In Vivo Biocompatibility Analysis of a Novel Barrier Membrane Based on Bovine Dermis-Derived Collagen for Guided Bone Regeneration (GBR)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Native Dermis-Derived Bovine Membrane
2.1.2. Native Dermis-Derived Porcine Membrane
2.1.3. Native Pericardium-Derived Porcine Membrane
2.2. In Vivo Experimental Procedure
2.2.1. Experiment Design
2.2.2. Animal Husbandry
2.2.3. Subcutaneous Implantation Model According to DIN EN ISO 10993-6
2.2.4. Explantation and Fixation
2.3. Histology and Immunohistochemistry
2.3.1. Histopathological Analysis
2.3.2. Histopathological Biomaterial Scoring
2.3.3. Histomorphometrical Analysis
2.4. Statistical Analysis
3. Results
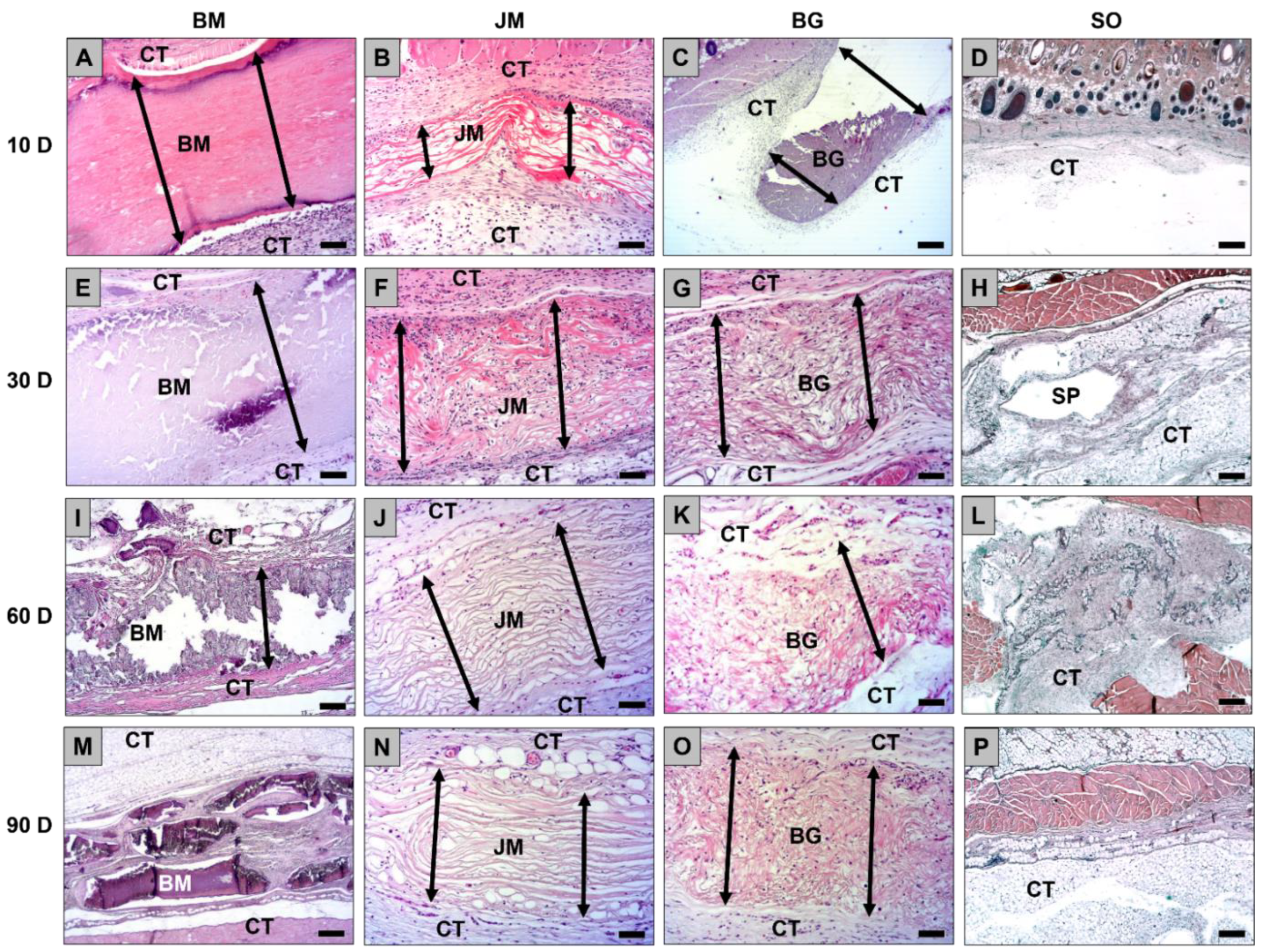
3.1. Histopathology Evaluation
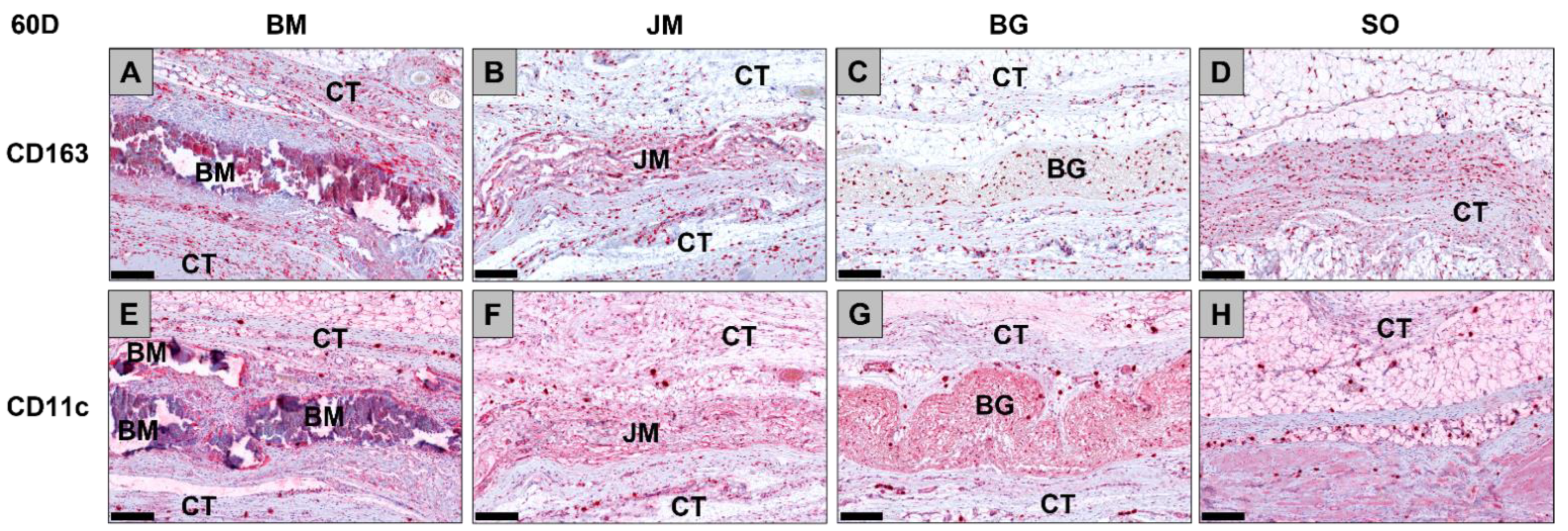
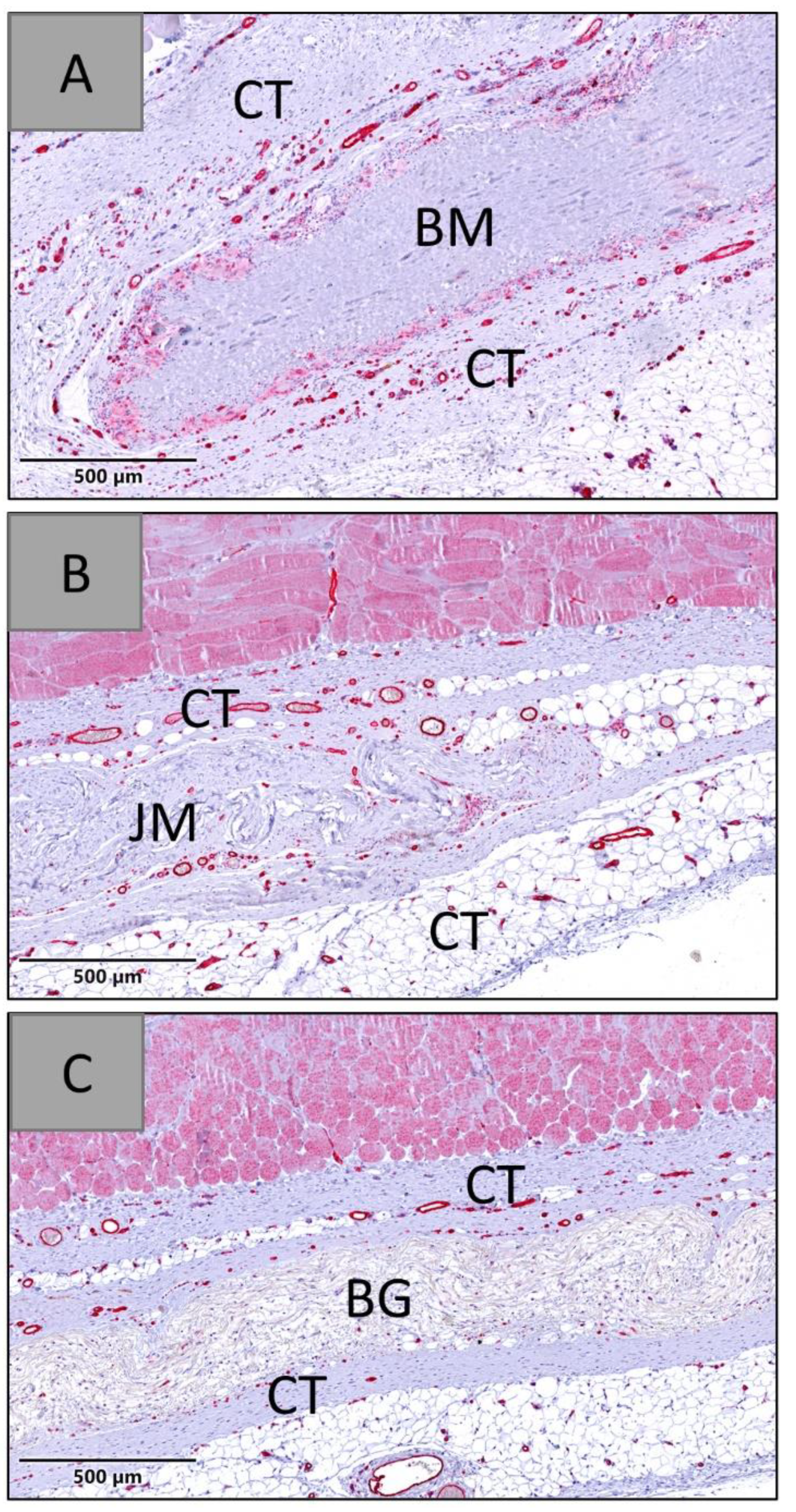
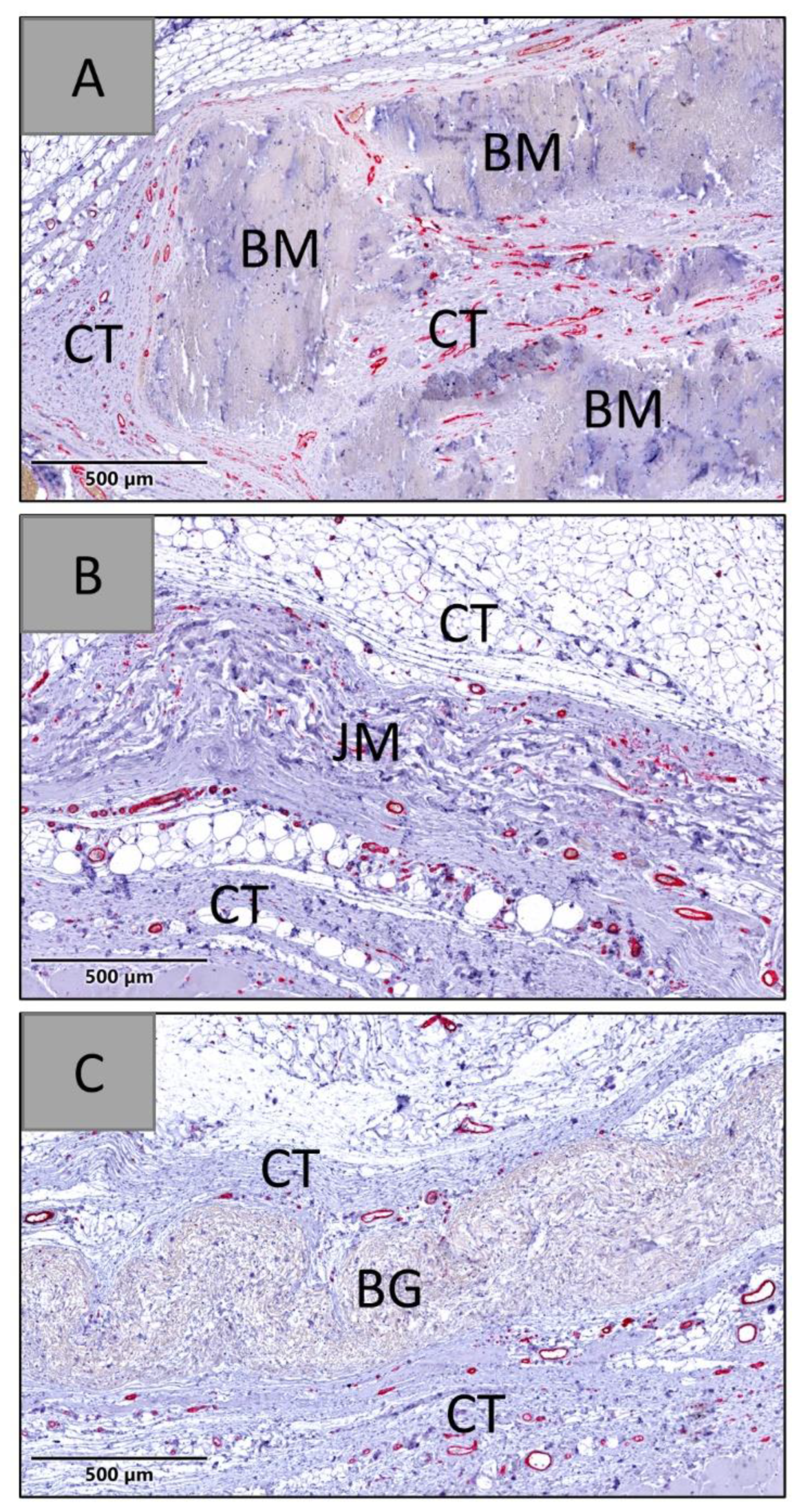
3.1.1. Tissue Response/Inflammation and Tissue Integration
3.1.2. Biomaterial Scoring Results and Irritancy Score Calculation
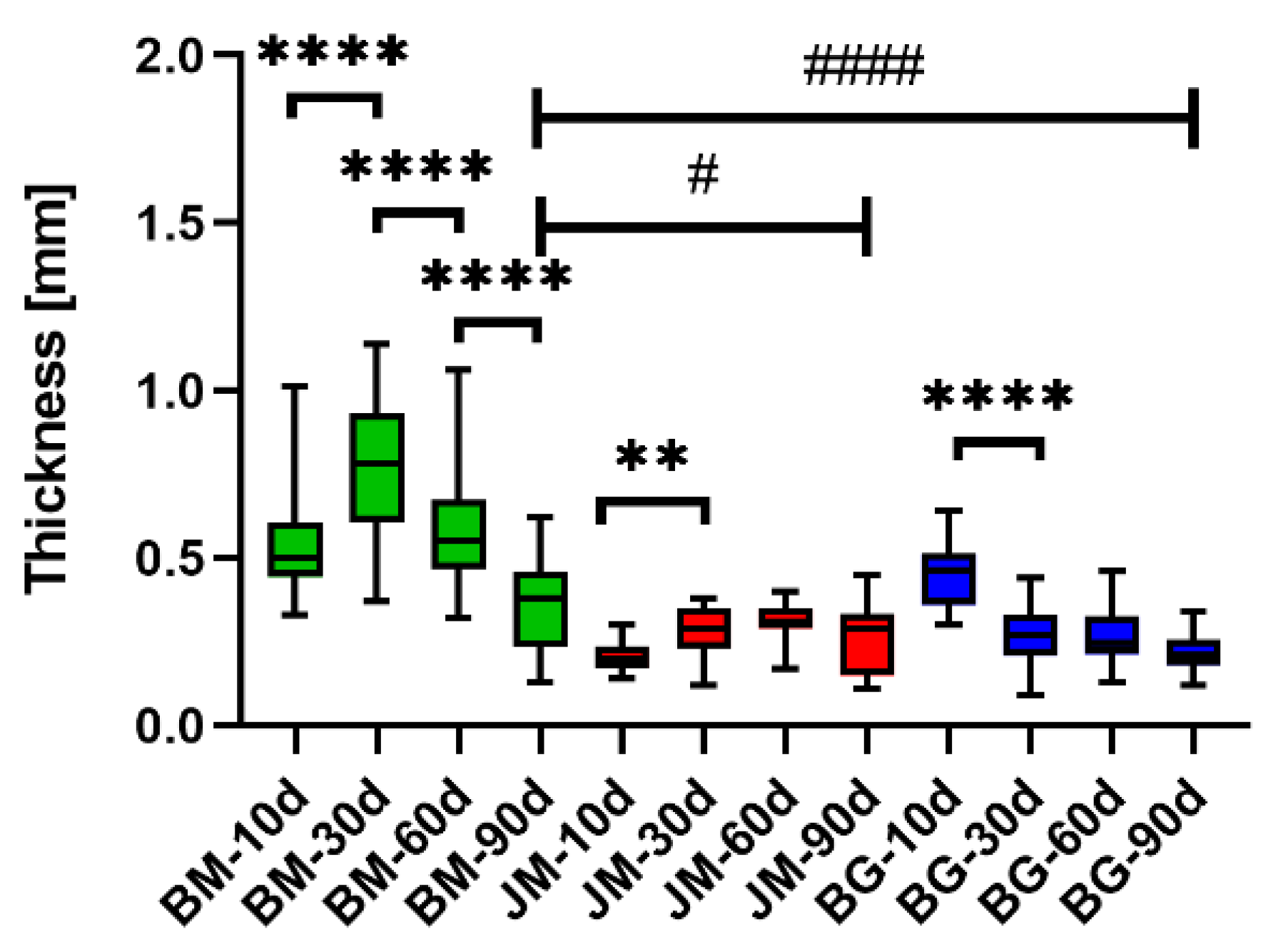
3.2. Histomorphometrical Results
3.2.1. Thickness Measurements
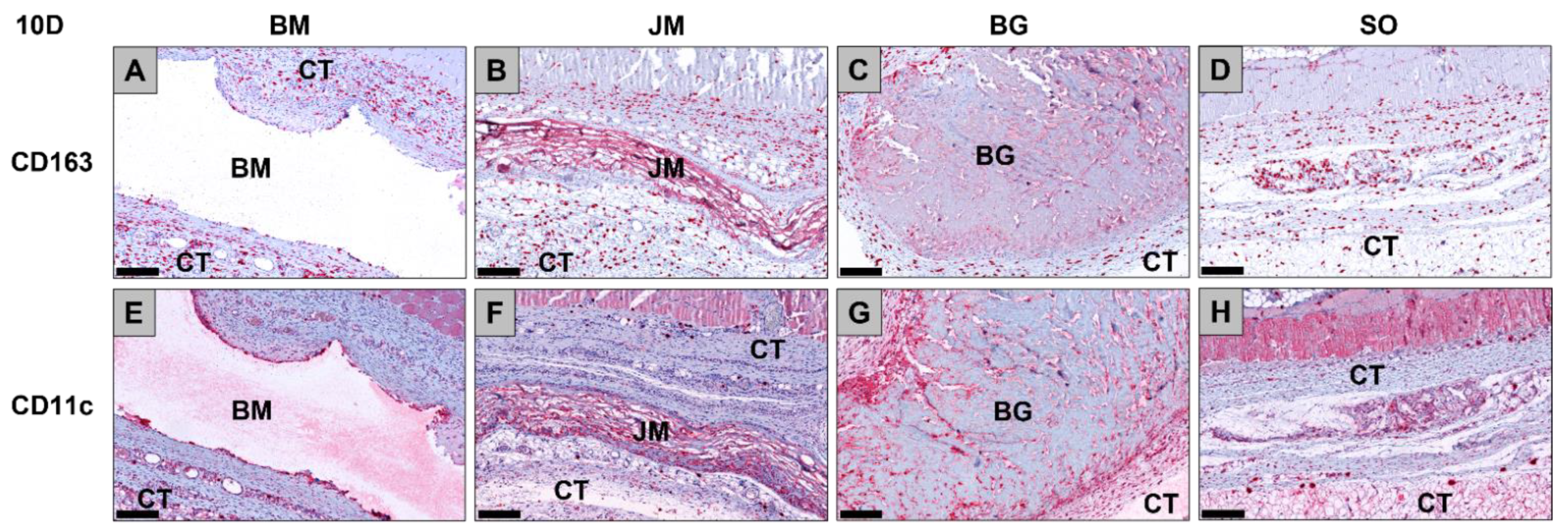
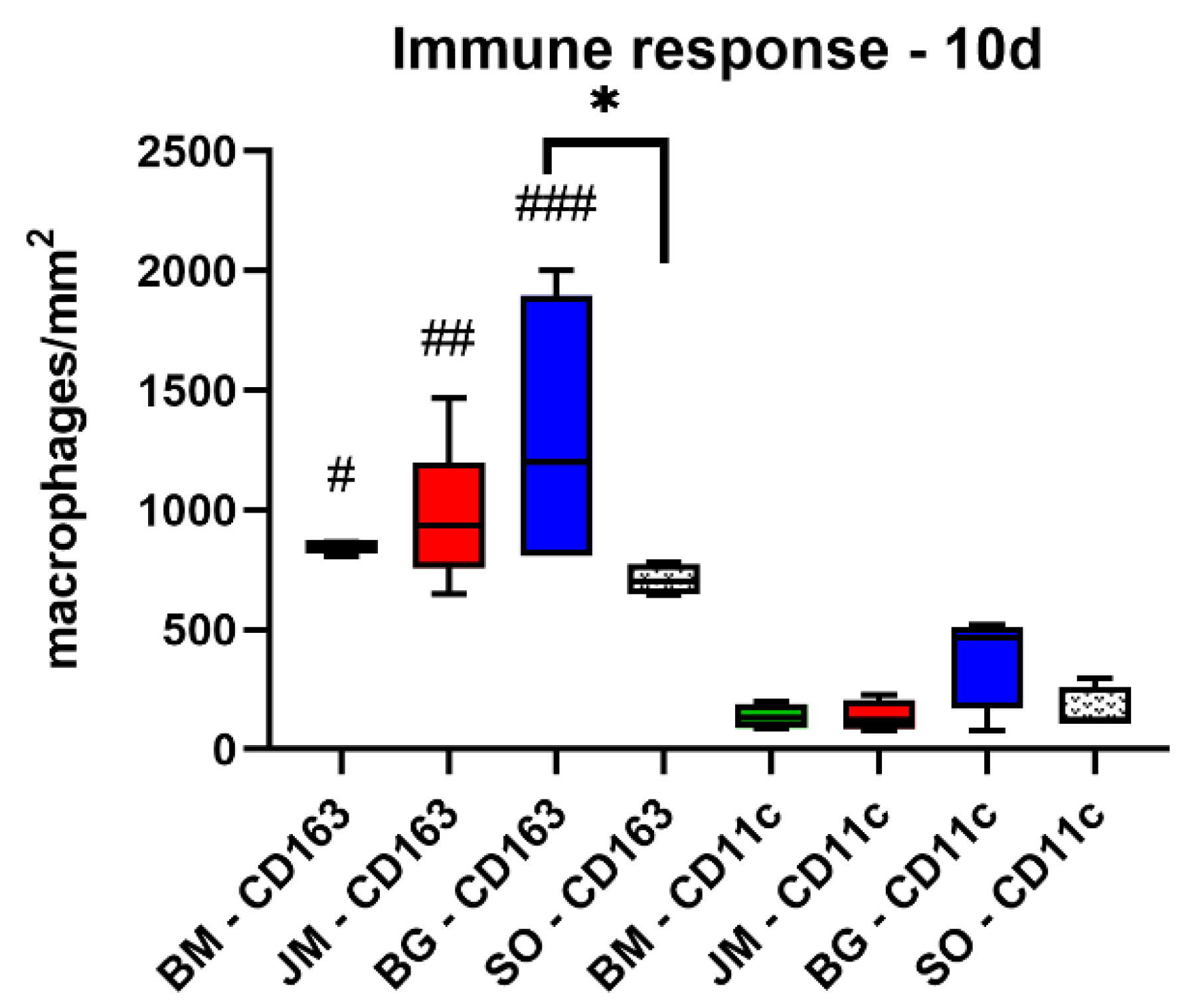
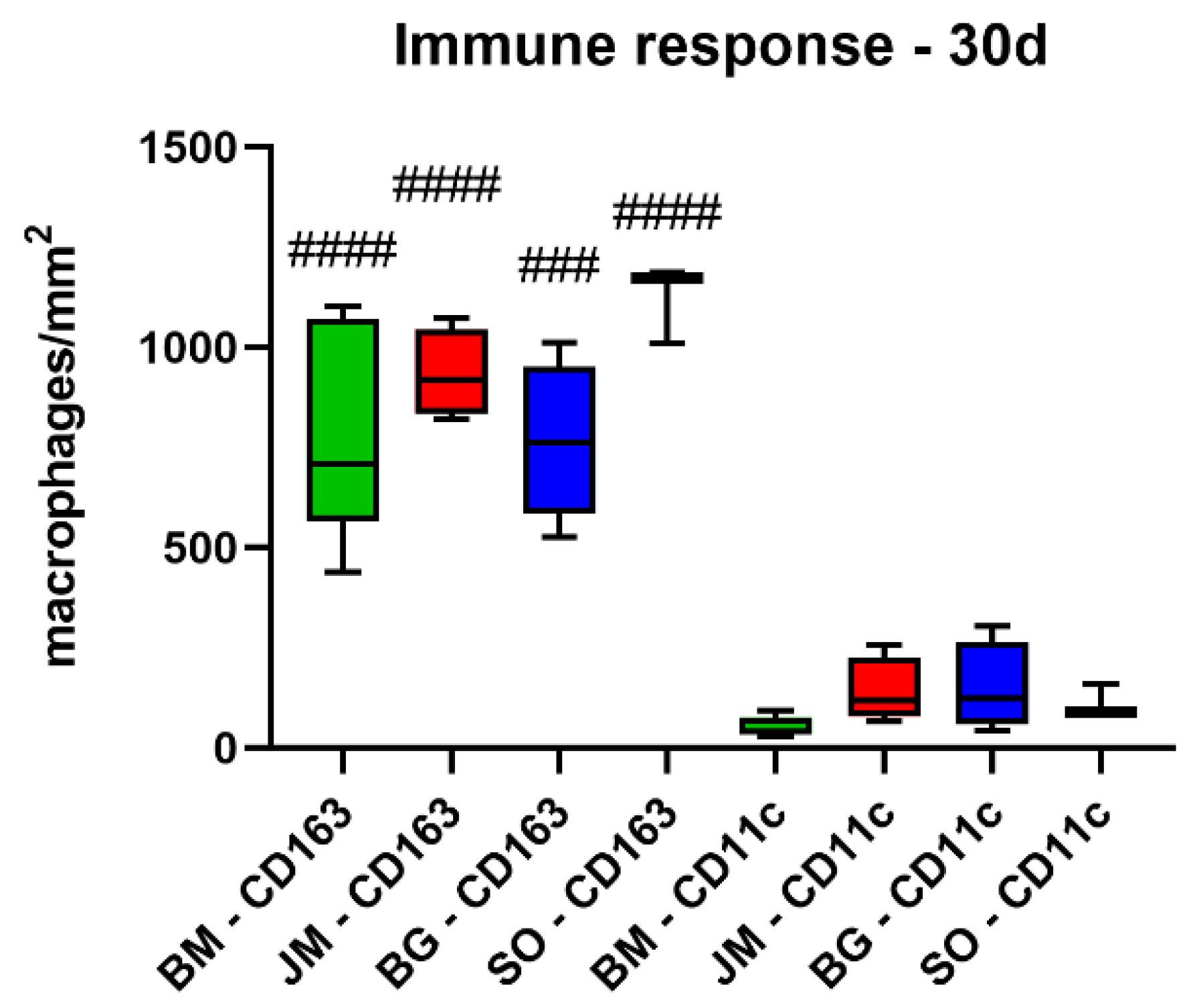
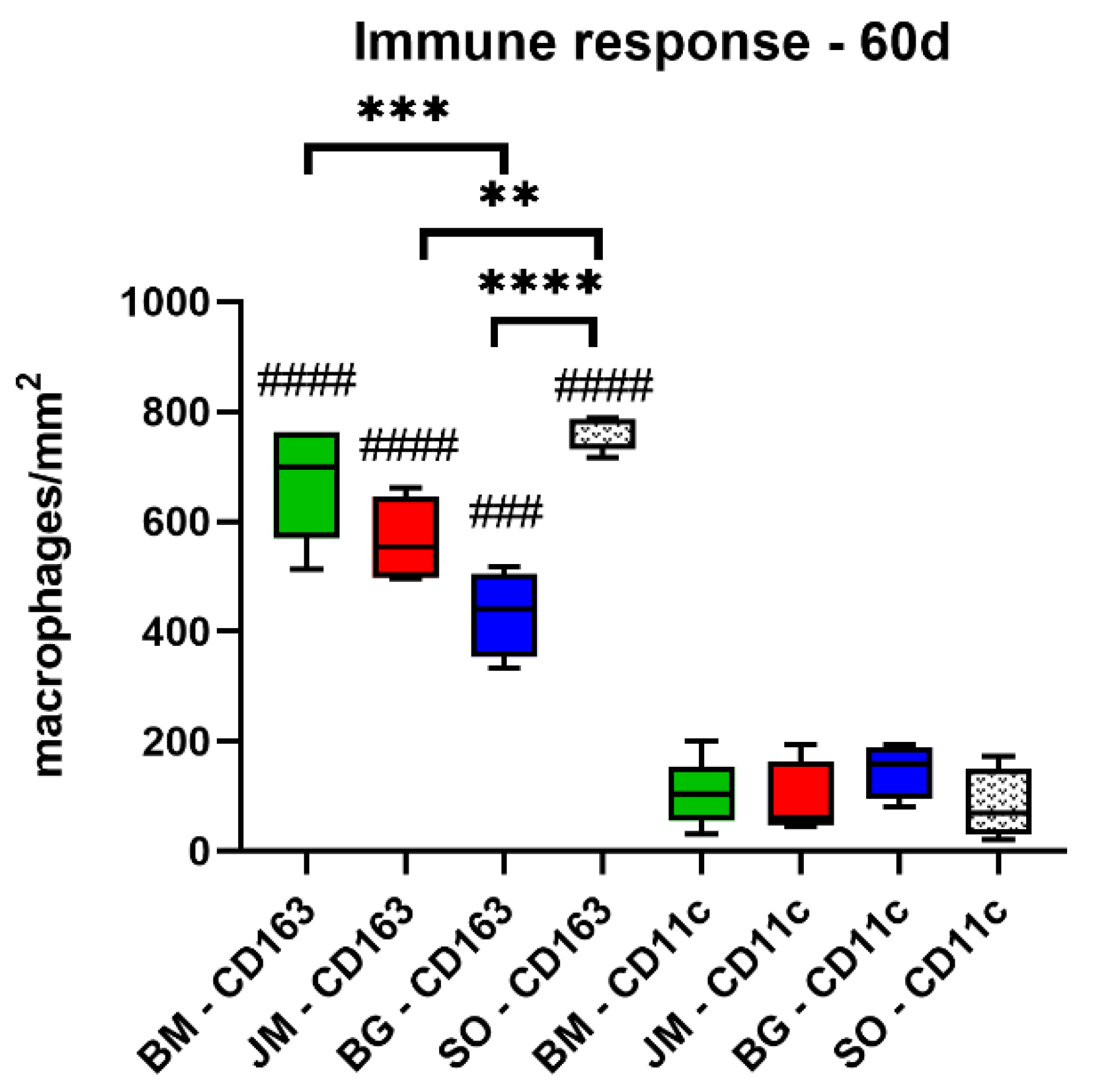
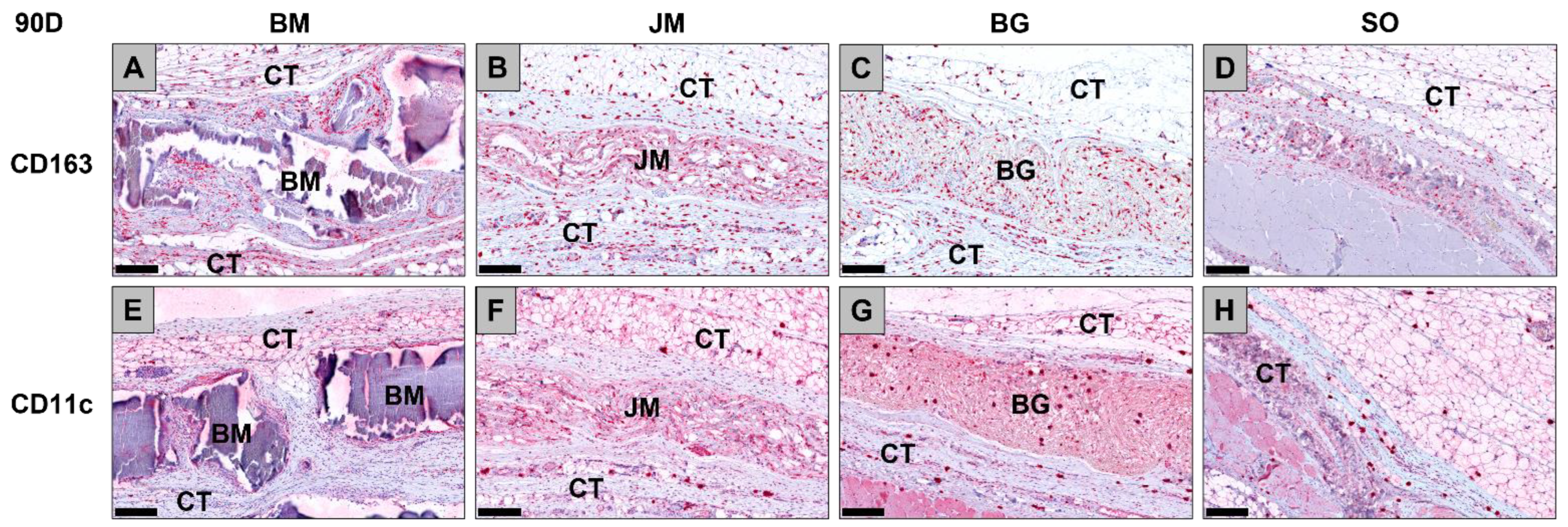
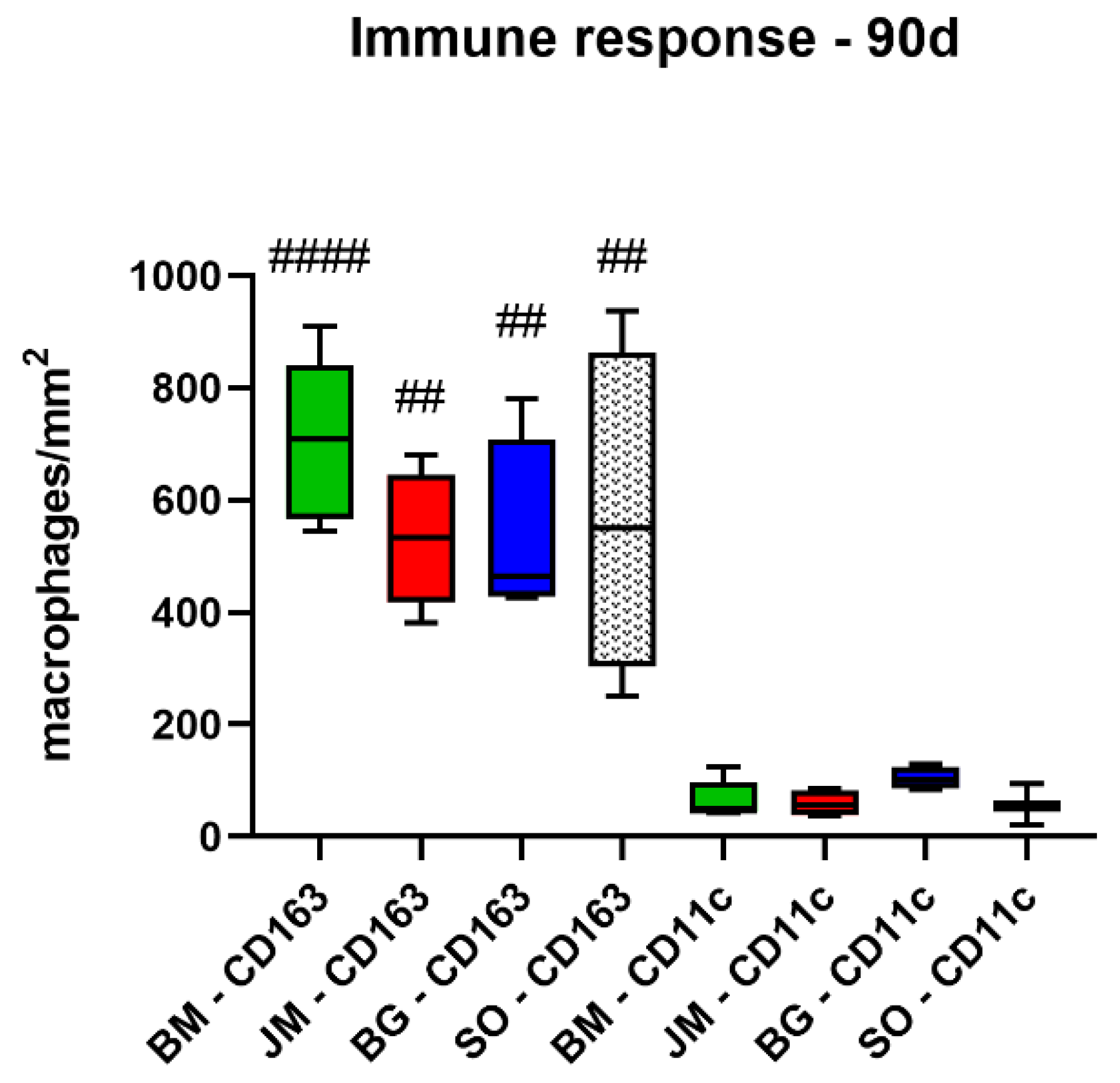
3.2.2. Immune Response Measurements
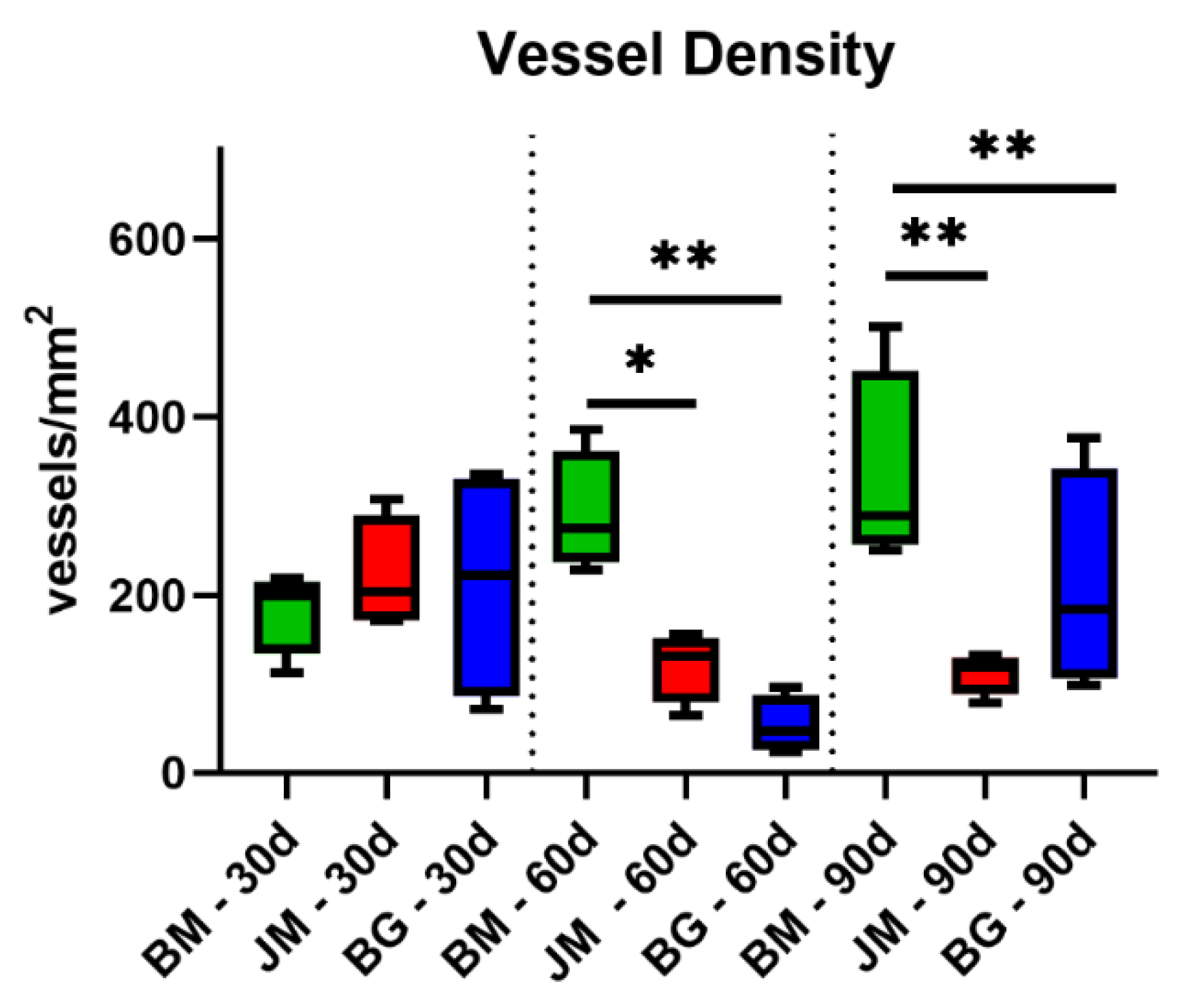
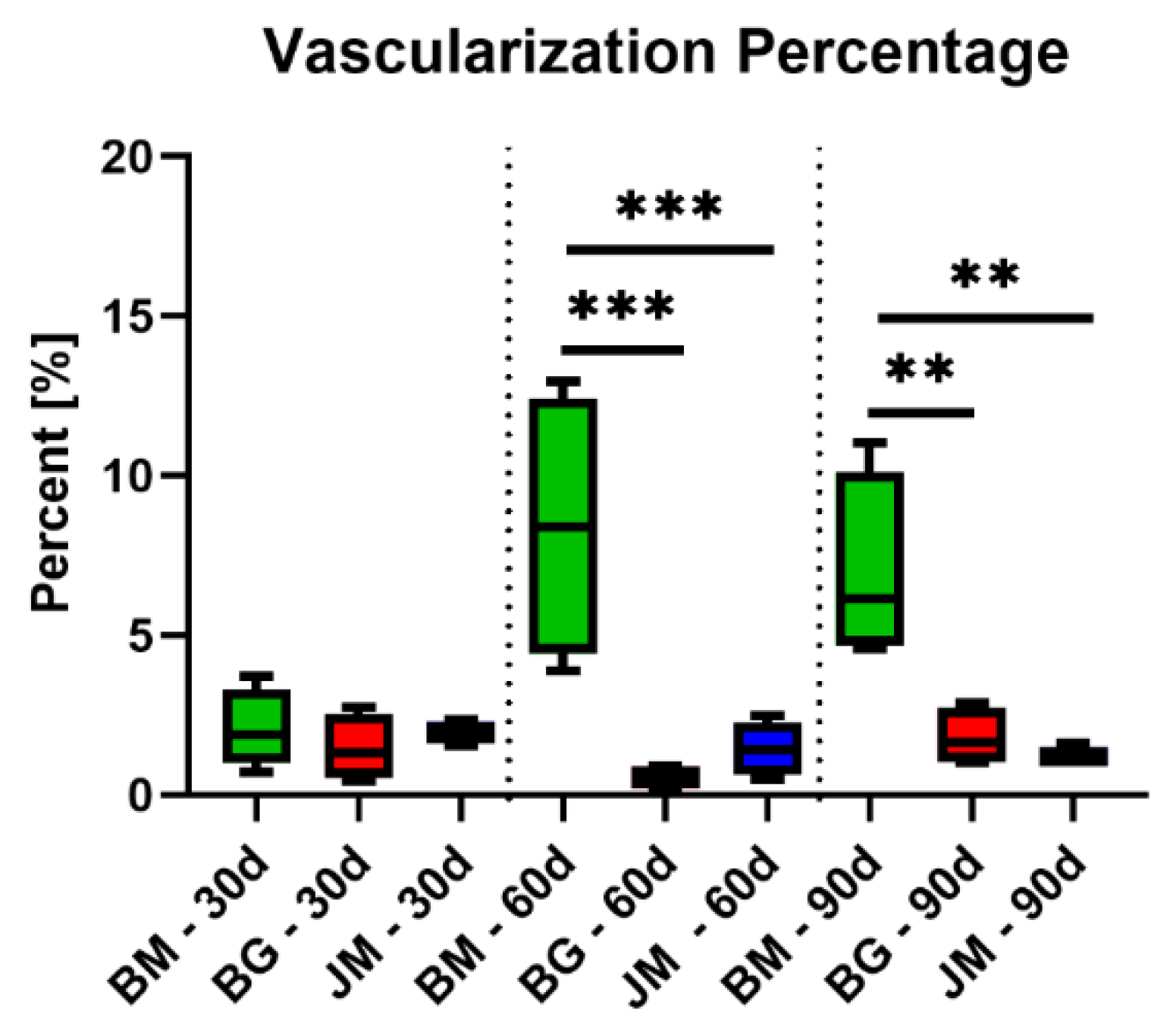
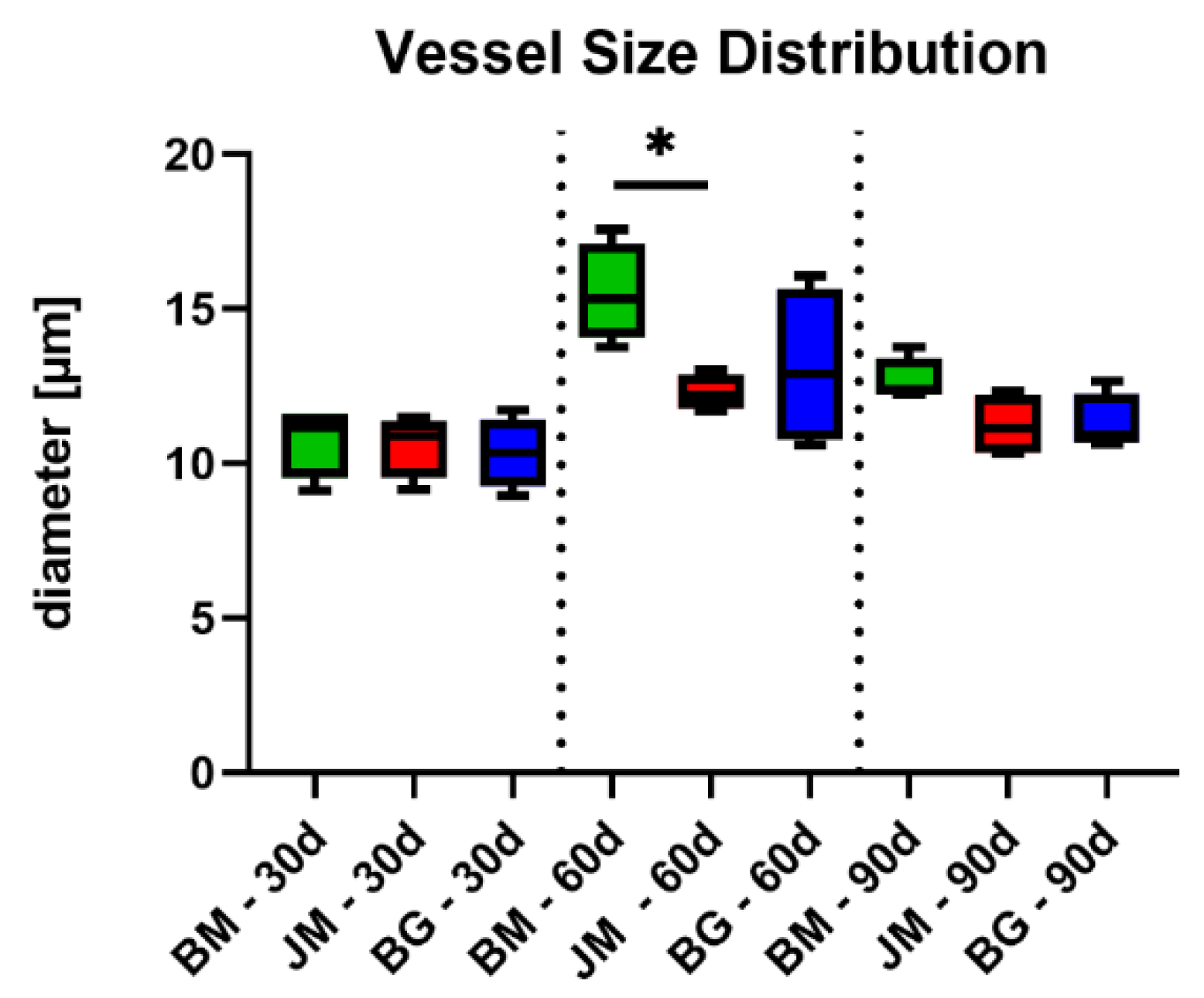
3.2.3. Vascularization Measurements
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ha, S.W.; Wintermantel, E.; Maier, G. Polymere. In Medizintechnik Life Science Engineering; Wintermantel, E., Ha, S.-W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 211–268. [Google Scholar]
