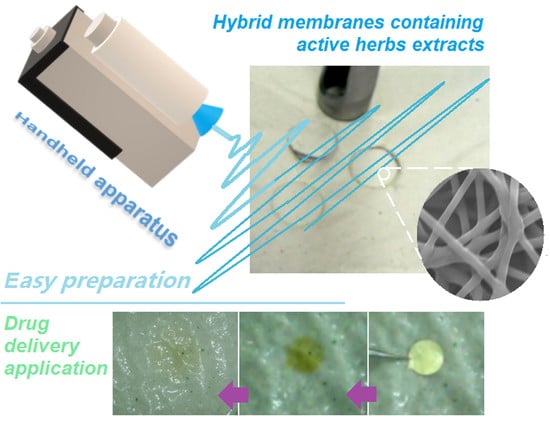
Electrospun Hybrid Films for Fast and Convenient Delivery of Active Herb Extracts
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Electrospun Films
2.3. Characterizations of Nanofibers
2.4. Functional Performances
3. Results and Discussion
3.1. Electrospinning Is Explored to Transfer LQK into Nanofiber Mats
3.2. The Morphologies of the LQK-Loaded Nanofibers and Their Transferring to Orodispersible Films
3.3. Physical State of the Components Loaded into the Films
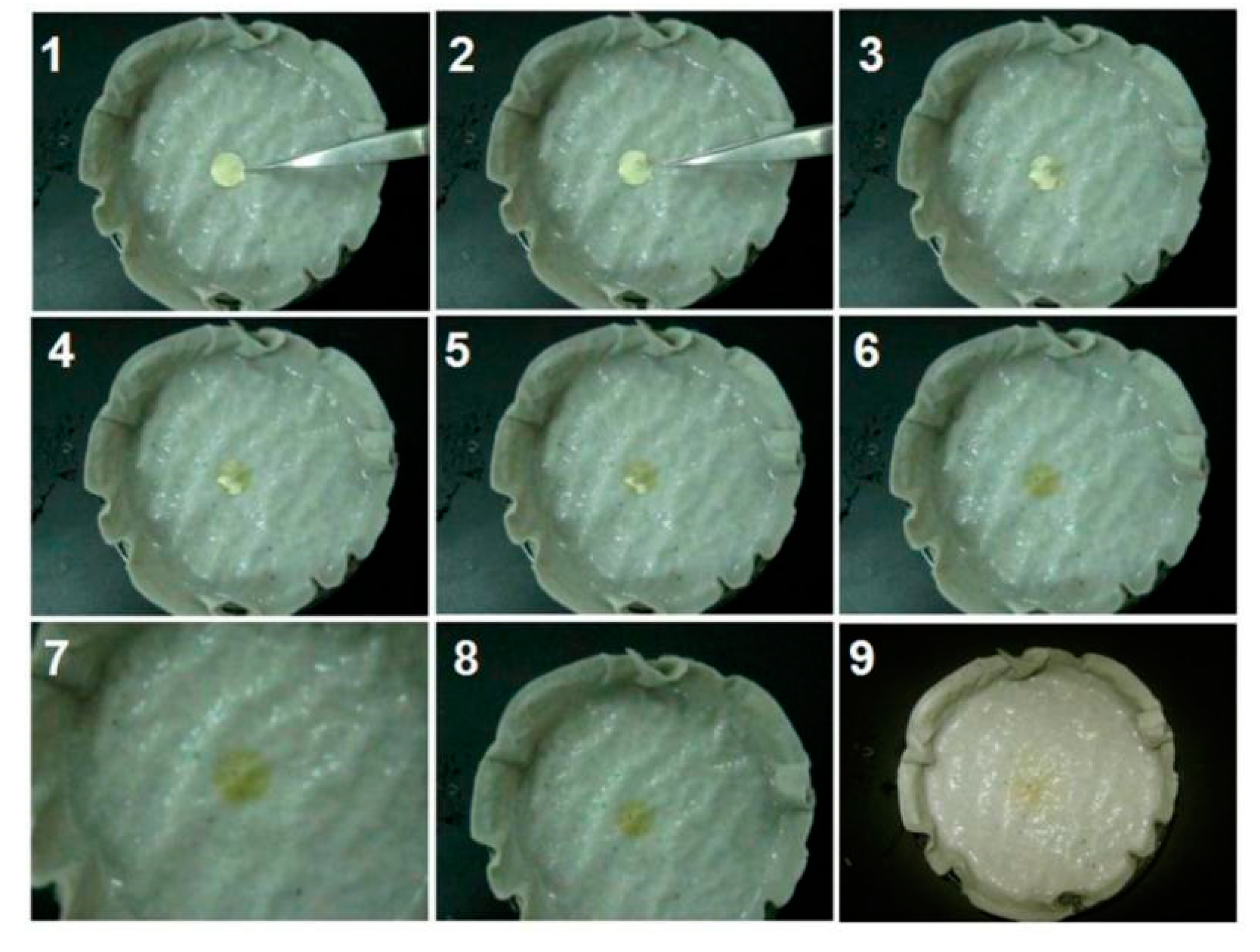
3.4. The Fast-Disintegrating Performances of the LQK-Loaded Films
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tubtimsri, S.; Weerapol, Y. Improvement in solubility and absorption of nifedipine using solid solution: Correlations beween surface free energy and drug dissolution. Polymers 2021, 13, 2963. [Google Scholar] [CrossRef]
- Leonarta, F.; Lee, C.K. Nanofibrous membrane with encapsulated glucose oxidase for self-sustained antimicrobial applications. Membranes 2021, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.B.; Zhou, C.X.; Fan, Y.Y.; Liu, Q.; Zhang, H.F.; Wu, Z.W. Electrospun polylactic acid/sulfadiazine sodium/proteinase nanofibers and their applications in treating frostbite. J. Appl. Polym. Sci. 2022, 139, e51716. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.G. Electrospun medicated nanofibers for wound healing—Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Dziemidowicz, K.; Williams, G.R.; Ramakrishna, S. Encapsulation of pharmaceutical and nutraceutical active ingredients using electrospinning processes. Nanomaterials 2021, 11, 1968. [Google Scholar] [CrossRef]
- Peres, R.M.; Sousa, J.M.L.; Oliveira, M.O.D.; Rossi, M.V.; Oliveira, R.R.D.; Lima, N.B.D.; Bernussi, A.; Warzywoda, J.; Sarmento, B.; Munhoz, A.H., Jr. Pseudoboehmite as a drug delivery system for acyclovir. Sci. Rep. 2021, 11, 15448. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Rasekh, M.; Patel, M.; Onaiwu, E.; Nazari, K.; Kucuk, I.; Wilson, P.B.; Arshad, M.S.; Ahmad, Z.; Chang, M.W. Recent applications of electrical, centrifugal, and pressurised emerging technologies for fibrous structure engineering in drug delivery, regenerative medicine and theranostics. Adv. Drug Deliv. Rev. 2021, 175, 113823. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, M.Y.G.; Kim, J.; Chitara, B.; Wymer, N.; Yan, F. N-halamine-decorated electrospun polyacrylonitrile nanofibrous membranes: Characterization and antimicrobial properties. React. Funct. Polym. 2021, 168, 105058. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, S.; Kumar, S.; Rohatgi, S.; Kundu, P.P. Synthesis of rifaximin loaded chitosan-alginate core-shell nanoparticles (Rif@CS/Alg-NPs) for antibacterial applications. Int. J. Biol. Macromol. 2021, 183, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk, J.; Stojko, M.; Musial-Kulik, M.; Karpeta-Jarzabek, P.; Pastusiak, M.; Janeczek, H.; Dobrzynski, P.; Sobota, M.; Kasperczyk, J. Dual-jet electrospun PDLGA/PCU nonwovens and their mechanical and hydrolytic degradation properties. J. Mech. Behav. Biomed. 2022, 126, 105050. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G. Preface-bettering drug delivery knowledge from pharmaceutical techniques and excipients. Curr. Drug Deliv. 2021, 18, 2–3. [Google Scholar] [CrossRef]
