Moving beyond 90% Carbon Capture by Highly Selective Membrane Processes
Abstract
:1. Introduction
2. Methods
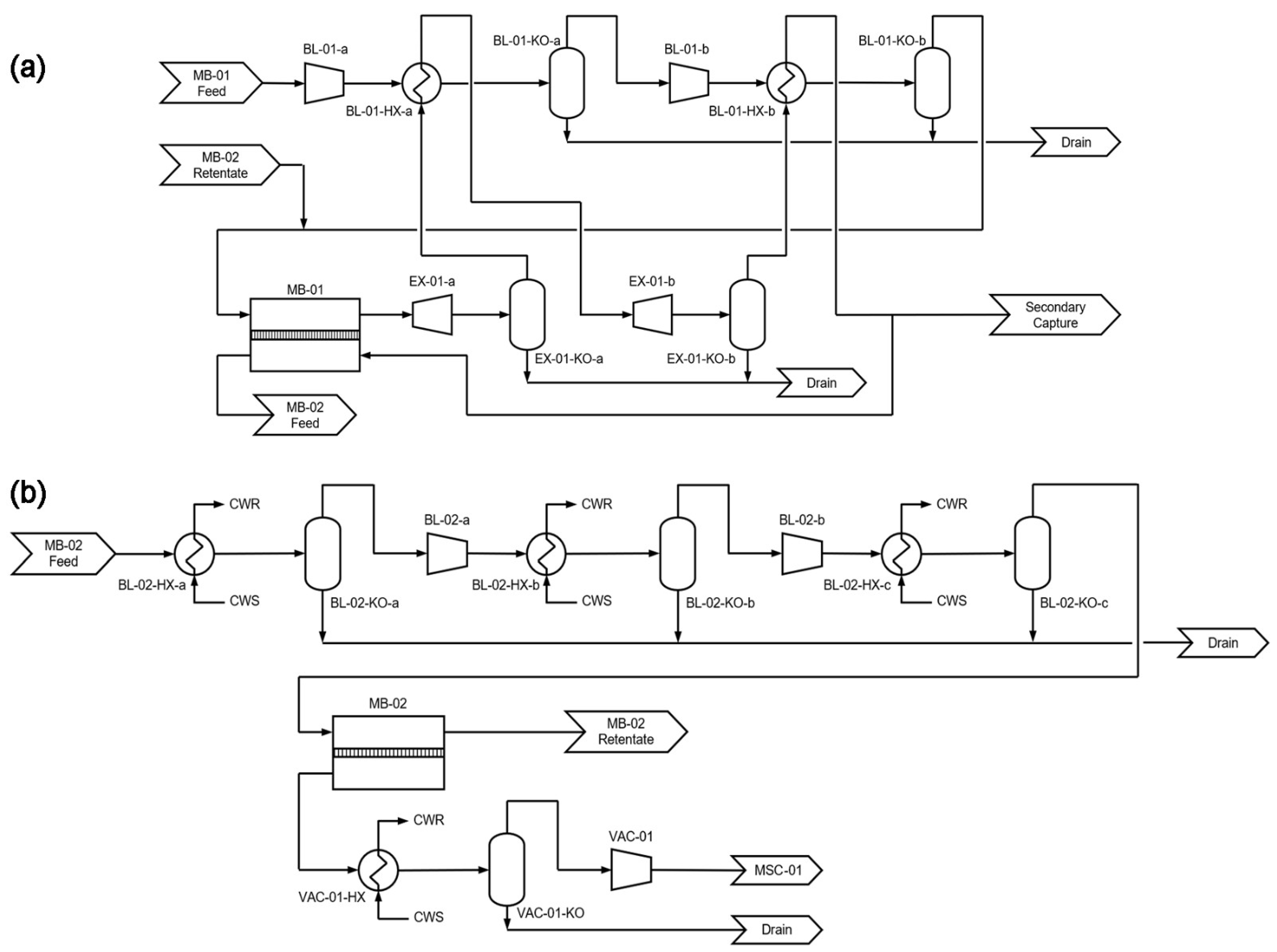
2.1. Process Description
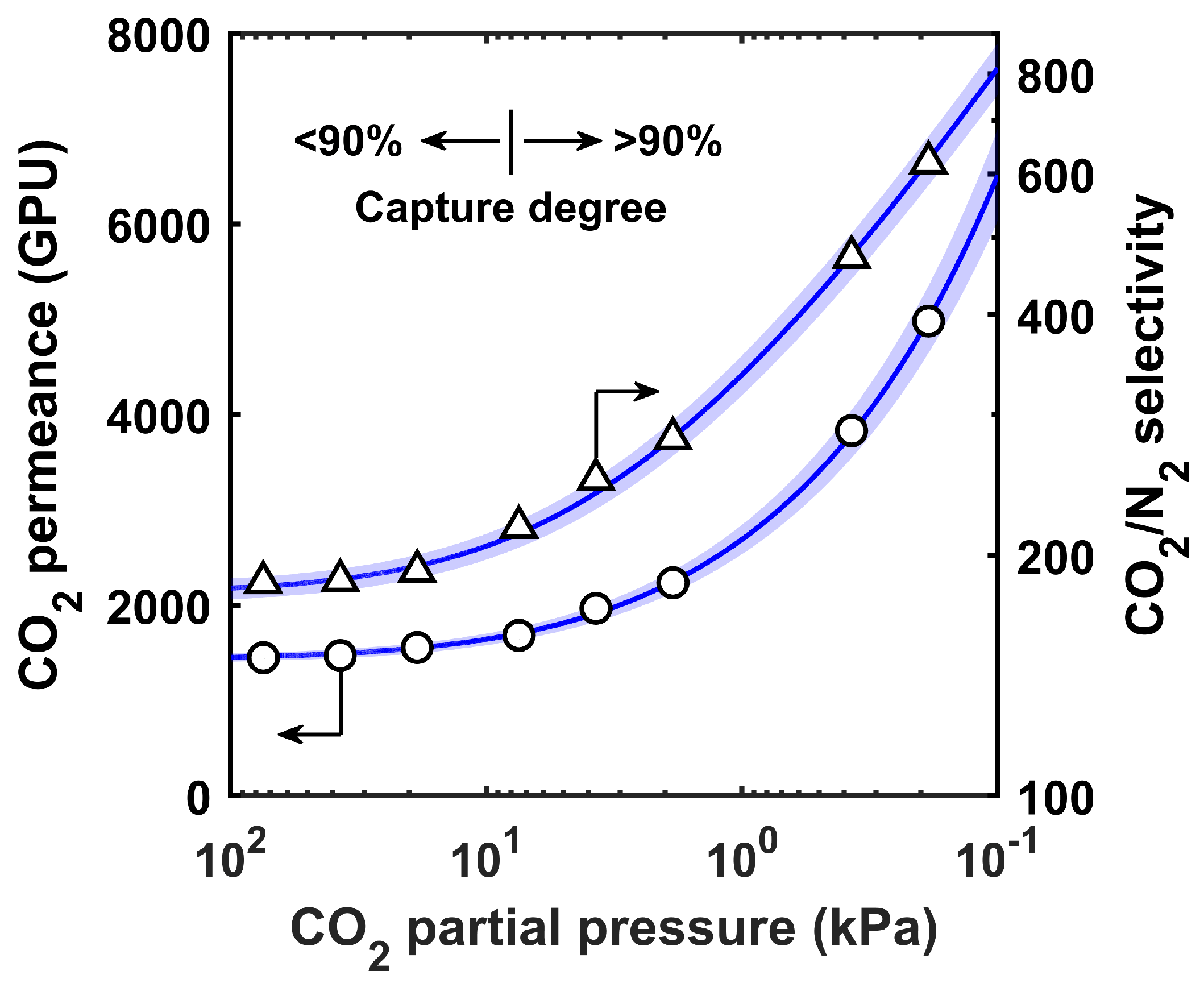
2.2. FTM Modeling
2.3. Process Modeling
2.4. Costing Modeling
- 1.
- An installed membrane skid cost of USD 44.6/m2 membrane area was assigned, including USD 21.5/m2 membrane element cost, USD 5.4/m2 housing cost, and 17.7/m2 installation cost, based on commercial-scale reverse osmosis plants [44];
- 2.
- A membrane lifetime of 4 years was assumed with a membrane replacement cost of USD 5.4/m2/yr;
- 3.
- A capital charge factor of 0.125 was applied to calculate the capital cost [5].
