Edible Clusteroluminogenic Films Obtained from Starch of Different Botanical Origins for Food Packaging and Quality Management of Frozen Foods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Starch Films
2.3. Determination of Film Thickness
2.4. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.5. Nuclear Magnetic Resonance (NMR)
2.6. Molecular Weight Determination
2.7. Amylose Content Determination
2.8. Thermogravimetric Analysis (TGA) and Derivative Thermogravimetry (DTG)
2.9. Determination of the Tensile Strength
2.10. Scanning Electron Microscopy (SEM) Analysis
2.11. Characterization of Optical Properties of the Films
2.12. Toxicity Assessment
2.13. Contact Angle Measurement
2.14. Determination of Water Vapor Permeability and Erosion Susceptibility
2.15. Evaluation of Film Performance in Food Packaging
2.16. Statistical Analysis
3. Results and Discussion
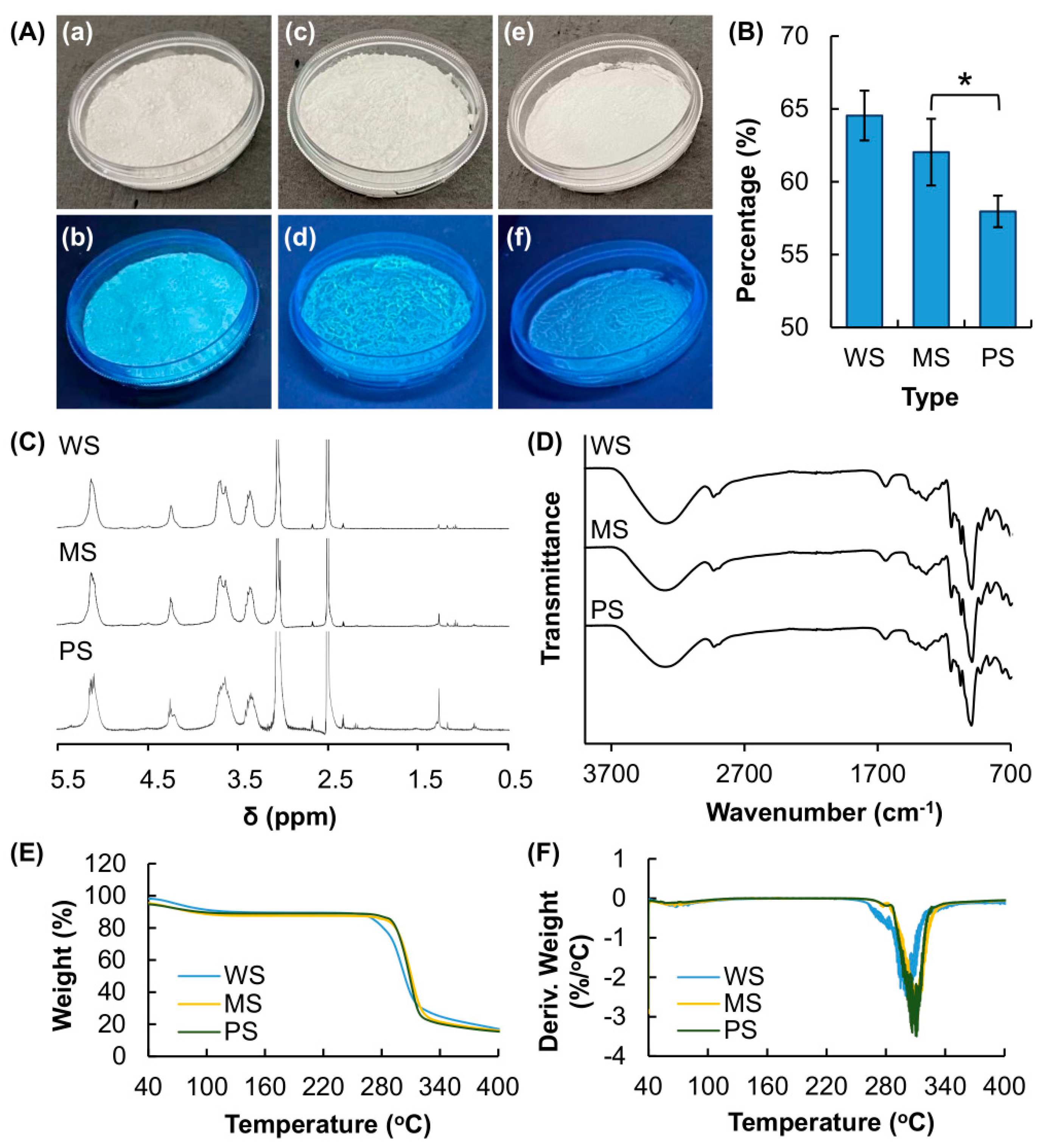
3.1. Structural and Thermal Characterization of Starch of Different Botanical Sources
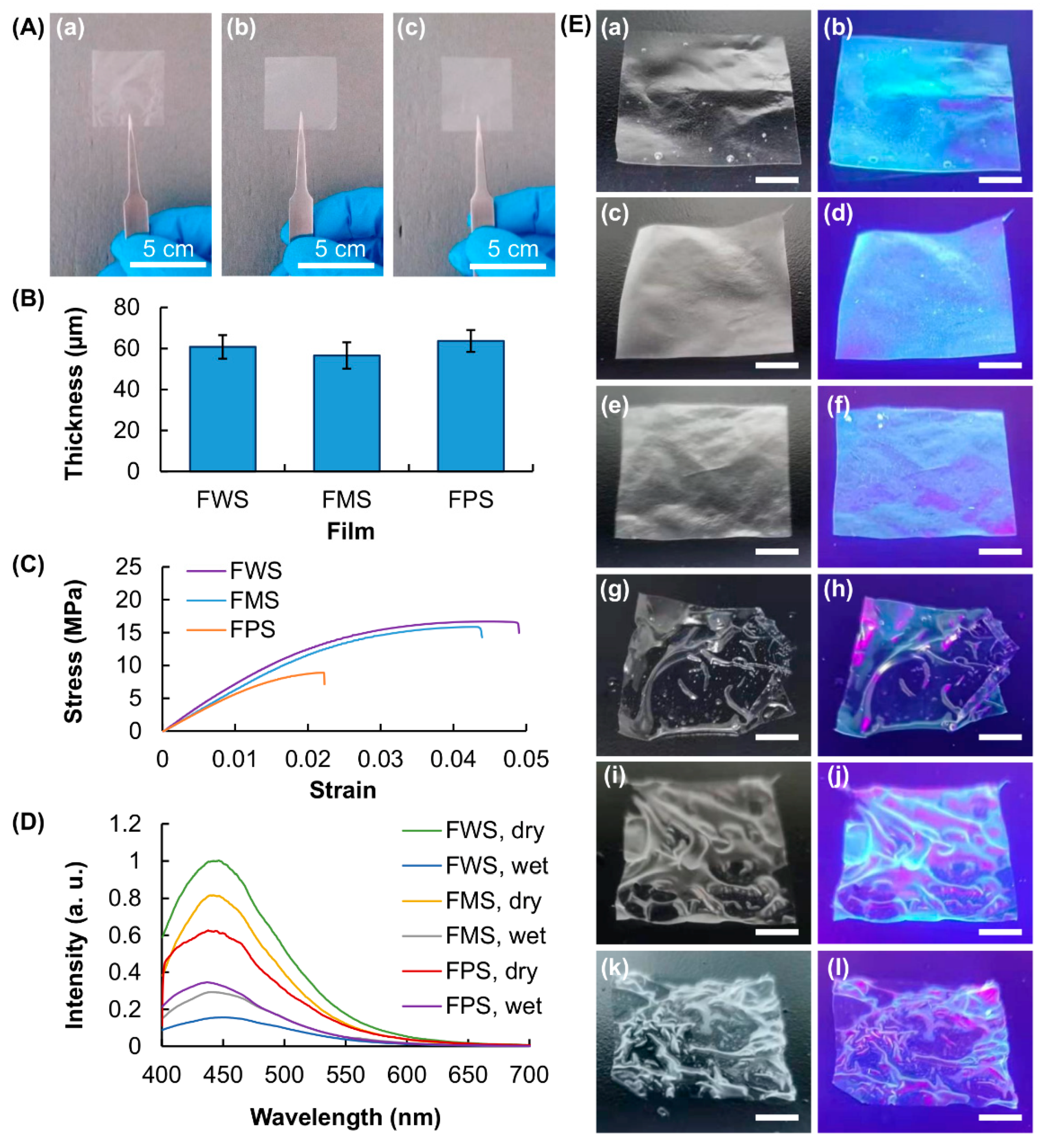
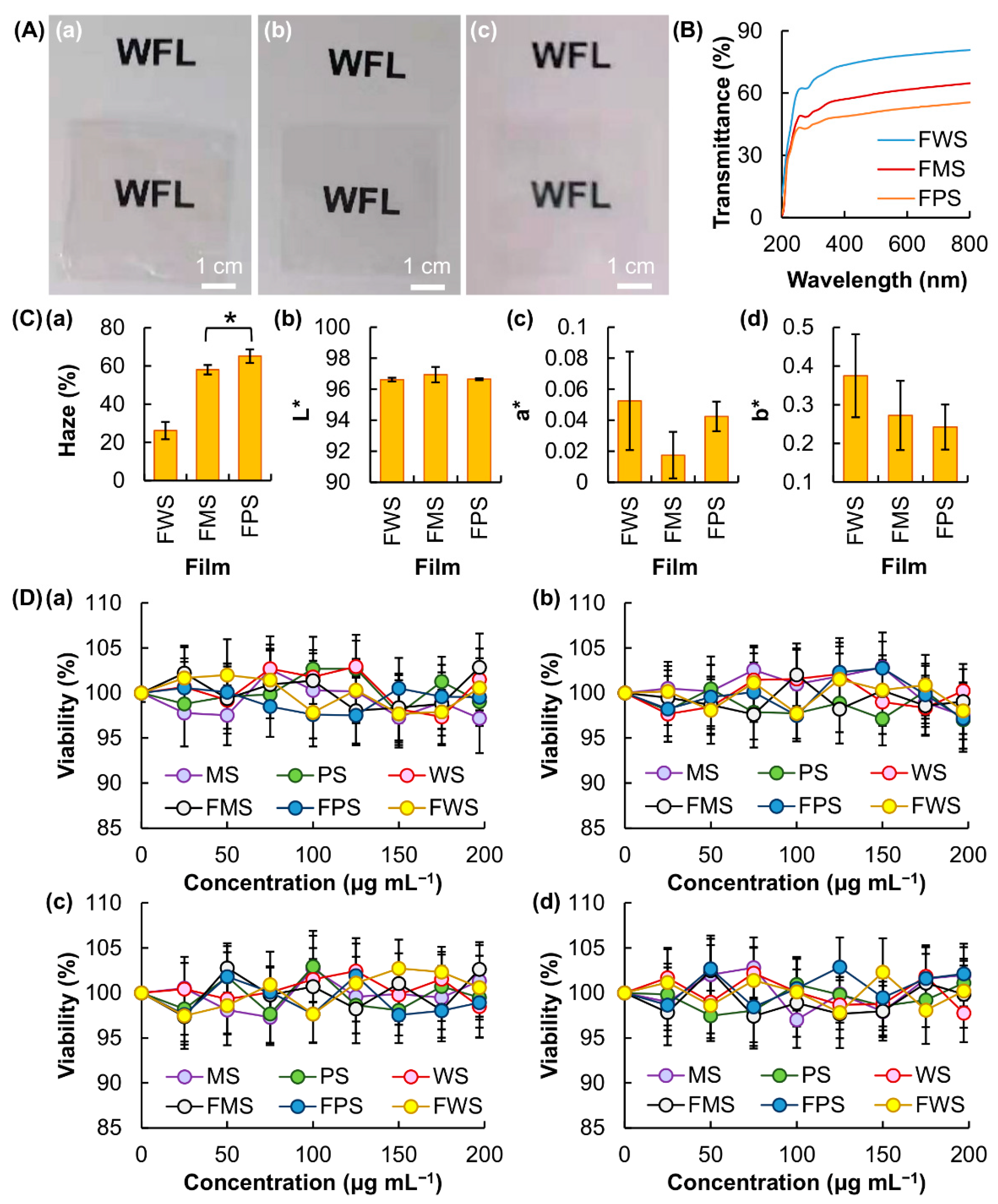
3.2. Characterization of Physical Properties of the Generated Films
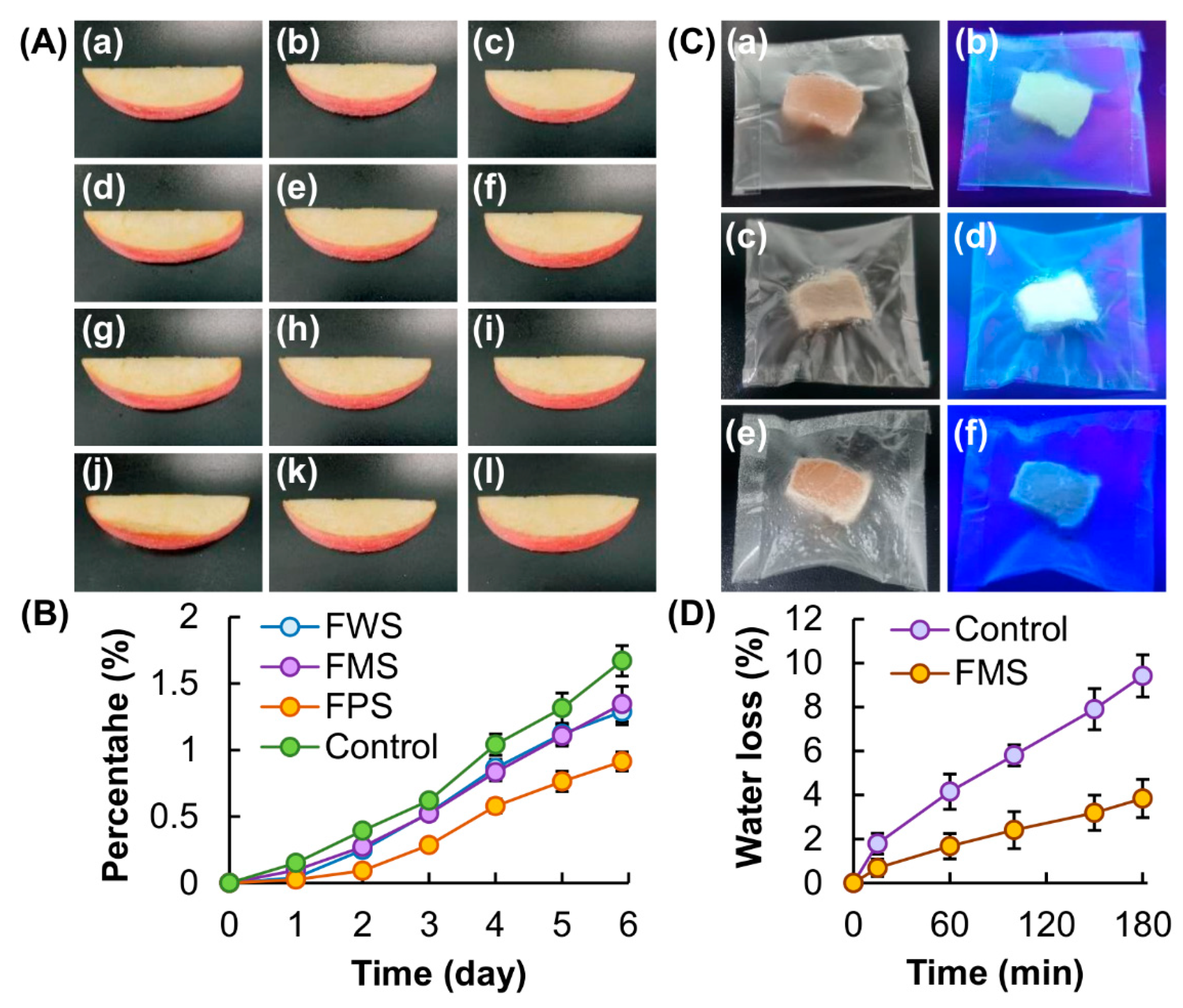
3.3. Performance as Edible and Indicating Films in Food Packaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prabhu, T.N.; Prashantha, K. A review on present status and future challenges of starch based polymer films and their composites in food packaging applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- Lai, W.F. Design of polymeric films for antioxidant active food packaging. Int. J. Mol. Sci. 2021, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Wongphan, P.; Khowthong, M.; Supatrawiporn, T.; Harnkarnsujarit, N. Novel edible starch films incorporating papain for meat tenderization. Food Packag. Shelf Life 2021, 31, 100787. [Google Scholar] [CrossRef]
