Antioxidant and Antimicrobial Properties of Mung Bean Phyto-Film Combined with Longkong Pericarp Extract and Sonication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Longkong Pericarp Extraction (LPE)
2.2. Film Formation
2.3. Quality Analysis
2.3.1. Physical Properties
2.3.2. Mechanical Properties
2.3.3. Phytochemicals and Antioxidant Activities
2.3.4. Antimicrobial Activity
2.4. Statistical Analysis
3. Results and Discussion
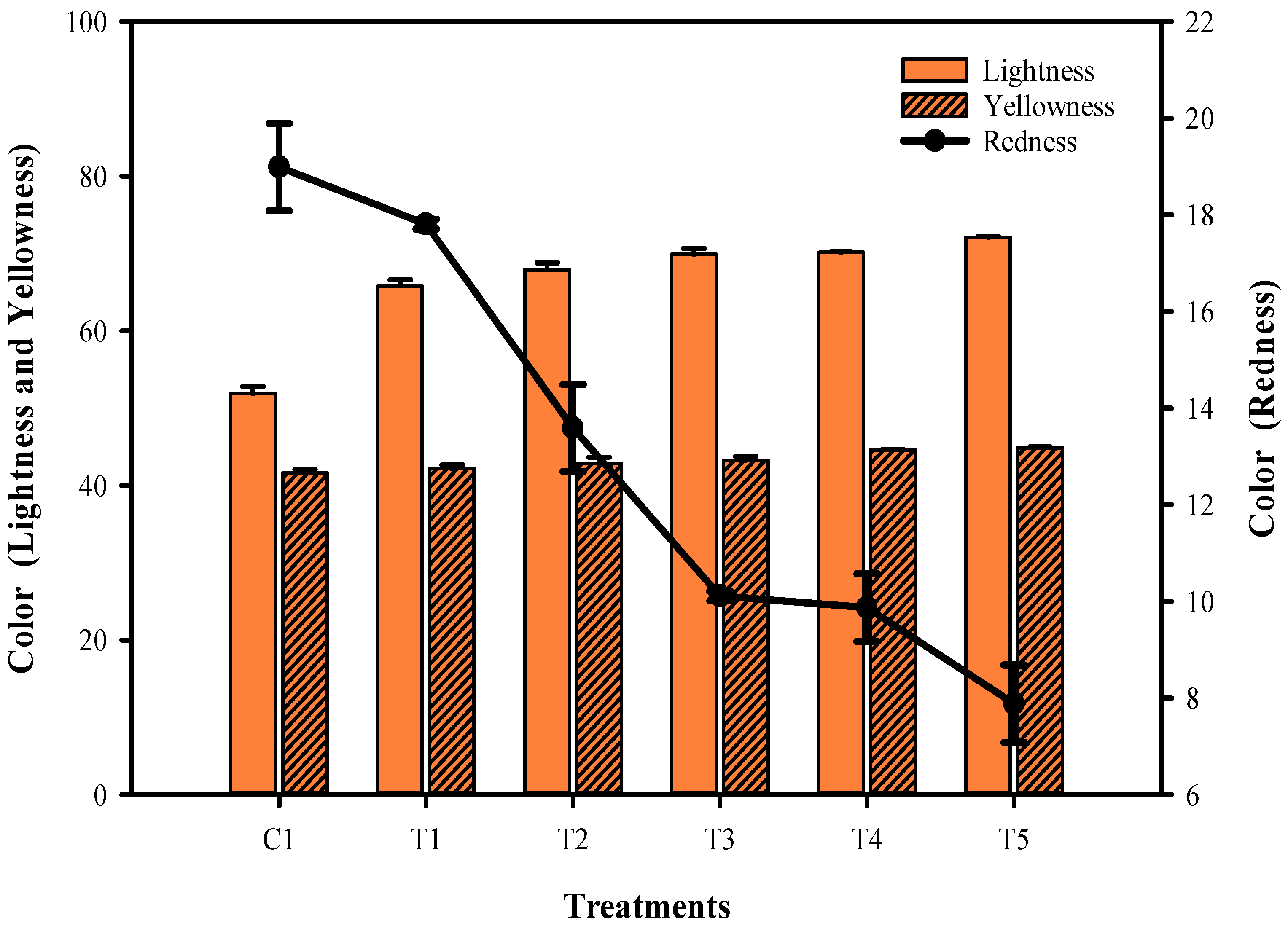
3.1. Color Characteristics
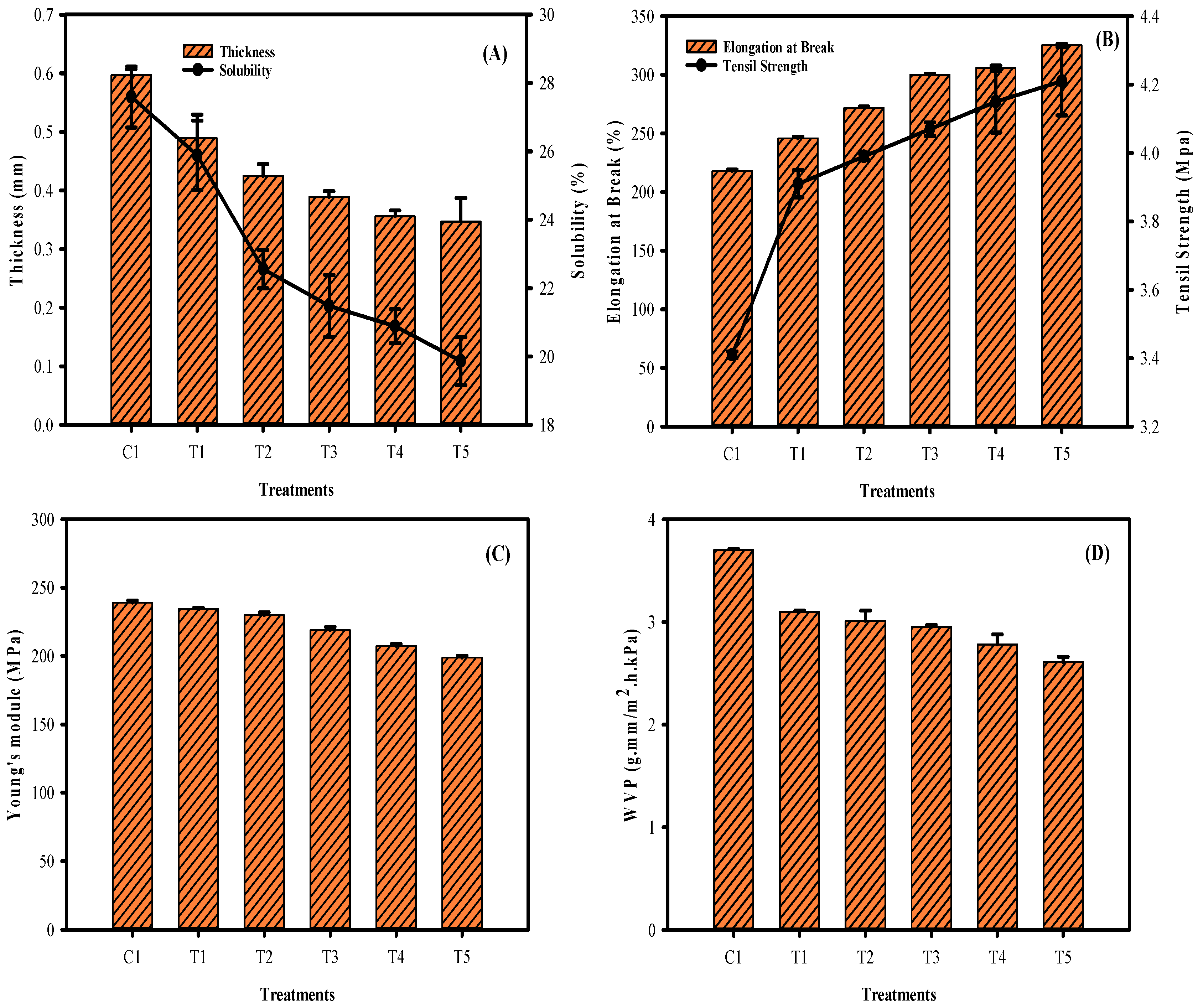
3.2. Film Thickness, Solubility, EAB, TS, Young’s Modulus, and WVP
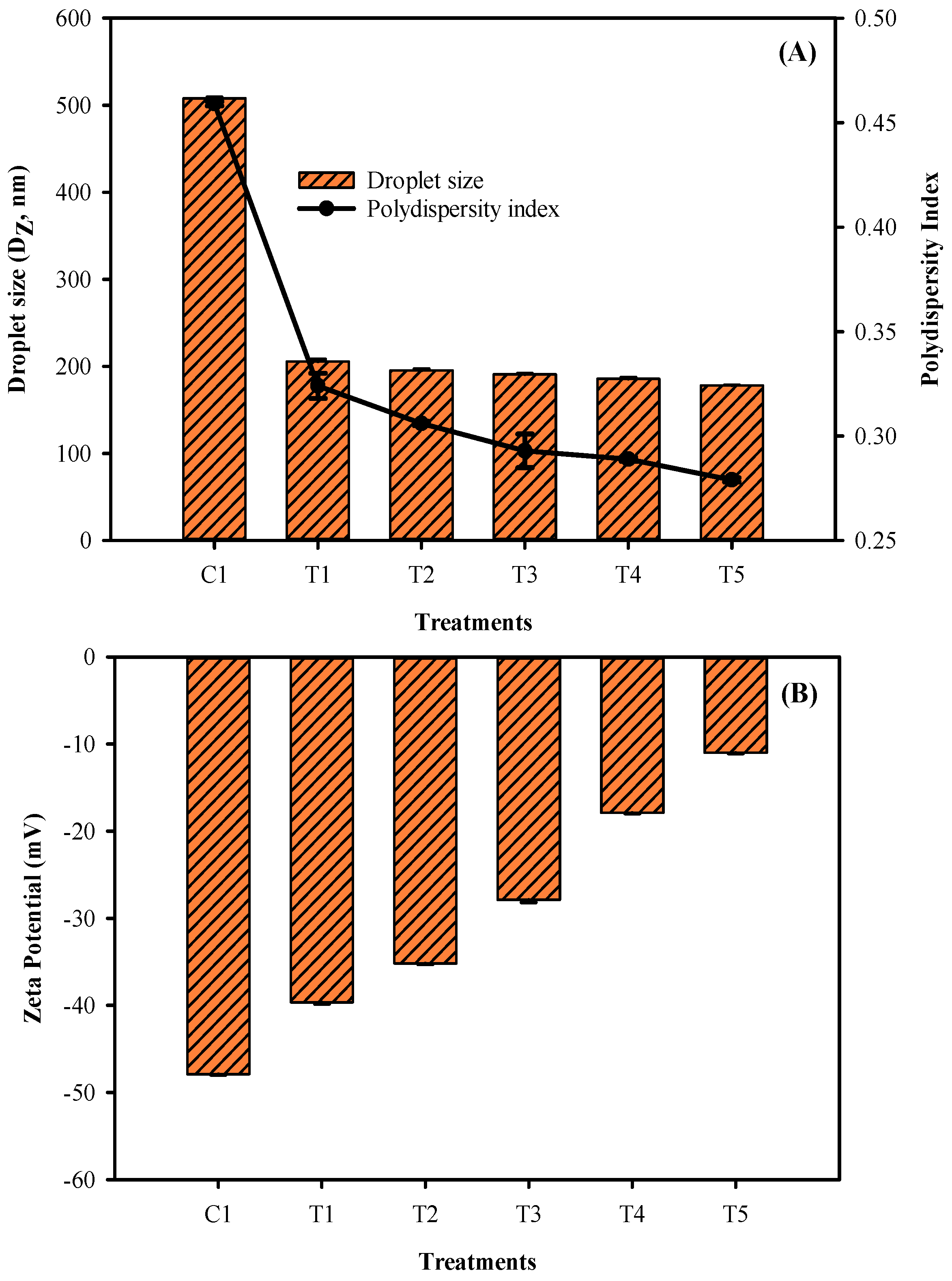
3.3. Film Droplet Size, Polydispersity Index, and Zeta Potential
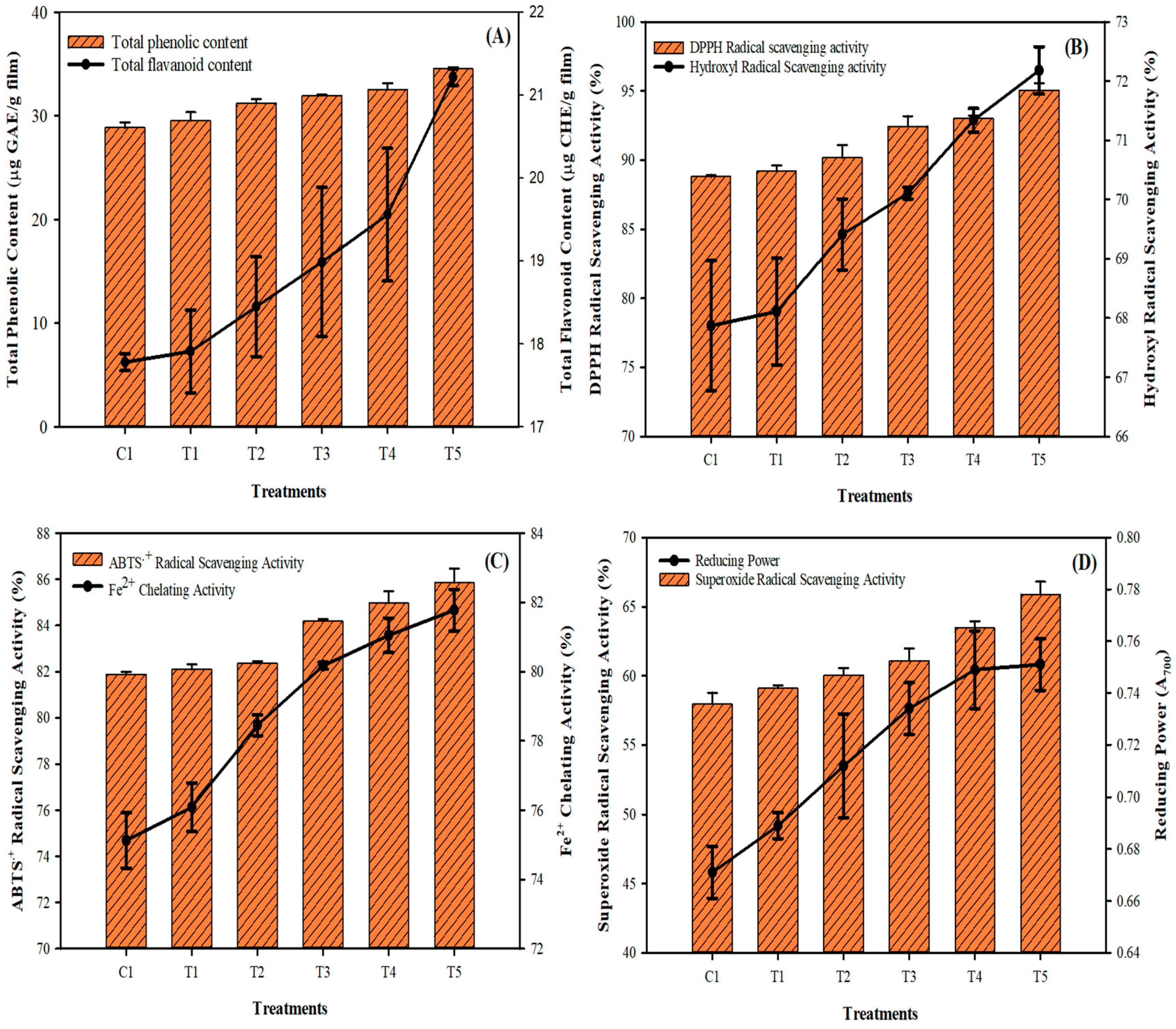
3.4. Phytochemicals and Antioxidant Activities
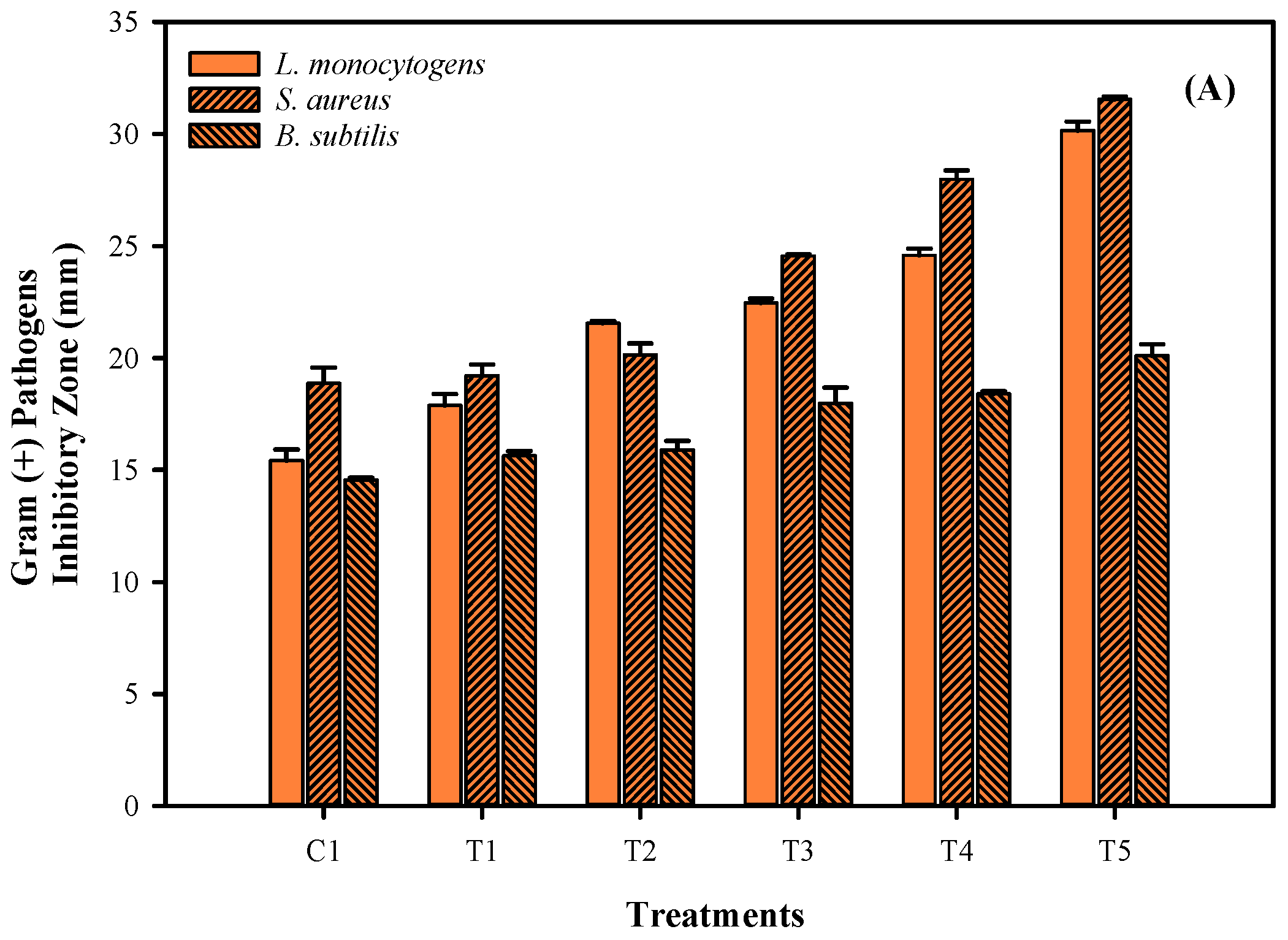
3.5. Antimicrobial Activities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joshi, M.; Timilsena, Y.; Adhikari, B. Global production, processing and utilization of lentil: A review. J. Integr. Agric. 2017, 16, 2898–2913. [Google Scholar] [CrossRef]
- Grela, E.R.; Kiczorowska, B.; Samolinska, W.; Matras, J.; Kiczorowski, P.; Rybinski, W.; Hanczakowska, E. Chemical composition of leguminous seeds: Part I—content of basic nutrients, amino acids, phytochemical compounds, and antioxidant activity. Eur. Food Res. Technol. 2017, 243, 1385–1395. [Google Scholar] [CrossRef]
