Enhancing the Dye-Rejection Efficiencies and Stability of Graphene Oxide-Based Nanofiltration Membranes via Divalent Cation Intercalation and Mild Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Fabrication
2.3. Membrane Characterization
2.4. Nanofiltration Performance Evaluation
2.5. Membrane Stability Assessment
3. Results and Discussion
3.1. Morphological Properties of GOM Series
3.2. Effect of Mg2+ Crosslinking on the Chemical Structure of GOM Series
3.3. Effect of Thermal Reduction on the Chemical Structure of GOM Series
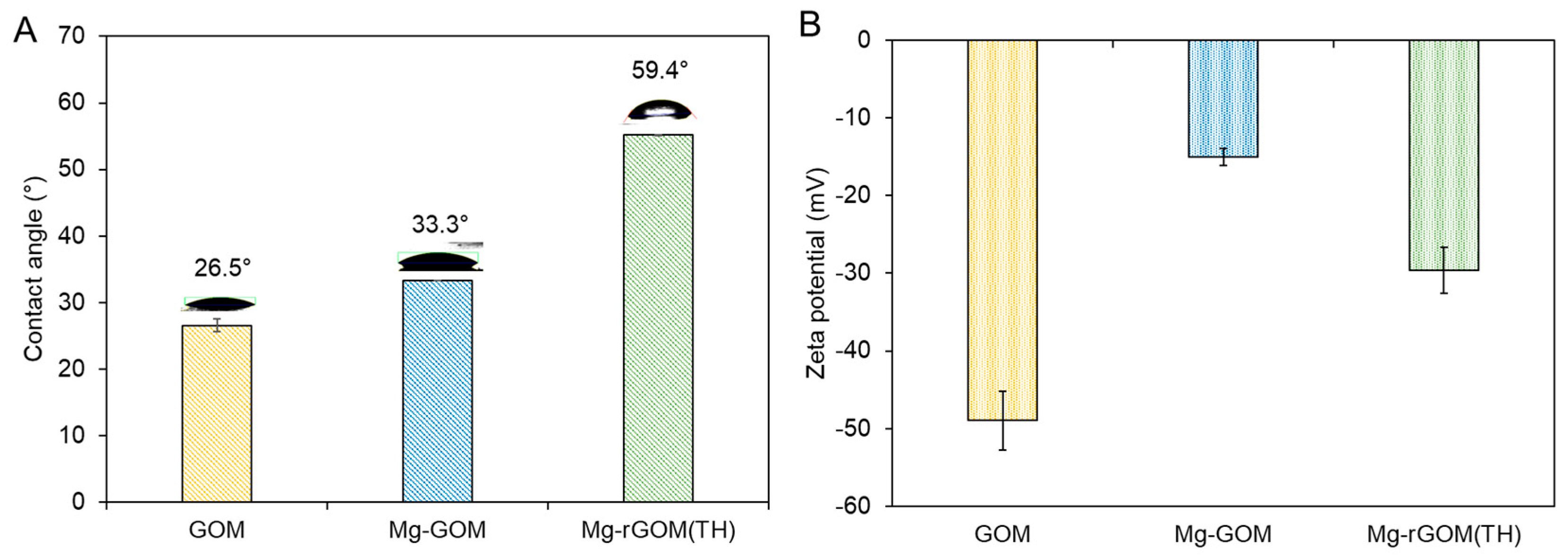
3.4. Surface Characteristics of GOM Series
3.5. The Nanochannel Size of the GOM Series
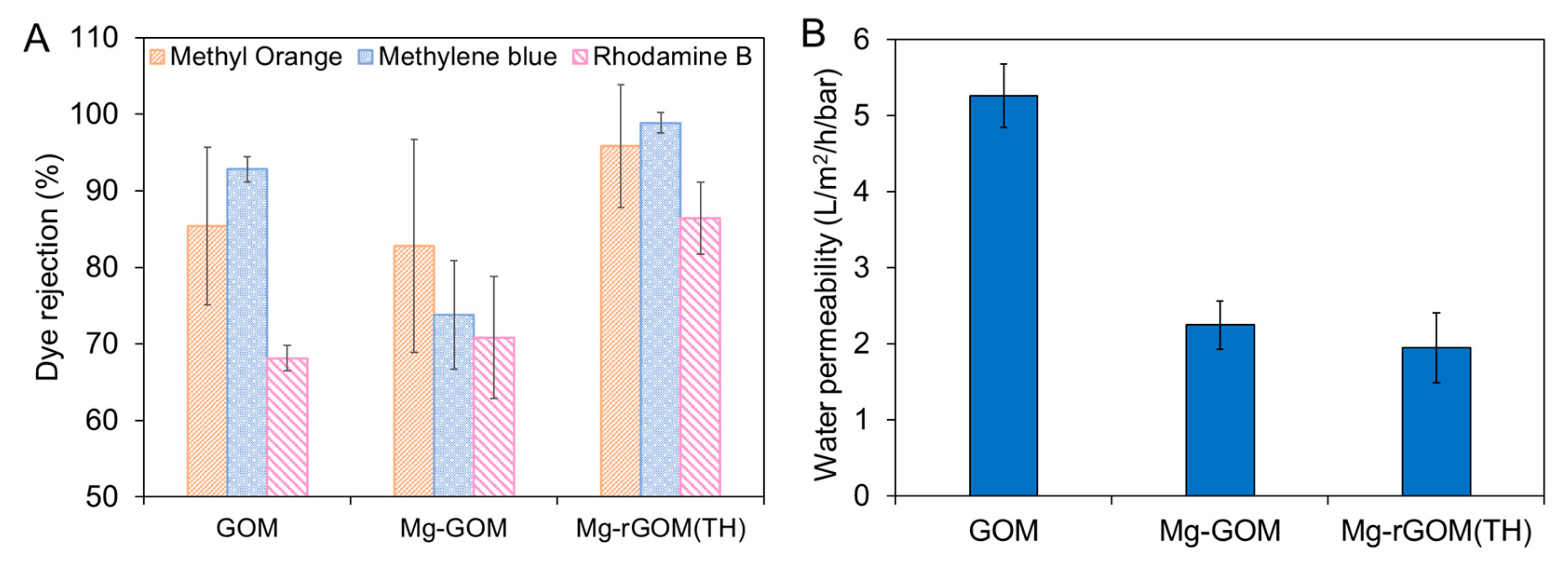
3.6. The NF Performance of the GOM Series
3.7. The Stability of the GOM Series
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Greve, P.; Kahil, T.; Mochizuki, J.; Schinko, T.; Satoh, Y.; Burek, P.; Fischer, G.; Tramberend, S.; Burtscher, R.; Langan, S. Global assessment of water challenges under uncertainty in water scarcity projections. Nat. Sustain. 2018, 1, 486–494. [Google Scholar] [CrossRef]
- Tang, C.Y.; Yang, Z.; Guo, H.; Wen, J.J.; Nghiem, L.D.; Cornelissen, E. Potable water reuse through advanced membrane technology. Environ. Sci. Technol. 2018, 52, 10215–10223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R.-H.; Lee, S.; Jeong, J.; Lee, J.-H.; Kim, Y.-K. Reuse of greywater and rainwater using fiber filter media and metal membrane. Desalination 2007, 202, 326–332. [Google Scholar] [CrossRef]
- Amy, G.; Ghaffour, N.; Li, Z.; Francis, L.; Linares, R.V.; Missimer, T.; Lattemann, S. Membrane-based seawater desalination: Present and future prospects. Desalination 2017, 401, 16–21. [Google Scholar] [CrossRef]
- Nikooe, N.; Saljoughi, E. Preparation and characterization of novel PVDF nanofiltration membranes with hydrophilic property for filtration of dye aqueous solution. Appl. Surf. Sci. 2017, 413, 41–49. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Z.; Cheng, Q.; Lü, Z.; Liu, M.; Gao, C. Application of thin-film composite hollow fiber membrane to submerged nanofiltration of anionic dye aqueous solutions. Sep. Purif. Technol. 2012, 88, 121–129. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Anokhina, T.S.; Volkov, A.V. Application of cellophane films as nanofiltration membranes. Pet. Chem. 2015, 55, 746–752. [Google Scholar] [CrossRef]
- Anokhina, T.; Dmitrieva, E.; Volkov, A. Recovery of Model Pharmaceutical Compounds from Water and Organic Solutions with Alginate-Based Composite Membranes. Membranes 2022, 12, 235. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Mulcahy, D. Brackish water desalination by a hybrid forward osmosis–nanofiltration system using divalent draw solute. Desalination 2012, 284, 175–181. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Mänttäri, M.; Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol. 2008, 63, 251–263. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, X.-H.; Tang, C.Y. Recent development of novel membranes for desalination. Desalination 2018, 434, 37–59. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Xia, Y.; Yuan, S.; Zhang, X. Tailoring the physicochemical and geometric properties of two-dimensional graphene membranes for aqueous separation. Desalination 2022, 530, 115621. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Y.; Xia, Y.; Selomulya, C.; Zhang, X. Stable cation-controlled reduced graphene oxide membranes for improved NaCl rejection. J. Membr. Sci. 2021, 621, 118995. [Google Scholar] [CrossRef]
- Lee, S.E.; Jang, J.; Kim, J.; Woo, J.Y.; Seo, S.; Jo, S.; Kim, J.-W.; Jeon, E.-S.; Jung, Y.; Han, C.-S. Tunable sieving of small gas molecules using horizontal graphene oxide membrane. J. Membr. Sci. 2020, 610, 118178. [Google Scholar] [CrossRef]
- Xi, Y.-H.; Liu, Z.; Ji, J.; Wang, Y.; Faraj, Y.; Zhu, Y.; Xie, R.; Ju, X.-J.; Wang, W.; Lu, X. Graphene-based membranes with uniform 2D nanochannels for precise sieving of mono-/multi-valent metal ions. J. Membr. Sci. Sci. 2018, 550, 208–218. [Google Scholar] [CrossRef]
- Yang, E.; Ham, M.-H.; Park, H.B.; Kim, C.-M.; Song, J.-h.; Kim, I.S. Tunable semi-permeability of graphene-based membranes by adjusting reduction degree of laminar graphene oxide layer. J. Membr. Sci. 2018, 547, 73–79. [Google Scholar] [CrossRef]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotech. 2017, 12, 546–550. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.E.; Seo, S.; Woo, J.Y.; Han, C.-S. Near-complete blocking of multivalent anions in graphene oxide membranes with tunable interlayer spacing from 3.7 to 8.0 angstrom. J. Membr. Sci. 2019, 592, 117394. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, K.; Ji, Y.; Liu, G.; Jin, W.; Xu, N. Controllable ion transport by surface-charged graphene oxide membrane. Nat. Commun. 2019, 10, 1253. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Chen, Q.; Li, X.; Liu, H. Controlling covalent functionalization of graphene oxide membranes to improve enantioseparation performances. J. Membr. Sci. 2019, 582, 83–90. [Google Scholar] [CrossRef]
- Yin, Z.; Wan, S.; Yang, J.; Kurmoo, M.; Zeng, M.-H. Recent advances in post-synthetic modification of metal–organic frameworks: New types and tandem reactions. Coord. Chem. Rev. 2019, 378, 500–512. [Google Scholar] [CrossRef]
- Ching, K.; Lian, B.; Leslie, G.; Chen, X.; Zhao, C. Metal-cation-modified graphene oxide membranes for water permeation. Carbon 2020, 170, 646–657. [Google Scholar] [CrossRef]