- Sen, F.; Uzunsoy, I.; Basturk, E.; Kahraman, M.V. Antimicrobial agent-free hybrid cationic starch/sodium alginate polyelectrolyte films for food packaging materials. Carbohyd. Polym. 2017, 170, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.; Scarlett, C.J.; Stathopoulos, C. Characterization of pea starch-guar gum biocomposite edible films enriched by natural antimicrobial agents for active food packaging. Food Bioprod. Process. 2017, 105, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Muller, J.; González-Martínez, C.; Chiralt, A. Combination of poly(lactic) acid and starch for biodegradable food packaging. Materials 2017, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of ecofriendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.A.; Farenzena, S.; Pintos, E.; Rodríguez, M.S.; Villar, M.A.; García, M.A.; López, O.V. Active films based on thermoplastic corn starch and chitosan oligomer for food packaging applications. Food Packag. Shelf Life 2017, 14, 128–136. [Google Scholar] [CrossRef]
- Heydari, A.; Alemzadeh, I.; Vossoughi, M. Functional properties of biodegradable corn starch nanocomposites for food packaging applications. Mater. Des. 2013, 50, 954–961. [Google Scholar] [CrossRef]
- Romani, V.P.; Prentice, C.; Martins, V.G. Active and sustainable materials from rice starch, fish protein and oregano essential oil for food packaging. Ind. Crop. Prod. 2017, 97, 268–274. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Vaidya, R.; Balasubramaniyam, K.; Asha, D.; Sudha, V.; Anjana, R.M.; Mohan, V. Resistant starch and dietary fibre content of common indian cooked foods and its effect on glycemic index. Ann. Nutr. Metab. 2013, 63, 1308. [Google Scholar]
