A Short Overview of Biological Fuel Cells
Abstract
1. Introduction
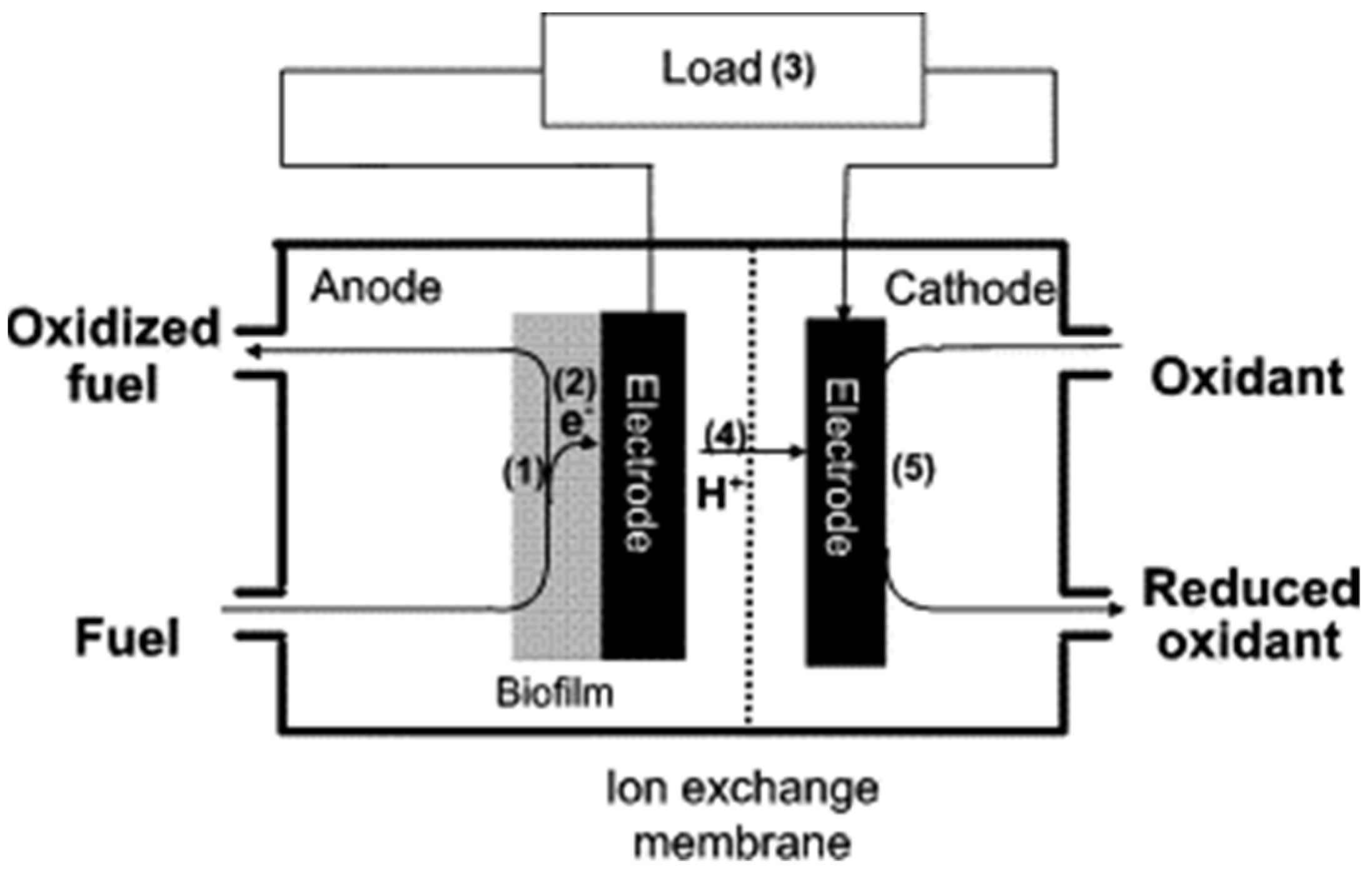
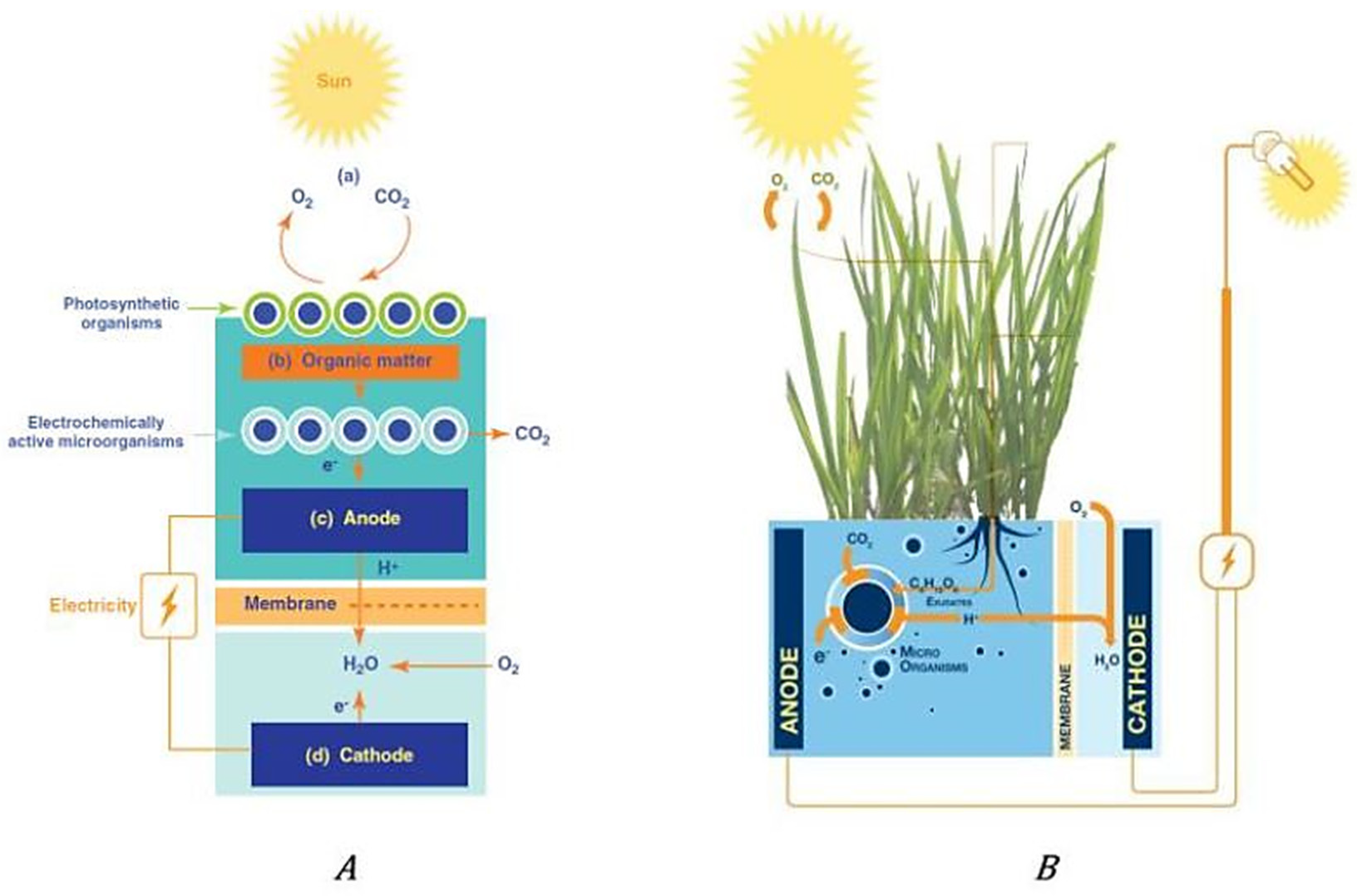
2. Microbial Fuel Cells (MFCs)
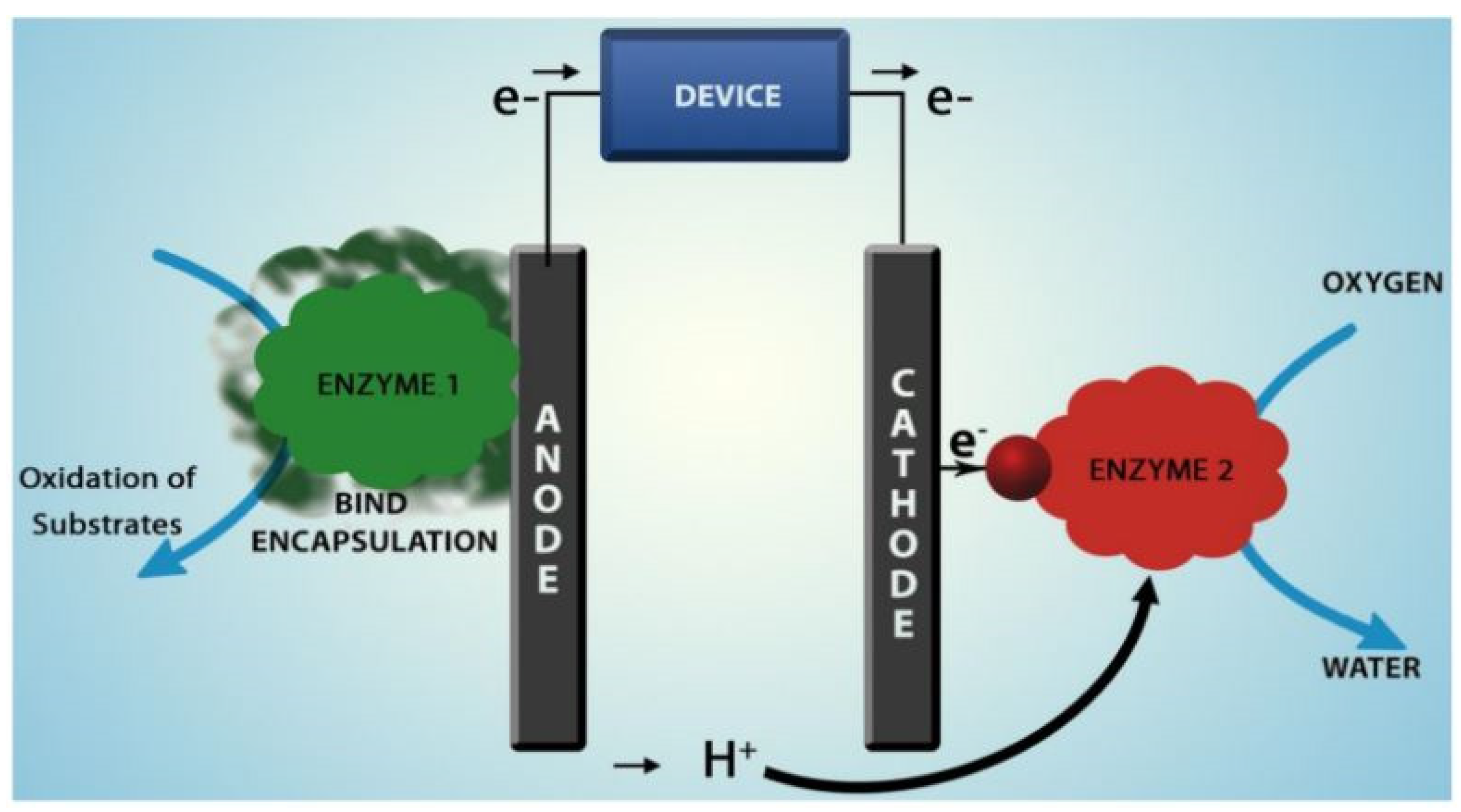
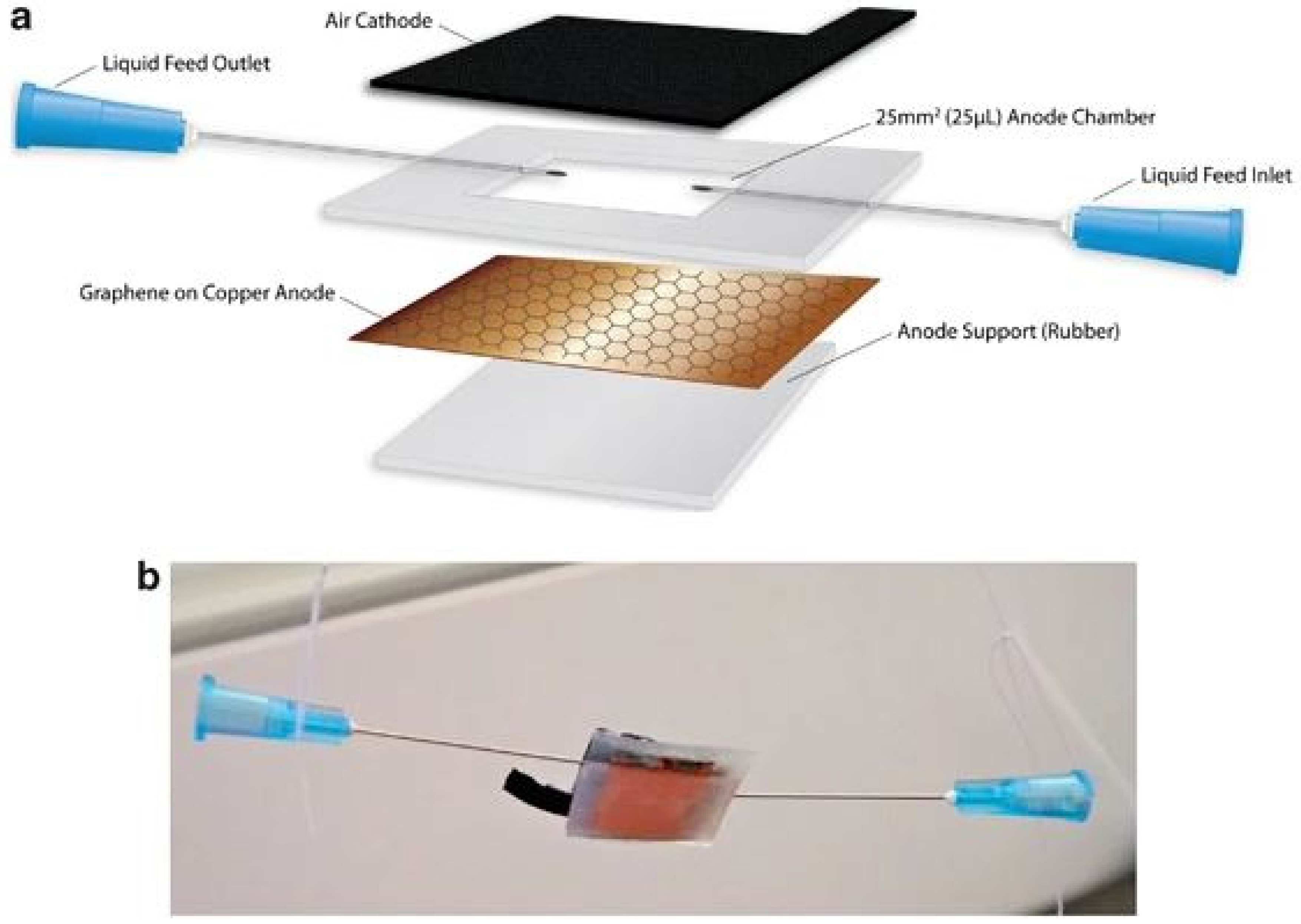
3. Enzymatic Fuel Cells (EFCs)
- Wide possibility of enzymes production based on sustainable biological processes: a huge variety of living organisms can be used as a source to extract enzymes in a renewable way [63].
- Specificity of catalytic redox reactions for their natural substrates that enables in some cases to work in a single chamber cell (i.e., without separation membrane), and make preliminary fuel purification steps unnecessary [65].
- Possibility to efficiently catalyze reactions under mild and safe conditions at physiological pH, ambient temperature and pressure [63].