3. Results and Discussion
3.1. Performance of the Benchmark FTM
3.2. Capture Using the Primary System Only
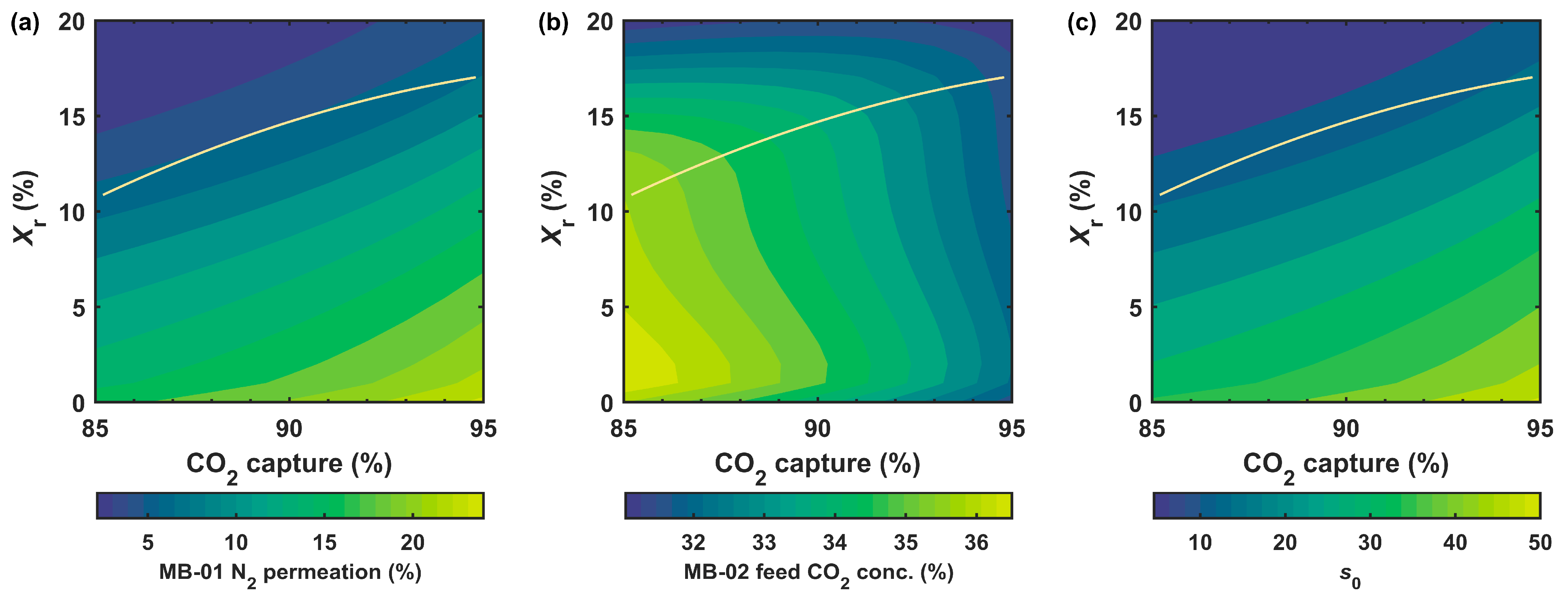