- Daramola, B.; Makanju, O.O. Assessment of selected cooking characteristics of prime starch and food grade fibre isolated from cassava (Manihot esculenta) pulp. Afr. J. Biotechnol. 2008, 7, 2717–2720. [Google Scholar] [CrossRef]
- Carcea, M.; Salvatorelli, S.; Turfani, V. Measurement of resistant starch in cooked cereal-based foods. Qual. Assur. Saf. Crop. Foods 2009, 1, 240–245. [Google Scholar] [CrossRef]
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Dhull, S.B.; Malik, T.; Kaur, R.; Kumar, P.; Kaushal, N.; Singh, A. Banana starch: Properties illustration and food applications—A review. Starch 2020, 73, 2000085. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Observations on the impact of amylopectin and amylose structure on the swelling of starch granules. Food Hydrocoll. 2020, 103, 105663. [Google Scholar] [CrossRef]
- Ulbrich, M.; Flöter, E. Modification of starches with different amylose/amylopectin ratios using the dual approach with hydroxypropylation and subsequent acid thinning—III: Impacts on gel characteristics. Starch 2020, 73, 2000146. [Google Scholar] [CrossRef]
- Nivelle, M.A.; Remmerie, E.; Bosmans, G.M.; Vrinten, P.; Nakamura, T.; Delcour, J.A. Amylose and amylopectin functionality during baking and cooling of bread prepared from flour of wheat containing unusual starches: A temperature-controlled time domain 1H NMR study. Food Chem. 2019, 295, 110–119. [Google Scholar] [CrossRef]
- Nivelle, M.A.; Beghin, A.S.; Vrinten, P.; Nakamura, T.; Delcour, J.A. Amylose and amylopectin functionality during storage of bread prepared from flour of wheat containing unique starches. Food Chem. 2020, 320, 126609. [Google Scholar] [CrossRef] [PubMed]
- Karakelle, B.; Kian-Pour, N.; Toker, O.S.; Palabiyik, I. Effect of process conditions and amylose/amylopectin ratio on the pasting behavior of maize starch: A modeling approach. J. Cereal Sci. 2020, 94, 102998. [Google Scholar] [CrossRef]