- Dahiya, P.K.; Linnemann, A.R.; Van Boekel, M.A.J.S.; Khetarpaul, N.; Grewal, R.B.; Nout, M.J.R. Mung bean: Technological and nutritional potential. Crit. Rev. Food Sci. Nutr. 2015, 55, 670–688. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Yousaf, L.; Xue, Y.; Hu, J.; Wu, J.; Hu, X.; Feng, N.; Shen, Q. Mung bean (Vigna radiata L.): Bioactive polyphenols, polysaccharides, peptides and health benefits. Nutrients 2019, 11, 1238. [Google Scholar] [CrossRef] [Green Version]
- Edgar, V.; Fabian, F.; Mario, R.J.; Ileana, V. Coupling plant biomass derived from phytoremediation of potential toxic-metal-polluted soils to bioenergy production and high-value by-products—A review. Appl. Sci. 2021, 11, 2982. [Google Scholar] [CrossRef]
- Larotonda, F.D.S.; Torres, M.D.; Gocalves, M.P.; Sereno, A.M.; Hilliou, L. Hybrid carrageenan-based formulations for edible film preparation: Benchmarking with kappa carrageenan. J. Appl. Polym. Sci. 2016, 132, 1–10. [Google Scholar] [CrossRef]
- Irkin, R.; Esmer, O.K. Novel food packaging systems with natural antimicrobial agents. J. Food Sci. Technol. 2015, 52, 6095–6111. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Meenune, M. Changes in physiochemical quality and browning related enzyme activity of longkong fruit during four different weeks of on-tree maturation. Food Chem. 2012, 131, 1437–1442. [Google Scholar] [CrossRef]
- Venkatachalam, K. Bioactive compounds of longkong fruit (Lansium domesticum Corr.). In Bioactive Compounds in Underutilized Fruits and Nuts; Reference Series in Phytochemicals; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Nagarajan, M.; Rajasekaran, B.; Benjakul, S.; Venkatachalam, K. Influence of chitosan-gelatin edible coating incorporated with longkong pericarp extract on refrigerated black tiger shrimp (Penaeus monodon). Curr. Res. Food Sci. 2021, 4, 345–353. [Google Scholar] [CrossRef]
