Preparation and Characterization of a Thin-Film Composite Membrane Modified by MXene Nano-Sheets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
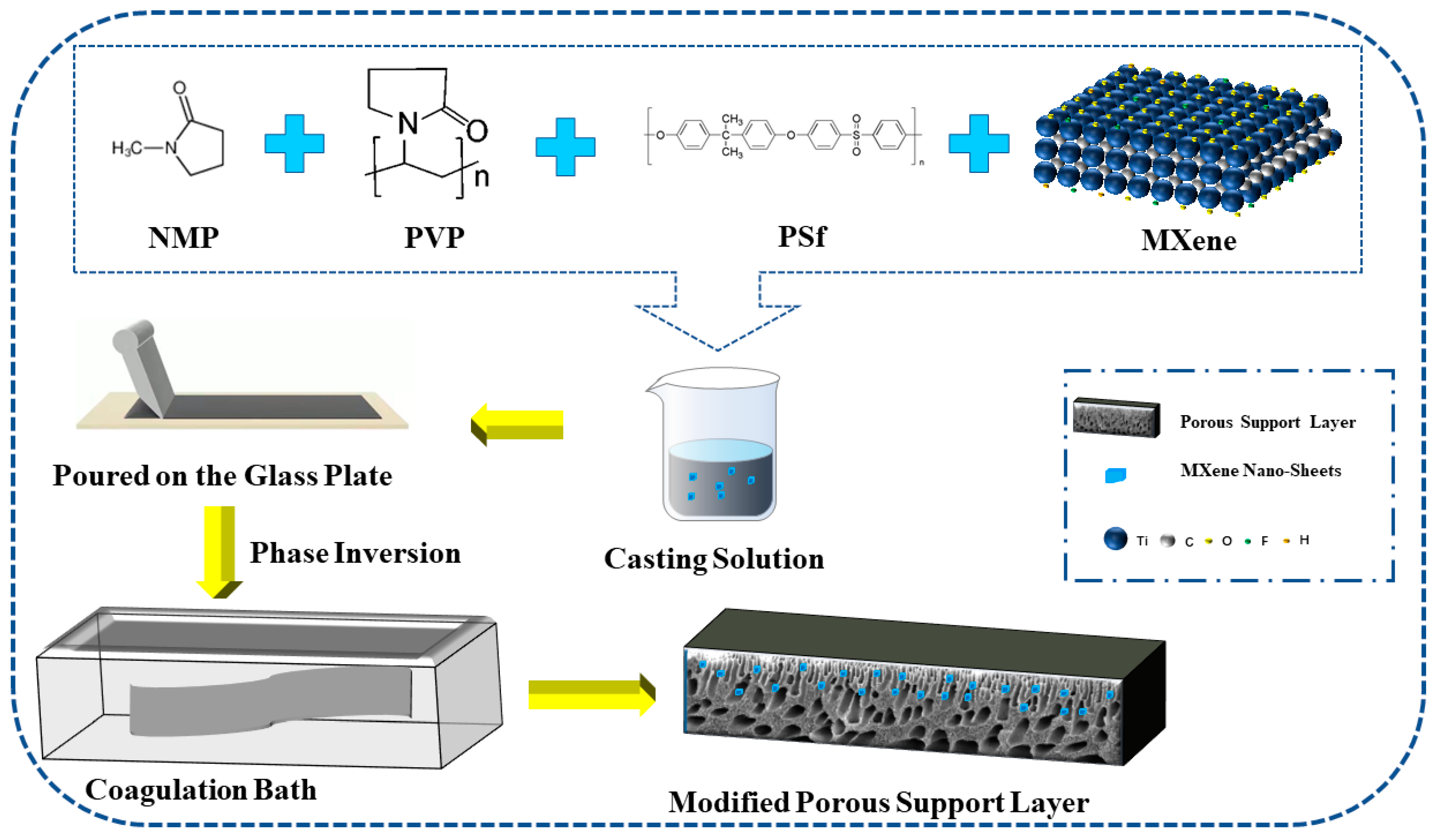
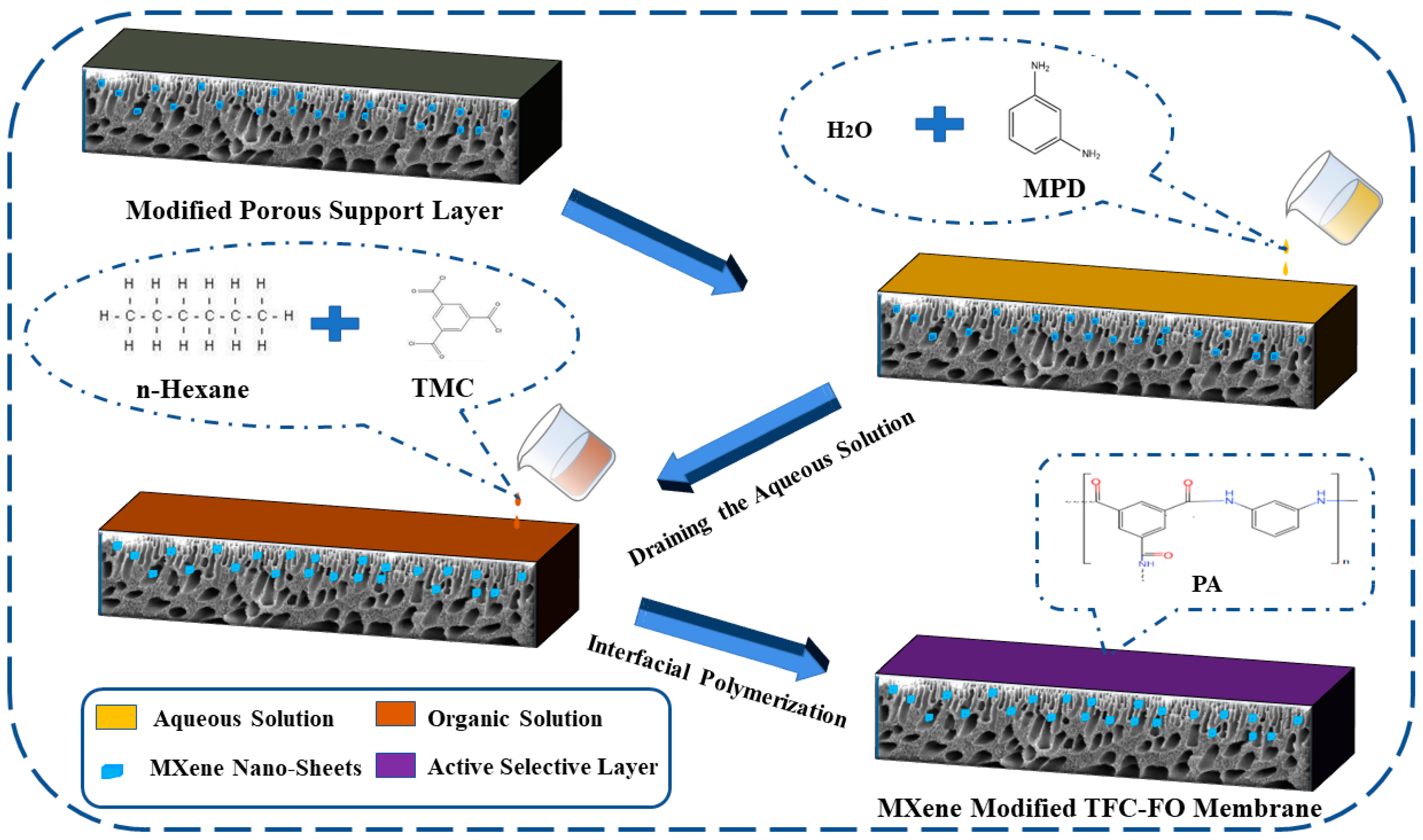
2.2. Preparation of the MXene Nano-Sheets Modified TFC-FO Membranes
2.2.1. The Preparation of the Modified Porous Support Layer
2.2.2. Preparation of the Active Selective Layer
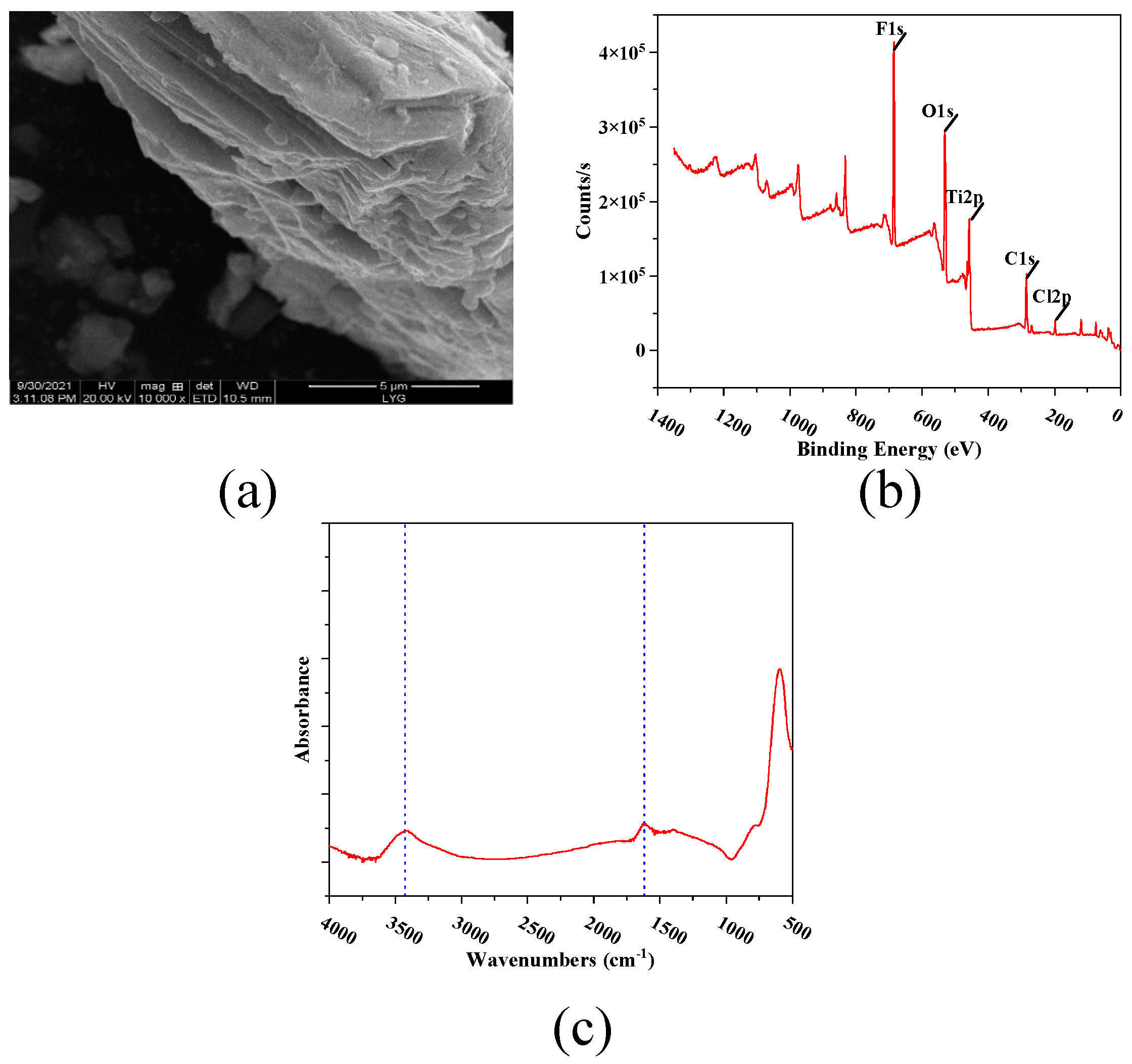
2.3. Characterization of the MXene Nano-Sheets
2.4. The Characterization of the MXene Nano-Sheets Modified TFC-FO Membranes
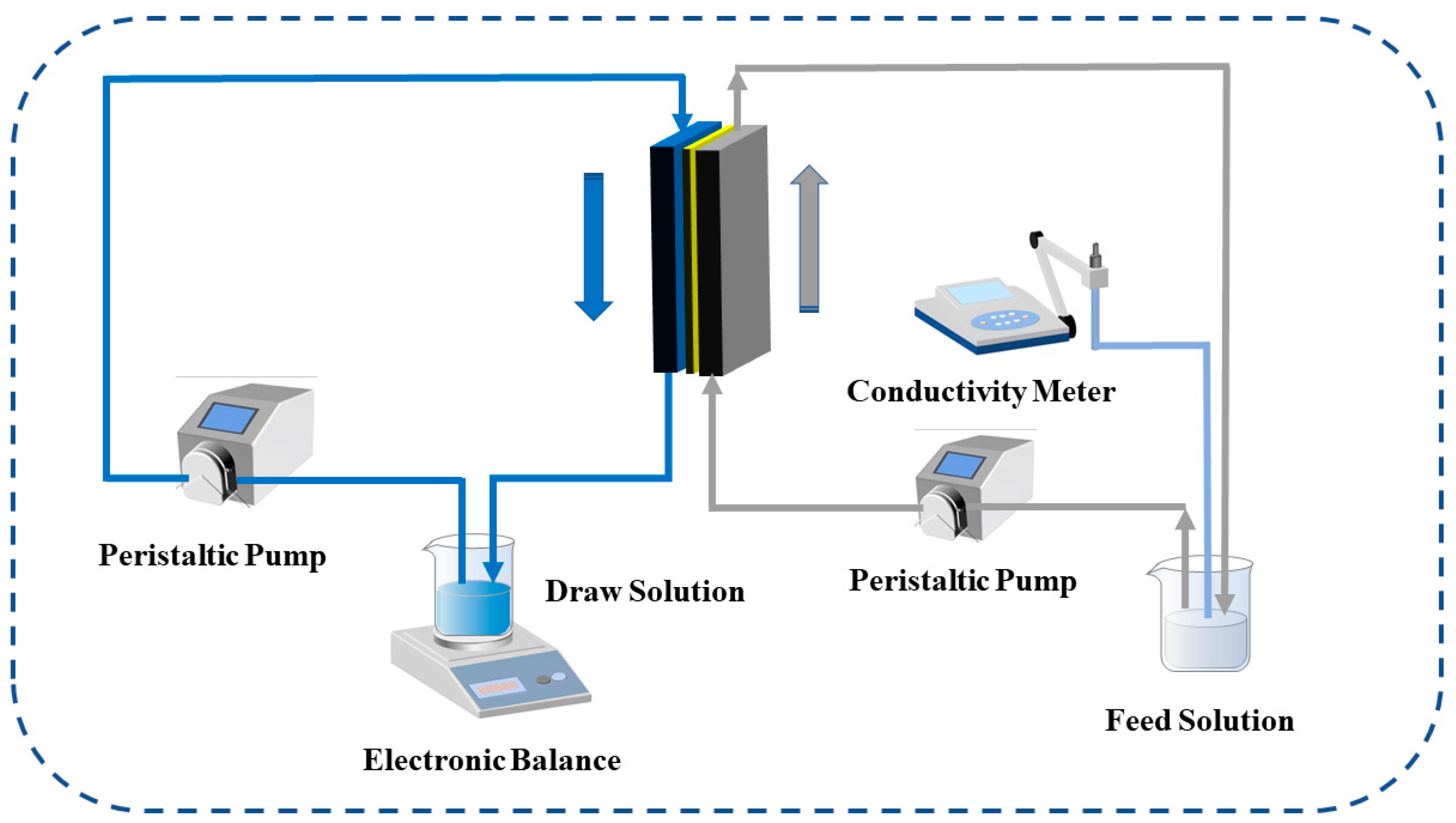
2.5. Permeability Test of the MXene Nano-Sheets Modified TFC-FO Membranes
2.5.1. Test Equipment
2.5.2. Test Parameters
3. Results and Discussion
3.1. Characterization of MXene Nano-Sheets
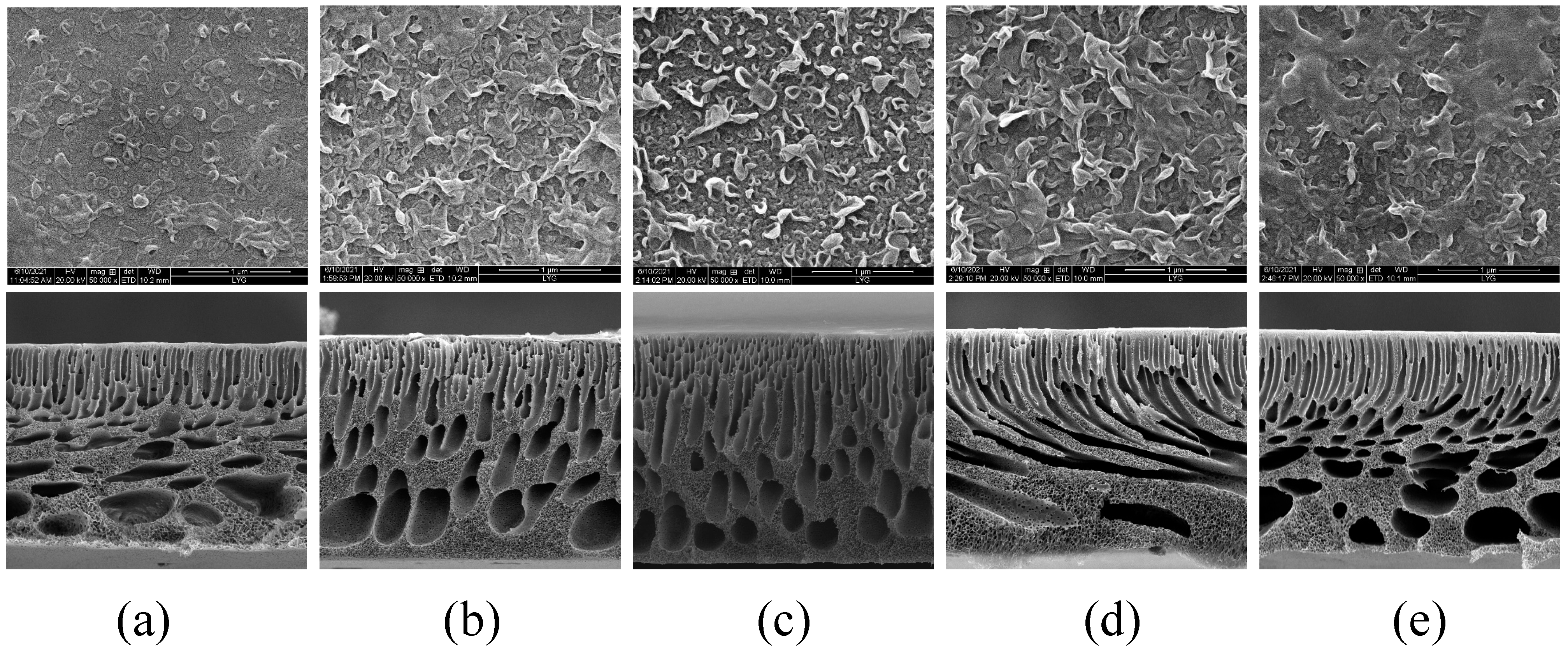
3.2. Characterization of the Physical Morphology of the TFC-FO Membranes Modified by MXene Nano-Sheets
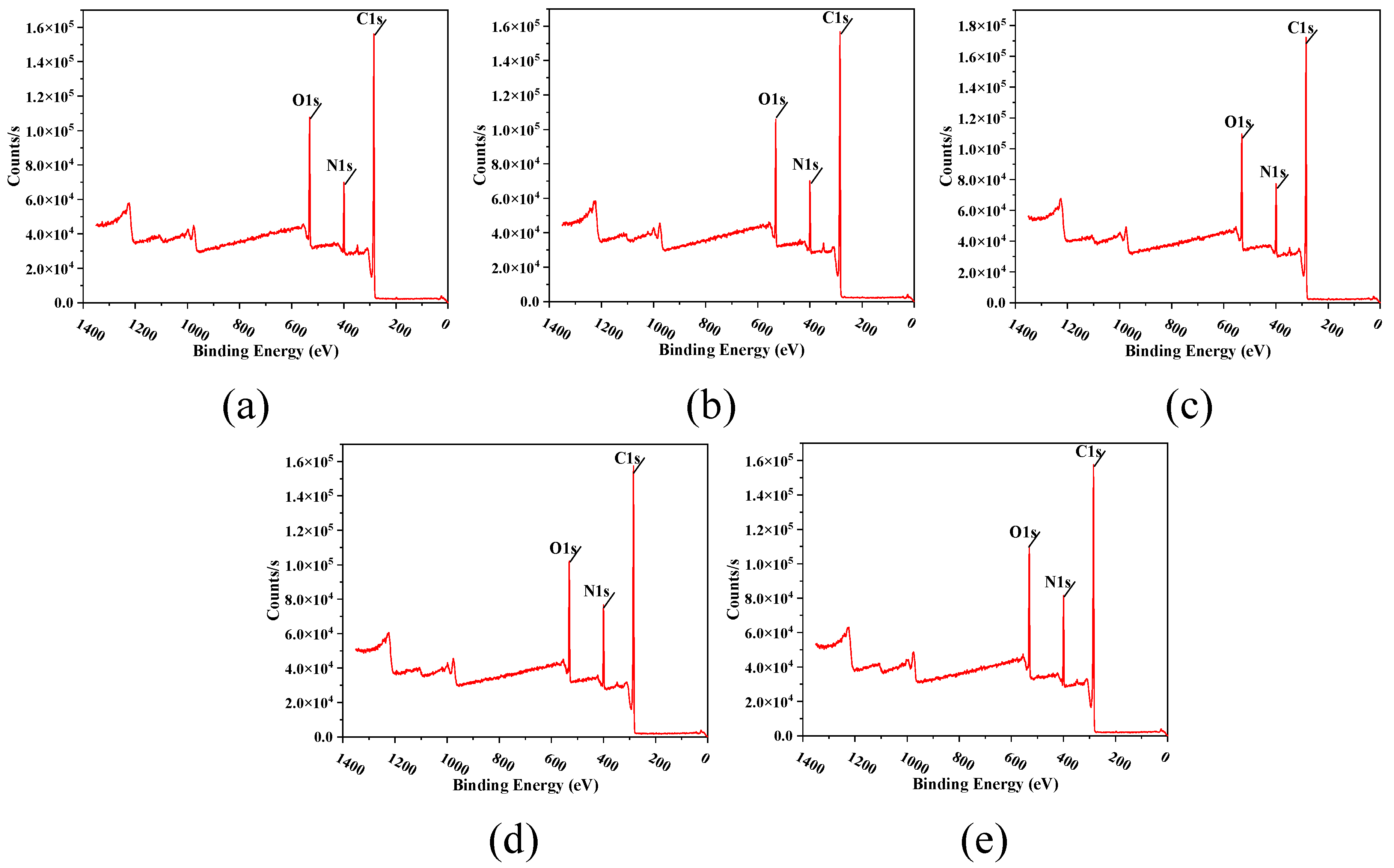
3.3. Chemical Element Composition Characterization of the TFC-FO Membranes Modified by MXene Nano-Sheets
3.4. Permeability Test of the MXene Nano-Sheets Modified TFC-FO Membranes
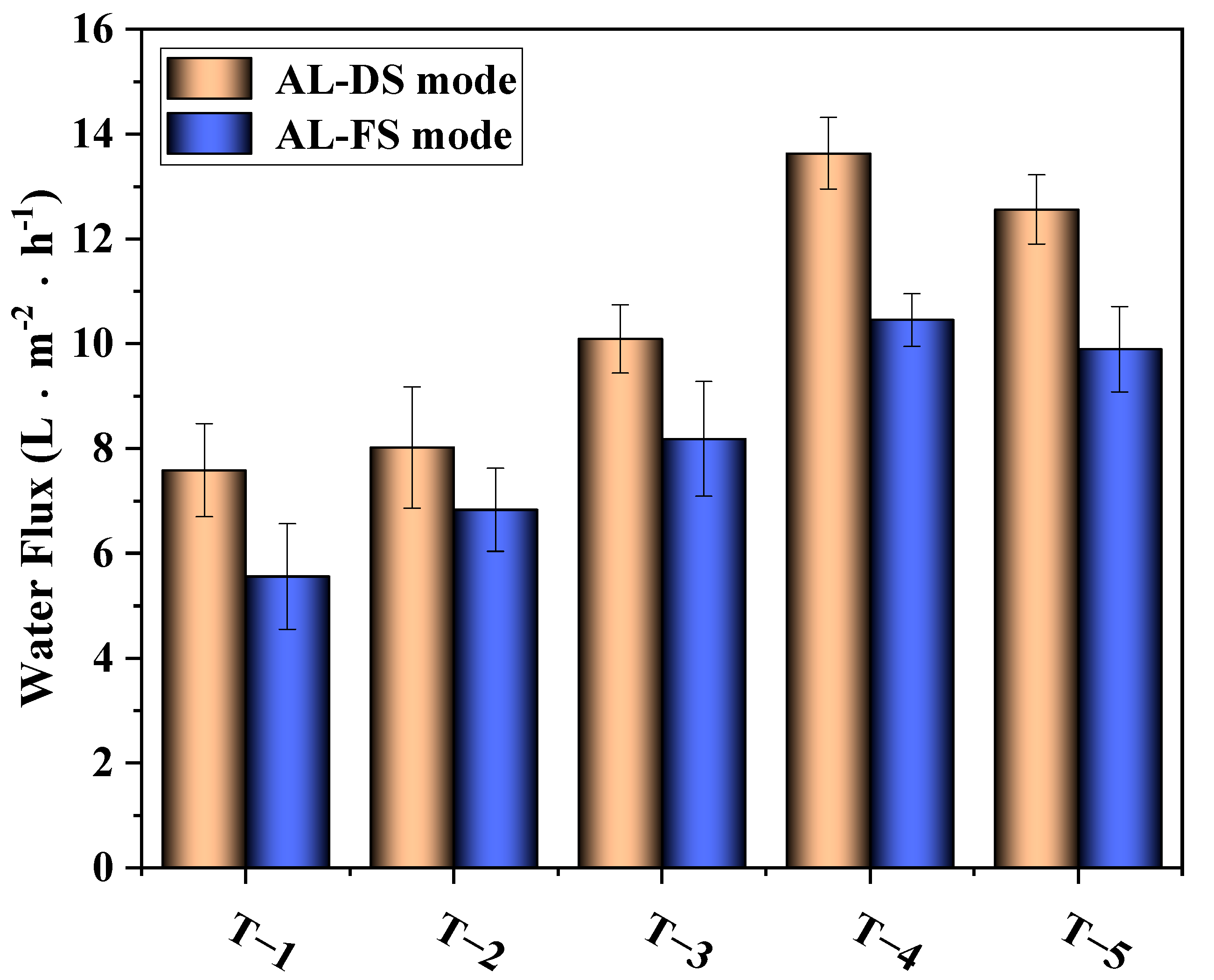
3.4.1. Effects of MXene Nano-Sheets on the Water Flux of the TFC-FO Membranes
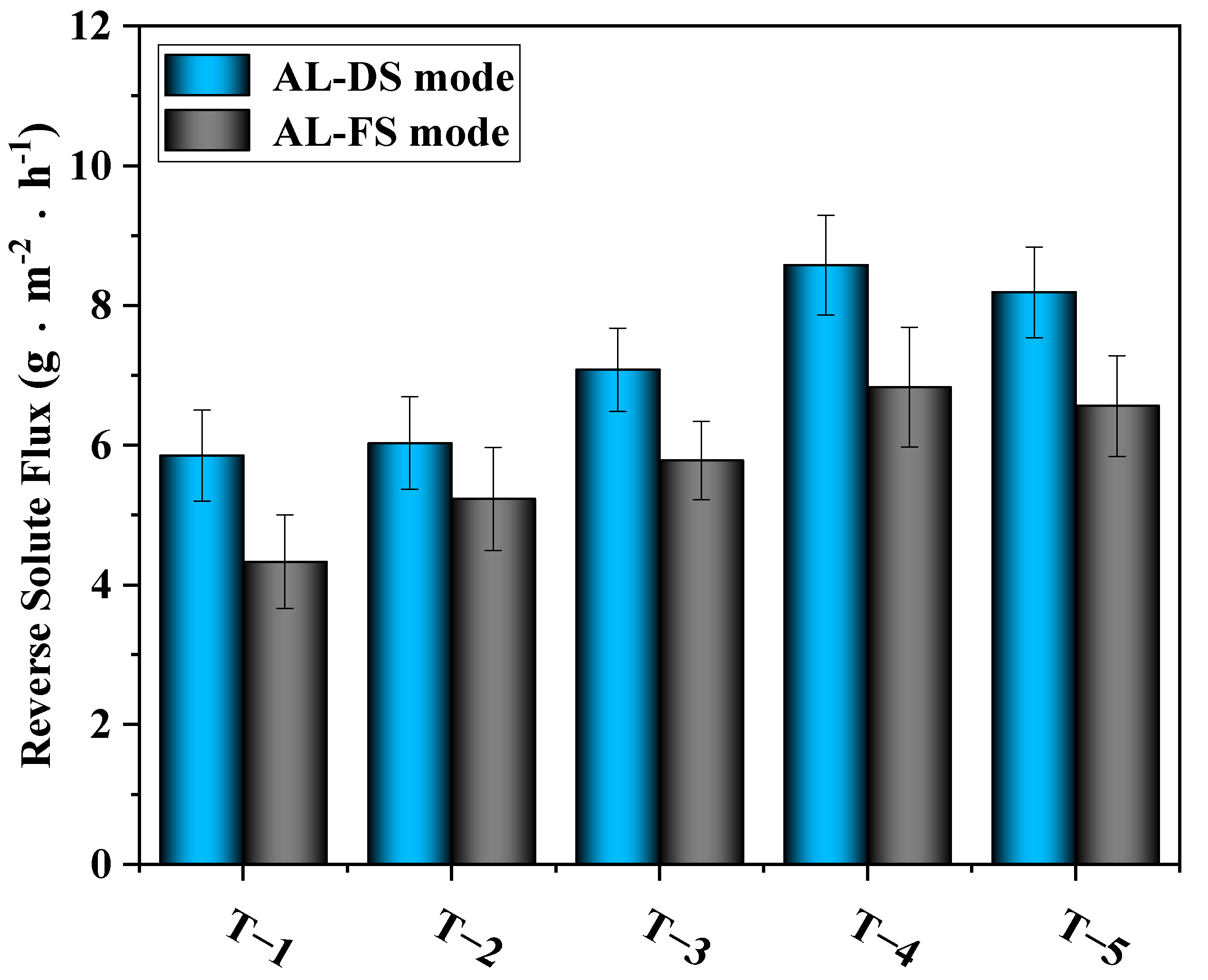
3.4.2. Effects of MXene Nano-Sheets on the Reverse Solute Flux of the TFC-FO Membranes