- Escobar-Puentes, A.A.; García-Gurrola, A.; Rincón, S.; Zepeda, A.; Martínez-Bustos, F. Effect of amylose/amylopectin content and succinylation on properties of corn starch nanoparticles as encapsulants of anthocyanins. Carbohydr. Polym. 2020, 250, 116972. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Kayitesi, E.; Adebo, O.A.; Oyedeji, A.B.; Ogundele, O.M.; Obilana, A.; Njobeh, P.B. A review on the physicochemical properties and potential food applications of cowpea (Vigna unguiculata) starch. Int. J. Food Sci. Technol. 2020, 56, 52–60. [Google Scholar] [CrossRef]
- Lai, W.F. Non-aromatic clusteroluminogenic polymers: Structural design and applications in bioactive agent delivery. Mater. Today Chem. 2021, 23, 100712. [Google Scholar] [CrossRef]
- Lai, W.F. Non-conjugated polymers with intrinsic luminescence for drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101916. [Google Scholar] [CrossRef]
- Lai, W.F.; Deng, R.; He, T.; Wong, W. A bioinspired, sustained-release material in response to internal signals for biphasic chemical sensing in wound therapy. Adv. Heal. Mater. 2020, 10, e2001267. [Google Scholar] [CrossRef]
- Lai, W.F.; Gui, D.; Wong, M.; Döring, A.; Rogach, A.L.; He, T.; Wong, W.T. A self-indicating cellulose-based gel with tunable performance for bioactive agent delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102428. [Google Scholar] [CrossRef]
- Lai, W.F.; Huang, E.; Wong, W.T. A gel-forming clusteroluminogenic polymer with tunable emission behavior as a sustained-release carrier enabling real-time tracking during bioactive agent delivery. Appl. Mater. Today 2020, 21, 100876. [Google Scholar] [CrossRef]