- Bunyaratavej, P.; Wang, H.-L. Collagen membranes: A review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beretta, M.; Cicciù, M.; Poli, P.P.; Rancitelli, D.; Bassi, G.; Grossi, G.B.; Maiorana, C. A Retrospective Evaluation of 192 Implants Placed in Augmented Bone: Long-Term Follow-Up Study. J. Oral Implant. 2015, 41, 669–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Corbella, S.; Taschieri, S.; Elkabbany, A.; Del Fabbro, M.; Von Arx, T. Guided tissue regeneration using a barrier membrane in endodontic surgery. Swiss Dent. J. 2016, 126, 13–25. [Google Scholar]
- Ghanaati, S. Non-cross-linked porcine-based collagen i-iii membranes do not require high vascularization rates for their integration within the implantation bed: A paradigm shift. Acta Biomater. 2012, 8, 3061–3072. [Google Scholar] [CrossRef]
- Tanneberger, A.M.; Al-Maawi, S.; Herrera-Vizcaino, C.; Orlowska, A.; Kubesch, A.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated giant cells within the in vivo implantation bed of a collagen-based biomaterial determine its degradation pattern. Clin. Oral Investig. 2020, 25, 859–873. [Google Scholar] [CrossRef]
- Moses, O.; Kozlovsky, A.; Nemcovsky, C. Bioresorbable Collagen Membranes for Guided Bone Regeneration. J. Periodontol. 2012, 78, 1943. [Google Scholar] [CrossRef] [Green Version]
- Barbeck, M.; Lorenz, J.; Kubesch, A.; Böhm, N.; Booms, P.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Porcine Dermis-Derived Collagen Membranes Induce Implantation Bed Vascularization Via Multinucleated Giant Cells: A Physiological Reaction? J. Oral Implant. 2015, 41, e238–e251. [Google Scholar] [CrossRef]