- Lv, X.-B.; Xie, R.; Ji, J.-Y.; Liu, Z.; Wen, X.-Y.; Liu, L.-Y.; Hu, J.-Q.; Ju, X.-J.; Wang, W.; Chu, L.-Y. A novel strategy to fabricate cation-cross-linked graphene oxide membrane with high aqueous stability and high separation performance. ACS Appl. Mater. Interfaces 2020, 12, 56269–56280. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene oxide papers modified by divalent ions—enhancing mechanical properties via chemical cross-linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380–383. [Google Scholar] [CrossRef]
- Yu, W.; Graham, N. Development of a stable cation modified graphene oxide membrane for water treatment. 2D Mater. 2017, 4, 045006. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, X.; Thebo, K.H.; Cheng, H.-M.; Ren, W. Controlling reduction degree of graphene oxide membranes for improved water permeance. Sci. Bull. 2018, 63, 788–794. [Google Scholar] [CrossRef] [Green Version]
- Zheng, A.L.T.; Boonyuen, S.; Ohno, T.; Andou, Y. Hydrothermally reduced graphene hydrogel intercalated with divalent ions for dye adsorption studies. Processes 2021, 9, 169. [Google Scholar] [CrossRef]
- Yang, E.; Kim, C.-M.; Song, J.-h.; Ki, H.; Ham, M.-H.; Kim, I.S. Enhanced desalination performance of forward osmosis membranes based on reduced graphene oxide laminates coated with hydrophilic polydopamine. Carbon 2017, 117, 293–300. [Google Scholar] [CrossRef]
- Geng, J.; Liu, L.; Yang, S.B.; Youn, S.-C.; Kim, D.W.; Lee, J.-S.; Choi, J.-K.; Jung, H.-T. A simple approach for preparing transparent conductive graphene films using the controlled chemical reduction of exfoliated graphene oxide in an aqueous suspension. J. Phys. Chem. C 2010, 114, 14433–14440. [Google Scholar] [CrossRef]
- Sitohang, H.; Pasaribu, N.; Siburian, R.; Simanjuntak, C. Model of formation graphene from graphite with ammonia. In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018)—Research in Industry 4.0, Medan, Indonesia, 30–31 August 2018; pp. 1036–1038. [Google Scholar]
- Yu, H.; He, Y.; Xiao, G.; Fan, Y.; Ma, J.; Gao, Y.; Hou, R.; Chen, J. Weak-reduction graphene oxide membrane for improving water purification performance. J. Mater. Sci. Technol. 2020, 39, 106–112. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, F.; Yi, R.; He, Z.; Wang, Z.; Chen, J.; Liu, W.; Xu, J.; Chen, L. Effect of physical and chemical structures of graphene oxide on water permeation in graphene oxide membranes. Appl. Surf. Sci. 2020, 520, 146308. [Google Scholar] [CrossRef]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through pKa Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, C.-B.; Kumar, S.R.; Chang, C.-W.; Chen, C.-H.; Chen, D.W.; Lue, S.J. Graphene oxide-cation interaction: Inter-layer spacing and zeta potential changes in response to various salt solutions. J. Membr. Sci. 2018, 554, 253–263. [Google Scholar] [CrossRef]
- Baskoro, F.; Rajesh Kumar, S.; Jessie Lue, S. Grafting thin layered graphene oxide onto the surface of nonwoven/PVDF-PAA composite membrane for efficient dye and macromolecule separations. Nanomaterials 2020, 10, 792. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.V.; Kumar, S.R.; Lue, S.J. Separation mechanisms of binary dye mixtures using a PVDF ultrafiltration membrane: Donnan effect and intermolecular interaction. J. Membr. Sci. 2019, 575, 38–49. [Google Scholar] [CrossRef]