- Bae, Y.; Kim, Y.; Lee, E.S. Endosomal pH-responsive Fe-based hyaluronate nanoparticles for doxorubicin delivery. Molecules 2021, 26, 3547. [Google Scholar] [CrossRef] [PubMed]
- Hajjari, M.M.; Golmakani, M.T.; Sharif, N.; Niakousari, M. In-vitro and in-silico characterization of zein fiber incorporating cuminaldehyde. Food Bioprod. Process. 2021, 128, 166–176. [Google Scholar] [CrossRef]
- Ullah, A.; Saito, Y.; Ullah, S.; Haider, M.K.; Nawaz, H.; Duy-Nam, P.; Kharaghani, D.; SooKim, I. Bioactive sambong oil-loaded electrospun cellulose acetate nanofibers: Preparation, characterization, and in-vitro biocompatibility. Int. J. Biol. Macromol. 2021, 166, 1009–1021. [Google Scholar] [CrossRef]
- Feng, X.C.; Hao, J.S. Identifying new pathways and targets for wound healing and therapeutics from natural sources. Curr. Drug Deliv. 2021, 18, 1052–1072. [Google Scholar] [CrossRef] [PubMed]
- Vineis, C.; Maya, I.C.; Mowafi, S.; Varesano, A.; Ramírez, D.O.S.; Taleb, M.A.; Tonetti, C.; Guarino, V.; El-Sayed, H. Synergistic effect of sericin and keratin in gelatin based nanofibers for in vitro applications. Int. J. Biol. Macromol. 2021, 190, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Bagliotti, M.A.; Miguel, S.R.; Chaves, D.S.M.P.; Perosa, F.R.; Gomes, D.O.A.; Marlus, C. Cellulose nanofibers improve the performance of retrograded starch/pectin microparticles for colon-specific delivery of 5-ASA. Pharmaceutics 2021, 13, 1515. [Google Scholar]
- Song, Y.; Huang, H.; He, D.; Yang, M.; Wang, H.; Zhang, H.; Li, J.; Li, Y.; Wang, C. Gallic acid/2-hydroxypropyl-β-cyclodextrin inclusion complexes electrospun nanofibrous webs: Fast dissolution, improved aqueous solubility and antioxidant property of gallic acid. Chem. Res. Chin. Univ. 2021, 37, 450–455. [Google Scholar] [CrossRef]
- Saraogi, G.K.; Tholiya, S.; Mishra, Y.; Mishra, V.; Albuttim, A.; Nayak, P.; Tambuwala, M.M. Formulation development and evaluation of pravastatin-loaded nanogel for hyperlipidemia management. Gels 2022, 8, 81. [Google Scholar] [CrossRef]
- Naidoo, S.; Daniels, A.; Habib, S.; Singh, M. Poly-l-lysine–lactobionic acid-capped selenium nanoparticles for liver-targeted gene delivery. Int. J. Mol. Sci. 2022, 23, 1492. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Itagaki, N.; Kobayashi, H.; Tanaka, K.; Takarada, W.; Kikutani, T.; Takasaki, M. Bamboo charcoal/poly(l-lactide) fiber webs prepared using laser-heated melt electrospinning. Polymers 2021, 13, 2776. [Google Scholar] [CrossRef] [PubMed]
- Hardt, J.C.; Pellá, M.C.G.; Meira, A.C.R.; Rosenberger, A.G.; Caetano, J.; Dragunski, D.C. Potential wound dressings from electrospun medicated poly(butylene-adipate-co-terephthalate)/poly-(ε-caprolactone) microfibers. J. Mol. Liq. 2021, 339, 116694. [Google Scholar] [CrossRef]
- Vu, Q.M.; Nguyen, T.C.; Dam, D.M.N.; Vu, Q.T.; Le, T.L.; Hoang, T.D.; Tran, T.K.N.; Nguyen, T.A.; Nguyen, P.H.; Thai, H. A novel method for preparation of carrageenan/fish scale collagen/allopurinol biocomposite film. Int. J. Polym. Sci. 2021, 2021, 9960233. [Google Scholar] [CrossRef]
