The Impact of Operational Parameters on Polypropylene Membrane Performance during the Separation of Oily Saline Wastewaters by the Membrane Distillation Process
Abstract
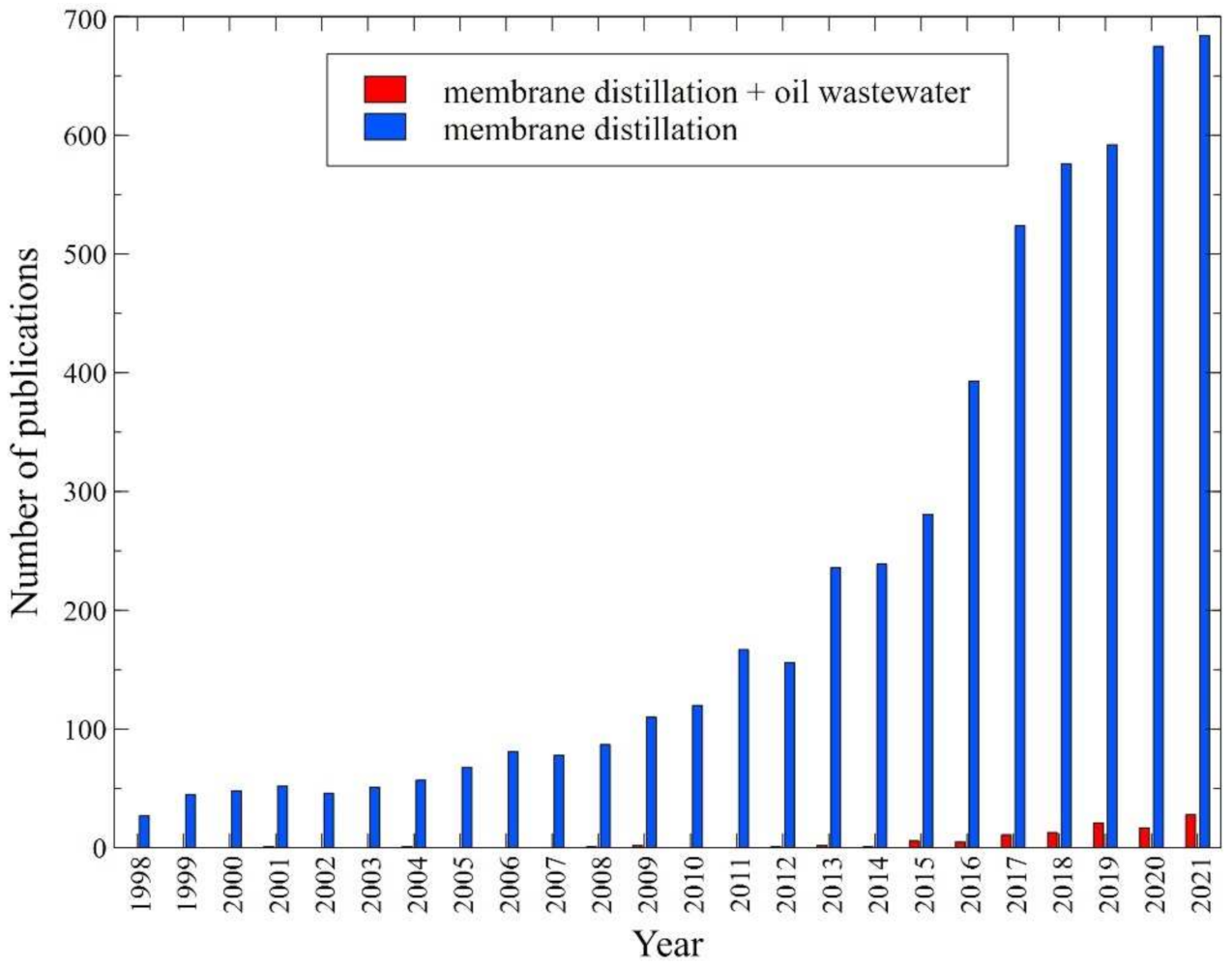
:1. Introduction
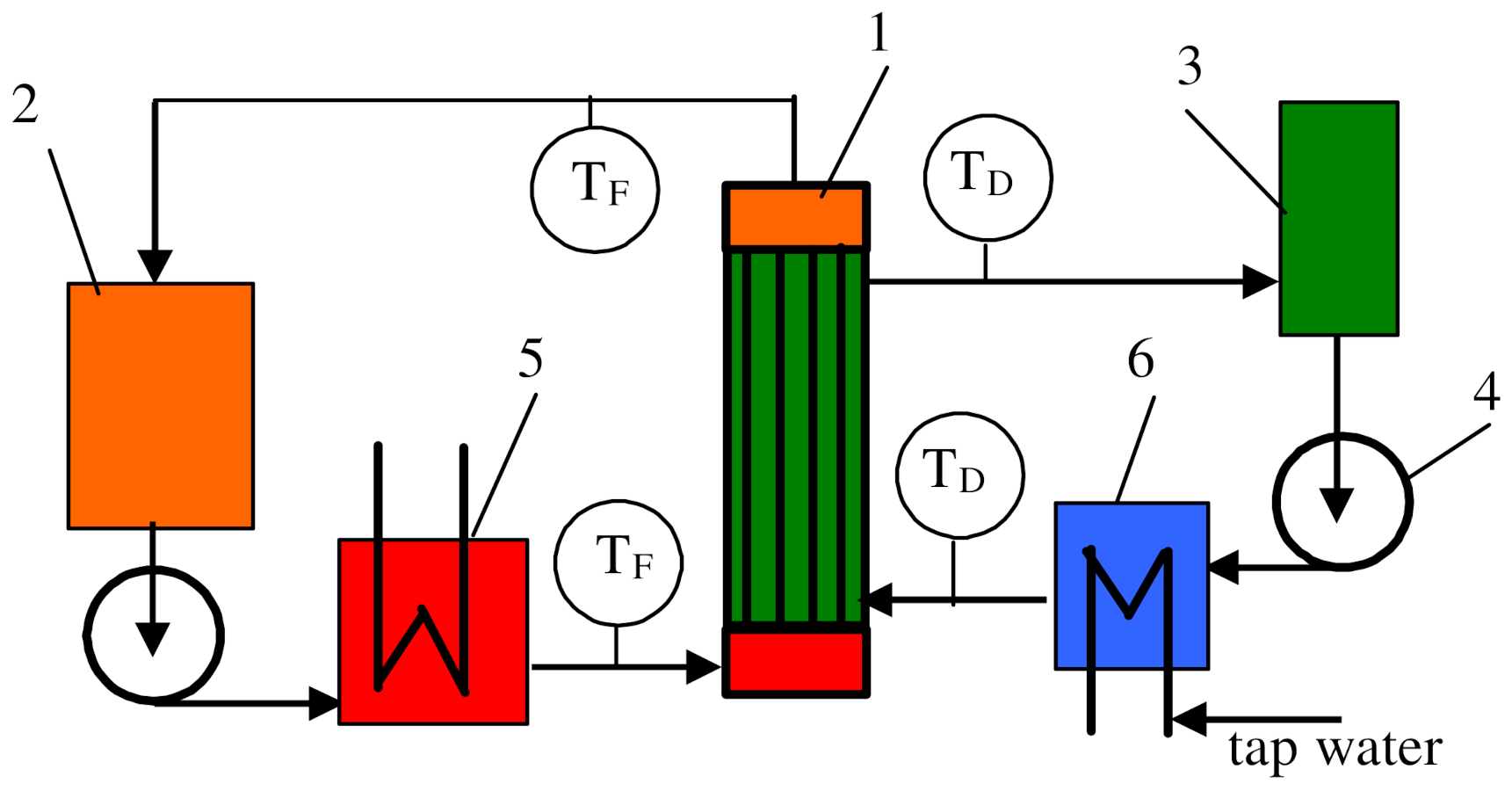
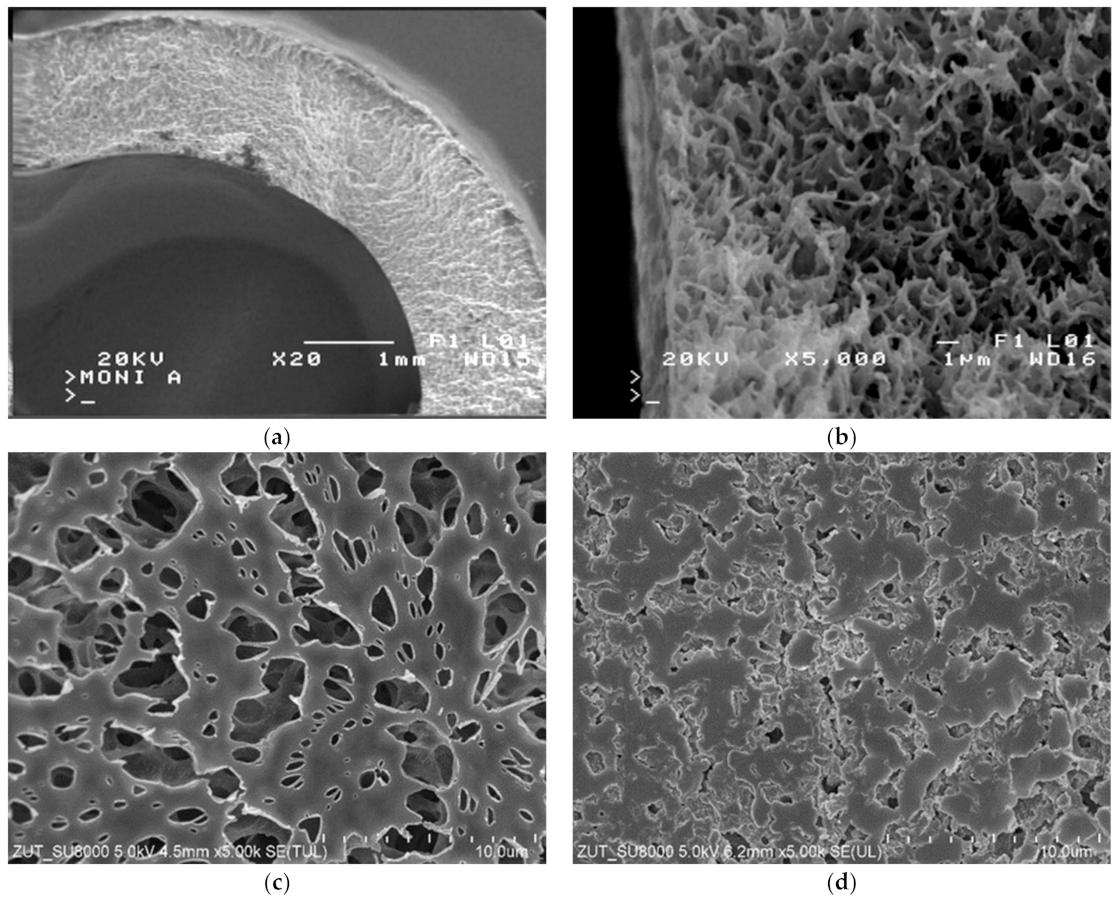
2. Materials and Methods
3. Results and Discussion
3.1. MD Process of Salt Solution and Oil Emulsion
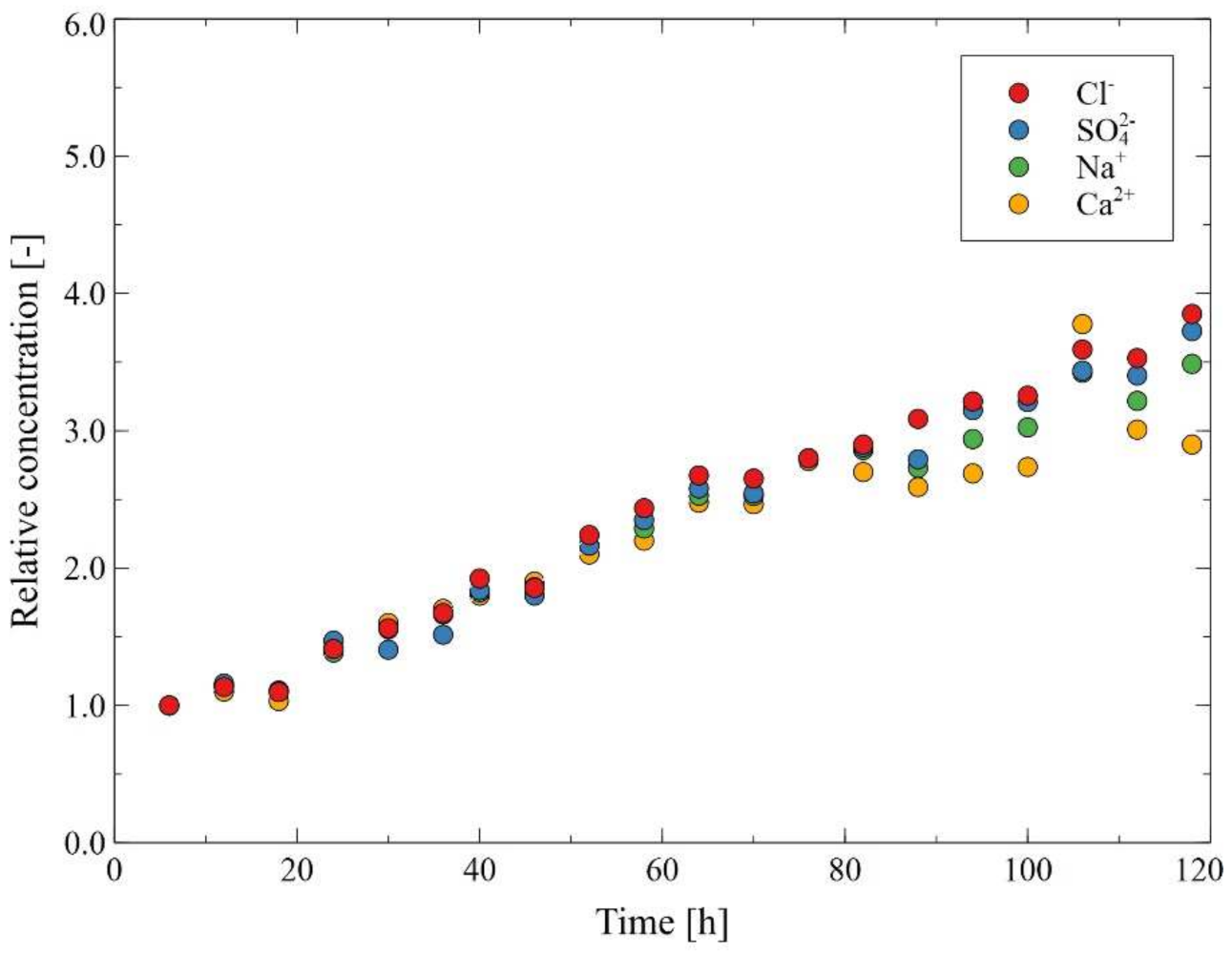
3.2. MD Process of the Baltic Seawater
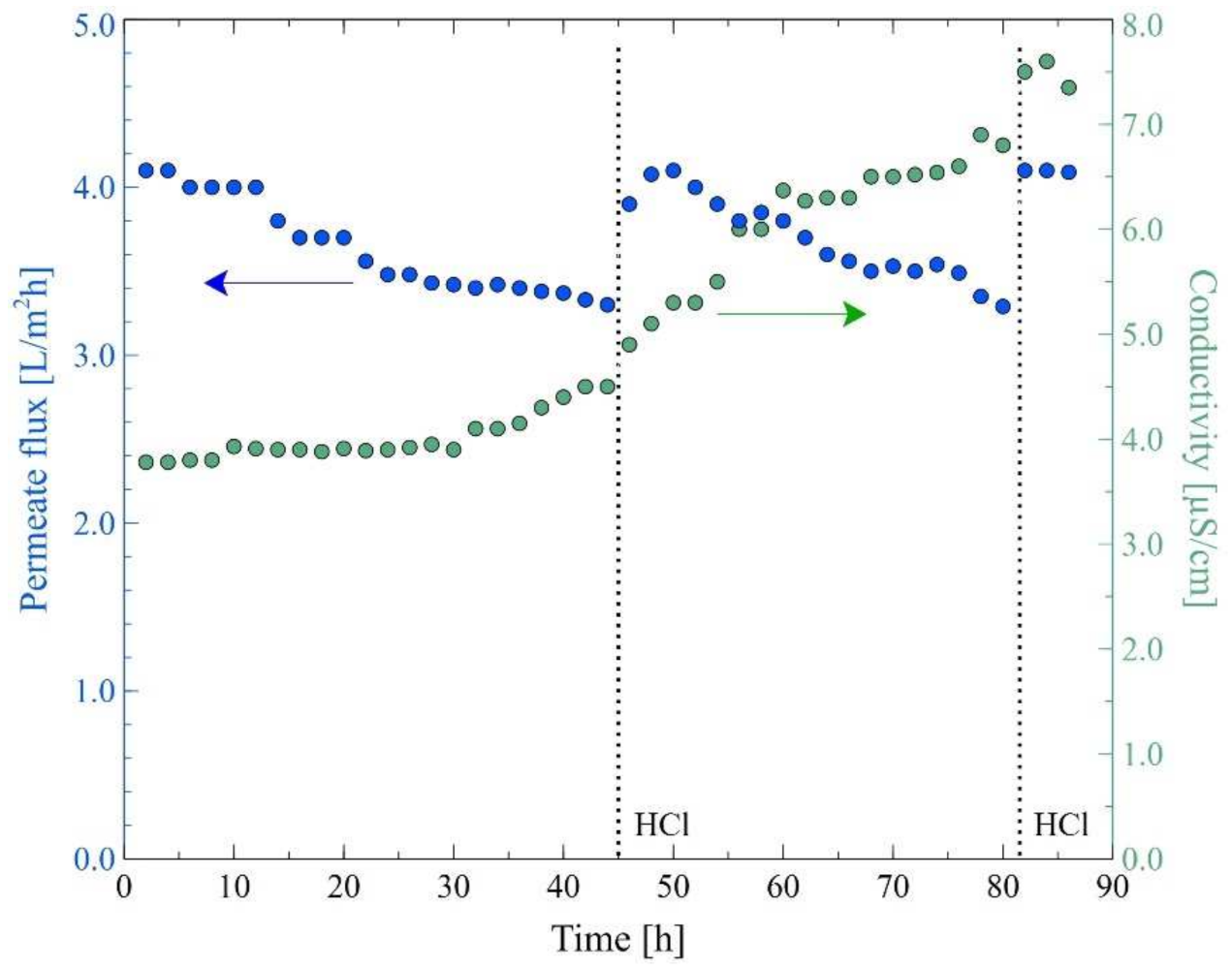