3.2.1. Effect of Retentate Recycle
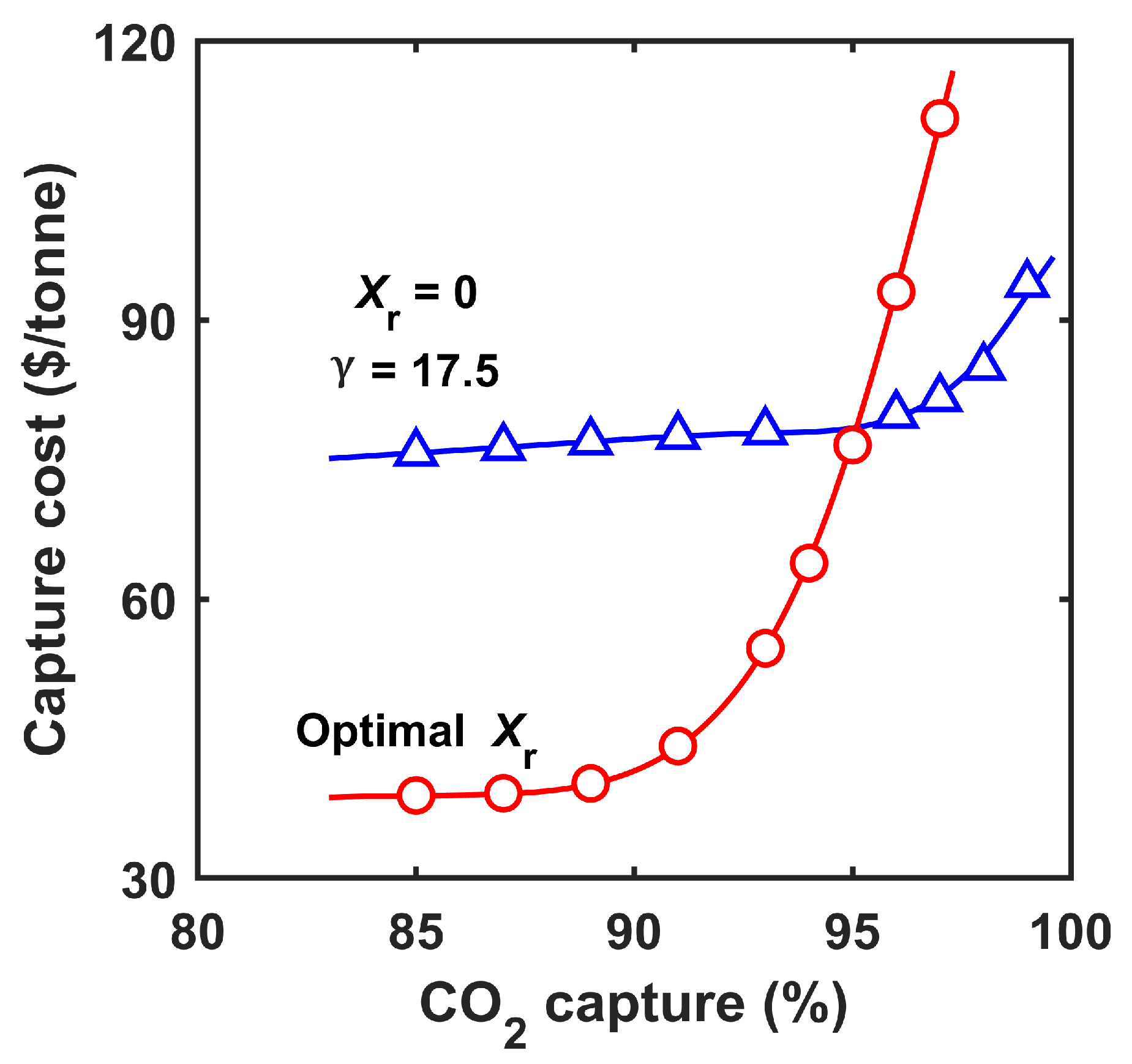
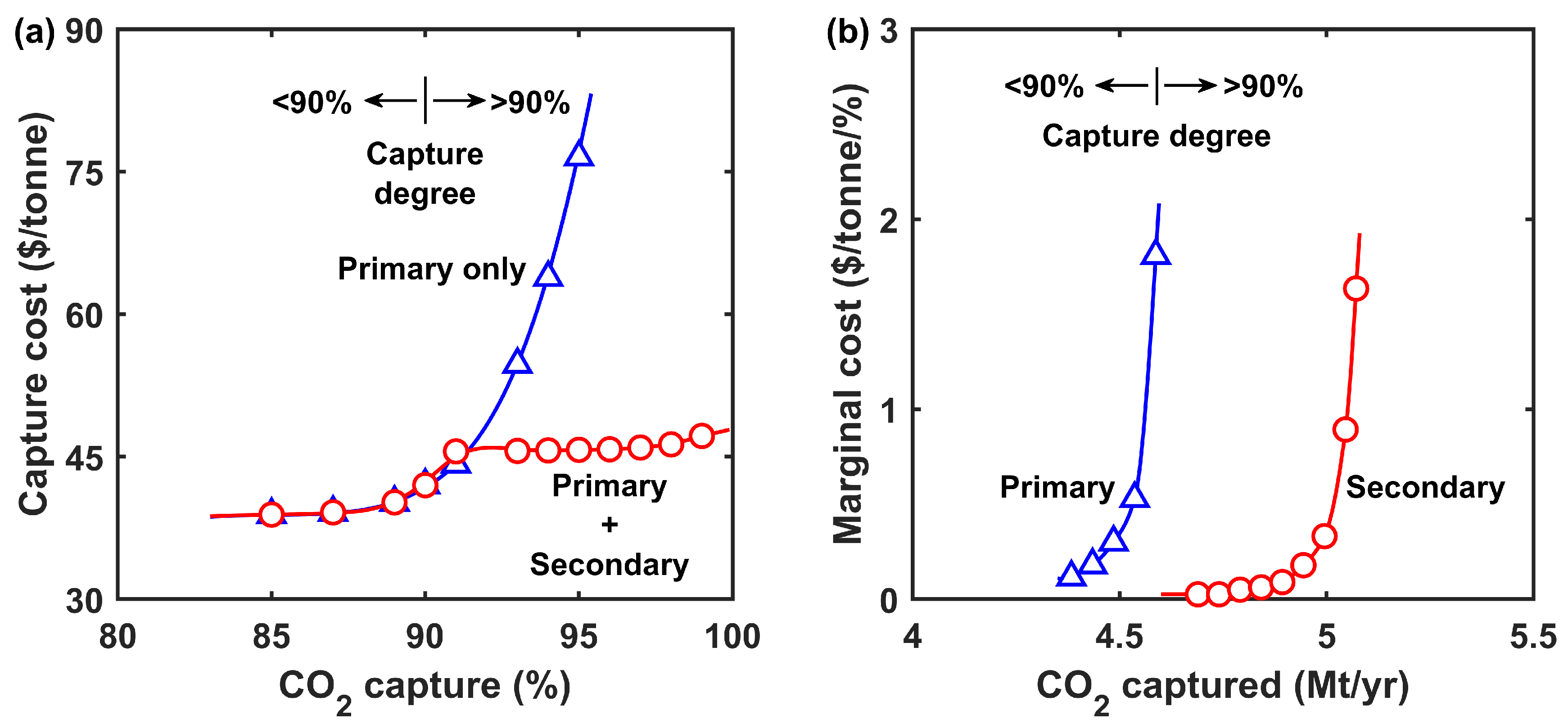
3.2.2. Costs at Different Capture Degrees