- Łyszczarz, E.; Brniak, W.; Szafraniec-Szczęsny, J.; Majka, T.M.; Majda, D.; Zych, M.; Pielichowski, K.; Jachowicz, R. The impact of the preparation method on the properties of orodispersible films with aripiprazole: Electrospinning vs. casting and 3D printing methods. Pharmaceutics 2021, 13, 1122. [Google Scholar] [CrossRef]
- Sa’adon, S.; Ansari, M.N.M.; Razak, S.I.A.; Anand, J.S.; Nayan, N.H.M.; Ismail, A.E.; Khan, M.U.A.; Haider, A. Preparation and physicochemical characterization of a diclofenac sodium-dual layer polyvinyl alcohol patch. Polymers 2021, 13, 2459. [Google Scholar] [CrossRef] [PubMed]
- Enikov, R.E.T.; Anton, R. Method for production of aligned nanofibers and fiber elasticity measurement. J. Mech. Behav. Biomed. Mater. 2021, 113, 104151. [Google Scholar]
- Liu, Y.; Chen, X.; Liu, Y.; Gao, Y.; Liu, P. Electrospun coaxial fibers to optimize the release of poorly water-soluble drug. Polymers 2022, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Yu, D.G.; Liu, H.; Liu, Y.; Liu, P. Electrospun PVP-core/PHBV-shell nanofibers to eliminate tailing off for an improved sustained release of curcumin. Mol. Pharm. 2021, 18, 4170–4178. [Google Scholar] [CrossRef]
- Yu, D.G.; Lv, H. Preface-striding into nano drug delivery. Curr. Drug. Deliv. 2022, 19, 1–3. [Google Scholar] [CrossRef]
- Kiss, K.; Hegedüs, K.; Vass, P.; Vári-Mező, D.; Farkas, A.; Nagy, Z.K.; Molnár, L.; Tóvári, J.; Mező, G.; Marosi, G. Development of fast-dissolving dosage forms of curcuminoids by electrospinning for potential tumor therapy application. Int. J. Pharm. 2021, 611, 121327. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y. Engineering of hollow polymeric nanosphere-supported imidazolium-based ionic liquids with enhanced antimicrobial activities. Nano Res. 2022. [Google Scholar] [CrossRef]
- Lukiev, I.V.; Antipina, L.S.; Goreninskii, S.I.; Tverdokhlebova, T.S.; Vasilchenko, D.V.; Nemoykina, A.L.; Goncharova, D.A.; Svetlichnyi, V.A.; Dambaev, G.T.; Bouznik, V.M.; et al. Antibacterial ferroelectric hybrid membranes fabricated via electrospinning for wound healing. Membranes 2021, 11, 986. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.F.; Wang, L.C.; Nie, J.; Ma, G.P. Multilayer electrospun nanofibrous membranes with antibacterial property for air filtration. Appl. Surf. Sci. 2020, 515, 145962. [Google Scholar] [CrossRef]
- Salim, S.A.; Kamoun, E.A.; Evans, S.; EL-Moslamy, S.H.; El-Fakharany, E.M.; Elmazar, M.M.; Abdel-Aziz, A.F.; Abou-Saleh, R.H.; Salaheldin, T.A. Mercaptopurine-loaded sandwiched tri-layered composed of electrospun polycaprolactone/poly(methyl methacrylate) nanofibrous scaffolds as anticancer carrier with antimicrobial and antibiotic features: Sandwich configuration nanofibers, release study and in vitro bioevaluation tests. Int. J. Nanomed. 2021, 16, 6937–6955. [Google Scholar]
- Manakhov, A.M.; Sitnikova, N.A.; Tsygankova, A.R.; Alekseev, A.Y.; Adamenko, L.S.; Permyakova, E.; Baidyshev, V.S.; Popov, Z.I.; Blahová, L.; Eliáš, M.; et al. Electrospun biodegradable nanofibers coated homogenously by Cu magnetron sputtering exhibit fast ion release. Computational and experimental study. Membranes 2021, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Mirzaie, Z.; Reisi-Vanani, A.; Barati, M.; Atyabi, S.M. The drug release kinetics and anticancer activity of the GO/PVA-curcumin nanostructures: The effects of the preparation method and the GO amount. J. Pharm. Sci. 2021, 110, 3715–3725. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.C.; Wang, M.L.; Zhou, Y.Q.; Sun, M.J.; Xie, Y.F.; Yu, D.G. Comparisons of antibacterial performances between electrospun polymer@drug nanohybrids with drug-polymer films. Adv. Compos. Hybrid Mater. 2022. [Google Scholar] [CrossRef]