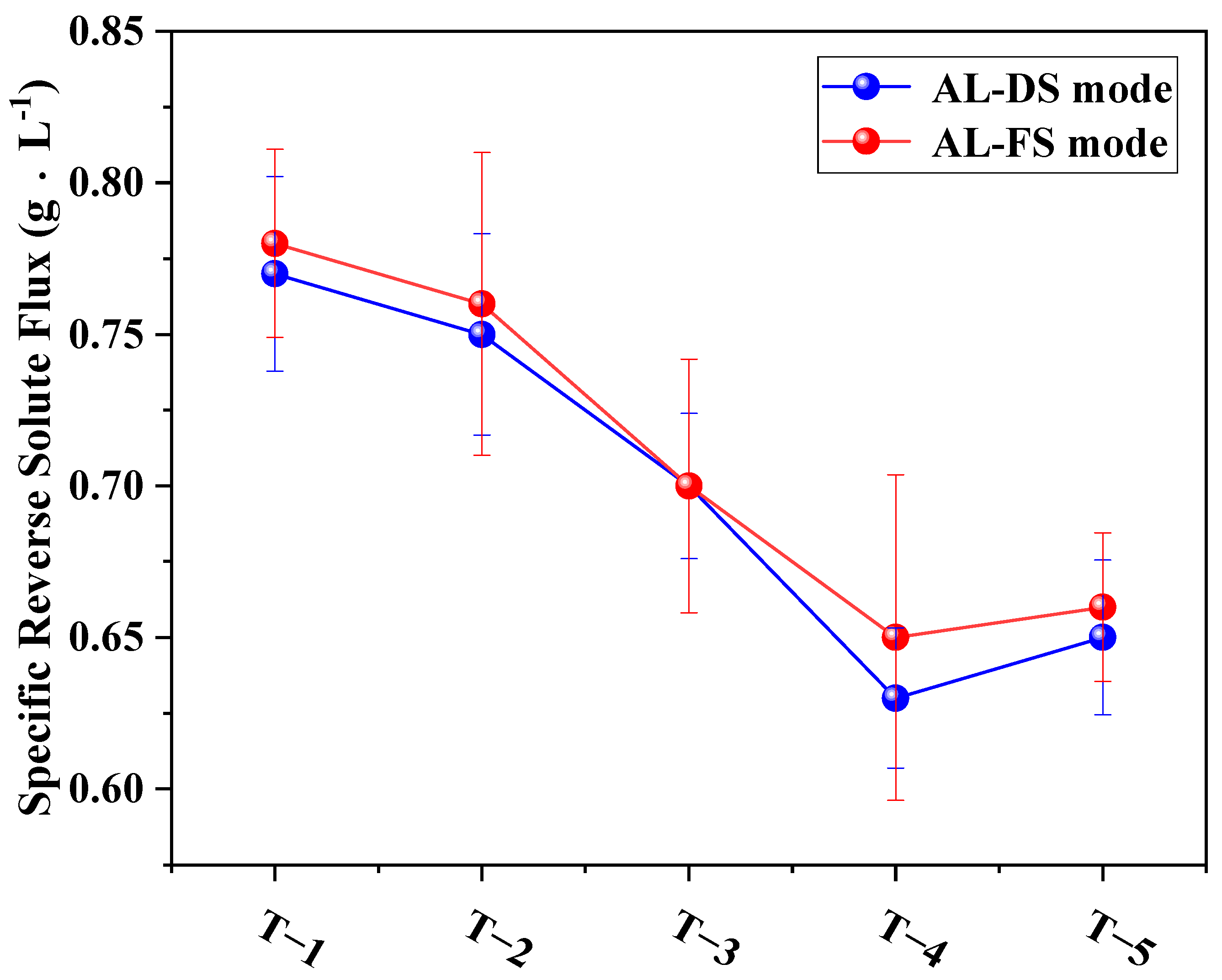
3.4.3. Effects of MXene Nano-Sheets on the Specific Reverse Solute Flux of TFC-FO Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alihemati, Z.; Hashemifard, S.A.; Matsuura, T.; Ismail, A.F.; Hilal, N. Current Status and Challenges of Fabricating Thin Film Composite Forward Osmosis Membrane: A Comprehensive Roadmap. Desalination 2020, 491, 114557. [Google Scholar] [CrossRef]
- Chia, W.Y.; Chia, S.R.; Khoo, K.S.; Chew, K.W.; Show, P.L. Sustainable Membrane Technology for Resource Recovery from Wastewater: Forward Osmosis and Pressure Retarded Osmosis. J. Water Process Eng. 2021, 39, 101758. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X. Forward Osmosis Technology for Water Treatment: Recent Advances and Future Perspectives. J. Clean. Prod. 2021, 280, 124354. [Google Scholar] [CrossRef]
- Lugito, G.; Ariono, D.; Rizqy Trihutama Putra, M.; Nabilla Zafra, Z. Progress, Challenges, and Prospects of Forward Osmosis (FO) and Pressure Retarded Osmosis (PRO) as An Alternative Solution for Water and Energy Crisis. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1143, 012060. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z.; Sun, D.D. Nano Gives the Answer: Breaking the Bottleneck of Internal Concentration Polarization with a Nanofiber Composite Forward Osmosis Membrane for a High Water Production Rate. Adv. Mater. 2011, 23, 3256–3260. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Yu, M.; Wang, Y.; Gao, T.; Yang, F. Conductive Thin Film Nanocomposite Forward Osmosis Membrane (TFN-FO) Blended with Carbon Nanoparticles for Membrane Fouling Control. Sci. Total Environ. 2019, 697, 134050. [Google Scholar] [CrossRef]
- Niksefat, N.; Jahanshahi, M.; Rahimpour, A. The Effect of SiO2 Nanoparticles on Morphology and Performance of Thin Film Composite Membranes for Forward Osmosis Application. Desalination 2014, 343, 140–146. [Google Scholar] [CrossRef]
- Rezaei-DashtArzhandi, M.; Sarrafzadeh, M.H.; Goh, P.S.; Lau, W.J.; Ismail, A.F.; Wong, K.C.; Mohamed, M.A. Enhancing the Desalination Performance of Forward Osmosis Membrane through the Incorporation of Green Nanocrystalline Cellulose and Halloysite Dual Nanofillers. J. Chem. Technol. Biotechnol. 2020, 95, 2359–2370. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Li, J.; Li, J.; Fan, M.; Han, M.; Liu, Z.; Li, Z.; Kong, F. Metal Organic Framework UiO-66 Incorporated Ultrafiltration Membranes for Simultaneous Organic Matter and Heavy Metal Ions Removal. Environ. Res. 2022, 112651. [Google Scholar] [CrossRef]
- Ahmed, Z.; Rehman, F.; Ali, U.; Ali, A.; Iqbal, M.; Thebo, K.H. Recent Advances in MXene-Based Separation Membranes. ChemBioEng Rev. 2021, 8, 110–120. [Google Scholar] [CrossRef]