3.3. Capture Using Combined Systems in Tandem
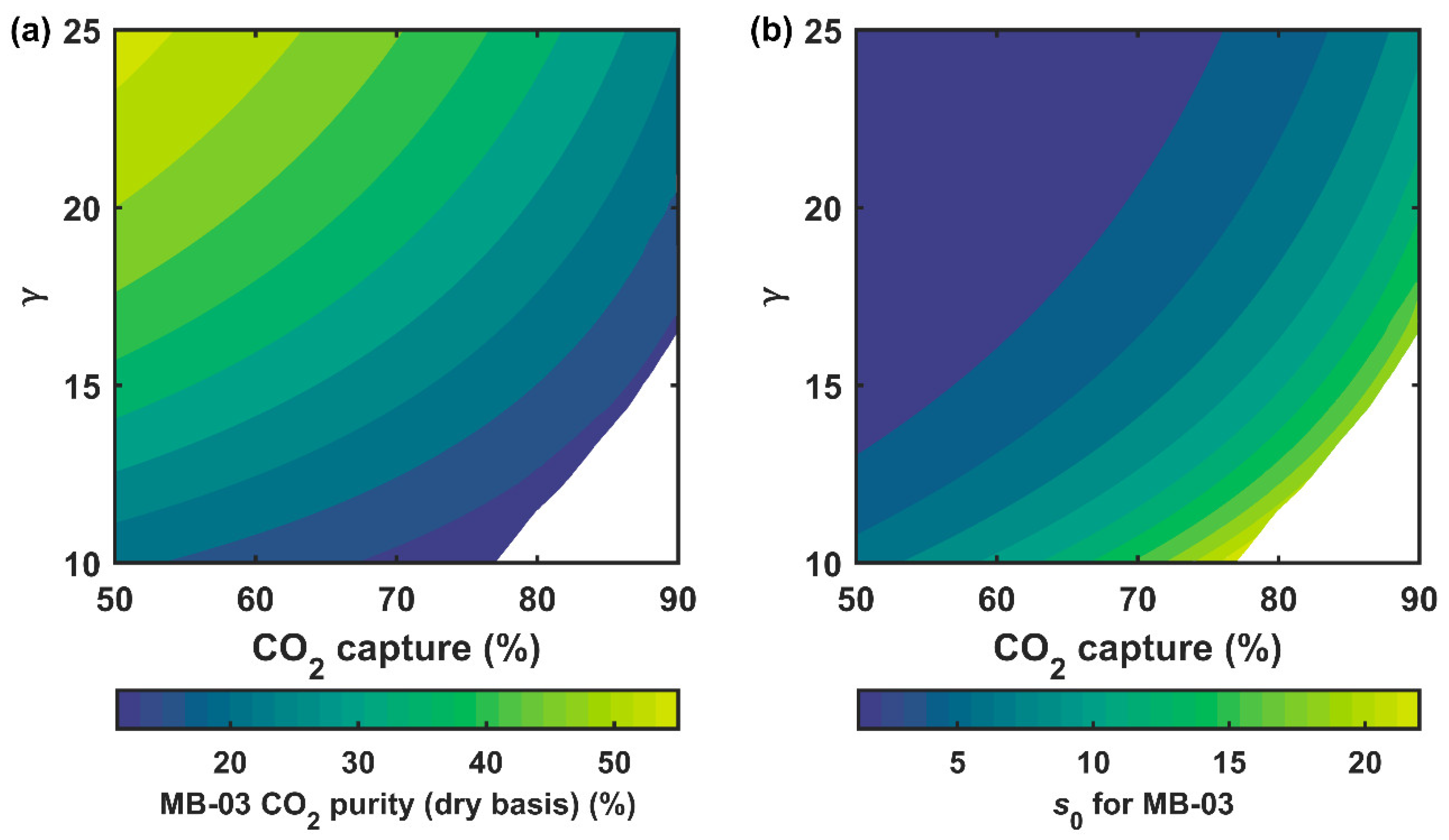
3.3.1. Separation Performance of the Secondary System
3.3.2. Process Economics
4. Conclusions
- 1.