- Man, J.; Cai, J.; Cai, C.; Xu, B.; Huai, H.; Wei, C. Comparison of physicochemical properties of starches from seed and rhizome of lotus. Carbohydr. Polym. 2012, 88, 676–683. [Google Scholar] [CrossRef]
- Lai, W.-F.; Zhao, S.; Chiou, J. Antibacterial and clusteroluminogenic hypromellose-graft-chitosan-based polyelectrolyte complex films with high functional flexibility for food packaging. Carbohydr. Polym. 2021, 271, 118447. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F.; Yip, W.; Wong, W. UV-shielding and clusteroluminogenic cellulose-based films with tuneable wettability and permeability for dually self-indicating food packaging. Adv. Mater. Technol. 2021, 6, 2100120. [Google Scholar] [CrossRef]
- Wongphan, P.; Panrong, T.; Harnkarnsujarit, N. Effect of different modified starches on physical, morphological, thermomechanical, barrier and biodegradation properties of cassava starch and polybutylene adipate terephthalate blend film. Food Packag. Shelf Life 2022, 32, 100844. [Google Scholar] [CrossRef]
- Mou, J.; Li, X.; Wang, H.; Fei, G.; Liu, Q. Preparation, characterization, and water resistance of cationic acetylated starch-g-poly(styrene-butyl acrylate) surfactant-free emulsion. Starch 2012, 64, 826–834. [Google Scholar] [CrossRef]
- Leloup, V.; Colonna, P.; Buleon, A. Influence of amylose-amylopectin ratio on gel properties. J. Cereal Sci. 1991, 13, 1–13. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Li, Y. Structure-property modification of microcrystalline cellulose film using agar and propylene glycol alginate. J. Appl. Polym. Sci. 2017, 134, 45533. [Google Scholar] [CrossRef]
- Gillgren, T.; Blennow, A.; Pettersson, A.J.; Stading, M. Modulating rheo-kinetics of native starch films towards improved wet-strength. Carbohydr. Polym. 2011, 83, 383–391. [Google Scholar] [CrossRef]