- Gueldenpfennig, T.; Houshmand, A.; Najman, S.; Stojanovic, S.; Korzinskas, T.; Smeets, R.; Gosau, M.; Pissarek, J.; Emmert, S.; Jung, O.; et al. The Condensation of Collagen Leads to an Extended Standing Time and a Decreased Pro-inflammatory Tissue Response to a Newly Developed Pericardium-based Barrier Membrane for Guided Bone Regeneration. In Vivo 2020, 34, 985–1000. [Google Scholar] [CrossRef]
- McAllister, B.S.; Haghighat, K. Bone Augmentation Techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef] [Green Version]
- Caballe-Serrano, J.; Munar-Frau, A.; Ortiz-Puigpelat, O.; Soto-Penaloza, D.; Penarrocha, M.; Hernandez-Alfaro, F. On the search of the ideal barrier membrane for guided bone regeneration. J. Clin. Exp. Dent. 2018, 10, e477–e483. [Google Scholar] [CrossRef] [PubMed]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46 (Suppl. S21), 103–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dau, M.; Volprich, L.; Grambow, E.; Vollmar, B.; Frerich, B.; Al-Nawas, B.; Kämmerer, P.W. Collagen membranes of dermal and pericardial origin—In vivo evolvement of vascularization over time. J. Biomed. Mater. Res. Part A 2020, 108, 2368–2378. [Google Scholar] [CrossRef] [PubMed]
- Kapogianni, E.; Alkildani, S.; Radenkovic, M.; Xiong, X.; Krastev, R.; Stöwe, I.; Bielenstein, J.; Jung, O.; Najman, S.; Barbeck, M.; et al. The Early Fragmentation of a Bovine Dermis-Derived Collagen Barrier Membrane Contributes to Transmembraneous Vascularization—A Possible Paradigm Shift for Guided Bone Regeneration. Membranes 2021, 11, 185. [Google Scholar] [CrossRef]
- Sowjanya, N.P. Versitality of the Use of Collagen Membrane in Oral Cavity. J. Clin. Diagn. Res. 2016, 10, ZC30–ZC33. [Google Scholar] [CrossRef]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. To Cross-Link or Not to Cross-Link? Cross-Linking Associated Foreign Body Response of Collagen-Based Devices. Tissue Eng. Part B Rev. 2015, 21, 298–313. [Google Scholar] [CrossRef] [Green Version]