- Aljohani, M.; Alkabli, J.; Abualnaja, M.M.; Alrefaei, A.F.; Almehmadi, S.J.; Mahmoud, M.H.H.; El-Metwaly, N.M. Electrospun AgNPs-polylactate nanofibers and their antimicrobial applications. React. Funct. Polym. 2021, 167, 104999. [Google Scholar] [CrossRef]
- Wu, C.; Wei, X.H.; Zhao, K.; Jiang, J.Y.; Jiao, M.Z.; Cheng, J.H.; Tang, Z.; Guo, Z.; Tang, Y.F. Novel dam-like effect based on piezoelectric energy conversion for drug sustained release of drug-loaded TiO2 @ BaTiO3 coaxial nanotube coating. Ceram. Int. 2021, 47, 17550–17559. [Google Scholar] [CrossRef]
- Wable, V.; Biswas, P.K.; Moheimani, R.; Aliahmad, N.; Omole, P.; Siegel, A.P.; Agarwal, M.; Dalir, H. Engineering the electrospinning of MWCNTs/epoxy nanofiber scaffolds to enhance physical and mechanical properties of CFRPs. Compos. Sci. Technol. 2021, 213, 108941. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yao, D.; Zhao, W.Y.; Zhang, R.; Yu, B.R.; Ma, G.P.; Li, Y.; Hao, D.F.; Xu, F.J. Engineering platelet-rich plasma based dual-network hydrogel as a bioactive wound dressing with potential clinical translational value. Adv. Funct. Mater. 2021, 31, 2009258. [Google Scholar] [CrossRef]
- Haidar, M.K.; Timur, S.S.; Demirbolat, G.M.; Nemutlu, E.; Gürsoy, R.N.; Ulubayram, K.; Öner, L.; Eroğlu, H. Electrospun nanofibers for dual and local delivery of neuroprotective drugs. Fiber. Polym. 2021, 22, 334–344. [Google Scholar] [CrossRef]
- Mouro, C.; Fangueiro, R.; Gouveia, I.C. Preparation and characterization of electrospun double-layered films membranes as a carrier for Centella asiatica (L.). Polymers 2020, 12, 2653. [Google Scholar] [CrossRef]
- Zhang, M.X.; Song, W.L.; Tang, Y.X.; Xu, X.Z.; Huang, Y.N.; Yu, D.G. Polymer-based nanofifiber-nanoparticle hybrids and their medical applications. Polymers 2022, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wu, M.; Zhu, J.W.; Yang, Y.Y.; Ge, R.L.; Yu, D.G. Engineered spindles of little molecules around electrospun nanofibers for biphasic drug release. Adv. Fiber Mater. 2021. [Google Scholar] [CrossRef]
- Lee, B.; Song, Y.; Park, C.; Kim, J.; Kang, J.; Lee, H.; Yoon, J.; Cho, S. Focused patterning of electrospun nanofibers using a dielectric guide structure. Polymers 2021, 13, 1505. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Xu, G.C.; Qiao, L.; Li, Y.; Gong, H.Y.; Shi, L.; Li, D.W.; Gao, M.; Liu, G.R.; et al. ZIF-8/PI nanofibrous membranes with high-temperature resistance for highly efficient PM0.3 air filtration and oil-water separation. Front. Chem. 2021, 9, 810861. [Google Scholar] [CrossRef]
- Kazsoki, A.; Palcsó, B.; Alpár, A.; Snoeck, R.; Andrei, G.; Zelkó, R. Formulation of acyclovir (core)-dexpanthenol (sheath) nanofibrous patches for the treatment of herpes labialis. Int. J. Pharm. 2022, 611, 121354. [Google Scholar] [CrossRef]