- Kang, X.; Liu, P.; Gao, W.; Wu, Z.; Yu, B.; Wang, R.; Cui, B.; Qiu, L.; Sun, C. Preparation of starch lipid complex by ultrasonication and its film forming capacity. Food Hydrocoll. 2020, 99, 105340. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, B.H.; Hatton, A.T.; Doyle, P.S. Controlling and predicting droplet size of nano emulsions: Scaling relations with experimental validation. Soft Mat. 2015, 12, 1452–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratap-Singh, A.; Guo, Y.; Ochoa, S.L.; Fathordoobady, F.; Singh, A. Optimal ultrasonication process time remains constant for a specific nano-emulsion size reduction system. Sci. Rep. 2021, 11, 9241. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, A.; Sumnu, G.; Sahin, S. A novel electrospun hydroxypropyl methylcellulose/polyethylene oxide blend nanofibers: Morphology and physicochemical properties. Carbohydr. Polym. 2018, 181, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Zielinski, F.A.A.; Zardo, M.D.; Demiate, M.I.; Nogueira, I.L. Optimization of the extraction of phenolic compounds from apples using response surface methodology. Food Chem. 2014, 149, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.; Song, K.B. Development of a chicken feet protein film containing essential oils. Food Hydrocoll. 2015, 46, 208–215. [Google Scholar] [CrossRef]
- Halliwell, N.; Guteridge, J.M.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constant for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Ahmed, J.; Hiremath, N.; Jacob, H. Antimicrobial, rheological, thermal properties of plasticized polylactide films incorporated with essential oils to inhibit staphylococcus aureus and campylobacter jejuni. J. Food Sci. 2016, 81, E419–E429. [Google Scholar] [CrossRef]