- Turhan, K.N.; Sahbaz, F. A simple method for determining light transmittance of polymer films used for packaging foods. Polym. Int. 2001, 50, 1138–1142. [Google Scholar] [CrossRef]
- Gürler, N.; Paşa, S.; Erdoğan, Ö.; Cevik, O. Physicochemical properties for food packaging and toxicity behaviors against healthy cells of environmentally friendly biocompatible starch/citric acid/polyvinyl alcohol biocomposite films. Starch 2021, 2100074. [Google Scholar] [CrossRef]
- Lai, W.F.; Rogach, A.L.; Wong, W.-T. One-pot synthesis of an emulsion-templated hydrogel-microsphere composite with tunable properties. Compos. Part A Appl. Sci. Manuf. 2018, 113, 318–329. [Google Scholar] [CrossRef]
- Lai, W.F.; Susha, A.S.; Rogach, A.L. Multicompartment microgel beads for co-delivery of multiple drugs at individual release rates. ACS Appl. Mater. Inter. 2016, 8, 871–880. [Google Scholar] [CrossRef]
- Lai, W.F.; Susha, A.S.; Rogach, A.L.; Wang, G.; Huang, M.; Hu, W.; Wong, W.T. Electrospray-mediated preparation of compositionally homogeneous core–shell hydrogel microspheres for sustained drug release. RSC Adv. 2017, 7, 44482–44491. [Google Scholar] [CrossRef] [Green Version]
- Lai, W.F.; Tang, R.; Wong, W.-T. Ionically crosslinked complex gels loaded with oleic acid-containing vesicles for transdermal drug delivery. Pharmaceutics 2020, 12, 725. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F.; Wong, E.; Wong, W.-T. Multilayered composite-coated ionically crosslinked food-grade hydrogel beads generated from algal alginate for controlled and sustained release of bioactive compounds. RSC Adv. 2020, 10, 44522–44532. [Google Scholar] [CrossRef]
- Bowker, B.; Zhuang, H. Freezing-thawing and sub-sampling influence the marination performance of chicken breast meat. Poult. Sci. 2017, 96, 3482–3488. [Google Scholar] [CrossRef]