- Nie, Y.; Xie, C.; Wang, Y. Preparation and Characterization of the Forward Osmosis Membrane Modified by MXene Nano-Sheets. Membranes 2022, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, J.; Liu, Y.; Xu, Y.; Li, R.; Hong, H.; Shen, L.; Lin, H.; Liao, B.Q. Enhanced Permeability and Antifouling Performance of Polyether Sulfone (PES) Membrane via Elevating Magnetic Ni@MXene Nanoparticles to Upper Layer in Phase Inversion Process. J. Membr. Sci. 2021, 623, 119080. [Google Scholar] [CrossRef]
- Alfahel, R.; Azzam, R.S.; Hafiz, M.; Hawari, A.H.; Pandey, R.P.; Mahmoud, K.A.; Hassan, M.K.; Elzatahry, A.A. Fabrication of Fouling Resistant Ti3C2Tx (MXene)/Cellulose Acetate Nanocomposite Membrane for Forward Osmosis Application. J. Water Process Eng. 2020, 38, 101551. [Google Scholar] [CrossRef]
- Wu, X.; Ding, M.; Xu, H.; Yang, W.; Zhang, K.; Tian, H.; Wang, H.; Xie, Z. Scalable Ti3 C2Tx MXene Interlayered Forward Osmosis Membranes for Enhanced Water Purification and Organic Solvent Recovery. ACS Nano 2020, 14, 9125–9135. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhao, S.; Fang, Z.; Ng, D.; Xie, C.; Wang, H.; Xie, Z. Thin-Film Composite Membrane with Interlayer Decorated Metal–Organic Framework UiO-66 toward Enhanced Forward Osmosis Performance. Ind. Eng. Chem. Res. 2019, 58, 195–206. [Google Scholar] [CrossRef]
