sáez, C.A.; Sánchez-Lizaso, J.L. Evaluating environmental and socio-economic requirements for improving desalination development. J. Clean. Prod. 2021, 324, 129296. [Google Scholar] [CrossRef]
- IDA Water Security Handbook, 2019–2020 and GWI DesalData. Available online: https://idadesal.org/e-library/ida-water-security-handbook/ (accessed on 19 January 2021).
- Cherif, H.; Belhadj, J. Chapter 15—Environmental Life Cycle Analysis of Water Desalination Processes. In Sustainable Desalination Handbook; Gude, V.G., Ed.; Butterworth-Heinemann: Oxford, UK, 2018. [Google Scholar]
- Curto, D.; Franzitta, V.; Guercio, A. A Review of the Water Desalination Technologies. Appl. Sci. 2021, 11, 670. [Google Scholar] [CrossRef]
- Do Thi, H.T.; Pasztor, T.; Fozer, D.; Manenti, F.; Toth, A.J. Comparison of Desalination Technologies Using Renewable Energy Sources with Life Cycle, PESTLE, and Multi-Criteria Decision Analyses. Water 2021, 13, 3023. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Chen, D.; Wang, Q.; Ying, Y.; Gao, W.; Xie, L. Recent advances in applications of carbon nanotubes for desalination: A review. Nanomaterials 2020, 10, 1203. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Khachatryan, K.; Krzan, M.; Ciesielski, W.; Żarska, S.; Szczepankowska, J. Polysaccharides Composite Materials as Carbon Nanoparticles Carrier. Polymers 2022, 14, 948. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, D.L.; Chung, T.-S. Nanoclays-Incorporated Thin-Film Nanocomposite Membranes for Reverse Osmosis Desalination. Adv. Mater. Interfaces 2020, 7, 7. [Google Scholar] [CrossRef]
- Bayer, T.; Selyanchyn, R.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. Spray-painted graphene oxide membrane fuel cells. J. Membr. Sci. 2017, 541, 347–357. [Google Scholar] [CrossRef]
- Ion-Ebrasu, D.; Pollet, B.G.; Caprarescu, S.; Chitu, A.; Trusca, R.; Niculescu, V.; Gabor, R.; Carcadea, E.; Varlam, M.; Vasile, B.S. Graphene inclusion effect on anion-exchange membranes properties for alkaline water electrolyzers. Int. J. Hydrog. Energy 2020, 45, 17057–17066. [Google Scholar] [CrossRef]
- El-Din, L.A.N.; El-Gendi, A.; Ismail, N.; Abed, K.A.; Ahmed, A.I. Evaluation of cellulose acetate membrane with carbon nanotubes additives. J. Ind. Eng. Chem. 2015, 26, 259–264. [Google Scholar] [CrossRef]