- Gupta, K.; Yasa, S.R.; Khan, A.; Sharma, O.P.; Khatri, O.P. Charge-driven interaction for adsorptive removal of organic dyes using ionic liquid-modified graphene oxide. J. Colloid Interface Sci. 2022, 607, 1973–1985. [Google Scholar] [CrossRef]
- Xu, T.; He, Y.; Qin, Y.; Zhao, C.; Peng, C.; Hu, J.; Liu, H. Facile preparation of porous organic copolymer based on triptycene and crown ether for efficient organic dye adsorption. RSC Adv. 2018, 8, 4963–4968. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Pan, T.; Hong, S.; Zhang, K.; Zhu, X.; Chen, B. Ultrathin graphene oxide membrane with constructed tent-shaped structures for efficient and tunable molecular sieving. Environ. Sci. Nano 2020, 7, 2373–2384. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, K.; Shen, J.; Liu, G.; Fan, Y.; Jin, W. Nanoparticles@ rGO membrane enabling highly enhanced water permeability and structural stability with preserved selectivity. AIChE J. 2017, 63, 5054–5063. [Google Scholar] [CrossRef]
- Dong, L.; Li, M.; Zhang, S.; Si, X.; Bai, Y.; Zhang, C. NH2-Fe3O4-regulated graphene oxide membranes with well-defined laminar nanochannels for desalination of dye solutions. Desalination 2020, 476, 114227. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, L.; Wang, J.; Zhao, F.; Fan, L.; Hua, D.; Japip, S.; Xiao, J.; Zhang, X.; Zhou, S.-F. Preparation of glycine mediated graphene oxide/g-C3N4 lamellar membranes for nanofiltration. J. Membr. Sci. 2020, 601, 117948. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Chen, J.; Cui, L.; Jing, W. Solvothermal-induced assembly of 2D-2D rGO-TiO2 nanocomposite for the construction of nanochannel membrane. J. Membr. Sci. 2020, 600, 117870. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Y.; Shi, W.; Bao, C.; Hu, X. Graphene oxide/methylene blue composite membrane for dyes separation: Formation mechanism and separation performance. Appl. Surf. Sci. 2020, 505, 144145. [Google Scholar] [CrossRef]
- Chang, Y.; Shen, Y.; Kong, D.; Ning, J.; Xiao, Z.; Liang, J.; Zhi, L. Fabrication of the reduced preoxidized graphene-based nanofiltration membranes with tunable porosity and good performance. RSC Adv. 2017, 7, 2544–2549. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Gong, J.-L.; Zeng, G.-M.; Deng, C.-H.; Yang, H.-C.; Liu, H.-Y.; Huan, S.-Y. Cross-linking to prepare composite graphene oxide-framework membranes with high-flux for dyes and heavy metal ions removal. Chem. Eng. J. 2017, 322, 657–666. [Google Scholar] [CrossRef]
- Deng, H.; Huang, J.; Qin, C.; Xu, T.; Ni, H.; Ye, P. Preparation of high-performance nanocomposite membranes with hydroxylated graphene and graphene oxide. J. Water Process Eng. 2021, 40, 101945. [Google Scholar] [CrossRef]