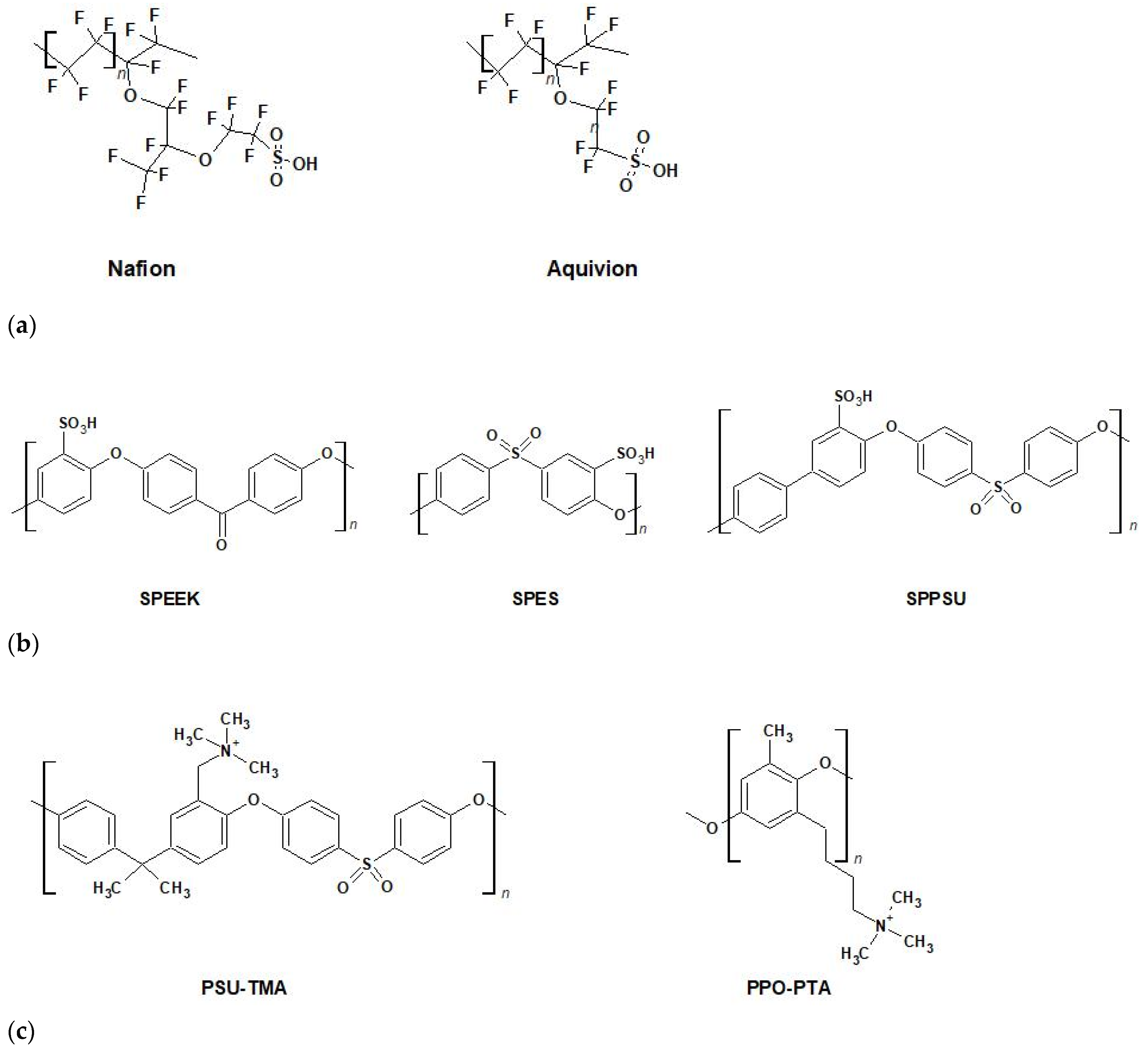
4. Ion Exchange Membranes for BioFCs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badwal:, S.P.S.; Giddey, S.S.; Munnings, C.; Bhatt, A.I.; Hollenkamp, A.F. Emerging electrochemical energy conversion and storage technologies. Front. Chem. 2014, 2, 79. [Google Scholar] [CrossRef]
- Kordesch, K.; Simader, G. Fuel Cells and Their Applications; VCH Verlagsges: Weinheim, Germany, 1996; 389p. [Google Scholar]
- Vielstich, W.; Iwasita, T. Brennstoffzellen. Naturwissenschaften 1982, 69, 372–382. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Price, A. Electrical energly storage—A review of technology options. Proc. Inst. Civ. Eng. Civ. Eng. 2005, 158, 52–58. [Google Scholar] [CrossRef]
- Tanc, B.; Arat, H.T.; Baltacioglu, E.; Aydin, K. Overview of the next quarter century vision of hydrogen fuel cell electric vehicles. Int. J. Hydrogen Energy 2019, 44, 10120–10128. [Google Scholar] [CrossRef]
- Millington, B.; Du, S.F.; Pollet, B.G. The effect of materials on proton exchange membrane fuel cell electrode performance. J. Power Sources 2011, 196, 9013–9017. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.B.; Yang, Z.J.; Wang, Y.M.; Jiang, C.X.; Ge, L.; Bakangura, E.; Xu, T.W. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Perry, M.L.; Fuller, T.F. A historical perspective of fuel cell technology in the 20th century. J. Electrochem. Soc. 2002, 149, S59–S67. [Google Scholar] [CrossRef]
- Andujar, J.M.; Segura, F. Fuel cells: History and updating. A walk along two centuries. Renew. Sustain. Energy Rev. 2009, 13, 2309–2322. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Mohan, S.V.; Pandey, A.; Varjani, S. Biomass, Biofuels, Biochemicals: Microbial Electrochemical Technology: Sustainable Platform for Fuels, Chemicals and Remediation; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Butti, S.K.; Velvizhi, G.; Sulonen, M.L.; Haavisto, J.M.; Koroglu, E.O.; Cetinkaya, A.Y.; Singh, S.; Arya, D.; Modestra, J.A.; Krishna, K.V. Microbial electrochemical technologies with the perspective of harnessing bioenergy: Maneuvering towards upscaling. Renew. Sustain. Energy Rev. 2016, 53, 462–476. [Google Scholar] [CrossRef]
- Ramírez-Vargas, C.A.; Prado, A.; Arias, C.A.; Carvalho, P.N.; Esteve-Núñez, A.; Brix, H. Microbial electrochemical technologies for wastewater treatment: Principles and evolution from microbial fuel cells to bioelectrochemical-based constructed wetlands. Water 2018, 10, 1128. [Google Scholar] [CrossRef]
- Cao, X.; Huang, X.; Liang, P.; Xiao, K.; Zhou, Y.; Zhang, X.; Logan, B.E. A new method for water desalination using microbial desalination cells. Environ. Sci. Technol. 2009, 43, 7148–7152. [Google Scholar] [CrossRef] [PubMed]
- Ivars-Barceló, F.; Zuliani, A.; Fallah, M.; Mashkour, M.; Rahimnejad, M.; Luque, R. Novel applications of microbial fuel cells in sensors and biosensors. Appl. Sci. 2018, 8, 1184. [Google Scholar] [CrossRef]
- Logan, B.E.; Wallack, M.J.; Kim, K.-Y.; He, W.; Feng, Y.; Saikaly, P.E. Assessment of microbial fuel cell configurations and power densities. Environ. Sci. Technol. Lett. 2015, 2, 206–214. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, J.M. Electricity-producing bacterial communities in microbial fuel cells. TRENDS Microbiol. 2006, 14, 512–518. [Google Scholar] [CrossRef]
- Bennetto, H.P. Microbial fuel cells. Life Chem. Rep. 1984, 2, 363–453. [Google Scholar]
- Wu, Y.-c.; Wang, Z.-j.; Zheng, Y.; Xiao, Y.; Yang, Z.-h.; Zhao, F. Light intensity affects the performance of photo microbial fuel cells with Desmodesmus sp. A8 as cathodic microorganism. Appl. Energy 2014, 116, 86–90. [Google Scholar] [CrossRef]
- Himmel, M.; Baker, J.; Overend, R.; Palmore, G.; Whitesides, G. Microbial and enzymatic biofuel cells, Enzymatic conversion of biomass for fuels production. ACS Books Am. Chem. Soc. 1994, 14, 271–290. [Google Scholar]
- Stirling, J.L.; Bennetto, H.P.; Delaney, G.M.; Mason, J.R.; Roller, S.D.; Tanaka, K.; Thurston, C.F. Microbial Fuel Cells; Portland Press: London, UK, 1983. [Google Scholar]
- Bullen, R.A.; Arnot, T.; Lakeman, J.; Walsh, F. Biofuel cells and their development. Biosens. Bioelectron. 2006, 21, 2015–2045. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Oh, S.T.; Kim, J.R.; Premier, G.C.; Lee, T.H.; Kim, C.; Sloan, W.T. Sustainable wastewater treatment: How might microbial fuel cells contribute. Biotechnol. Adv. 2010, 28, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.; Mishra, V.; Agrawal, S. Production of bio-electricity during wastewater treatment using a single chamber microbial fuel cell. Int. J. Eng. Sci. Technol. 2011, 3. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, V.; Malyan, S.K.; Sharma, J.; Mathimani, T.; Maskarenj, M.S.; Ghosh, P.C.; Pugazhendhi, A. Microbial fuel cells (MFCs) for bioelectrochemical treatment of different wastewater streams. Fuel 2019, 254, 115526. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Wang, K.; Lv, S.; Wang, X.; Zhuo, L.; Zhang, J. Effects of Concentration Variations on the Performance and Microbial Community in Microbial Fuel Cell Using Swine Wastewater. Energies 2020, 13, 2231. [Google Scholar] [CrossRef]
- Ni, H.; Wang, K.; Lv, S.; Wang, X.; Zhang, J.; Zhuo, L.; Li, F. Effects of Modified Anodes on the Performance and Microbial Community of Microbial Fuel Cells Using Swine Wastewater. Energies 2020, 13, 3980. [Google Scholar] [CrossRef]
- Chang, I.S.; Jang, J.K.; Gil, G.C.; Kim, M.; Kim, H.J.; Cho, B.W.; Kim, B.H. Continuous determination of biochemical oxygen demand using microbial fuel cell type biosensor. Biosens. Bioelectron. 2004, 19, 607–613. [Google Scholar] [CrossRef]
- Wu, Q.; Jiao, S.; Ma, M.; Peng, S. Microbial fuel cell system: A promising technology for pollutant removal and environmental remediation. Environ. Sci. Pollut. Res. 2020, 27, 6749–6764. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Adhami, A.; Darvari, S.; Zirepour, A.; Oh, S.-E. Microbial fuel cell as new technology for bioelectricity generation: A review. Alex. Eng. J. 2015, 54, 745–756. [Google Scholar] [CrossRef]
- Allenand, R.; Bennetto, H. Microbial fuel-cells, Applied Biochemistry and Biotechnology. Appl. Biochem. Biotechnol. 1993, 39, 27–40. [Google Scholar] [CrossRef]