- Nurkhamidah, S.; Rahmawati, Y.; Gumilang, R.; Riswanda, M.; Fahrizal, Y.; Ramadhan, I. Enhanced Performance of Celluloce Acetate/Polyethylene Glycol (CA/PEG) with the Addition of Functionalized Carbon Nanotube (CNT) Prepared by Wet-Dry Method; AIP Publishing: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Vatanpour, V.; Esmaeili, M.; Farahani, M.H.D.A. Fouling reduction and retention increment of polyethersulfone nanofiltration membranes embedded by amine-functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2014, 466, 70–81. [Google Scholar] [CrossRef]
- El-Aassar, A.-H.M. Study on polyamide thin film nano-composite membranes using different types of multi-walled carbon nanotubes at different pH feed solutions. J. Am. Sci. 2016, 12. [Google Scholar] [CrossRef]
- Choi, H.-g.; Yoon, S.H.; Son, M.; Celik, E.; Park, H.; Choi, H. Efficacy of synthesis conditions on functionalized carbon nanotube blended cellulose acetate membrane for desalination. Desalination Water Treat. 2016, 57, 7545–7554. [Google Scholar] [CrossRef]
- Rashed, A.O.; Esawi, A.M.K.; Ramadan, A.R. Novel polysulfone/carbon nanotube-polyamide thin film nanocomposite membranes with improved water flux for forward osmosis desalination. ACS Omega 2020, 5, 14427–14436. [Google Scholar] [CrossRef]
- Jin, H.; Huang, Y.; Wang, X.; Yu, P.; Luo, Y. Preparation of modified cellulose acetate membranes using functionalized multi-walled carbon nanotubes for forward osmosis. Desalination Water Treat. 2016, 57, 7166–7174. [Google Scholar] [CrossRef]
- Silva, M.; Hilliou, L.; Amorim, M.T. Fabrication of pristine-multiwalled carbon nanotubes/cellulose acetate composites for removal of methylene blue. Polym. Bull. 2020, 77, 623–653. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Su, Y.; Zhang, R.; Fan, L.; Liu, Y.; Ma, T.; Jiang, Z. Fabrication and characterization of antifouling carbon nanotube/polyethersulfone ultrafiltration membranes. RSC Adv. 2016, 6, 35532–35538. [Google Scholar] [CrossRef]
- Van der Bruggen, B. The separation power of nanotubes in membranes: A review. Int. Sch. Res. Netw. Nanotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- Jose, T.; George, S.C.; MG, M.; Thomas, S. Functionalized MWCNT and PVA nanocomposite membranes for dielectric and pervaporation applications. Chem. Eng. Process Technol. 2015, 6, 1. [Google Scholar]
- Manawi, Y.; Wang, K.; Kochkodan, V.; Johnson, D.; Atieh, M.; Khraisheh, M. Engineering the surface and mechanical properties of water desalination membranes using ultralong carbon nanotubes. Membranes 2018, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Eslami, J.; Abdi, Y.; Khamsavi, A.; EbrahimNataj, Z.; Kazemi, A.S. Effect of surface area of carbon nanotubes on membrane performance for effective water desalination. Appl. Phys. A Mater. Sci. Processing 2018, 124, 791. [Google Scholar] [CrossRef]