- Chi, Z.M.; Zhao, S.Q.; Feng, Y.X.; Yang, L. On-line dissolution analysis of multiple drugs encapsulated in electrospun nanofibers. Int. J. Pharm. 2020, 588, 119800. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, G.; Zhao, Y.; Zhou, M.; Zhong, A.; Sun, J. Promotion of skin regeneration through co-axial electrospun fibers loaded with basic fibroblast growth factor. Adv. Compos. Hybrid Mater. 2022. [Google Scholar] [CrossRef]
- Kalous, T.; Holec, P.; Erben, J.; Bilek, M.; Batka, O.; Pokorny, P.; Chaloupek, J.; Chvojka, J. The optimization of alternating current electrospun PA 6 solutions using a visual analysis system. Polymers 2021, 13, 2098. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Cheng, W.; Zhu, J.J.; Li, W.Y.; Li, D.Y.; Yang, X.; Zhao, W.X.; Ren, M.J.; Ren, J.J.; Mo, X.M.; et al. Electrospun nanoyarn and exosomes of adipose-derived stem cells for urethral regeneration: Evaluations in vitro and in vivo. Colloid Surf. B Biointerface 2022, 209, 112218. [Google Scholar] [CrossRef] [PubMed]
- Terra, A.L.M.; Moreira, J.B.; Costa, J.A.V.; Morais, M.G.D. Development of time-pH indicator nanofibers from natural pigments: An emerging processing technology to monitor the quality of foods. LWT 2021, 142, 111020. [Google Scholar] [CrossRef]
- Li, J.K.; Guan, S.M.; Su, J.J.; Liang, J.H.; Cui, L.L.; Zhang, K. The Development of hyaluronic acids used for skin tissue regeneration. Curr. Drug Deliv. 2020, 18, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Panigrahi, G.K.; Sahoo, J.K.; Pradhan, A.K.; Purohit, A.K.; Dhal, J.P. Electrospun magnetic polyacrylonitrile-GO hybrid nanofibers for removing Cr(VI) from water. J. Mol. Liq. 2021, 326, 115364. [Google Scholar] [CrossRef]
- Wang, K.L.; Wang, X.Y.; Jiang, D.; Pei, Y.F.; Wang, Z.; Zhou, X.J.; Wu, J.L.; Mo, X.M.; Wang, H.S. Delivery of mRNA vaccines and anti-PDL1 siRNA through non-invasive transcutaneous route effectively inhibits tumor growth. Compos. Part B-Eng. 2022, 233, 109648. [Google Scholar] [CrossRef]
- Yuan, Z.C.; Sheng, D.D.; Jiang, L.P.; Shafiq, M.; Khan, A.U.R.; Hashim, R.; Chen, Y.J.; Li, B.J.; Xie, X.R.; Chen, J.; et al. Vascular Endothelial Growth Factor-Capturing Aligned Electrospun Polycaprolactone/Gelatin Nanofibers Promote Patellar Ligament Regeneration. Acta Biomater. 2022, 140, 233–246. [Google Scholar] [CrossRef]
- Song, X.R.; Jiang, Y.X.; Zhang, W.X.; Elfawal, G.; Wang, K.L.; Jiang, D.; Hong, H.Y.; Wu, J.L.; He, C.L.; Mo, X.M.; et al. Transcutaneous tumor vaccination combined with anti-programmed death-1 monoclonal antibody treatment produces a synergistic antitumor effect. Acta Biomater. 2022, 140, 247–260. [Google Scholar] [CrossRef]
- Darbasizadeh, B.; Mortazavi, S.A.; Kobarfard, F.; Jaafari, M.R.; Hashemi, A.; Farhadnejad, H.; Feyzi-barnaji, B. Electrospun doxorubicin-loaded PEO/PCL core/sheath nanofibers for chemopreventive action against breast cancer cells. J. Drug. Deliv. Sci. Technol. 2021, 64, 102576. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating current electrospinning: The impacts of various high-voltage signal shapes and frequencies on the spinnability and productivity of polycaprolactone nanofibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Kang, S.X.; Hou, S.C.; Chen, X.W.; Yu, D.G.; Wang, L.; Li, X.Y.; Williams, G.R. Energy-saving electrospinning with a concentric Teflon-core rod spinneret to create medicated nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, M.; Hussain, S.; Gao, B.B.; Shah, K.A.; Mahar, F.K.; Yousif, M.; Hussain, S.; Ahmed, F. Fabrication and characterization of rizatriptan loaded pullulan nanofibers as oral fast-dissolving drug system. Mater. Res. Express. 2021, 8, 055404. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O. A scoping review on the biomedical applications of polymeric particles. Int. J. Polym. Mater. Polym. Biomater. 2022, 1–23. [Google Scholar] [CrossRef]
- Bukhary, H.; Williams, G.R.; Orlu, M. Fabrication of electrospun levodopa-carbidopa fixed-dose combinations. Adv. Fiber Mater. 2020, 2, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Miranda, C.S.; Silva, A.F.G.; Pereira-Lima, S.M.M.A.; Costa, S.P.G.; Homem, N.C.; Felgueiras, H.P. Tunable spun fiber constructs in biomedicine: Influence of processing parameters in the fibers’ architecture. Pharmaceutics 2022, 14, 164. [Google Scholar] [CrossRef]
- Ismail, I.; Bakar, N.F.A.; Tan, H.L.; Ideris, N.; Zain, Z.M.; Idris, S.S.; Radacsi, N. Ultra-sensitive electrosprayed AuNPs-decorated PAA/PAN electrospun nanofibers as glucose sensor. J. Mater. Res. 2021, 36, 4317–4328. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Qin, Y.; Li, C. Functional electrospun films for efficient oxygen reduction reaction. Chem. Res. Chin. Univ. 2021, 37, 379–393. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, K.; Yu, D.G.; Zheng, X.; Huang, C. Advances in biosensing and environmental monitoring based on electrospun nanofibers. Adv. Fiber Mater. 2022. [Google Scholar] [CrossRef]
- El-Shanshory, A.A.; Agwa, M.M.; Abd-Elhamid, A.I.; Soliman, H.M.A.; Mo, X.M.; Kenawy, E.R. Metronidazole Topically Immobilized Electrospun Nanofibrous Scaffold: Novel Secondary Intention Wound Healing Accelerator. Polymers 2022, 14, 454. [Google Scholar] [CrossRef]
- Bhusnure, O.G.; Gholve, S.B.; Giram, P.S.; Gaikwad, A.V.; Udumansha, U.; Mani, G.; Tae, J.H. Novel 5-flurouracil-Embedded non-woven PVA-PVP electrospun nanofibers with enhanced anti-cancer efficacy: Formulation, evaluation and in vitro anti-cancer activity. J. Drug. Deliv. Sci. Technol. 2021, 64, 102654. [Google Scholar] [CrossRef]
- Brimo, N.; Serdaroğlu, D. Çökeliler; Uysal, B. Comparing Antibiotic Pastes with Electrospun Nanofibers as Modern Drug Delivery Systems for Regenerative Endodontics. Curr. Drug Deliv. 2021, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Z.; Zhang, M.X.; Lv, H.; Yang, Y.Y.; Yu, D.G. Electrospun polyacrylonitrile-based lace nanostructures and their Cu(II) adsorption. Sep. Purif. Technol. 2022, 288, 120643. [Google Scholar] [CrossRef]
- Malihe, G.; Shahnoosh, A.; Amir, R.; Shiva, R.; Hajir, B.S. Fabrication and characterization of chitosan-polycaprolactone core-shell nanofibers containing tetracycline hydrochloride. Colloid Surf. A Physicochem. Eng. Asp. 2022, 636, 128163. [Google Scholar]
- Zhang, Z.; Zhao, Y.; Li, Z.; Zhang, L.; Liu, Z.; Long, Z.; Li, Y.; Liu, Y.; Fan, R.; Sun, K.; et al. Synthesis of carbon/SiO2 core-sheath nanofibers with Co-Fe nanoparticles embedded in via electrospinning for high-performance microwave absorption. Adv. Compos. Hybrid Mater. 2021, 5, 513–524. [Google Scholar] [CrossRef]