- Guo, K.; Hassett, D.J.; Gu, T.Y. Microbial Fuel Cells: Electricity Generation from Organic Wastes by Microbes; CABI: Wallingford, UK, 2012; Volume 9, pp. 162–189. [Google Scholar]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Hamelers, H.V.; Ter Heijne, A.; Sleutels, T.H.; Jeremiasse, A.W.; Strik, D.P.; Buisman, C.J. New applications and performance of bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Dewan, A.; Donovan, C.; Heo, D.; Beyenal, H. Evaluating the performance of microbial fuel cells powering electronic devices. J. Power Sources 2010, 195, 90–96. [Google Scholar] [CrossRef]
- Gil, G.-C.; Chang, I.-S.; Kim, B.H.; Kim, M.; Jang, J.-K.; Park, H.S.; Kim, H.J. Operational parameters affecting the performannce of a mediator-less microbial fuel cell. Biosens. Bioelectron. 2003, 18, 327–334. [Google Scholar] [CrossRef]
- Kim, J.R.; Min, B.; Logan, B.E. Evaluation of procedures to acclimate a microbial fuel cell for electricity production. Appl. Microbiol. Biotechnol. 2005, 68, 23–30. [Google Scholar] [CrossRef]
- Kim, B.H.; Chang, I.S.; Gadd, G.M. Challenges in microbial fuel cell development and operation. Appl. Microbiol. Biotechnol. 2007, 76, 485–494. [Google Scholar] [CrossRef]
- Delaney, G.M.; Bennetto, H.P.; Mason, J.R.; Roller, S.D.; Stirling, J.L.; Thurston, C.F. Electron-transfer coupling in microbial fuel cells. 2. performance of fuel cells containing selected microorganism—mediator—substrate combinations. J. Chem. Technol. Biotechnol. Biotechnol. 1984, 34, 13–27. [Google Scholar] [CrossRef]
- Lithgow, A.; Romero, L.; Sanchez, I.; Souto, F.; Vega, C. Interception of the electron-transport chain in bacteria with hydrophilic redox mediators. I: Selective improvement of the performance of biofuel cells with 2, 6-disulphonated thionine as mediator. J. Chem. Res. Synop. 1986, 5, 178–179. [Google Scholar]
- Ghasemi, M.; Daud, W.R.W.; Ismail, M.; Rahimnejad, M.; Ismail, A.F.; Leong, J.X.; Miskan, M.; Liew, K.B. Effect of pre-treatment and biofouling of proton exchange membrane on microbial fuel cell performance. Int. J. Hydrogen Energy 2013, 38, 5480–5484. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Stamatelatou, K.; Bebelis, S.; Lyberatos, G. Electricity generation from synthetic substrates and cheese whey using a two chamber microbial fuel cell. Biochem. Eng. J. 2010, 50, 10–15. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Najafpour, G.; Ghoreyshi, A.A. Effect of mass transfer on performance of microbial fuel cell. Intech 2011, 5, 233–250. [Google Scholar]
- Chaudhuri, S.K.; Lovley, D.R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 2003, 21, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.K.; Pham, T.H.; Chang, I.S.; Kang, K.H.; Moon, H.; Cho, K.S.; Kim, B.H. Construction and operation of a novel mediator-and membrane-less microbial fuel cell. Process Biochem. 2004, 39, 1007–1012. [Google Scholar] [CrossRef]
- Ieropoulos, I.A.; Greenman, J.; Melhuish, C.; Hart, J. Comparative study of three types of microbial fuel cell. Enzym. Microb. Technol. 2005, 37, 238–245. [Google Scholar] [CrossRef]
- Yasri, N.; Roberts, E.P.; Gunasekaran, S. The electrochemical perspective of bioelectrocatalytic activities in microbial electrolysis and microbial fuel cells. Energy Rep. 2019, 5, 1116–1136. [Google Scholar] [CrossRef]
- Ter Heijne, A.; Pereira, M.; Pereira, J.; Sleutels, T. Electron Storage in Electroactive Biofilms. Trends Biotechnol. 2020, 39, 34–42. [Google Scholar] [CrossRef]
- Mink, J.E.; Qaisi, R.M.; Logan, B.E.; Hussain, M.M. Energy harvesting from organic liquids in micro-sized microbial fuel cells. Npg Asia Mater. 2014, 6, e89. [Google Scholar] [CrossRef][Green Version]
- Zaman, B.; Samadikun, B.P.; Budihardjo, M.A.; Hardyanti, N.; Rachma, A.F.; Hasna, S.I. Potential of phytotechnology in wastewater treatments to produce alternative electrical energy: A review. J. Phys. Conf. Ser. 2020, 1524, 012082. [Google Scholar] [CrossRef]
- Das, S.; Mangwani, N. Recent developments in microbial fuel cells: A review. J. Sci. Ind. Res. 2010, 69, 727–731. [Google Scholar]
- Guarnieri, M.T.; Chou, Y.-C.; Salvachúa, D.; Mohagheghi, A.; St. John, P.C.; Peterson, D.J.; Bomble, Y.J.; Beckham, G.T. Metabolic engineering of Actinobacillus succinogenes provides insights into succinic acid biosynthesis. Appl. Environ. Microbiol. 2017, 83, e00996-17. [Google Scholar] [CrossRef]
- Nkiruka, A.; Adeoye, M.; Omojokun, A.O.; Fatile, J.A. The effect of electrodes on the voltage generation of microbial fuel cell. Niger. J. Pure Appl. Phys. 2020, 10, 8–11. [Google Scholar] [CrossRef]
- Qiao, Y.-J.; Qiao, Y.; Zou, L.; Wu, X.-S.; Liu, J.-H. Biofilm promoted current generation of Pseudomonas aeruginosa microbial fuel cell via improving the interfacial redox reaction of phenazines. Bioelectrochemistry 2017, 117, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Arkatkar, A.; Mungray, A.K.; Sharma, P. Study of electrochemical activity zone of Pseudomonas aeruginosa in microbial fuel cell. Process Biochem. 2021, 101, 213–217. [Google Scholar] [CrossRef]
- Vega, C.A.; Fernández, I. Mediating effect of ferric chelate compounds in microbial fuel cells with Lactobacillus plantarum, Streptococcus lactis, and Erwinia dissolvens. Bioelectrochem. Bioenerg. 1987, 17, 217–222. [Google Scholar] [CrossRef]
- Lovley, D.R.; Giovannoni, S.J.; White, D.C.; Champine, J.E.; Phillips, E.; Gorby, Y.A.; Goodwin, S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch. Microbiol. 1993, 159, 336–344. [Google Scholar] [CrossRef]
- Finneran, K.T.; Johnsen, C.V.; Lovley, D.R. Rhodoferax ferrireducens sp. nov., a psychrotolerant, facultatively anaerobic bacterium that oxidizes acetate with the reduction of Fe(III). Int. J. Syst. Evol. Microbiol. 2003, 53, 669–673. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, S.; Zhuang, L.; Li, W.; Zhang, J.; Lu, N.; Deng, L. Microbial fuel cell based on Klebsiella pneumoniae biofilm. Electrochem. Commun. 2008, 10, 1641–1643. [Google Scholar] [CrossRef]
- Xiao, X.; Xia, H.-q.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A. Tackling the Challenges of Enzymatic (Bio)Fuel Cells. Chem. Rev. 2019, 119, 9509–9558. [Google Scholar] [CrossRef]
- Ivanov, I.; Vidaković-Koch, T.; Sundmacher, K. Recent Advances in Enzymatic Fuel Cells: Experiments and Modeling. Energies 2010, 3, 803–846. [Google Scholar] [CrossRef]
- Leech, D.; Kavanagh, P.; Schuhmann, W. Enzymatic fuel cells: Recent progress. Electrochim. Acta 2012, 84, 223–234. [Google Scholar] [CrossRef]