- Raaijmakers, M.J.T.; Benes, N.E. Current Trends in Interfacial Polymerization Chemistry. Prog. Polym. Sci. 2016, 63, 86–142. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent Developments in Forward Osmosis: Opportunities and Challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Zhang, S.; Liao, S.; Qi, F.; Liu, R.; Xiao, T.; Hu, J.; Li, K.; Wang, R.; Min, Y. Direct Deposition of Two-Dimensional MXene Nanosheets on Commercially Available Filter for Fast and Efficient Dye Removal. J. Hazard. Mater. 2020, 384, 121367. [Google Scholar] [CrossRef]
- Liu, G.; Shen, J.; Liu, Q.; Liu, G.; Xiong, J.; Yang, J.; Jin, W. Ultrathin Two-Dimensional MXene Membrane for Pervaporation Desalination. J. Membr. Sci. 2018, 548, 548–558. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, W.; Xu, H.; Yang, W.; Kong, Q.; Wang, A.; Ding, M.; Shang, J. Fabrication of a Novel Antifouling Polysulfone Membrane with in Situ Embedment of Mxene Nanosheets. Int. J. Environ. Res. Public Health 2019, 16, 4659. [Google Scholar] [CrossRef] [Green Version]
- Han, R.; Xie, Y.; Ma, X. Crosslinked P84 Copolyimide/MXene Mixed Matrix Membrane with Excellent Solvent Resistance and Permselectivity. Chin. J. Chem. Eng. 2019, 27, 877–883. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Abdel-Ghafar, H.M.; Abdel-Aal, E.S.A.; Huang, M.; Gul, S.; Jiang, H. Polyamide Membrane with an Ultrathin GO Interlayer on Macroporous Substrate for Minimizing Internal Concentration Polarization in Forward Osmosis. Chem. Eng. J. 2021, 412, 128607. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, C.; Tang, C.Y.; Wang, R.; Fane, A.G. Synthesis and Characterization of Flat-Sheet Thin Film Composite Forward Osmosis Membranes. J. Membr. Sci. 2011, 372, 292–302. [Google Scholar] [CrossRef]