- The retentate recycle process was advantageous for ≤90% capture owing to the reduced parasitic energy consumption and membrane area. In comparison, the enriching cascade was inferior for the partial capture scenario;
- 2.
- At >90% capture, the enriching cascade outperformed the retentate recycle pro-cess since a higher feed-to-permeate pressure ratio could be applied;
- 3.
- The combined process with primary and secondary capture systems in tandem could achieve a low capture cost of USD 47.2/tonne at 99% capture. The FTM-based deep CCS approach complements DAC.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Global Warming of 1.5 °C: An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty, Report Number: IPCC SR1.5; Intergovernmental Panel on Climate Change: Incheon, Korea, 2018. [Google Scholar]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. Department of Energy’s carbon sequestration program. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Lackner, K.S.; Grimes, P.; Ziock, H.-J. Capturing Carbon Dioxide from Air. In Carbon Capture and Storage: CO2 Management Technologies; Apple Academic Press, Inc.: Oakvile, ON, Canada, 2001; pp. 363–376. [Google Scholar]
- Fout, T.; Zoelle, A.; Keairns, D.; Turner, M.; Woods, M.; Kuehn, N.; Shah, V.; Chou, V.; Pinkerton, L. Cost and Performance Baseline for Fossil Energy Plants Volume 1a: Bituminous Coal (PC) and Natural Gas to Electricity, Revision 3; Report Number: DOE/NETL-2015/1723; U.S. Department of Energy: Washington, DC, USA, 2015. [Google Scholar]
- James, R.; Zoelle, A.; Keairns, D.; Turner, M.; Woods, M.; Kuehn, N. Cost and Performance Baseline for Fossil Energy Plants Volume 1: Bituminous Coal and Natural Gas to Electricity, Revision 4; Report Number: DOE/NETL-PUB-22638; U.S. Department of Energy: Washington, DC, USA, 2019. [Google Scholar]
- Keith, D.W. Why capture CO2 from the atmosphere? Science 2009, 325, 1654–1655. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Sanchez, D.L.; Mazzotti, M. Assessment of carbon dioxide removal potential via BECCS in a carbon-neutral Europe. Energy Environ. Sci. 2021, 14, 3086–3097. [Google Scholar] [CrossRef]
- Deutz, S.; Bardow, A. Life-cycle assessment of an industrial direct air capture process based on temperature–vacuum swing adsorption. Nat. Energy 2021, 6, 203–213. [Google Scholar] [CrossRef]
- Sanz-Perez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct capture of CO2 from ambient air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Holmes, H.E.; Lively, R.P.; Realff, M.J. Defining targets for adsorbent material performance to enable viable BECCS processes. JACS Au 2021, 1, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Dods, M.N.; Kim, E.J.; Long, J.R.; Weston, S.C. Deep CCS: Moving beyond 90% carbon dioxide capture. Environ. Sci. Technol. 2021, 55, 8524–8534. [Google Scholar] [CrossRef]
- McGlashan, N.; Marquis, A. Simultaneous removal of CO2, SO2, and NOx from flue gas by liquid phase dehumidification at cryogenic temperatures and low pressure. Proc. Inst. Mech.Eng. Part A J. Power Energy 2008, 222, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Hägg, M.-B. A feasibility study of CO2 capture from flue gas by a facilitated transport membrane. J. Membr. Sci. 2010, 359, 140–148. [Google Scholar] [CrossRef]
- Brandl, P.; Bui, M.; Hallett, J.P.; Mac Dowell, N. Beyond 90% capture: Possible, but at what cost? Int. J. Greenh. Gas Control 2021, 105, 103239. [Google Scholar] [CrossRef]
- Danaci, D.; Bui, M.; Petit, C.; Dowell, N.M. En route to zero emissions for power and industry with amine-based post-combustion capture. Environ. Sci. Technol. 2021, 55, 10619–10632. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gao, T.; Rochelle, G.T.; Bhown, A.S. Zero- and negative-emissions fossil-fired power plants using CO2 capture by conventional aqueous amines. Int. J. Greenh. Gas Control 2021, 111, 103473. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Ramasubramanian, K.; Verweij, H.; Ho, W.S.W. Membrane processes for carbon capture from coal-fired power plant flue gas: A modeling and cost study. J. Membr. Sci. 2012, 421–422, 299–310. [Google Scholar] [CrossRef]