- Lyn, F.H.; Tan, C.P.; Zawawi, R.M.; Nur Hanani, Z.A. Effect of sonication time and heat treatment on the structural and physical properties of chitosan/graphene oxide nanocomposite films. Food Packag. Shelf Life 2021, 28, 100663. [Google Scholar]
- Gomes, L.P.; Souza, H.K.S.; Campina, J.M.; Andrade, C.T.; Silva, A.E.; Goncalves, M.P.; Paschoalin, V.M.F. Edible chitosan films and their nanosized counterparts exhibit antimicrobial activity and enhanced mechanical and barrier properties. Molecules 2019, 24, 127. [Google Scholar] [CrossRef] [Green Version]
- Tavares, L.; Souza, H.K.S.; Goncalves, M.P.; Rocha, C.M.R. Physicochemical and microstructural properties of composite edible film obtained by complex coacervation between chitosan and whey protein isolate. Food Hydrocoll. 2021, 113, 106471. [Google Scholar] [CrossRef]
- Beikzadeh, S.; Ghorbani, M.; Shahbazi, N.; Izadi, F.; Pilevar, Z.; Mortazavian, A.M. The effect of novel thermal and nonthermal technologies on the properties of edible food packaging. Food Eng. Rev. 2020, 12, 333–345. [Google Scholar] [CrossRef]
- Ji, T.; Zhang, R.; Dong, X.; Sameen, D.E.; Ahmed, S.; Li, S.; Liu, Y. Effect of ultrasonication time on the properties of polyvinyl alcohol sodium carboxymethyl cellulose/nano-ZnO/ multilayer graphene nanoplatelet composite films. Nanomaterials 2020, 10, 1797. [Google Scholar] [CrossRef]
- Guo, Z.; Ge, X.; Yang, L.; Gou, Q.; Han, L.; Yu, Q. Utilization of watermelon peel as a pectin source and the effect of ultrasound treatment on pectin film properties. LWT Food Sci. Technol. 2021, 147, 111569. [Google Scholar] [CrossRef]