- Mazurenko, I.; Hitaishi, V.P.; Lojou, E. Recent advances in surface chemistry of electrodes to promote direct enzymatic bioelectrocatalysis. Curr. Opin. Electrochem. 2020, 19, 113–121. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Calzoni, E.; Cesaretti, A.; Di Michele, A.; Emiliani, C.; Gammaitoni, L.; Sisani, E. Enzymatic fuel cell technology for energy production from bio-sources. In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2019; Volume 2191, p. 020014. [Google Scholar]
- Christwardana, M.; Kim, K.J.; Kwon, Y. Fabrication of Mediatorless/Membraneless Glucose/Oxygen Based Biofuel Cell using Biocatalysts Including Glucose Oxidase and Laccase Enzymes. Sci. Rep. 2016, 6, 30128. [Google Scholar] [CrossRef] [PubMed]
- Zebda, A.; Alcaraz, J.P.; Vadgama, P.; Shleev, S.; Minteer, S.D.; Boucher, F.; Cinquin, P.; Martin, D.K. Challenges for successful implantation of biofuel cells. Bioelectrochemistry 2018, 124, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Aquino Neto, S.; De Andrade, A.R. New energy sources: The enzymatic biofuel cell. J. Braz. Chem. Soc. 2013, 24, 1891–1912. [Google Scholar] [CrossRef]
- Holmberg, S.; Perebikovsky, A.; Kulinsky, L.; Madou, M. 3-D Micro and Nano Technologies for Improvements in Electrochemical Power Devices. Micromachines 2014, 5, 171–203. [Google Scholar] [CrossRef]
- Yahiro, A.T.; Lee, S.M.; Kimble, D.O. Bioelectrochemistry: I. Enzyme utilizing bio-fuel cell studies. Biochim. Et Biophys. Acta (BBA) Spec. Sect. Biophys. Subj. 1964, 88, 375–383. [Google Scholar] [CrossRef]
- Naidoo, S.; Naidoo, Q.; Blottnitz, H.; Vaivars, G. Glucose oxidase as a biocatalytic enzyme-based bio-fuel cell using Nafion membrane limiting crossover. IOP Conf. Ser. Mater. Sci. Eng. 2013, 49, 012062. [Google Scholar] [CrossRef]
- Sokic-Lazic, D.; de Andrade, A.R.; Minteer, S.D. Utilization of enzyme cascades for complete oxidation of lactate in an enzymatic biofuel cell. Electrochim. Acta 2011, 56, 10772–10775. [Google Scholar] [CrossRef]
- Willner, I.; Yan, Y.M.; Willner, B.; Tel-Vered, R. Integrated Enzyme-Based Biofuel Cells—A Review. Fuel Cells 2009, 9, 7–24. [Google Scholar] [CrossRef]
- Yan, Y.M.; Yehezkeli, O.; Willner, I. Integrated, electrically contacted NAD(P)(+)-dependent enzyme-carbon nanotube electrodes for biosensors and biofuel cell applications. Chem. A Eur. J. 2007, 13, 10168–10175. [Google Scholar] [CrossRef]
- Mazurenko, I.; Wang, X.; de Poulpiquet, A.; Lojou, E. H2/O2 enzymatic fuel cells: From proof-of-concept to powerful devices. Sustain. Energy Fuels 2017, 1, 1475–1501. [Google Scholar] [CrossRef]
- Cosnier, S.; Gross, A.J.; Giroud, F.; Holzinger, M. Beyond the hype surrounding biofuel cells: What’s the future of enzymatic fuel cells? Curr. Opin. Electrochem. 2018, 12, 148–155. [Google Scholar] [CrossRef]
- Pasquini, L.; Zhakisheva, B.; Sgreccia, E.; Narducci, R.; Di Vona, M.L.; Knauth, P. Stability of Proton Exchange Membranes in Phosphate Buffer for Enzymatic Fuel Cell Application: Hydration, Conductivity and Mechanical Properties. Polymers 2021, 13, 475. [Google Scholar] [CrossRef] [PubMed]
- de Poulpiquet, A.; Ranava, D.; Monsalve, K.; Giudici-Orticoni, M.-T.; Lojou, E. Biohydrogen for a New Generation of H2/O2 Biofuel Cells: A Sustainable Energy Perspective. ChemElectroChem 2014, 1, 1724–1750. [Google Scholar] [CrossRef]
- Mazurenko, I.; Monsalve, K.; Infossi, P.; Giudici-Orticoni, M.-T.; Topin, F.; Mano, N.; Lojou, E. Impact of substrate diffusion and enzyme distribution in 3D-porous electrodes: A combined electrochemical and modelling study of a thermostable H2/O2 enzymatic fuel cell. Energy Environ. Sci. 2017, 10, 1966–1982. [Google Scholar] [CrossRef]
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Akers, N.L.; Moore, C.M.; Minteer, S.D. Development of alcohol/O2 biofuel cells using salt-extracted tetrabutylammonium bromide/Nafion membranes to immobilize dehydrogenase enzymes. Electrochim. Acta 2005, 50, 2521–2525. [Google Scholar] [CrossRef]
- Ivnitski, D.; Branch, B.; Atanassov, P.; Apblett, C. Glucose oxidase anode for biofuel cell based on direct electron transfer. Electrochem. Commun. 2006, 8, 1204–1210. [Google Scholar] [CrossRef]
- Chen, T.; Barton, S.C.; Binyamin, G.; Gao, Z.; Zhang, Y.; Kim, H.-H.; Heller, A. A Miniature Biofuel Cell. J. Am. Chem. Soc. 2001, 123, 8630–8631. [Google Scholar] [CrossRef]
- Heller, A. Miniature biofuel cells. Phys. Chem. Chem. Phys. 2004, 6, 209–216. [Google Scholar] [CrossRef]
- Sakai, H.; Nakagawa, T.; Tokita, Y.; Hatazawa, T.; Ikeda, T.; Tsujimura, S.; Kano, K. A high-power glucose/oxygen biofuel cell operating under quiescent conditions. Energy Environ. Sci. 2009, 2, 133–138. [Google Scholar] [CrossRef]
- Cosnier, S.; Gross, A.J.; Le Goff, A.; Holzinger, M. Recent advances on enzymatic glucose/oxygen and hydrogen/oxygen biofuel cells: Achievements and limitations. J. Power Sources 2016, 325, 252–263. [Google Scholar] [CrossRef]
- Xu, S.; Minteer, S.D. Enzymatic Biofuel Cell for Oxidation of Glucose to CO2. ACS Catal. 2012, 2, 91–94. [Google Scholar] [CrossRef]
- Ghassemi, Z.; Slaughter, G. Biological Fuel Cells and Membranes. Membranes 2017, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Vincent, K.A.; Cracknell, J.A.; Lenz, O.; Zebger, I.; Friedrich, B.; Armstrong, F.A. Electrocatalytic hydrogen oxidation by an enzyme at high carbon monoxide or oxygen levels. Proc. Natl. Acad. Sci. USA 2005, 102, 16951–16954. [Google Scholar] [CrossRef] [PubMed]
- Besic, S.; Minteer, S.D. Micellar Polymer Encapsulation of Enzymes. In Enzyme Stabilization and Immobilization: Methods and Protocols; Minteer, S.D., Ed.; Springer: New York, NY, USA, 2017; pp. 93–108. [Google Scholar]
- Ahmad, R.; Sardar, M. Enzyme Immobilization: An Overview on Nanoparticles as ImmobilizationMatrix. Biochem. Anal. Biochem. 2015, 4, 1. [Google Scholar]
- Mokhtar, N.F.; Abd. Rahman, R.N.Z.R.; Muhd Noor, N.D.; Mohd Shariff, F.; Mohamad Ali, M.S. The Immobilization of Lipases on Porous Support by Adsorption and Hydrophobic Interaction Method. Catalysts 2020, 10, 744. [Google Scholar] [CrossRef]
- Korkut, S.; Kilic, M.S. Power improvement of enzymatic fuel cells used for sustainable energy generation. Environ. Prog. Sustain. Energy 2016, 35, 859–866. [Google Scholar] [CrossRef]
- Fischback, M.B.; Youn, J.K.; Zhao, X.; Wang, P.; Park, H.G.; Chang, H.N.; Kim, J.; Ha, S. Miniature Biofuel Cells with Improved Stability Under Continuous Operation. Electroanalysis 2006, 18, 2016–2022. [Google Scholar] [CrossRef]