- Barbeck, M.; Lorenz, J.; Holthaus, M.G.; Raetscho, N.; Kubesch, A.; Booms, P.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Porcine Dermis and Pericardium-Based, Non–Cross-Linked Materials Induce Multinucleated Giant Cells After Their In Vivo Implantation: A Physiological Reaction? J. Oral Implant. 2015, 41, e267–e281. [Google Scholar] [CrossRef]
- Moura, C.C.G.; Soares, P.B.F.; Carneiro, K.F.; De Souza, M.A.; Magalhães, D. Cytotoxicity of bovine and porcine collagen membranes in mononuclear cells. Braz. Dent. J. 2012, 23, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Petrova, A.; Westphal, G.; Valbuena, R.; Ringe, J. Struktur und Technofunktionelle Eigenschaften von Kollagenen Tierischen Ursprungs. 2011. Available online: https://www.leibniz-institut.de/archiv/petrova_13_09_11.pdf (accessed on 13 March 2022).
- Behring, J.; Junker, R.; Walboomers, X.F.; Chessnut, B.; Jansen, J.A. Toward guided tissue and bone regeneration: Morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review. Odontology 2008, 96, 1–11. [Google Scholar] [CrossRef]
- Rothamel, D.; Schwarz, F.; Fienitz, T.; Smeets, R.; Dreiseidler, T.; Ritter, L.; Happe, A.; Zöller, J. Biocompatibility and biodegradation of a native porcine pericardium membrane: Results of in vitro and in vivo examinations. Int. J. Oral Maxillofac. Implant. 2012, 27, 146–154. [Google Scholar]
- Willershausen, I.; Barbeck, M.; Boehm, N.; Sader, R.; Willershausen, B.; Kirkpatrick, C.J.; Ghanaati, S. Non-cross-linked collagen type i/iii materials enhance cell proliferation: In vitro and in vivo evidence. J. Appl. Oral Sci. Rev. FOB 2014, 22, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; E London, R.; Guenat, C.; Hashimoto, F.; Mechanic, G.L. Structure and formation of a stable histidine-based trifunctional cross-link in skin collagen. J. Biol. Chem. 1987, 262, 11428–11434. [Google Scholar] [CrossRef]