- Arcan, I.; Yemenicioglu, A. Incorporating phenolic compounds opens a new perspective to use zein films as flexible bioactive packaging materials. Food Res. Int. 2011, 44, 550–556. [Google Scholar] [CrossRef] [Green Version]
- Mangmee, K.; Homthawornchoo, W. Antioxidant activity and physicochemical properties of rice starch chitosan-based films containing green tea extract. Food Appl. Biosci. J. 2016, 4, 126–137. [Google Scholar]
- Wang, L.; Ding, J.; Fang, Y.; Pan, X.; Fan, F.; Li, P.; Hu, Q. Effect of ultrasonic power on properties of edible composite films based on rice protein hydrolysates and chitosan. Ultrason. Sonochem. 2020, 65, 105049. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, J.; Liu, D.; Ye, X.; Ke, F. Impact of ultrasonic treatment on properties of starch film-forming dispersion and the resulting films. Carbohydr. Polym. 2010, 81, 701–711. [Google Scholar] [CrossRef]
- Kurt, A.; Kahyaoglu, T. Characterization of a new biodegradable edible film made from salep glucomannan. Carbohydr. Polym. 2014, 104, 50–58. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Lorena Ramos-García, M.; del Carmen Martínez-González, M.; Hernández-Romano, J. Physicochemical characterization and antimicrobial activity of edible propolis-chitosan nanoparticle films. Prog. Org. Coat. 2019, 137, 105326. [Google Scholar] [CrossRef]
- Mustafa, P.; Niazi, M.B.; Jahan, Z.; Samin, G.; Hussain, A.; Ahmed, T.; Naqvi, S.R. Starch/propolis/anthocyanins rosemary extract composite films as active and intelligent food packaging materials. J. Food Saf. 2020, 40, e12725. [Google Scholar] [CrossRef]