- Trivedi, S.; Alameh, K. Effect of vertically aligned carbon nanotube density on the water flux and salt rejection in desalination membranes. SpringerPlus 2016, 5, 1158. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Liu, Y.; Prada Silvy, R.; Tan, Y.; Azad, S.; Krause, B.; Pötschke, P.; Grady, B. Aspect ratio effects of multi-walled carbon nanotubes on electrical, mechanical, and thermal properties of Polycarbonate/MWCNT composites. J. Polym. Sci. B Polym. Phys. 2014, 52, 73–83. [Google Scholar] [CrossRef]
- Defeng, W.; Wu, L.; Zhou, W.; Sun, Y.; Zhang, M. Relations between the aspect ratio of carbon nanotubes and the formation of percolation networks in biodegradable polylactide/carbon nanotube composites. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 479–489. [Google Scholar]
- Martone, A.; Faiella, G.; Antonucci, V.; Giordano, M.; Zarrelli, M. The effect of the aspect ratio of carbon nanotubes on their effective reinforcement modulus in an epoxy matrix. Compos. Sci. Technol. 2011, 71, 1117–1123. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; De, S.; Hussein, I. Impact of aspect ratio of carbon nanotubes on shear and extensional rheology of polyethylene nanocomposites. Appl. Rheol. 2013, 23. [Google Scholar] [CrossRef]
- Gao, C.; Vo, C.D.; Jin, Y.Z.; Li, W.; Armes, S.P. Multihydroxy Polymer-Functionalized Carbon Nanotubes: Synthesis, Derivatization, and Metal Loading. Macromolecules 2005, 38, 8634–8648. [Google Scholar] [CrossRef]
- Papp, I.Z.; Kozma, G.; Puskás, R.; Simon, T.; Kónya, Z.; Kukovecz, Á. Effect of planetary ball milling process parameters on the nitrogen adsorption properties of multiwall carbon nanotubes. Adsorption 2013, 19, 687–694. [Google Scholar] [CrossRef]
- Kierkowicz, M.; Pach, E.; Santidrián, A.; Sandoval, S.; Gonçalves, G.; Tobías-Rossell, E.; Kalbáč, M.; Ballesteros, B.; Tobias, G. Comparative study of shortening and cutting strategies of single-walled and multi-walled carbon nanotubes assessed by scanning electron microscopy. Carbon 2018, 139, 922–932. [Google Scholar] [CrossRef]