- Omar, O.; Dahlin, A.; Gasser, A.; Dahlin, C. Tissue dynamics and regenerative outcome in two resorbable non-cross-linked collagen membranes for guided bone regeneration: A preclinical molecular and histological study in vivo. Clin. Oral Implant. Res. 2017, 29, 7–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef] [Green Version]
- Pröhl, A.; Batinic, M.; Alkildani, S.; Hahn, M.; Radenkovic, M.; Najman, S.; Jung, O.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Bone Healing Capacity of a Novel Bone Grafting Material Combined with Hyaluronic Acid. Int. J. Mol. Sci. 2021, 22, 4818. [Google Scholar] [CrossRef]
- DIN EN ISO 10993-6; Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects after Implantation (ISO 10993-6:2016). German Version of EN ISO 10993-6:2016; International Organisation for Standardization: Geneva, Switzerland, 2016.
- Radenković, M.; Alkildani, S.; Stoewe, I.; Bielenstein, J.; Sundag, B.; Bellmann, O.; Jung, O.; Najman, S.; Stojanović, S.; Barbeck, M. Comparative In Vivo Analysis of the Integration Behavior and Immune Response of Collagen-Based Dental Barrier Membranes for Guided Bone Regeneration (GBR). Membranes 2021, 11, 712. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, X.; Sun, L.; She, Z.; Tan, R.; Wang, W. Immune response of bovine sourced cross-linked collagen sponge for hemostasis. J. Biomater. Appl. 2017, 32, 920–931. [Google Scholar] [CrossRef]