- Park, M.J.; Phuntsho, S.; He, T.; Nisola, G.M.; Tijing, L.D.; Li, X.M.; Chen, G.; Chung, W.J.; Shon, H.K. Graphene Oxide Incorporated Polysulfone Substrate for the Fabrication of Flat-Sheet Thin-Film Composite Forward Osmosis Membranes. J. Membr. Sci. 2015, 493, 496–507. [Google Scholar] [CrossRef]
- Peng, L.E.; Yang, Z.; Long, L.; Zhou, S.; Guo, H.; Tang, C.Y. A Critical Review on Porous Substrates of TFC Polyamide Membranes: Mechanisms, Membrane Performances, and Future Perspectives. J. Membr. Sci. 2022, 641, 119871. [Google Scholar] [CrossRef]
- Zhou, J.; He, H.L.; Sun, F.; Su, Y.; Yu, H.Y.; Gu, J.S. Structural Parameters Reduction in Polyamide Forward Osmosis Membranes via Click Modification of the Polysulfone Support. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124082. [Google Scholar] [CrossRef]
- Sun, H.; Luo, X.; Liu, J.; Li, G.; Zhang, Y.; Li, P.; Niu, Q.J. Novel Thin-Film Composite Pervaporation Membrane with Controllable Crosslinking Degree for Enhanced Water/Alcohol Separation Performance. Sep. Purif. Technol. 2020, 234, 116027. [Google Scholar] [CrossRef]