- Han, Y.; Yang, Y.; Ho, W.S.W. Recent progress in the engineering of polymeric membranes for CO2 capture from flue gas. Membranes 2020, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Alent’ev, A.Y.; Volkov, A.; Vorotyntsev, I.; Maksimov, A.; Yaroslavtsev, A. Membrane technologies for decarbonization. Membr. Membr. Technol. 2021, 3, 255–273. [Google Scholar] [CrossRef]
- Novitskii, E.; Bazhenov, S.; Volkov, A. Optimization of methods for purification of gas mixtures to remove carbon dioxide (a review). Pet. Chem. 2021, 61, 407–423. [Google Scholar] [CrossRef]
- Ho, W.S.W.; Sirkar, K.K. Membrane Handbook, reprint ed.; Kluwer Academic Publishers: Boston, MA, USA, 2001. [Google Scholar]
- Klingberg, P.; Wilkner, K.; Schluter, M.; Grunauer, J.; Shishatskiy, S. Separation of carbon dioxide from real power plant flue gases by gas permeation using a supported ionic liquid membrane: An investigation of membrane stability. Membranes 2019, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Shafiee, A.; Nomvar, M.; Liu, Z.; Abbas, A. Automated process synthesis for optimal flowsheet design of a hybrid membrane cryogenic carbon capture process. J. Clean. Prod. 2017, 150, 309–323. [Google Scholar] [CrossRef]
- Deng, X.; Zou, C.; Han, Y.; Lin, L.-C.; Ho, W.S.W. Computational evaluation of carriers in facilitated transport membranes for postcombustion carbon capture. J. Phys. Chem. C 2020, 124, 25322–25330. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Lasseuguette, E.; Janakiram, S.; Deng, L.; Ferrari, M.C. Analysis of CO2 facilitation transport effect through a hybrid poly(allyl amine) membrane: Pathways for further improvement. Membranes 2020, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Hägg, M.-B. Swelling behavior and gas permeation performance of PVAm/PVA blend FSC membrane. J. Membr. Sci. 2010, 363, 295–301. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, Z.; Zhang, C.; Yuan, S.; Zhu, Y.; Wang, J.; Wang, S. PVAm–PIP/PS composite membrane with high performance for CO2/N2 separation. AIChE J. 2013, 59, 215–228. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, L.; Wang, B.; Dutta, P.; Ho, W.S.W. Amine-containing polymer/zeolite Y composite membranes for CO2/N2 separation. J. Membr. Sci. 2016, 497, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ho, W.S.W. High-molecular-weight polyvinylamine/piperazine glycinate membranes for CO2 capture from flue gas. J. Membr. Sci. 2016, 514, 376–384. [Google Scholar] [CrossRef] [Green Version]
- Tong, Z.; Ho, W.S.W. New sterically hindered polyvinylamine membranes for CO2 separation and capture. J. Membr. Sci. 2017, 543, 202–211. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S.W. Design of amine-containing CO2-selective membrane process for carbon capture from flue gas. Ind. Eng. Chem. Res. 2020, 59, 5340–5350. [Google Scholar] [CrossRef]
- Matsuoka, A.; Otani, A.; Kamio, E.; Matsuyama, H. A gradient viscosity model for estimating CO2 permeability of amino acid ionic liquid-based facilitated transport membrane. Sep. Purif. Technol. 2022, 280, 119847. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Wei, Z.; Yuan, S.; Zhao, J.; Wang, J.; Wang, S. Novel tertiary amino containing thin film composite membranes prepared by interfacial polymerization for CO2 capture. J. Membr. Sci. 2010, 362, 265–278. [Google Scholar] [CrossRef]
- Yao, K.; Wang, Z.; Wang, J.; Wang, S. Biomimetic material—poly(N-vinylimidazole)–zinc complex for CO2 separation. Chem. Commun. 2012, 48, 1766–1768. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Cai, Y.; Yi, C.; Yang, D.; Yuan, S. Investigation of gas permeation behavior in facilitated transport membranes: Relationship between gas permeance and partial pressure. Chem. Eng. J. 2013, 225, 744–751. [Google Scholar] [CrossRef]
- Han, Y.; Wu, D.; Ho, W.S.W. Simultaneous effects of temperature and vacuum and feed pressures on facilitated transport membrane for CO2/N2 separation. J. Membr. Sci. 2019, 573, 476–484. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S.W. Mitigated carrier saturation of facilitated transport membranes for decarbonizing dilute CO2 sources: An experimental and techno-economic study. J. Membr. Sci. Lett. 2022, 2, 100014. [Google Scholar] [CrossRef]