- Yang, K.; Huang, L.-j.; Wang, Y.-x.; Du, Y.-c.; Zhang, Z.-j.; Wang, Y.; Kipper, M.J.; Belfiore, L.A.; Tang, J.-g. Graphene oxide nanofiltration membranes containing silver nanoparticles: Tuning separation efficiency via nanoparticle size. Nanomaterials 2020, 10, 454. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Membrane | Atomic Composition (wt.%) | C/O Ratio | ||
|---|---|---|---|---|
| C | O | Mg | ||
| GOM | 48.61 | 61.18 | 0.20 | 0.79 |
| Mg-GOM | 38.16 | 39.54 | 6.77 | 0.97 |
| Mg-rGOM(TH) | 46.24 | 31.75 | 7.15 | 1.46 |
| Organic Dye | Molecular Weight (g/mol) | Size (Å3) | Charge |
|---|---|---|---|
| Methyl orange (MO) | 327.34 | 17.93 × 7.54 × 6.02 | Negative |
| Methylene blue (MB) | 319.85 | 16.94 × 8.24 × 4.55 | Positive |
| Rhodamine B (RB) | 479.02 | 18.54 × 14.35 × 9.14 | Neutral |
| GOM | Permeability (L/m2/h/bar) | Organic Dye Rejection (%) | Ref. | ||
|---|---|---|---|---|---|
| MO | MB | RB | |||
| Mg-rGOM(TH) | 1.95 | 95.8 | 98.9 | 86.4 | This study |
| GO/SiO2 | 44.2 | 91.0 | [43] | ||
| GO/Fe3O4 | 296 | 98.0 | [44] | ||
| GO/NH2-Fe3O4 | 15.6 | 70.0 | [45] | ||
| GO/glycine/g-C3N4 | 207 | 87.0 | [46] | ||
| GO/TiO2 nanosheet | 9.36 | 97.3 | 98.8 | [47] | |
| GO/MB | 3.83 | 96.37 | [48] | ||
| Reduced preoxidized GO | 5.3 | 97.5 | [49] | ||
| GO/isophorone diisocyanate | 80–100 | 97 | 97.7 | 96.2 | [50] |
| GO/hydroxylated graphene | <24.4 | <99.7 | <99.7 | [51] | |
| GO/Ag | 20.8–33.9 | 94.6–96.8 | 77.9–84.2 | [52] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jee, H.; Jang, J.; Kang, Y.; Eisa, T.; Chae, K.-J.; Kim, I.S.; Yang, E. Enhancing the Dye-Rejection Efficiencies and Stability of Graphene Oxide-Based Nanofiltration Membranes via Divalent Cation Intercalation and Mild Reduction. Membranes 2022, 12, 402. https://doi.org/10.3390/membranes12040402
Jee H, Jang J, Kang Y, Eisa T, Chae K-J, Kim IS, Yang E. Enhancing the Dye-Rejection Efficiencies and Stability of Graphene Oxide-Based Nanofiltration Membranes via Divalent Cation Intercalation and Mild Reduction. Membranes. 2022; 12(4):402. https://doi.org/10.3390/membranes12040402
Chicago/Turabian StyleJee, Hobin, Jaewon Jang, Yesol Kang, Tasnim Eisa, Kyu-Jung Chae, In S. Kim, and Euntae Yang. 2022. "Enhancing the Dye-Rejection Efficiencies and Stability of Graphene Oxide-Based Nanofiltration Membranes via Divalent Cation Intercalation and Mild Reduction" Membranes 12, no. 4: 402. https://doi.org/10.3390/membranes12040402
APA StyleJee, H., Jang, J., Kang, Y., Eisa, T., Chae, K.-J., Kim, I. S., & Yang, E. (2022). Enhancing the Dye-Rejection Efficiencies and Stability of Graphene Oxide-Based Nanofiltration Membranes via Divalent Cation Intercalation and Mild Reduction. Membranes, 12(4), 402. https://doi.org/10.3390/membranes12040402