- Yin, L.J.; Chu, B.S.; Kobayashi, I.; Nakajima, M. Performance of selected emulsifiers and their combinations in the preparation of β-carotene nano dispersions. Food Hydrocoll. 2009, 23, 1617–1622. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Chen, J.C.; Liu, D.H.; Ye, X.Q. effect of ultrasonic treatment on the total phenolic and antioxidant activity of extracts from citrus peel. J. Food Sci. 2008, 73, T115–T120. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Marquez, D.B.; Martinez-Avila, G.C.; Wong-Paa, J.E.; Belmares-Cerda, R.; Rodriguez-Herrera, R.; Aguilar, C.N. Ultrasound-assisted extraction of phenolic compounds from Laurus nobilis L. and their antioxidant activity. Ultrason. Sonochem. 2013, 20, 1149–1154. [Google Scholar] [CrossRef]
- Karabegovic, I.T.; Veljkovic, V.B.; Lazic, M.L. Ultrasound-assisted extraction of total phenols and flavonoids from dry tobacco (Nicotiana tabacum) leaves. Nat. Prod. Commun. 2011, 6, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.; Li, H.; Yi, J.; Zhang, J.; Che, H.; Cao, J.; Yang, L.; Zhu, C.; Jiang, W. Antioxidant properties of the mung bean flavonoids on alleviating heat stress. PLoS ONE 2011, 6, e21071. [Google Scholar] [CrossRef] [Green Version]
- Aparicio-Fernandez, X.; Vega-Ahuatzin, A.; Ochoa-Velasco, C.E.; Cid-Perez, S.; Hernandez-Carranza, P.; Avila-Sosa, R. Physical and antioxidant characterization of edible films added with red prickly pear (Opuntia ficus-Indica L.) cv. San Martin peel and/or its aqueous extracts. Food Bioprocess Technol. 2017, 11, 368–379. [Google Scholar] [CrossRef]