- Barbeck, M.; Dard, M.; Kokkinopoulou, M.; Markl, J.; Booms, P.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Small-sized granules of biphasic bone substitutes support fast implant bed vascularization. Biomatter 2015, 5, e1056943. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; He, Y.; Yu, X.; Fu, W.; Wang, W.; Huang, H. Rapid vascularization of tissue-engineered vascular grafts in vivo by endothelial cells in co-culture with smooth muscle cells. J. Mater. Sci. Mater. Electron. 2012, 23, 1109–1117. [Google Scholar] [CrossRef]
- Barbeck, M.; Najman, S.; Stojanovic, S.; Mitić, Z.; Živković, J.M.; Choukroun, J.; Kovačević, P.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Addition of blood to a phycogenic bone substitute leads to increased in vivo vascularization. Biomed. Mater. 2015, 10, 055007. [Google Scholar] [CrossRef] [Green Version]
- Lindner, C.; Pröhl, A.; Abels, M.; Löffler, T.; Batinic, M.; Jung, O.; Barbeck, M. Specialized Histological and Histomorphometrical Analytical Methods for Biocompatibility Testing of Biomaterials for Maxillofacial Surgery in (Pre-) Clinical Studies. In Vivo 2020, 34, 3137–3152. [Google Scholar] [CrossRef] [PubMed]
- Geistlich Biomaterials “Bio-gide”. Available online: https://www.geistlich-pharma.com/fileadmin/content/Geistlich_Pharma/Pdf/pdfs_Dental_englisch/600331_BRO_Product_information_EN_2004_Original_71581.pdf (accessed on 1 July 2021).
- Geistlich Biomaterials Porcine Collagen Membranes and Matrices. Available online: https://www.geistlich-pharma.com/en/dental/further-sites/quality-safety/porcine-collagen-membranes-and-matrices/ (accessed on 13 March 2022).
- Rothamel, D.; Schwarz, F.; Sager, M.; Herten, M.; Sculean, A.; Becker, J. Biodegradation of differently cross-linked collagen membranes: An experimental study in the rat. Clin. Oral Implant. Res. 2005, 16, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Botiss Biomaterials “Jason Membrane”. Available online: https://www.botiss-dental.com/pdf/botiss_membranes_EN.pdf (accessed on 1 July 2021).