- Moore, C.M.; Akers, N.L.; Hill, A.D.; Johnson, Z.C.; Minteer, S.D. Improving the Environment for Immobilized Dehydrogenase Enzymes by Modifying Nafion with Tetraalkylammonium Bromides. Biomacromolecules 2004, 5, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Meredith, S.; Xu, S.; Meredith, M.T.; Minteer, S.D. Hydrophobic Salt-modified Nafion for Enzyme Immobilization and Stabilization. JoVE 2012, 65, e3949. [Google Scholar] [CrossRef] [PubMed]
- ElKaoutit, M.; Naranjo-Rodriguez, I.; Domínguez, M.; Hidalgo-Hidalgo-de-Cisneros, J.L. Bio-functionalization of electro-synthesized polypyrrole surface by heme enzyme using a mixture of Nafion and glutaraldehyde as synergetic immobilization matrix: Conformational characterization and electrocatalytic studies. Appl. Surf. Sci. 2011, 257, 10926–10935. [Google Scholar] [CrossRef]
- Klotzbach, T.; Watt, M.; Ansari, Y.; Minteer, S.D. Effects of hydrophobic modification of chitosan and Nafion on transport properties, ion-exchange capacities, and enzyme immobilization. J. Membr. Sci. 2006, 282, 276–283. [Google Scholar] [CrossRef]
- Klotzbach, T.L.; Watt, M.; Ansari, Y.; Minteer, S.D. Improving the microenvironment for enzyme immobilization at electrodes by hydrophobically modifying chitosan and Nafion® polymers. J. Membr. Sci. 2008, 311, 81–88. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef] [PubMed]
- Gierke, T. Ionic clustering in Nafion® perfluorosulfonic acid membranes and its relationship to hydroxyl rejection and chlor-alkali current efficiency. J. Electrochem. Soc. 1977, 124, 319C. [Google Scholar]
- Yeager, H.; Steck, A. Cation and water diffusion in Nafion ion exchange membranes: Influence of polymer structure. J. Electrochem. Soc. 1981, 128, 1880. [Google Scholar] [CrossRef]
- Gebel, G. Structural evolution of water swollen perfluorosulfonated ionomers from dry membrane to solution. Polymer 2000, 41, 5829–5838. [Google Scholar] [CrossRef]
- Iezzi, R.C.; Santos, R.D.; Silva, G.C.d.; Paganin, V.A.; Ticianelli, E.A. CO tolerance and stability of proton exchange membrane fuel cells with Nafion® and Aquivion® membranes and Mo-based anode electrocatalysts. J. Braz. Chem. Soc. 2018, 29, 1094–1104. [Google Scholar] [CrossRef]
- Giancola, S.; Arciniegas, R.A.B.; Fahs, A.; Chailan, J.-F.; Di Vona, M.L.; Knauth, P.; Narducci, R. Study of annealed Aquivion® ionomers with the INCA method. Membranes 2019, 9, 134. [Google Scholar] [CrossRef]
- Yandrasits, M.A.; Hamrock, S.J. Membranes for PEM Fuel Cells: 3M Research Activities. In Fuel Cell Chemistry and Operation; ACS Publications: Washington, DC, USA, 2010; pp. 15–29. [Google Scholar]
- Narducci, R.; Knauth, P.; Chailan, J.-F.; Di Vona, M.L. How to improve Nafion with tailor made annealing. RSC Adv. 2018, 8, 27268–27274. [Google Scholar] [CrossRef]
- Casciola, M.; Alberti, G.; Sganappa, M.; Narducci, R. On the decay of Nafion proton conductivity at high temperature and relative humidity. J. Power Sources 2006, 162, 141–145. [Google Scholar] [CrossRef]
- Ramirez-Nava, J.; Martínez-Castrejón, M.; García-Mesino, R.L.; López-Díaz, J.A.; Talavera-Mendoza, O.; Sarmiento-Villagrana, A.; Rojano, F.; Hernández-Flores, G. The Implications of Membranes Used as Separators in Microbial Fuel Cells. Membranes 2021, 11, 738. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.; Wacrenier, O.; Vona, M.L.D.; Knauth, P. Hydration and Ionic Conductivity of Model Cation and Anion-Conducting Ionomers in Buffer Solutions (Phosphate, Acetate, Citrate). J. Phys. Chem. B 2018, 122, 12009–12016. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, M.J.; Villigram, R.E.; Torrence, N.J.; Brancato, S.J.; Minteer, S.D. Effects of mixture casting Nafion® with quaternary ammonium bromide salts on the ion-exchange capacity and mass transport in the membranes. J. Membr. Sci. 2002, 205, 3–10. [Google Scholar] [CrossRef]
- Moehlenbrock, M.J.; Minteer, S.D. Extended lifetime biofuel cells. Chem. Soc. Rev. 2008, 37, 1188–1196. [Google Scholar] [CrossRef]
- Bahar, T. Development of Reasonably Stable Chitosan Based Proton Exchange Membranes for a Glucose Oxidase Based Enzymatic Biofuel Cell. Electroanalysis 2020, 32, 536–545. [Google Scholar] [CrossRef]
- Stenina, I.A.; Sistat, P.; Rebrov, A.I.; Pourcelly, G.; Yaroslavtsev, A.B. Ion mobility in Nafion-117 membranes. Desalination 2004, 170, 49–57. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Bakeri, G.; Ghasemi, M.; Zirepour, A. A review on the role of proton exchange membrane on the performance of microbial fuel cell. Polym. Adv. Technol. 2014, 25, 1426–1432. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.M.; Buisman, C.J.N. Effects of Membrane Cation Transport on pH and Microbial Fuel Cell Performance. Environ. Sci. Technol. 2006, 40, 5206–5211. [Google Scholar] [CrossRef]
- Roziere, J.; Jones, D.J. Non-fluorinated polymer materials for proton exchange membrane fuel cells. Annu. Rev. Mater. Res. 2003, 33, 503–555. [Google Scholar] [CrossRef]
- Hou, H.; Di Vona, M.L.; Knauth, P. Building bridges: Crosslinking of sulfonated aromatic polymers—A review. J. Membr. Sci. 2012, 423, 113–127. [Google Scholar] [CrossRef]
- Hou, H.; Di Vona, M.L.; Knauth, P. Durability of sulfonated aromatic polymers for proton-exchange-membrane fuel cells. ChemSusChem 2011, 4, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Shabani, M.; Younesi, H.; Pontié, M.; Rahimpour, A.; Rahimnejad, M.; Zinatizadeh, A.A. A critical review on recent proton exchange membranes applied in microbial fuel cells for renewable energy recovery. J. Clean. Prod. 2020, 264, 121446. [Google Scholar] [CrossRef]
- Kaliaguine, S.; Mikhailenko, S.; Wang, K.; Xing, P.; Robertson, G.; Guiver, M. Properties of SPEEK based PEMs for fuel cell application. Catal. Today 2003, 82, 213–222. [Google Scholar] [CrossRef]
- Beyraghi, F.; Mirfarsi, S.H.; Rowshanzamir, S.; Karimi, A.; Parnian, M.J. Optimal thermal treatment conditions for durability improvement of highly sulfonated poly (ether ether ketone) membrane for polymer electrolyte fuel cell applications. Int. J. Hydrogen Energy 2020, 45, 13441–13458. [Google Scholar] [CrossRef]
- Oh, Y.S.; Lee, H.J.; Yoo, M.; Kim, H.J.; Han, J.; Kim, T.H. Synthesis of novel crosslinked sulfonatod poly(ether sulfone)s using bisazide and their properties for fuel cell application. J. Membr. Sci. 2008, 323, 309–315. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Sgreccia, E.; Tamilvanan, M.; Khadhraoui, M.; Chassigneux, C.; Knauth, P. High ionic exchange capacity polyphenylsulfone (SPPSU) and polyethersulfone (SPES) cross-linked by annealing treatment: Thermal stability, hydration level and mechanical properties. J. Membr. Sci. 2010, 354, 134–141. [Google Scholar] [CrossRef]