- Gul, O.; Saricaoglu, F.T.; Besir, A.; Atalar, I.; Yazici, F. Effect of ultrasound treatment on the properties of nano-emulsion films obtained from hazelnut meal protein and clove essential oil. Ultrason. Sonochem. 2018, 41, 466–474. [Google Scholar] [CrossRef]
- Hamed, S.F.; Sadek, Z.; Edris, A. Antioxidant and antimicrobial activities of clove bud essential oil and eugenol nanoparticles in alcohol-free microemulsion. J. Oleo Sci. 2012, 61, 641–648. [Google Scholar] [CrossRef]
- Randhir, R.; Lin, Y.T.; Shetty, K. Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Proc. Biochem. 2004, 39, 637–646. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, S.K.; Dawoud, M.T.; Sholkamy, N.E.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Meenune, M. Effect of methyl jasmonate on physiological and biochemical quality changes of longkong fruit under low temperature storage. Fruits 2015, 70, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Alzagameem, A.; Klein, E.S.; Bergs, M.; Bergs, M.; Do, T.X.; Korte, I.; Dohlen, S.; Huwe, C.; Kreyenschmidt, J.; Kamm, B.; et al. Antimicrobial activity of lignin and lignin-derived cellulose and chitosan composites against selected pathogenic and spoilage microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef] [Green Version]
- Sukatta, U.; Rugthaworn, P.; Khanoonkon, N.; Anongjanya, P.; Kongsin, K.; Sukyai, P.; Harnkarnsujarit, N.; Sothornvit, R.; Chollakup, R. Rambutan (Nephelium lappaceum) peel extract: Antimicrobial and antioxidant activities and its application as a bioactive compound in whey protein isolate film. Songklanakarin J. Sci. Technol. 2021, 43, 37–44. [Google Scholar]