- Wang, R.; Shi, L.; Tang, C.Y.; Chou, S.; Qiu, C.; Fane, A.G. Characterization of Novel Forward Osmosis Hollow Fiber Membranes. J. Membr. Sci. 2010, 355, 158–167. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Guan, C.Y.; Liu, C.X.; Lang, W.Z.; Wang, Y. Construction of SiO2@MWNTs Incorporated PVDF Substrate for Reducing Internal Concentration Polarization in Forward Osmosis. J. Membr. Sci. 2018, 564, 328–341. [Google Scholar] [CrossRef]
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge- and Size-Selective Ion Sieving through Ti3C2Tx MXene Membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031. [Google Scholar] [CrossRef]
- Karahan, H.E.; Goh, K.; Zhang, C.; Yang, E.; Yıldırım, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, B.; Chen, Y.; et al. MXene Materials for Designing Advanced Separation Membranes. Adv. Mater. 2020, 32, 1906697. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wei, Y.; Deng, J.; Ding, L.; Li, Z.K.; Wang, H. Self-Crosslinked MXene (Ti3C2Tx) Membranes with Good Antiswelling Property for Monovalent Metal Ion Exclusion. ACS Nano 2019, 13, 10535–10544. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Fan, Y.; Meng, X.; Li, J.; Li, C.; Sunarso, J.; Yang, N.; Meng, B.; Zhang, W. Modeling of Hydrated Cations Transport through 2D MXene (Ti3C2Tx) Membranes for Water Purification. J. Membr. Sci. 2021, 631, 119346. [Google Scholar] [CrossRef]

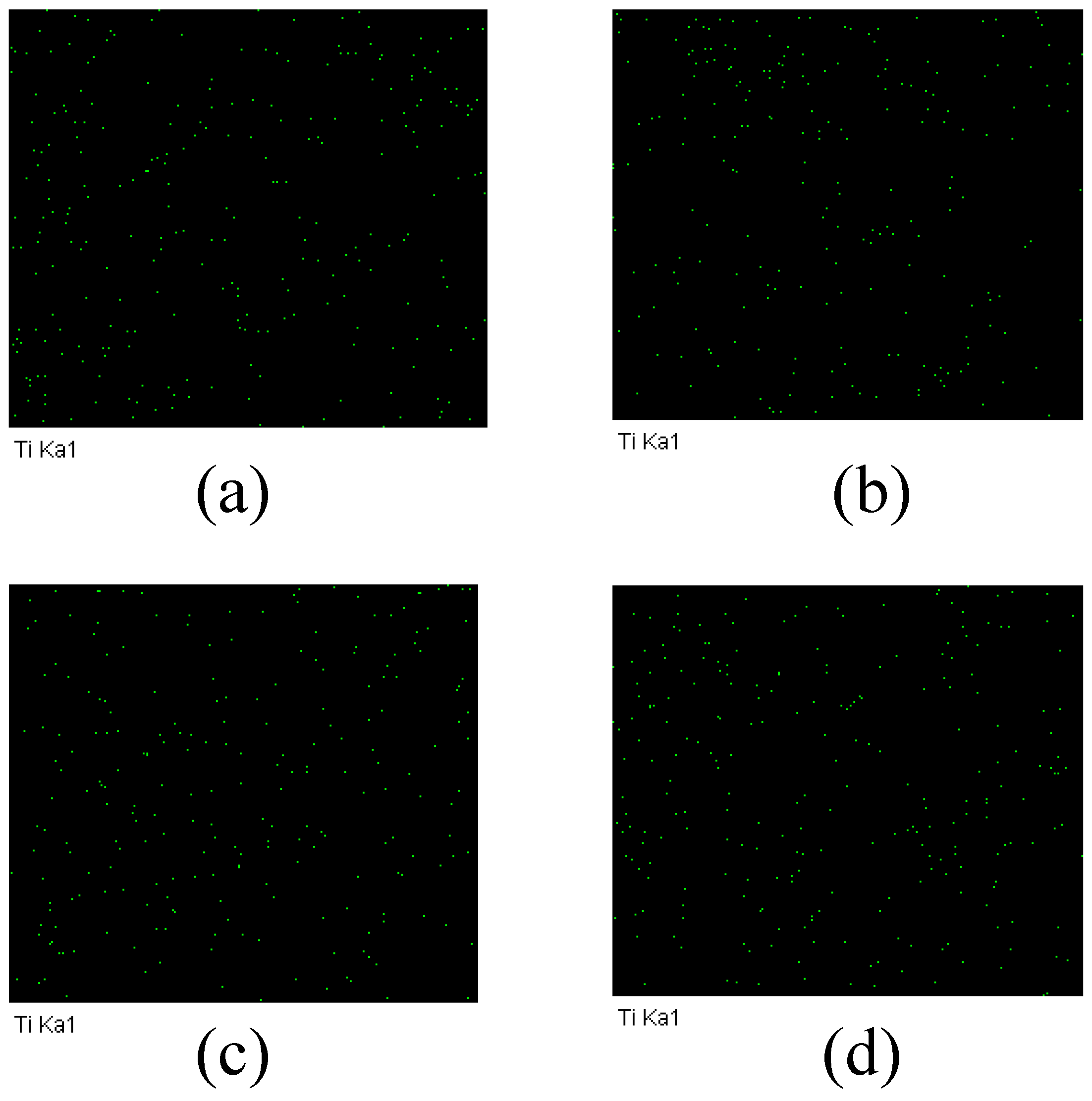
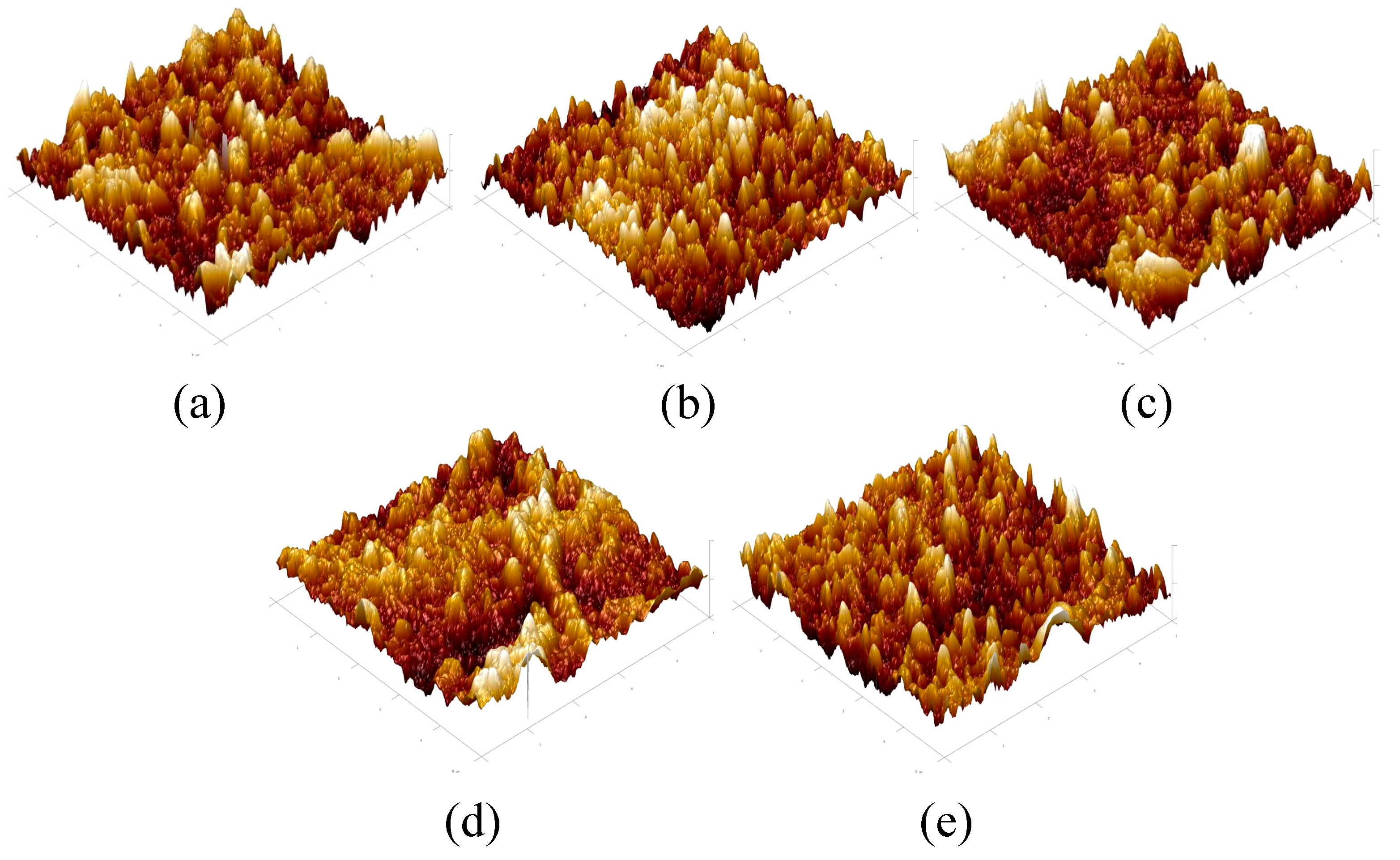
| TFC-FO Membranes | Ra (nm) | Rms (nm) |
|---|---|---|
| T-1 | 23.9 | 34.6 |
| T-2 | 32.4 | 40.3 |