- Ebrahimi, M. Biomaterials Application in Therapeutic and Regenerative Medicine From the Perspective of Patients’ Faith. Biomed. J. Sci. Tech. Res. 2018, 8, 1–3. [Google Scholar] [CrossRef]
- Almarzouqi, F.; Rennekampff, H.; AlMarzouki, M.; Lambertz, A.; Acharya, M.; Klink, C.; Popov, A.; Pallua, N. Porcine-Derived Biomaterials in Tissue Engineering and Reconstructive Surgery: Considerations and Alternatives in Muslim Patients. J. Tissue Eng. Regen. Med. 2018, 13, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Busenlechner, D.; Tangl, S.; Arnhart, C.; Redl, H.; Schuh, C.; Watzek, G.; Gruber, R. Resorption of deproteinized bovine bone mineral in a porcine calvaria augmentation model. Clin. Oral Implant. Res. 2011, 23, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.; Lim, H.-C.; Sapata, V.; Yoon, S.R.; Jung, R.E.; Jung, U.-W. Recombinant bone morphogenetic protein-2 and platelet-derived growth factor-BB for localized bone regeneration. Histologic and radiographic outcomes of a rabbit study. Clin. Oral Implant. Res. 2017, 28, e236–e243. [Google Scholar] [CrossRef] [Green Version]
- Abels, M.; Alkildani, S.; Pröhl, A.; Xiong, X.; Krastev, R.; Korzinskas, T.; Stojanovic, S.; Jung, O.; Najman, S.; Barbeck, M. The Granule Size Mediates the In Vivo Foreign Body Response and the Integration Behavior of Bone Substitutes. Materials 2021, 14, 7372. [Google Scholar] [CrossRef]
- Zizzari, V.L.; Zara, S.; Tetè, G.; Vinci, R.; Gherlone, E.; Cataldi, A. Biologic and clinical aspects of integration of different bone substitutes in oral surgery: A literature review. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 392–402. [Google Scholar] [CrossRef]
- Cardinal, M.; Eisenbud, D.E.; Armstrong, D.G. Wound shape geometry measurements correlate to eventual wound healing. Wound Repair Regen. 2009, 17, 173–178. [Google Scholar] [CrossRef]
- Farina, R.; Simonelli, A.; Rizzi, A.; Pramstraller, M.; Cucchi, A.; Trombelli, L. Early postoperative healing following buccal single flap approach to access intraosseous periodontal defects. Clin. Oral Investig. 2012, 17, 1573–1583. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Barbu, H.M.; Iancu, S.A.; Jarjour Mirea, I.; Mignogna, M.D.; Samet, N.; Calvo-Guirado, J.L. Management of Schneiderian Membrane Perforations during Sinus Augmentation Procedures: A Preliminary Comparison of Two Different Approaches. J. Clin. Med. 2019, 8, 1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viña-Almunia, J.; Peñarrocha-Diago, M. Influence of perforation of the sinus membrane on the survival rate of implants placed after direct sinus lift. Literature update. Med. Oral Patol. Oral Y Cir. Bucal 2009, 14, 6. [Google Scholar]
- Cho, S.-C.; Wallace, S.S.; Froum, S.J.; Tarnow, D.P. Influence of anatomy on Schneiderian membrane perforations during sinus elevation surgery: Three-dimensional analysis. Pract. Proced. Aesthetic Dent. PPAD 2001, 13, 160–163. [Google Scholar]
- Levin, L.; Herzberg, R.; Dolev, E.; Schwartz-Arad, D. Smoking and complications of onlay bone grafts and sinus lift operations. Int. J. Oral Maxillofac. Implant. 2004, 19, 369–373. [Google Scholar] [CrossRef]
- Schwartz-Arad, D.; Herzberg, R.; Dolev, E. The Prevalence of Surgical Complications of the Sinus Graft Procedure and Their Impact on Implant Survival. J. Periodontol. 2004, 75, 511–516. [Google Scholar] [CrossRef]
- Van Den Bergh, J.P.A.; Ten Bruggenkate, C.M.; Disch, F.J.M.; Tuinzing, D.B. Anatomical aspects of sinus floor elevations. Clin. Oral Implant. Res. 2000, 11, 256–265. [Google Scholar] [CrossRef]
- Proussaefs, P.; Lozada, J.; Kim, J.; Rohrer, M.D. Repair of the perforated sinus membrane with a resorbable collagen membrane: A human study. Int. J. Oral Maxillofac. Implant. 2004, 19, 413–420. [Google Scholar]
- Fotek, P.D.; Neiva, R.F.; Wang, H.-L. Comparison of Dermal Matrix and Polytetrafluoroethylene Membrane for Socket Bone Augmentation: A Clinical and Histologic Study. J. Periodontol. 2009, 80, 776–785. [Google Scholar] [CrossRef]
- Lorenz, J.; Kubesch, A.; Korzinskas, T.; Barbeck, M.; Landes, C.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. TRAP-Positive Multinucleated Giant Cells Are Foreign Body Giant Cells Rather Than Osteoclasts: Results From a Split-Mouth Study in Humans. J. Oral Implant. 2015, 41, e257–e266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbeck, M.; Motta, A.; Migliaresi, C.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Heterogeneity of biomaterial-induced multinucleated giant cells: Possible importance for the regeneration process? J. Biomed. Mater. Res. Part A 2016, 104, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Mcclain, P.E.; Wiley, E.R.; Mccague, K.E. Species variation in the cross-linking characteristics of collagen from the intramuscular connective tissues of striated muscles (bos taurus, ovis aries, sus scrofa). Int. J. Biochem. 1971, 2, 167–172. [Google Scholar] [CrossRef]
- Ghodbane, S.A.; Dunn, M. Physical and mechanical properties of cross-linked type I collagen scaffolds derived from bovine, porcine, and ovine tendons. J. Biomed. Mater. Res. Part A 2016, 104, 2685–2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angele, P.; Abke, J.; Kujat, R.; Faltermeier, H.; Schumann, D.; Nerlich, M.; Kinner, B.; Englert, C.; Ruszczak, Z.; Mehrl, R.; et al. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials 2004, 25, 2831–2841. [Google Scholar] [CrossRef]
- Lin, Y.K.; Liu, D.C. Comparison of physical–chemical properties of type I collagen from different species. Food Chem. 2006, 99, 244–251. [Google Scholar] [CrossRef]
- Rothamel, D.; Schwarz, F.; Sculean, A.; Herten, M.; Scherbaum, W.; Becker, J. Biocompatibility of various collagen membranes in cultures of human PDL fibroblasts and human osteoblast-like cells. Clin. Oral Implant. Res. 2004, 15, 443–449. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Orth, C.; Willershausen, I.; Thimm, B.W.; Hoffmann, C.; Rasic, A.; Sader, R.A.; Unger, R.E.; Peters, F.; et al. Influence of β-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 2010, 6, 4476–4487. [Google Scholar] [CrossRef]
- Ottenbacher, N.; Alkildani, S.; Korzinskas, T.; Pissarek, J.; Ulm, C.; Jung, O.; Sundag, B.; Bellmann, O.; Stojanovic, S.; Najman, S.; et al. Novel Histomorphometrical Approach to Evaluate the Integration Pattern and Functionality of Barrier Membranes. Dent. J. 2021, 9, 127. [Google Scholar] [CrossRef]



| Function | Name | Description | Implant Sizes (mm × mm) |
|---|---|---|---|
| Test Article | Bovine membrane | Native dermis-based Collagen membrane | 10.0 × 10.0 |
| Positive Control 1 | Bio-Gide® membrane | Native dermis-based collagen membrane | 10.0 × 10.0 |
| Positive Control 2 | Jason® membrane | Native pericardium-based collagen membrane | 10.0 × 10.0 |
| Negative Control | Sham operation | without biomaterial insertion | - |
| Response | Score (phf = Per High Powered (×400) Field) | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Polymorphonuclear cells | 0 | Rare, 1–5/phf | 6–10/phf | Heavy infiltrate | Packed |
| Lymphocytes | 0 | Rare, 1–5/phf | 6–10/phf | Heavy infiltrate | Packed |
| Plasma cells | 0 | Rare, 1–5/phf | 6–10/phf | Heavy infiltrate | Packed |
| Macrophages | 0 | Rare, 1–5/phf | 6–10/phf | Heavy infiltrate | Packed |
| Giant cells | 0 | Rare, 1–2/phf | 3–5/phf | Heavy infiltrate | Packed |
| Necrosis | 0 | Minimal | Mild | Moderate | Marked |
| Neovascularization | 0 | Minimal capillary proliferation focal, 1–3 buds | Groups of 4–7 capillaries with supporting fibroblastic structures | Broad band of capillaries with supporting structures | Extensive band of capillaries with supporting fibroblastic structures |