- Frelka, J.C.; Phinney, D.M.; Yang, X.; Knopp, M.V.; Heldman, D.R.; Wick, M.P.; Vodovotz, Y. Assessment of chicken breast meat quality after freeze/thaw abuse using magnetic resonance imaging techniques. J. Sci. Food Agric. 2018, 99, 844–853. [Google Scholar] [CrossRef]
- Yadav, D.N.; Patki, P.E.; Khan, M.A.; Sharma, G.K.; Bawa, A.S. Effect of freeze-thaw cycles and additives on rheological and sensory properties of ready to bake frozen chapaties. Int. J. Food Sci. Technol. 2008, 43, 1714–1720. [Google Scholar] [CrossRef]
- Wangtueai, S.; Maneerote, J. Effect of phosphate and freeze-thaw cycles on physicochemical and sensory properties of frozen nile tilapia (Oreochromis niloticus) fillets. Food. Appl. Biosci. J. 2018, 6, 117–132. [Google Scholar]
- Rahman, M.H.; Hossain, M.M.; Rahman, S.M.E.; Hashem, M.A.; Oh, D.H. Effect of repeated freeze-thaw cycles on beef quality and safety. Korean J. Food Sci. Anim. Resour. 2014, 34, 482–495. [Google Scholar] [CrossRef] [Green Version]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, C.; Rachtanapun, P. Biodegradable rice starch/carboxymethyl chitosan films with added propolis extract for potential use as active food packaging. Polymers 2018, 10, 954. [Google Scholar] [CrossRef] [Green Version]
- Iamareerat, B.; Singh, M.; Sadiq, M.B.; Anal, A.K. Reinforced cassava starch based edible film incorporated with essential oil and sodium bentonite nanoclay as food packaging material. J. Food Sci. Technol. 2018, 55, 1953–1959. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I. Photo-producible and photo-degradable starch/TiO2 bionanocomposite as a food packaging material: Development and characterization. Int. J. Biol. Macromol. 2018, 106, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Jonhed, A.; Andersson, C.; Järnström, L. Effects of film forming and hydrophobic properties of starches on surface sized packaging paper. Packag. Technol. Sci. 2007, 21, 123–135. [Google Scholar] [CrossRef]