- Ning, T.; Zhou, Y.; Xu, H.X.; Guo, S.; Yu, D.G. Orodispersible membranes from a modified coaxial electrospinning for fast dissolution of diclofenac sodium. Membranes 2021, 11, 802. [Google Scholar] [CrossRef]
- Huang, C.; Dong, J.; Zhang, Y.; Chai, S.; Wang, X.; Kang, S.; Yu, D.; Wang, P.; Jiang, Q. Gold Nanoparticles-Loaded Polyvinylpyrrolidone/Ethylcellulose Coaxial Electrospun Nanofibers with Enhanced Osteogenic Capability for Bone Tissue Regeneration. Mater. Des. 2021, 212, 110240. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Andriyana, A.; Muhamad, F.; Ang, B.C. Effect of core-to-shell flowrate ratio on morphology, crystallinity, mechanical properties and wettability of poly(lactic acid) fibers prepared via modified coaxial electrospinning. Polymer 2021, 237, 124378. [Google Scholar] [CrossRef]
- Zhao, K.; Lu, Z.H.; Zhao, P.; Kang, S.X.; Yang, Y.Y.; Yu, D.G. Modified tri-axial electrospun functional core–shell nanofibrous membranes for natural photodegradation of antibiotics. Chem. Eng. J. 2021, 425, 131455. [Google Scholar] [CrossRef]
- Ghosal, K.; Augustine, R.; Zaszczynska, A.; Barman, M.; Jain, A.; Hasan, A.; Kalarikkal, N.; Sajkiewicz, P.; Thomas, S. Novel drug delivery systems based on triaxial electrospinning based nanofibers. React. Funct. Polym. 2021, 163, 104895. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Y.; Li, D.; Xu, G.; Yin, D.; Xiao, Y.; Xu, T.; Chen, X.; Zhu, X.; Shi, X. Negative isolation of circulating tumor cells using a microfluidic platform integrated with streptavidin-functionalized PLGA nanofibers. Adv. Fiber Mater. 2021, 3, 192–202. [Google Scholar] [CrossRef]
- Wang, Y.B.; Xu, H.X.; Wu, M.; Yu, D.G. Nanofibers-Based Food Packaging. ES Food Agrofor. 2021. [Google Scholar] [CrossRef]
- Qi, H.N.; Wang, G.Y.; Ma, Q.L.; Li, D.; Dong, X.T.; Yu, W.S.; Wang, J.X.; Liu, G.X.; Zhang, X.J. Conjugative electrospinning towards Janus-type nanofibers array membrane concurrently displaying dual-functionality of improved red luminescence and tuneable superparamagnetism. J. Mater. Sci. Mater. Electron. 2022, 10, 4438–4449. [Google Scholar] [CrossRef]
- Qi, H.N.; Xie, Y.R.; Yang, L.; Tang, X.H.; Ma, Q.L.; Yu, W.S.; Dong, X.T.; Li, D.; Liu, G.X.; Wang, J.X. Electrospun polyfunctional switch-typed anisotropic photoconductive film endued with superparamagnetic-fluorescent performances. Appl. Mater. Today 2021, 24, 101086. [Google Scholar] [CrossRef]
- Yu, D.G.; Wang, M.L.; Ge, R. Strategies for sustained drug release from electrospun multi-layer nanostructures. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1722. [Google Scholar] [CrossRef]
- Wang, M.L.; Hou, J.S.; Yu, D.G.; Li, S.Y.; Zhu, J.W.; Chen, Z.Z. Electrospun tri-layer nanodepots for sustained release of acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Lee, J.; Moon, S.; Han, Y.B.; Yang, S.J.; Lahann, J.; Lee, K.J. Facile fabrication of anisotropic multicompartmental microfibers using charge reversal electrohydrodynamic co-jetting. Macromol. Rapid Commun. 2021, 43, 2100560. [Google Scholar] [CrossRef]