3.3. MD Process of Baltic Seawater Contaminated with Oil
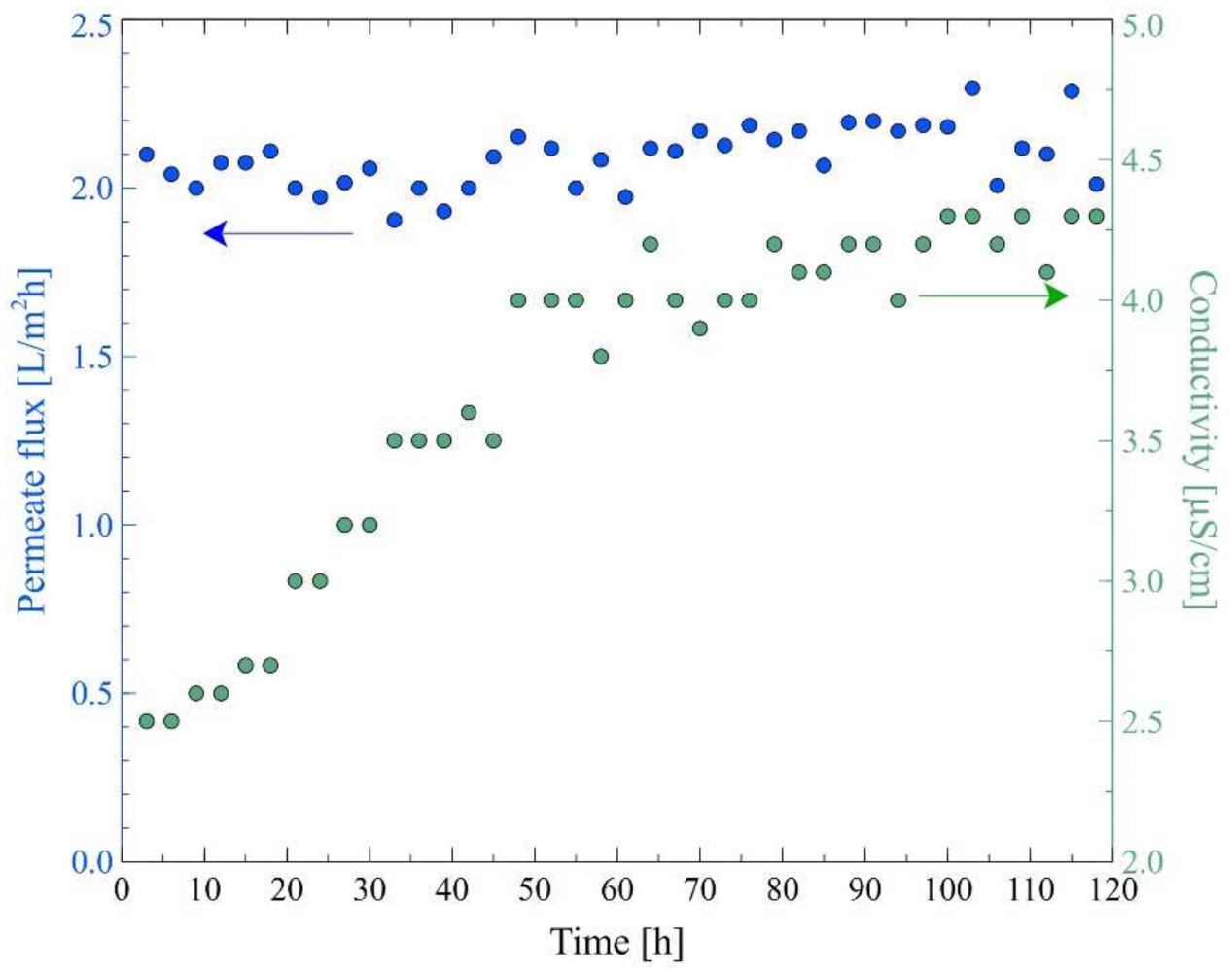
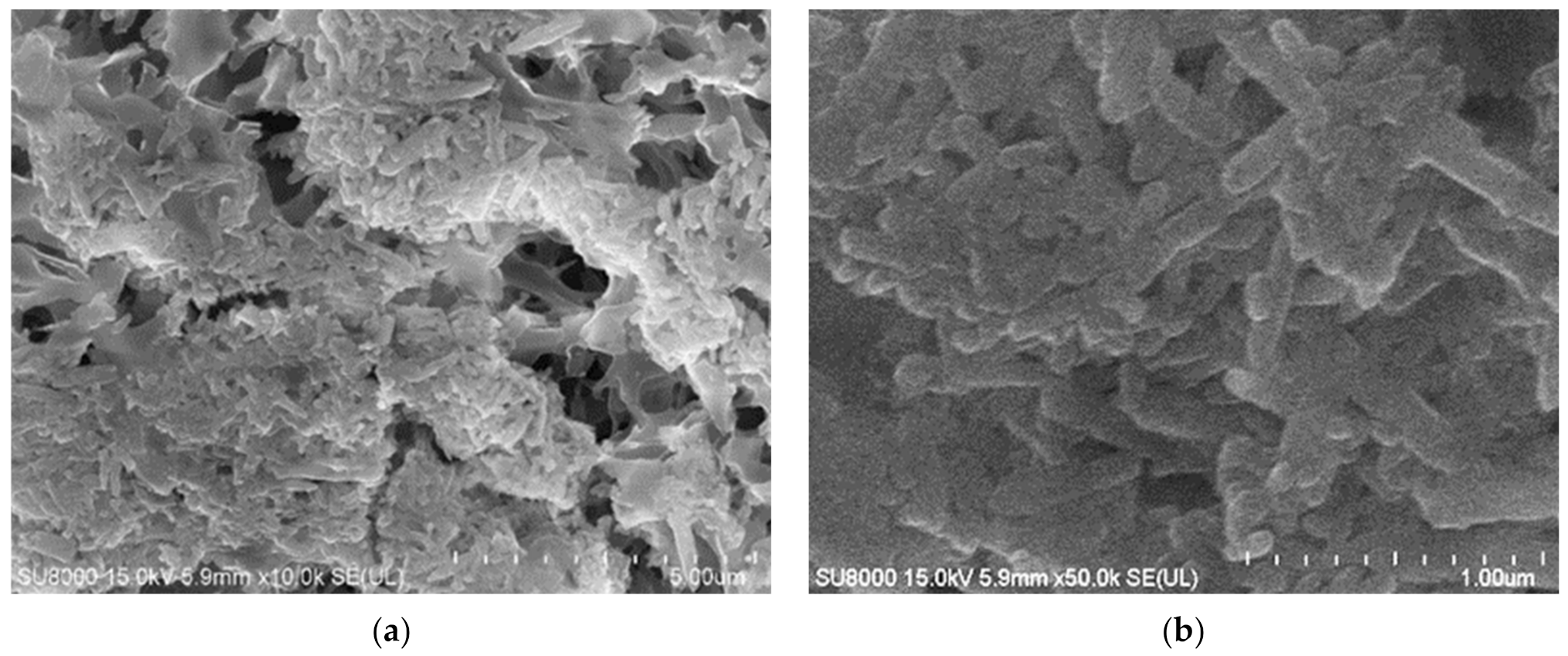
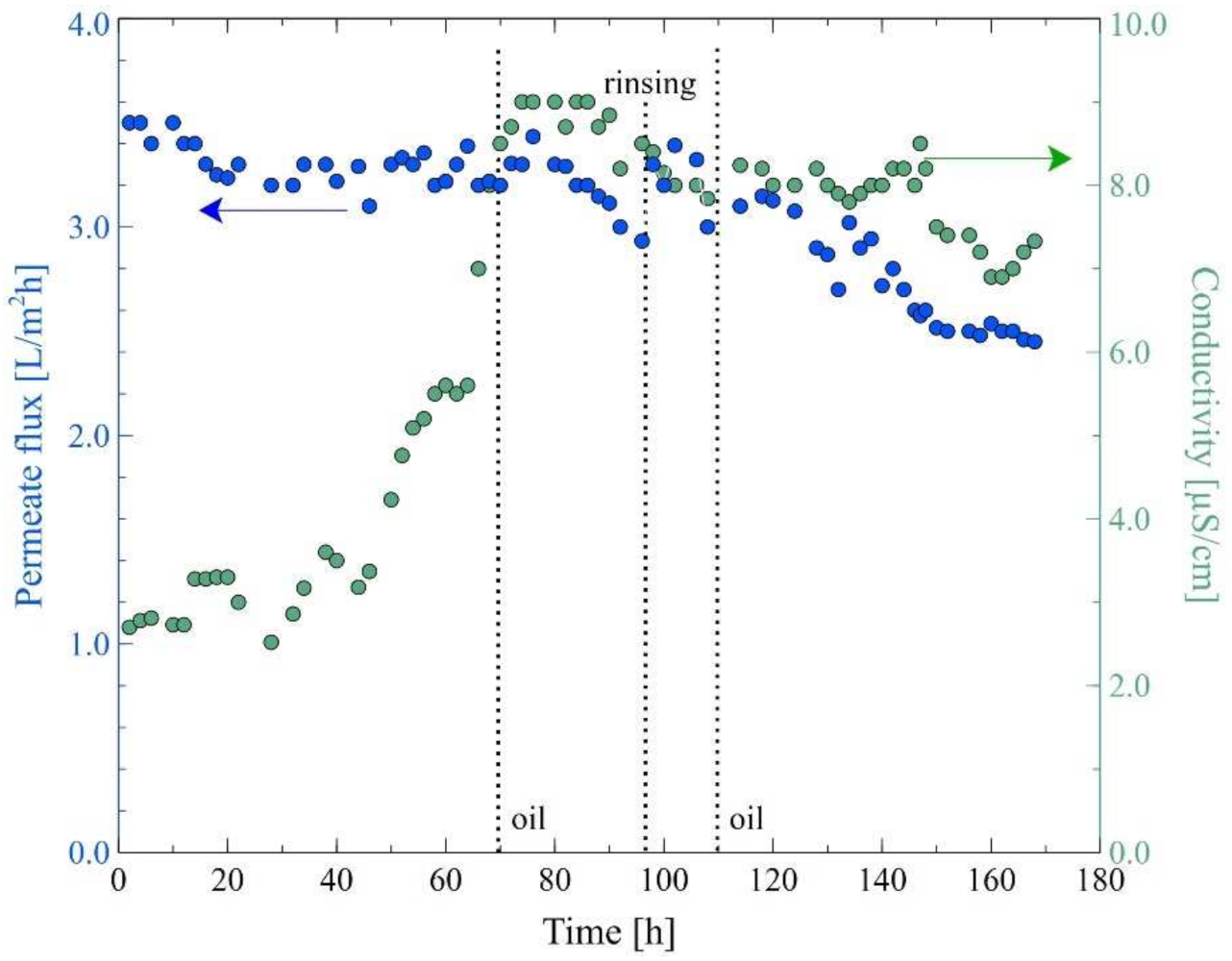
3.4. MD Process of Oily Wastewaters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Curcio, E.; Ji, X.; Di Profio, G.; Sulaiman, A.O.; Fontananova, E.; Drioli, E. Membrane Distillation Operated at High Seawater Concentration Factors: Role of the Membrane on CaCO3 Scaling in Presence of Humic Acid. J. Membr. Sci. 2010, 346, 263–269. [Google Scholar] [CrossRef]