- Han, Y.; Wu, D.; Ho, W.S.W. Nanotube-reinforced facilitated transport membrane for CO2/N2 separation with vacuum operation. J. Membr. Sci. 2018, 567, 261–271. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S.W. Facilitated transport membranes for H2 purification from coal-derived syngas: A techno-economic analysis. J. Membr. Sci. 2021, 636, 119549. [Google Scholar] [CrossRef]
- Nasri, Z.; Binous, H. Applications of the Soave–Redlich–Kwong equation of state using Mathematica®. J. Chem. Eng. Jpn. 2007, 40, 534–538. [Google Scholar] [CrossRef] [Green Version]
- Scholz, M.; Harlacher, T.; Melin, T.; Wessling, M. Modeling gas permeation by linking nonideal effects. Ind. Eng. Chem. Res. 2012, 52, 1079–1088. [Google Scholar] [CrossRef]
- Couper, J.R.; Penney, W.R.; Fair, J.R. Chemical Process Equipment: Selection and Design, revised 2nd ed.; Elsevier Inc.: Oxford, UK, 2009. [Google Scholar]
- Wilf, M.; Awerbuch, L.; Bartels, C. The Guidebook to Membrane Desalination Technology: Reverse Osmosis, Nanofiltration and Hybrid Systems Process, Design, Applications and Economics; Desalination Publications: L’Aquila, Italy, 2007. [Google Scholar]
- Zhao, L.; Riensche, E.; Blum, L.; Stolten, D. Multi-stage gas separation membrane processes used in post-combustion capture: Energetic and economic analyses. J. Membr. Sci. 2010, 359, 160–172. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Qiao, Z.; Wu, H.; Dong, S.; Zhao, S.; Wang, J. Post-combustion CO2 capture with membrane process: Practical membrane performance and appropriate pressure. J. Membr. Sci. 2019, 581, 195–213. [Google Scholar] [CrossRef]
- Sinha, A.; Darunte, L.A.; Jones, C.W.; Realff, M.J.; Kawajiri, Y. Systems design and economic analysis of direct air capture of CO2 through temperature vacuum swing adsorption using MIL-101(Cr)-PEI-800 and mmen-Mg2(dobpdc) MOF adsorbents. Ind. Eng. Chem. Res. 2017, 56, 750–764. [Google Scholar] [CrossRef]
- Azarabadi, H.; Lackner, K.S. A sorbent-focused techno-economic analysis of direct air capture. Appl. Energy 2019, 250, 959–975. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Power plant net power | 650 MWe (supercritical coal-fired power plant) [5] |
| Power plant capacity factor | 0.85 [5] |
| Flue gas flow rate | 32.71 kmol/s |
| Flue gas composition | 13.21% CO2, 65.21% N2, 17.25% H2O with balancing O2 at 57 °C and 101.3 kPa (1 atm) |
| Primary CO2 capture spec | 90% CO2 recovery, ≥95% CO2 purity |
| Residual flue gas flow rate | 24.65 kmol/s |
| Residual flue gas composition | 1.85% CO2, 85.70% N2, 7.72% H2O † with balancing O2 |
| Secondary CO2 capture spec | 90% CO2 recovery, ≥95% CO2 purity |
| Membrane temperature | 67 °C |
| Feed pressure ‡ | 354.6 kPa (3.5 atm) for both MB-01 and MB-02; 456.0 kPa (4.5 atm) for both MB-03 and MB-04 |
| Feed water content ‡ | 100% relative humidity at given feed temperature and pressure |
| Percentage of retentate recycle | 15% |
| Vacuum pressure ‡ | 81.0 kPa (0.8 atm) for MB-02; 20.3 kPa (0.2 atm) for both MB-03 and MB-04 |
| Heat transfer coefficient‡ | 60 W m–2 K–1 for BL-01 and BL-03; 100 W m–2 K–1 for BL-02 and BL-04 * |
| 1431 GPU | |
| 183 | |
| 0.46 | |
| 7.5 kPa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Ho, W.S.W. Moving beyond 90% Carbon Capture by Highly Selective Membrane Processes. Membranes 2022, 12, 399. https://doi.org/10.3390/membranes12040399
Han Y, Ho WSW. Moving beyond 90% Carbon Capture by Highly Selective Membrane Processes. Membranes. 2022; 12(4):399. https://doi.org/10.3390/membranes12040399
Chicago/Turabian StyleHan, Yang, and W. S. Winston Ho. 2022. "Moving beyond 90% Carbon Capture by Highly Selective Membrane Processes" Membranes 12, no. 4: 399. https://doi.org/10.3390/membranes12040399
APA StyleHan, Y., & Ho, W. S. W. (2022). Moving beyond 90% Carbon Capture by Highly Selective Membrane Processes. Membranes, 12(4), 399. https://doi.org/10.3390/membranes12040399