| T-3 | 35.7 | 45.3 |
| T-4 | 40.2 | 53.4 |
| T-5 | 38.3 | 51.2 |
| TFC-FO Membranes | C Content (%) | O Content (%) | N Content (%) | O/N Value |
|---|---|---|---|---|
| T-1 | 75.41 | 13.91 | 10.68 | 1.30 |
| T-2 | 74.82 | 13.68 | 11.39 | 1.20 |
| T-3 | 73.93 | 13.84 | 12.08 | 1.15 |
| T-4 | 74.43 | 13.48 | 11.97 | 1.13 |
| T-5 | 72.21 | 14.84 | 12.78 | 1.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Nie, Y.; Chen, C.; Zhao, H.; Zhao, Y.; Jia, Y.; Li, J.; Li, Z. Preparation and Characterization of a Thin-Film Composite Membrane Modified by MXene Nano-Sheets. Membranes 2022, 12, 368. https://doi.org/10.3390/membranes12040368
Wang Y, Nie Y, Chen C, Zhao H, Zhao Y, Jia Y, Li J, Li Z. Preparation and Characterization of a Thin-Film Composite Membrane Modified by MXene Nano-Sheets. Membranes. 2022; 12(4):368. https://doi.org/10.3390/membranes12040368
Chicago/Turabian StyleWang, Yi, Yuqi Nie, Chunhong Chen, Hongjie Zhao, Ye Zhao, Yujin Jia, Jun Li, and Zhanguo Li. 2022. "Preparation and Characterization of a Thin-Film Composite Membrane Modified by MXene Nano-Sheets" Membranes 12, no. 4: 368. https://doi.org/10.3390/membranes12040368
APA StyleWang, Y., Nie, Y., Chen, C., Zhao, H., Zhao, Y., Jia, Y., Li, J., & Li, Z. (2022). Preparation and Characterization of a Thin-Film Composite Membrane Modified by MXene Nano-Sheets. Membranes, 12(4), 368. https://doi.org/10.3390/membranes12040368