- Biniaz, P.; Torabi Ardekani, N.; Makarem, M.; Rahimpour, M. Water and Wastewater Treatment Systems by Novel Integrated Membrane Distillation (MD). ChemEngineering 2019, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Julian, H.; Nurgirisia, N.; Qiu, G.; Ting, Y.-P.; Wenten, I.G. Membrane Distillation for Wastewater Treatment: Current Trends, Challenges and Prospects of Dense Membrane Distillation. J. Water Process Eng. 2022, 46, 102615. [Google Scholar] [CrossRef]
- Hussain, A.; Janson, A.; Matar, J.M.; Adham, S. Membrane Distillation: Recent Technological Developments and Advancements in Membrane Materials. Emergent Mater. 2021, 1–21. [Google Scholar] [CrossRef]
- Azeem, M.A.; Lawal, D.U.; Al Abdulgader, H.; Baroud, T.N. Enhanced Performance of Superhydrophobic Polyvinylidene Fluoride Membrane with Sandpaper Texture for Highly Saline Water Desalination in Air-Gap Membrane Distillation. Desalination 2022, 528, 115603. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Suga, Y.; Takagi, R.; Matsuyama, H. Effect of Hollow Fiber Membrane Properties and Operating Conditions on Preventing Scale Precipitation in Seawater Desalination with Vacuum Membrane Distillation. Desalination 2022, 527, 115578. [Google Scholar] [CrossRef]
- Jiang, K.; Chamani, H.; Matsuura, T.; Rana, D.; Lan, C. Effect of Temperature and Salt on PVDF Membrane Wetting Properties. JCM 2021. [Google Scholar] [CrossRef]
- Zuo, J.; Bonyadi, S.; Chung, T.-S. Exploring the Potential of Commercial Polyethylene Membranes for Desalination by Membrane Distillation. J. Membr. Sci. 2016, 497, 239–247. [Google Scholar] [CrossRef]