- Manea, C.; Mulder, M. Characterization of polymer blends of polyethersulfone/sulfonated polysulfone and polyethersulfone/sulfonated polyetheretherketone for direct methanol fuel cell applications. J. Membr. Sci. 2002, 206, 443–453. [Google Scholar] [CrossRef]
- Maier, G.; Meier-Haack, J. Sulfonated aromatic polymers for fuel cell membranes. In Fuel Cells II; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–62. [Google Scholar]
- Di Vona, M.L.; Sgreccia, E.; Licoccia, S.; Alberti, G.; Tortet, L.; Knauth, P. Analysis of temperature-promoted and solvent-assisted cross-linking in sulfonated poly (ether ether ketone)(SPEEK) proton-conducting membranes. J. Phys. Chem. B 2009, 113, 7505–7512. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Slade, R.C.; Yee, E.L.H. An alkaline polymer electrochemical interface: A breakthrough in application of alkaline anion-exchange membranes in fuel cells. Chem. Commun. 2006, 13, 1428–1429. [Google Scholar] [CrossRef] [PubMed]
- Varcoe, J.R.; Slade, R.C. Prospects for alkaline anion-exchange membranes in low temperature fuel cells. Fuel Cells 2005, 5, 187–200. [Google Scholar] [CrossRef]
- Danks, T.N.; Slade, R.C.; Varcoe, J.R. Alkaline anion-exchange radiation-grafted membranes for possible electrochemical application in fuel cells. J. Mater. Chem. 2003, 13, 712–721. [Google Scholar] [CrossRef]
- Adams, L.A.; Poynton, S.D.; Tamain, C.; Slade, R.C.; Varcoe, J.R. A carbon dioxide tolerant aqueous-electrolyte-free anion-exchange membrane alkaline fuel cell. ChemSusChem Chem. Sustain. Energy Mater. 2008, 1, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, H.; Fukuta, K. Anion exchange membrane and ionomer for alkaline membrane fuel cells (AMFCs). ECS Trans. 2008, 16, 257. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Fang, J.; Shen, P.K. Quaternized poly (phthalazinon ether sulfone ketone) membrane for anion exchange membrane fuel cells. J. Membr. Sci. 2006, 285, 317–322. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y. Quaternized polyethersulfone Cardo anion exchange membranes for direct methanol alkaline fuel cells. J. Membr. Sci. 2005, 262, 1–4. [Google Scholar] [CrossRef]
- Yi, F.; Yang, X.; Li, Y.; Fang, S. Synthesis and ion conductivity of poly (oxyethylene) methacrylates containing a quaternary ammonium group. Polym. Adv. Technol. 1999, 10, 473–475. [Google Scholar] [CrossRef]
- Kang, J.J.; Li, W.Y.; Lin, Y.; Li, X.P.; Xiao, X.R.; Fang, S.B. Synthesis and ionic conductivity of a polysiloxane containing quaternary ammonium groups. Polym. Adv. Technol. 2004, 15, 61–64. [Google Scholar] [CrossRef]
- Chen, N.; Lee, Y.M. Anion exchange polyelectrolytes for membranes and ionomers. Prog. Polym. Sci. 2021, 113, 101345. [Google Scholar] [CrossRef]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-based polymer electrolyte membranes: Importance of morphology on ion transport and membrane stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Noonan, K.J.; Coates, G.W. Alkaline-stable anion exchange membranes: A review of synthetic approaches. Prog. Polym. Sci. 2020, 100, 101177. [Google Scholar] [CrossRef]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, Y.S. Durability challenges of anion exchange membrane fuel cells. Energy Environ. Sci. 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- Knauth, P.; Pasquini, L.; Narducci, R.; Sgreccia, E.; Becerra-Arciniegas, R.-A.; Di Vona, M. Effective ion mobility in anion exchange ionomers: Relations with hydration, porosity, tortuosity, and percolation. J. Membr. Sci. 2021, 617, 118622. [Google Scholar] [CrossRef]
- Han, K.W.; Ko, K.H.; Abu-Hakmeh, K.; Bae, C.; Sohn, Y.J.; Jang, S.S. Molecular dynamics simulation study of a polysulfone-based anion exchange membrane in comparison with the proton exchange membrane. J. Phys. Chem. C 2014, 118, 12577–12587. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Wang, C.; Lee, Y.M.; Guiver, M.D. Phenyltrimethylammonium functionalized polysulfone anion exchange membranes. Macromolecules 2012, 45, 2411–2419. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Qu, C. Imidazolium functionalized polysulfone anion exchange membrane for fuel cell application. J. Mater. Chem. 2011, 21, 12744–12752. [Google Scholar] [CrossRef]
- Du, R.; Gao, B.; Li, Y.; Wang, L. Preparation and structure/property relationship of polysulfone anion-exchange membranes. Acta Polym. Sin. 2010, 7, 924–931. [Google Scholar] [CrossRef]
- Di Vona, M.; Narducci, R.; Pasquini, L.; Pelzer, K.; Knauth, P. Anion-conducting ionomers: Study of type of functionalizing amine and macromolecular cross-linking. Int. J. Hydrogen Energy 2014, 39, 14039–14049. [Google Scholar] [CrossRef]
- Huang, T.; He, G.; Xue, J.; Otoo, O.; He, X.; Jiang, H.; Zhang, J.; Yin, Y.; Jiang, Z.; Douglin, J.C. Self-crosslinked blend alkaline anion exchange membranes with bi-continuous phase separated morphology to enhance ion conductivity. J. Membr. Sci. 2020, 597, 117769. [Google Scholar] [CrossRef]
- Wu, X.; Chen, W.; Yan, X.; He, G.; Wang, J.; Zhang, Y.; Zhu, X. Enhancement of hydroxide conductivity by the di-quaternization strategy for poly (ether ether ketone) based anion exchange membranes. J. Mater. Chem. A 2014, 2, 12222–12231. [Google Scholar] [CrossRef]
- Ge, Q.; Ran, J.; Miao, J.; Yang, Z.; Xu, T. Click chemistry finds its way in constructing an ionic highway in anion-exchange membrane. ACS Appl. Mater. Interfaces 2015, 7, 28545–28553. [Google Scholar] [CrossRef]
- Pandey, T.P.; Sarode, H.N.; Yang, Y.; Yang, Y.; Vezzu, K.; Di Noto, V.; Seifert, S.; Knauss, D.M.; Liberatore, M.W.; Herring, A.M. A highly hydroxide conductive, chemically stable anion exchange membrane, poly (2, 6 dimethyl 1, 4 phenylene oxide)-b-poly (vinyl benzyl trimethyl ammonium), for electrochemical applications. J. Electrochem. Soc. 2016, 163, H513. [Google Scholar] [CrossRef]
- Narducci, R.; Ercolani, G.; Becerra-Arciniegas, R.A.; Pasquini, L.; Knauth, P.; Di Vona, M.L. “Intrinsic” anion exchange polymers through the dissociation of strong basic groups: PPO with grafted bicyclic guanidines. Membranes 2019, 9, 57. [Google Scholar] [CrossRef]
- Gangrade, A.S.; Cassegrain, S.; Ghosh, P.C.; Holdcroft, S. Permselectivity of ionene-based, Aemion (R) anion exchange membranes. J. Membr. Sci. 2022, 641, 12. [Google Scholar] [CrossRef]