- Kamath, S.M.; Sridhar, K.; Jaison, D.; Gopinath, V.; Ibrahim, B.K.M.; Gupta, N.; Sundaram, A.; Sivaperumal, P.; Padmapriya, S.; Patil, S.S. Fabrication of tri-layered electrospun polycaprolactone mats with improved sustained drug release profile. Sci. Rep. 2020, 10, 18179. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, J.L.; Shao, Y.; Deng, R.H.; Zhu, J.T.; Yang, Z.Z. Recent advances in scalable synthesis and performance of Janus polymer/inorganic films. Prog. Mater. Sci. 2022, 124, 100888. [Google Scholar] [CrossRef]
- Bertani, R.; Bartolozzi, A.; Pontefisso, A.; Quaresimin, M.; Zappalorto, M. Improving the antimicrobial and mechanical properties of epoxy resins via nanomodification: An Overview. Molecules 2021, 26, 5426. [Google Scholar] [CrossRef]
- Drug Administration Instruction: Lianhua Qingwen Keli; Beijing Yiling Pharmaceutical Co., Ltd.: Beijing, China, 2021.
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.-G. Electrospun structural hybrids of acyclovir-polyacrylonitrile at acyclovir for modifying drug release. Polymers 2021, 13, 4286. [Google Scholar] [CrossRef] [PubMed]
- Vass, P.; Szabó, E.; Domokos, A.; Hirsch, E.; Galata, D.; Farkas, B.; Démuth, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; et al. Scale-up of electrospinning technology: Applications in the ph armaceutical industry. Wiley Interdiscip. Rev. Nanomed. Nanbiotechnol. 2020, 12, e1611. [Google Scholar]
- Khalid, G.M.; Selmin, F.; Musazzi, U.M.; Gennari, C.G.M.; Minghetti, P.; Cilurzo, F. Trends in the characterization methods of orodispersible films. Curr. Drug Deliv. 2021, 18, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Lawson, D.; Onyekuru, L.; Dziemidowicz, K.; Angkawinitwong, U.; Costa, P.F.; Radacsi, N.; Williams, G.R. Protein encapsulation by electrospinning and electrospraying. J. Control. Release 2021, 329, 1172–1197. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Rostamabadi, M.M.; Assadpour, E.; Jafari, S.M. Electrospraying as a novel process for the synthesis of particles/nanoparticles loaded with poorly water-soluble bioactive molecules. Adv. Colloid Interface Sci. 2021, 290, 102384. [Google Scholar] [CrossRef]
- Tanhaei, A.; Mohammadi, M.; Hamishehkar, H.; Hamblin, M.R. Electrospraying as a novel method of particle engineering for drug delivery vehicles. J. Control. Release 2021, 330, 851–865. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Jiang, W.; Shen, L.; Zhang, G.; Gao, Y.; Yang, Y.; Yu, D.-G. Electrospun Hybrid Films for Fast and Convenient Delivery of Active Herb Extracts. Membranes 2022, 12, 398. https://doi.org/10.3390/membranes12040398
Guo S, Jiang W, Shen L, Zhang G, Gao Y, Yang Y, Yu D-G. Electrospun Hybrid Films for Fast and Convenient Delivery of Active Herb Extracts. Membranes. 2022; 12(4):398. https://doi.org/10.3390/membranes12040398
Chicago/Turabian StyleGuo, Shiri, Wenlai Jiang, Liangfei Shen, Gaoyi Zhang, Yiman Gao, Yaoyao Yang, and Deng-Guang Yu. 2022. "Electrospun Hybrid Films for Fast and Convenient Delivery of Active Herb Extracts" Membranes 12, no. 4: 398. https://doi.org/10.3390/membranes12040398
APA StyleGuo, S., Jiang, W., Shen, L., Zhang, G., Gao, Y., Yang, Y., & Yu, D.-G. (2022). Electrospun Hybrid Films for Fast and Convenient Delivery of Active Herb Extracts. Membranes, 12(4), 398. https://doi.org/10.3390/membranes12040398