- Singaram, A.J.V.; Guruchandran, S.; Bakshi, A.; Muninathan, C.; Ganesan, N.D. Study on enhanced mechanical, barrier and optical properties of chemically modified mango kernel starch films. Packag. Technol. Sci. 2021, 34, 485–495. [Google Scholar] [CrossRef]

| Sample | Mn (Da) | Mw (Da) | Mp (Da) | Mz (Da) | Mz + 1 (Da) | PDI | Mz/Mw | Mz + 1/Mw |
|---|---|---|---|---|---|---|---|---|
| WS | 61,534 | 120,120 | 92,946 | 218,567 | 355,251 | 1.952097 | 1.819572 | 2.957467 |
| MS | 77,835 | 141,681 | 119,804 | 223,719 | 311,611 | 1.820281 | 1.579039 | 2.199390 |
| PS | 72,787 | 144,307 | 106,446 | 267,456 | 430,320 | 1.982601 | 1.853384 | 2.981977 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, W.-F.; Wong, W.-T. Edible Clusteroluminogenic Films Obtained from Starch of Different Botanical Origins for Food Packaging and Quality Management of Frozen Foods. Membranes 2022, 12, 437. https://doi.org/10.3390/membranes12040437
Lai W-F, Wong W-T. Edible Clusteroluminogenic Films Obtained from Starch of Different Botanical Origins for Food Packaging and Quality Management of Frozen Foods. Membranes. 2022; 12(4):437. https://doi.org/10.3390/membranes12040437
Chicago/Turabian StyleLai, Wing-Fu, and Wing-Tak Wong. 2022. "Edible Clusteroluminogenic Films Obtained from Starch of Different Botanical Origins for Food Packaging and Quality Management of Frozen Foods" Membranes 12, no. 4: 437. https://doi.org/10.3390/membranes12040437
APA StyleLai, W.-F., & Wong, W.-T. (2022). Edible Clusteroluminogenic Films Obtained from Starch of Different Botanical Origins for Food Packaging and Quality Management of Frozen Foods. Membranes, 12(4), 437. https://doi.org/10.3390/membranes12040437