- Endrodi, B.; Kecsenovity, E.; Samu, A.; Halmagyi, T.; Rojas-Carbonell, S.; Wang, L.; Yan, Y.; Janaky, C. High carbonate ion conductance of a robust PiperION membrane allows industrial current density and conversion in a zero-gap carbon dioxide electrolyzer cell. Energy Environ. Sci. 2020, 13, 4098–4105. [Google Scholar] [CrossRef]
- Galvan, V.; Shrimant, B.; Bae, C.; Prakash, G.K.S. Ionomer Significance in Alkaline Direct Methanol Fuel Cell to Achieve High Power with a Quarternized Poly(terphenylene) Membrane. ACS Appl. Energy Mater. 2021, 4, 5858–5867. [Google Scholar] [CrossRef]
- Becerra-Arciniegas, R.-A.; Narducci, R.; Ercolani, G.; Antonaroli, S.; Sgreccia, E.; Pasquini, L.; Knauth, P.; Di Vona, M. Alkaline stability of model anion exchange membranes based on poly (phenylene oxide)(PPO) with grafted quaternary ammonium groups: Influence of the functionalization route. Polymer 2019, 185, 121931. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Wang, Y.; An, L.; Guiver, M.D.; Li, N. Highly stable anion exchange membranes based on quaternized polypropylene. J. Mater. Chem. A 2015, 3, 12284–12296. [Google Scholar] [CrossRef]
- Lin, C.X.; Wang, X.Q.; Hu, E.N.; Yang, Q.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Quaternized triblock polymer anion exchange membranes with enhanced alkaline stability. J. Membr. Sci. 2017, 541, 358–366. [Google Scholar]
- Becerra-Arciniegas, R.A.; Narducci, R.; Ercolani, G.; Pasquini, L.; Knauth, P.; Di Vona, M.L. Aliphatic Anion Exchange Ionomers with Long Spacers and No Ether Links by Ziegler–Natta Polymerization: Properties and Alkaline Stability. Molecules 2022, 27, 395. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.-S.; Jannasch, P. Exploring different cationic alkyl side chain designs for enhanced alkaline stability and hydroxide ion conductivity of anion-exchange membranes. Macromolecules 2015, 48, 5742–5751. [Google Scholar] [CrossRef]
- Long, H.; Kim, K.; Pivovar, B.S. Hydroxide degradation pathways for substituted trimethylammonium cations: A DFT study. J. Phys. Chem. C 2012, 116, 9419–9426. [Google Scholar] [CrossRef]
- Becerra-Arciniegas, R.-A.; Narducci, R.; Ercolani, G.; Sgreccia, E.; Pasquini, L.; Di Vona, M.; Knauth, P. Model long side-chain PPO-based anion exchange ionomers: Properties and alkaline stability. J. Phys. Chem. C 2019, 124, 1309–1316. [Google Scholar] [CrossRef]
- Marino, M.; Kreuer, K. Alkaline stability of quaternary ammonium cations for alkaline fuel cell membranes and ionic liquids. ChemSusChem 2015, 8, 513–523. [Google Scholar] [CrossRef]
- Park, A.M.; Wycisk, R.J.; Ren, X.; Turley, F.E.; Pintauro, P.N. Crosslinked poly (phenylene oxide)-based nanofiber composite membranes for alkaline fuel cells. J. Mater. Chem. A 2016, 4, 132–141. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, Y.; Zhang, M.; Wang, Y.; Tang, H.; Li, N. Olefin metathesis-crosslinked, bulky imidazolium-based anion exchange membranes with excellent base stability and mechanical properties. J. Membr. Sci. 2020, 598, 117793. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Yan, X.; Zhang, F.; Wang, X.; Wu, X.; Pang, B.; Wang, J.; He, G. Ether spaced N-spirocyclic quaternary ammonium functionalized crosslinked polysulfone for high alkaline stable anion exchange membranes. J. Membr. Sci. 2020, 598, 117650. [Google Scholar] [CrossRef]
- Sgreccia, E.; Narducci, R.; Knauth, P.; Di Vona, M.L. Silica containing composite anion exchange membranes by sol–gel synthesis: A short review. Polymers 2021, 13, 1874. [Google Scholar] [CrossRef]
- Derbali, Z.; Fahs, A.; Chailan, J.-F.; Ferrari, I.; Di Vona, M.; Knauth, P. Composite anion exchange membranes with functionalized hydrophilic or hydrophobic titanium dioxide. Int. J. Hydrogen Energy 2017, 42, 19178–19189. [Google Scholar] [CrossRef]
- Pasquini, L.; Becerra-Arciniegas, R.A.; Narducci, R.; Sgreccia, E.; Gressel, V.; Di Vona, M.L.; Knauth, P. Properties and alkaline stability of composite anion conducting ionomers based on poly (phenylene oxide) grafted with DABCO and Mg/Al lamellar double hydroxide. ChemElectroChem 2020, 7, 2917–2924. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Casciola, M.; Donnadio, A.; Nocchetti, M.; Pasquini, L.; Narducci, R.; Knauth, P. Anionic conducting composite membranes based on aromatic polymer and layered double hydroxides. Int. J. Hydrogen Energy 2017, 42, 3197–3205. [Google Scholar] [CrossRef]
- Narducci, R.; Sgreccia, E.; Knauth, P.; Di Vona, M.L. Anion Exchange Membranes with 1D, 2D and 3D Fillers: A Review. Polymers 2021, 13, 3887. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.; Son, T.Y.; Nam, S.Y. Recent advances in composite polymer electrolyte membranes for fuel cell. Appl. Chem. Eng. 2019, 30, 1–10. [Google Scholar]
- Mandal, M.; Huang, G.; Ul Hassan, N.; Mustain, W.E.; Kohl, P.A. Poly(norbornene) anion conductive membranes: Homopolymer, block copolymer and random copolymer properties and performance. J. Mater. Chem. A 2020, 8, 17568–17578. [Google Scholar] [CrossRef]
- Narducci, R.; Sgreccia, E.; Ercolani, G.; Sette, M.; Antonaroli, S.; Pasquini, L.; Knauth, P.; Di Vona, M.L. Influence of the position of ionic groups in amphoteric polyelectrolytes on hydration and ionic conduction: Side chain vs. main chain. Eur. Polym. J. 2019, 119, 45–51. [Google Scholar] [CrossRef]
- Dharmalingam, S.; Kugarajah, V.; Sugumar, M. Membranes for Microbial Fuel Cells. In Microbial Electrochemical Technology: Sustainable Platform for Fuels, Chemicals and Remediation; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Biomass Biofuels Biochemicals; Elsevier Science Bv: Amsterdam, The Netherlands, 2019; pp. 143–194. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, I.V.; Pasquini, L.; Narducci, R.; Sgreccia, E.; Di Vona, M.L.; Knauth, P. A Short Overview of Biological Fuel Cells. Membranes 2022, 12, 427. https://doi.org/10.3390/membranes12040427
Ferrari IV, Pasquini L, Narducci R, Sgreccia E, Di Vona ML, Knauth P. A Short Overview of Biological Fuel Cells. Membranes. 2022; 12(4):427. https://doi.org/10.3390/membranes12040427
Chicago/Turabian StyleFerrari, Ivan Vito, Luca Pasquini, Riccardo Narducci, Emanuela Sgreccia, Maria Luisa Di Vona, and Philippe Knauth. 2022. "A Short Overview of Biological Fuel Cells" Membranes 12, no. 4: 427. https://doi.org/10.3390/membranes12040427
APA StyleFerrari, I. V., Pasquini, L., Narducci, R., Sgreccia, E., Di Vona, M. L., & Knauth, P. (2022). A Short Overview of Biological Fuel Cells. Membranes, 12(4), 427. https://doi.org/10.3390/membranes12040427