- Schönherr, J.; Buchheim, J.R.; Scholz, P.; Adelhelm, P. Boehm Titration Revisited (Part I): Practical Aspects for Achieving a High Precision in Quantifying Oxygen-Containing Surface Groups on Carbon Materials. C 2018, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- El-Badawi, N.; Ramadan, A.R.; Esawi, A.M.K.; El-Morsi, M. Novel carbon nanotube–cellulose acetate nanocomposite membranes for water filtration applications. Desalination 2014, 344, 79–85. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Das, R.; Abd Hamid, S.B.; Ali, M.; Yongzhi, W. Carbon Nanotubes Characterization by X-ray Powder Diffraction—A Review. Curr. Nanosci. 2015, 11, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Poirier, D.; Gauvin, R.; Drew, R.A.L. Structural characterization of a mechanically milled carbon nanotube/aluminum mixture. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1482–1489. [Google Scholar] [CrossRef]
- Jorio, A.; Saito, R. Raman spectroscopy for carbon nanotube applications. J. Appl. Phys. 2021, 129, 021102. [Google Scholar] [CrossRef]
- Zdrojek, M.; Gebicki, W.; Jastrzebski, C.; Melin, T.; Huczko, A. Studies of Multiwall Carbon Nanotubes Using Raman Spectroscopy and Atomic Force Microscopy. Solid State Phenom. 2004, 99, 265–268. [Google Scholar] [CrossRef]
- Kumar, A.; Pande, U.; Banerjee, M.K. Effect of high energy ball milling on the structure of iron-multiwall carbon nanotubes (MWCNT) composite. Adv. Mater. Res. 2017, 6, 245–255. [Google Scholar] [CrossRef]
- Hołda, A.K.; Vankelecom, I.F.J. Understanding and guiding the phase inversion process for synthesis of solvent resistant nanofiltration membranes. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Choi, H.-G.; Son, M.; Yoon, S.; Celik, E.; Kang, S.; Park, H.; Park, C.H.; Choi, H. Alginate fouling reduction of functionalized carbon nanotube blended cellulose acetate membrane in forward osmosis. Chemosphere 2015, 136, 204–210. [Google Scholar] [CrossRef]
- Fadl, E.; Noumir, H.; Morsy, A.; Kandil, S.; Ebrahim, S.; Khalil, M. Development of cellulose acetate membrane performance by carboxylate multiwalled carbon nanotubes. Adv. Nat. Sci. Nanosci. Nanotechnol. 2022, 13, 015006. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Shan, M.; Zhou, B.; Li, Y.; Li, B.; Niu, J.; Qian, X. Synergetic effects of oxidized carbon nanotubes and graphene oxide on fouling control and anti-fouling mechanism of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2013, 448, 81–92. [Google Scholar] [CrossRef]
- Jin, X.; Xu, J.; Wang, X.; Xie, Z.; Liu, Z.; Liang, B.; Chen, D.; Shen, G. Flexible TiO2/cellulose acetate hybrid film as a recyclable photocatalyst. RSC Adv. 2014, 4, 12640. [Google Scholar] [CrossRef]