| Fibrocytes/fibroconnective tissue, fibrosis | 0 | Narrow band | Moderately thick band | Thick band | Extensive band |
| Fatty infiltrate | 0 | Minimal amount of fat associated with fibrosis | Several layers of fat and fibrosis | Elongated and broad accumulation of fat cells about the implant site | Extensive fat completely surrounding the implant |
| Irritancy score = (Polymorphonuclear Cells + Lymphocytes + Plasma Cells + Macrophages + Giant Cells + Necrosis) × 2 + (Neovascularization + Fibrosis + Fatty Infiltrate). | |||||
| Overall Irritancy Score | Irritancy/Reactivity Status |
|---|---|
| 0.0 to 2.9 | Minimal or no reaction (non-irritant) |
| 3.0 to 8.9 | Slight reaction (slight irritant) |
| 9.0 to 15.0 | Moderate reaction (moderate irritant) |
| >15.1 | Severe reaction (severe irritant) |
| Parameter | Mean ± SD—Inflammation and Inflammatory Cell Types at Day 10 | |||
|---|---|---|---|---|
| BM | JM | BG | SO | |
| Polymorphonuclear Cells | 1.00 ± 0.8 | 0.30 ± 0.1 | 0.40 ± 0.1 | 0.38 ± 0.1 |
| Lymphocytes | 0.75 ± 0.3 | 0.40 ± 0.1 | 0.70 ± 0.3 | 0.63 ± 0.3 |
| Plasma Cells | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.21 ± 0.1 |
| Macrophages | 2.00 ± 0.0 | 1.50 ± 0.4 | 1.80 ± 0.3 | 1.58 ± 0.4 |
| Giant Cells | 0.67 ± 0.3 | 0.40 ± 0.2 | 0.25 ± 0.2 | 0.00 ± 0.0 |
| Neovascularization | 0.67 ± 0.3 | 0.90 ± 0.2 | 0.60 ± 0.2 | 1.08 ± 0.4 |
| Fibrosis | 0.00 ± 0.0 | 0.10 ± 0.2 | 0.10 ± 0.2 | 0.00 ± 0.0 |
| Fatty infiltrate | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| Necrosis | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| Parameter | Mean ± SD—Inflammation and Inflammatory Cell Types at Day 30 | |||
|---|---|---|---|---|
| BM | JM | BG | SO | |
| Polymorphonuclear Cells | 0.29 ± 0.1 | 0.29 ± 0.2 | 0.50 ± 0.3 | 0.45 ± 0.1 |
| Lymphocytes | 0.96 ± 0.7 | 1.00 ± 0.5 | 0.67 ± 0.5 | 0.60 ± 0.5 |
| Plasma Cells | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.1 ± 0.1 |
| Macrophages | 1.83 ± 0.3 | 2.08 ± 0.2 | 2.00 ± 0.7 | 1.80 ± 0.3 |
| Giant Cells | 0.58 ± 0.3 | 0.13 ± 0.1 | 0.58 ± 0.6 | 0.05 ± 0.1 |
| Neovascularization | 0.08 ± 0.1 | 0.17 ± 0.1 | 0.20 ± 0.2 | 1.30 ± 0.4 |
| Fibrosis | 0.00 ± 0.0 | 0.04 ± 0.1 | 0.20 ±0.2 | 0.00 ± 0.0 |
| Fatty infiltrate | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.08 ± 0.1 | 0.55 ± 0.3 |
| Necrosis | 0.04 ± 0.1 | 0.04 ± 0.1 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| Parameter | Mean ± SD—Inflammation and Inflammatory Cell Types at Day 60 | |||
|---|---|---|---|---|
| BM | JM | BG | SO | |
| Polymorphonuclear Cells | 0.25 ± 0.2 | 0.75 ± 0.5 | 0.50 ± 0.4 | 0.67 ± 0.2 |
| Lymphocytes | 0.58 ± 0.5 | 0.54 ± 0.2 | 0.33 ± 0.1 | 0.50 ± 0.2 |
| Plasma Cells | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.0 ± 0.0 |
| Macrophages | 2.08 ± 0.4 | 1.50 ± 0.4 | 1.08 ± 0.2 | 1.33 ± 0.4 |
| Giant Cells | 0.67 ± 0.5 | 0.08 ± 0.1 | 0.08 ± 0.1 | 0.00 ± 0.0 |
| Neovascularization | 0.42 ± 0.1 | 0.08 ± 0.1 | 0.08 ± 0.1 | 0.83 ± 0.4 |
| Fibrosis | 0.13 ± 0.1 | 0.00 ± 0.0 | 0.13 ± 0.14 | 0.00 ± 0.0 |
| Fatty infiltrate | 0.17 ± 0.1 | 0.00 ± 0.0 | 0.04 ± 0.1 | 0.33 ± 0.4 |
| Necrosis | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| Parameter | Mean ± SD—Inflammation and Inflammatory Cell Types at Day 90 | |||
|---|---|---|---|---|
| BM | JM | BG | SO | |
| Polymorphonuclear Cells | 0.33 ± 0.1 | 0.75 ± 0.4 | 0.96 ± 0.3 | 0.33 ± 0.1 |
| Lymphocytes | 0.83 ± 0.4 | 0.41 ± 0.3 | 0.54 ± 0.3 | 0.33 ± 0.1 |
| Plasma Cells | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.0 ± 0.0 |
| Macrophages | 1.92 ± 0.2 | 1.17 ± 0.3 | 1.08 ± 0.2 | 0.54 ± 0.3 |
| Giant Cells | 0.83 ± 0.4 | 0.08 ± 0.1 | 0.29 ± 0.2 | 0.00 ± 0.0 |
| Neovascularization | 0.58 ±0.2 | 0.13 ± 0.1 | 0.08 ± 0.1 | 0.46 ± 0.2 |
| Fibrosis | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.17 ± 0.1 | 0.00 ± 0.0 |
| Fatty infiltrate | 0.08 ± 0.1 | 0.17 ± 0.1 | 0.17 ± 0.1 | 0.42 ± 0.1 |
| Necrosis | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| Study Group | Treatment Irritancy Score | Overall Irritancy Score | Irritant Status | |
|---|---|---|---|---|
| Day 10 | BM | 9.50 | 2.90 | Non-irritant |
| JM | 6.20 | 6.60 | - | |
| BG | 7.00 | - | ||
| SO | 6.67 | - | - | |
| Day 30 | BM | 7.50 | 0.0 | Non-irritant |
| JM | 7.29 | 7.61 | - | |
| BG | 7.92 | - | ||
| SO | 7.85 | - | - | |
| Day 60 | BM | 7.88 | 2.84 | Non-irritant |
| JM | 5.83 | 5.04 | - | |
| BG | 4.25 | - | ||
| SO | 6.17 | - | - | |
| Day 90 | BM | 8.50 | 2.85 | Non-irritant |
| JM | 5.12 | 5.65 | - | |
| BG | 6.17 | - | ||
| SO | 3.29 | - | - |
| Membrane/Time Point | Thickness (mm) | |||
|---|---|---|---|---|
| Day 10 | Day 30 | Day 60 | Day 90 | |
| BM | 0.54 ± 0.15 | 0.77 ± 0.22 | 0.59 ± 0.18 | 0.36 ± 0.13 |
| JM | 0.20 ± 0.04 | 0.28 ± 0.07 | 0.31 ± 0.05 | 0.26 ± 0.11 |
| BG | 0.45 ± 0.10 | 0.26 ± 0.09 | 0.27 ± 0.08 | 0.22 ± 0.05 |
| Membrane/Time Point | Day 10 | Day 30 | Day 60 | Day 90 |
|---|---|---|---|---|
| CD163 (cells/mm2) | ||||
| BM | 848.9 ± 26.7 | 797.4 ± 273.4 | 673.0 ± 105.9 | 704.2 ± 146.3 |
| JM | 970.9 ± 300.4 | 933.3 ± 110.0 | 567.9 ± 75.3 | 531.8 ± 121.7 |
| BG | 1303.0 ± 592.1 | 766.5 ± 199.1 | 423.7 ± 78.2 | 534.2 ± 166.5 |
| SO | 708.7 ± 65.6 | 1123.0 ± 99.3 | 766.6 ± 34.0 | 572.3 ± 290.4 |
| CD11c (cells/mm2) | ||||
| BM | 173.3 ± 56.2 | 52.3 ± 26.2 | 104.3 ± 62.2 | 65.8 ± 34.7 |
| JM | 137.7 ± 65.6 | 140.3 ± 82.0 | 90.5 ± 70.0 | 59.1 ± 23.2 |
| BG | 382.2 ± 204.3 | 148.6 ± 111.0 | 148.5 ± 50.0 | 104.3 ± 20.4 |
| SO | 162.7 ± 91.5 | 109.6 ± 42.0 | 82.8 ± 64.2 | 56.3 ± 36.6 |
| Day 30 | Day 60 | Day 90 | |
|---|---|---|---|
| BM | Vessel Density (vessels/mm2) | ||
| 182.6 ± 47.73 | 291.0 ± 67.80 | 332.30 ± 114.70 | |
| Vessel Percentage (%) | |||
| 2.055 ± 1.246 | 8.405 ± 4.169 | 6.978 ± 2.955 | |
| Vessel Diameter (µm) | |||
| 10.78 ± 1.179 | 15.51 ± 1.587 | 12.67 ± 0.7209 | |
| JM | Vessel Density (vessels/mm2) | ||
| 221.4 ± 64.56 | 120.70 ± 39.53 | 112.20 ± 23.15 | |
| Vessel Percentage (%) | |||
| 1.978 ± 0.365 | 1.448 ± 0.8435 | 1.163 ± 0.3278 | |
| Vessel Diameter (µm) | |||
| 10.61 ± 1.019 | 12.29 ± 0.5697 | 11.23 ± 1.008 | |
| BG | Vessel Density (vessels/mm2) | ||
| 212.9 ± 131.7 | 53.52 ± 32.52 | 211.00 ± 125.80 | |
| Vessel Percentage (%) | |||
| 1.455 ± 1.065 | 0.5575 ± 0.4081 | 1.803 ± 0.9028 | |
| Vessel Diameter (µm) | |||
| 10.34 ± 1.065 | 13.13 ± 2.585 | 11.28 ± 0.9466 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindner, C.; Alkildani, S.; Stojanovic, S.; Najman, S.; Jung, O.; Barbeck, M. In Vivo Biocompatibility Analysis of a Novel Barrier Membrane Based on Bovine Dermis-Derived Collagen for Guided Bone Regeneration (GBR). Membranes 2022, 12, 378. https://doi.org/10.3390/membranes12040378
Lindner C, Alkildani S, Stojanovic S, Najman S, Jung O, Barbeck M. In Vivo Biocompatibility Analysis of a Novel Barrier Membrane Based on Bovine Dermis-Derived Collagen for Guided Bone Regeneration (GBR). Membranes. 2022; 12(4):378. https://doi.org/10.3390/membranes12040378
Chicago/Turabian StyleLindner, Carolin, Said Alkildani, Sanja Stojanovic, Stevo Najman, Ole Jung, and Mike Barbeck. 2022. "In Vivo Biocompatibility Analysis of a Novel Barrier Membrane Based on Bovine Dermis-Derived Collagen for Guided Bone Regeneration (GBR)" Membranes 12, no. 4: 378. https://doi.org/10.3390/membranes12040378
APA StyleLindner, C., Alkildani, S., Stojanovic, S., Najman, S., Jung, O., & Barbeck, M. (2022). In Vivo Biocompatibility Analysis of a Novel Barrier Membrane Based on Bovine Dermis-Derived Collagen for Guided Bone Regeneration (GBR). Membranes, 12(4), 378. https://doi.org/10.3390/membranes12040378