- Wang, D.; Lv, R.; Ma, X.; Zou, M.; Wang, W.; Yan, L.; Ding, T.; Ye, X.; Liu, D. Lysozyme immobilization on the calcium alginate film under sonication: Development of an antimicrobial film. Food Hydrocoll. 2018, 83, 1–8. [Google Scholar] [CrossRef]
- Ma, X.; Dong, Y.; Sun, H.; Chen, N. Highly fluorescent carbon dots from peanut shells as potential probes for copper ion: The optimization and analysis of the synthetic process. Mater. Chem. 2017, 5, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Development of ultrasound treated polyvinyl alcohol/tea polyphenol composite films and their physicochemical properties. Ultrason. Sonochem. 2019, 51, 386–394. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keawpeng, I.; Paulraj, B.; Venkatachalam, K. Antioxidant and Antimicrobial Properties of Mung Bean Phyto-Film Combined with Longkong Pericarp Extract and Sonication. Membranes 2022, 12, 379. https://doi.org/10.3390/membranes12040379
Keawpeng I, Paulraj B, Venkatachalam K. Antioxidant and Antimicrobial Properties of Mung Bean Phyto-Film Combined with Longkong Pericarp Extract and Sonication. Membranes. 2022; 12(4):379. https://doi.org/10.3390/membranes12040379
Chicago/Turabian StyleKeawpeng, Ittiporn, Balaji Paulraj, and Karthikeyan Venkatachalam. 2022. "Antioxidant and Antimicrobial Properties of Mung Bean Phyto-Film Combined with Longkong Pericarp Extract and Sonication" Membranes 12, no. 4: 379. https://doi.org/10.3390/membranes12040379
APA StyleKeawpeng, I., Paulraj, B., & Venkatachalam, K. (2022). Antioxidant and Antimicrobial Properties of Mung Bean Phyto-Film Combined with Longkong Pericarp Extract and Sonication. Membranes, 12(4), 379. https://doi.org/10.3390/membranes12040379