- De Menezes, B.R.C.; Ferreira, F.V.; Silva, B.C.; Simonetti, E.A.N.; Bastos, T.M.; Cividanes, L.S.; Thim, G.P. Effects of octadecylamine functionalization of carbon nanotubes on dispersion, polarity, and mechanical properties of CNT/HDPE nanocomposites. J. Mater. Sci. 2018, 53, 14311–14327. [Google Scholar] [CrossRef]



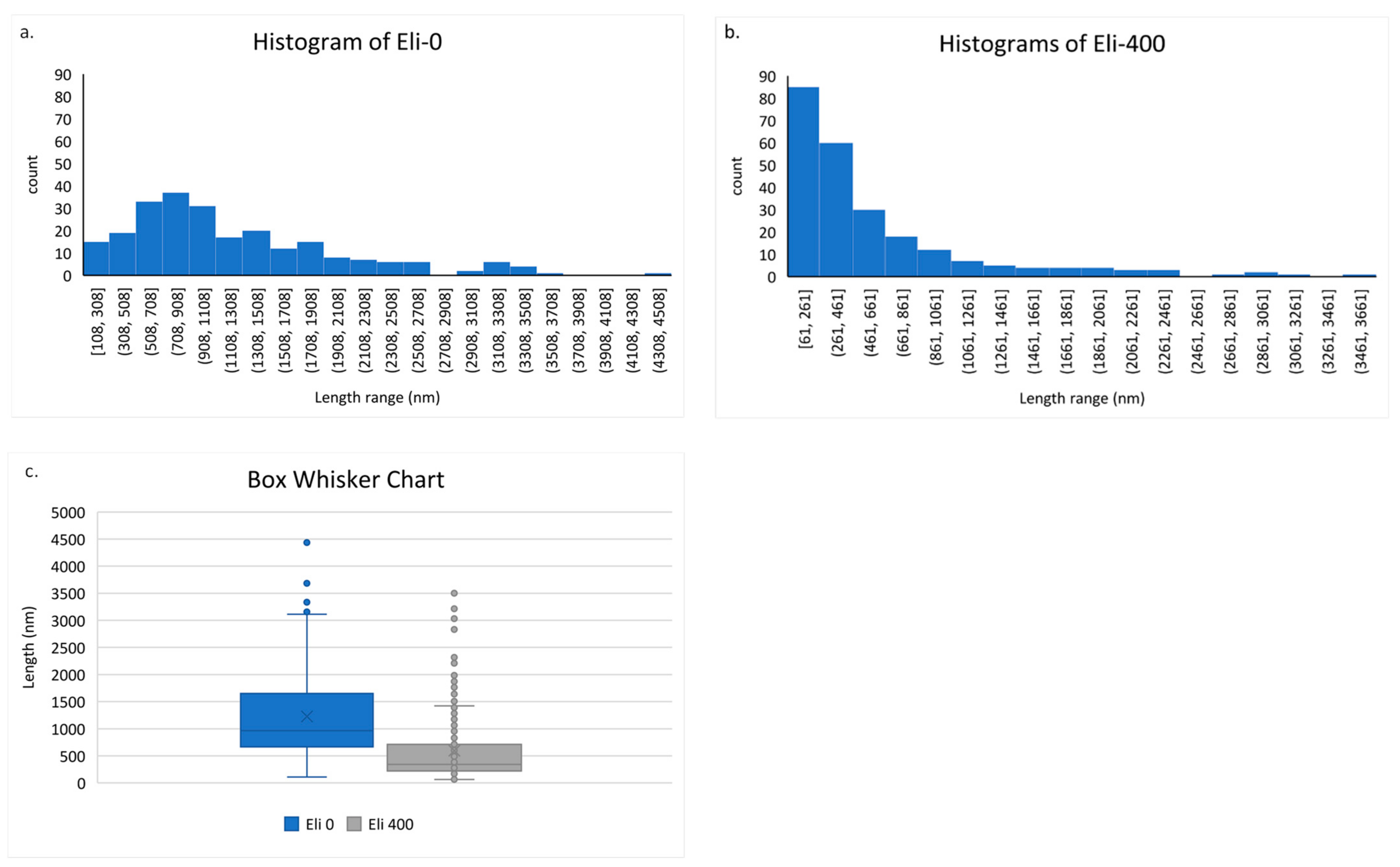


| Quartile 1 (25% of Population) | Quartile 3 (75% of the Total Population) | Quartile 2 (Median) | Mean Excluding Outliers | BET SA (m2/g) | |
|---|---|---|---|---|---|
| Eli-0 CNTs | 666 | 1649 | 965 | 1132 | 79 |
| Eli-400 CNTs | 223 | 710 | 342 | 421 | 113 |
| D | ID | G | IG | ID/IG | |
|---|---|---|---|---|---|
| Eli-0 CNTs | 1340 | 368.0 | 1580 | 357.5 | 1.03 |
| Eli-400 CNTs | 1340 | 112.8 | 1584 | 73.9 | 1.53 |
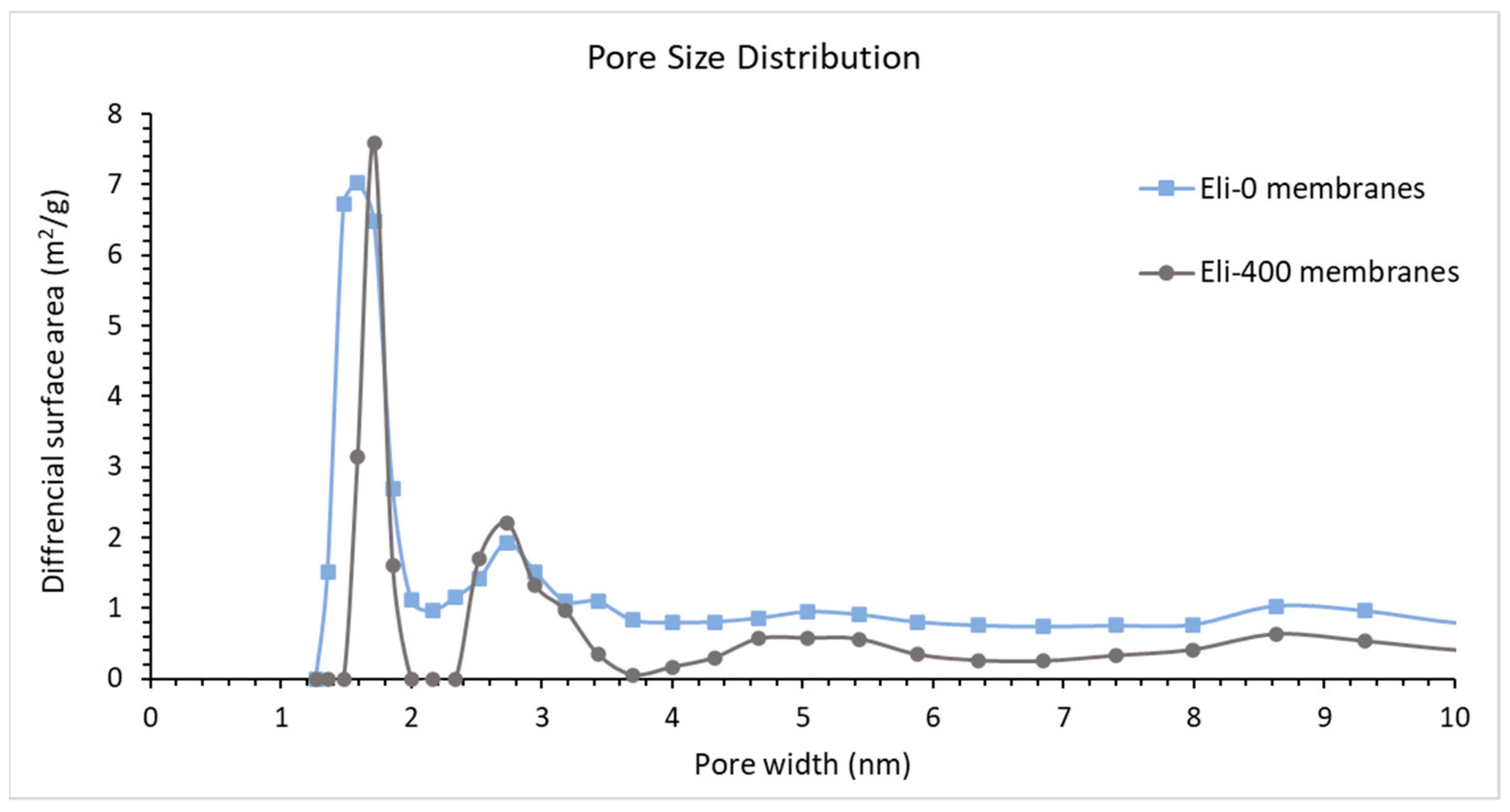
| BET SA of Membranes (m2/g) | Contact Angle | |
|---|---|---|
| Eli-0 membranes | 5.8 ± 1.3 | 64 ± 4 |
| Eli-400 membranes | 7.2 ± 0.3 | 61 ± 1 |
| CNTs Length (nm) | CNT Diameter (nm) | CNT BET SA (m2/g) | Membranes BET SA (m2/g) | Membranes Contact Angle | J Na2SO4 (L/m2h·bar) | Salt Retention % | |
|---|---|---|---|---|---|---|---|
| Eli-0 | 1132 ± 661 | 11 ± 1 | 79 | 5.8 ± 1.3 | 64 ± 4 | 0.41 ± 0.01 | 97.5 ± 0.3 |
| Eli-400 | 421 ± 299 | 11 ± 1 | 113 | 7.2 ± 0.3 | 61 ± 1 | 0.67 ± 0.02 | 96.5 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbadawi, N.A.; Ramadan, A.R.; Esawi, A.M.K. Studying the Effect of Shortening Carbon Nanotubes via Ball Milling on Cellulose Acetate Nanocomposite Membranes for Desalination Applications. Membranes 2022, 12, 474. https://doi.org/10.3390/membranes12050474
Elbadawi NA, Ramadan AR, Esawi AMK. Studying the Effect of Shortening Carbon Nanotubes via Ball Milling on Cellulose Acetate Nanocomposite Membranes for Desalination Applications. Membranes. 2022; 12(5):474. https://doi.org/10.3390/membranes12050474
Chicago/Turabian StyleElbadawi, Nouran A., Adham R. Ramadan, and Amal M. K. Esawi. 2022. "Studying the Effect of Shortening Carbon Nanotubes via Ball Milling on Cellulose Acetate Nanocomposite Membranes for Desalination Applications" Membranes 12, no. 5: 474. https://doi.org/10.3390/membranes12050474
APA StyleElbadawi, N. A., Ramadan, A. R., & Esawi, A. M. K. (2022). Studying the Effect of Shortening Carbon Nanotubes via Ball Milling on Cellulose Acetate Nanocomposite Membranes for Desalination Applications. Membranes, 12(5), 474. https://doi.org/10.3390/membranes12050474