- Kim, H.-W.; Yun, T.; Hong, S.; Lee, S.; Jeong, S. Retardation of Wetting for Membrane Distillation by Adjusting Major Components of Seawater. Water Res. 2020, 175, 115677. [Google Scholar] [CrossRef]
- Lu, C.H.; Fabuss, B.M. Calcium Sulfate Scaling in Saline Water Distillation. Ind. Eng. Chem. Proc. Des. Dev. 1968, 7, 206–212. [Google Scholar] [CrossRef]
- Swaminathan, J.; Lienhard, J.H. Design and Operation of Membrane Distillation with Feed Recirculation for High Recovery Brine Concentration. Desalination 2018, 445, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Park, K.-D.; Kim, B.; Lee, C.; Lee, S.; Choi, J.-S. Effect of Brine Concentration on Membrane Distillation Process for Seawater Desalination. DWT 2017, 77, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Elcik, H.; Fortunato, L.; Alpatova, A.; Soukane, S.; Orfi, J.; Ali, E.; AlAnsary, H.; Leiknes, T.; Ghaffour, N. Multi-Effect Distillation Brine Treatment by Membrane Distillation: Effect of Antiscalant and Antifoaming Agents on Membrane Performance and Scaling Control. Desalination 2020, 493, 114653. [Google Scholar] [CrossRef]
- Criscuoli, A. Thermal Performance of Integrated Direct Contact and Vacuum Membrane Distillation Units. Energies 2021, 14, 7405. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Conidi, C.; Cassano, A.; Aljlil, S.; Alharbi, O.A.; Drioli, E. Direct Contact Membrane Distillation for the Concentration of Clarified Orange Juice. J. Food Eng. 2016, 187, 37–43. [Google Scholar] [CrossRef]
- Gunko, S.; Verbych, S.; Bryk, M.; Hilal, N. Concentration of Apple Juice Using Direct Contact Membrane Distillation. Desalination 2006, 190, 117–124. [Google Scholar] [CrossRef]
- Chen, Q.; Burhan, M.; Akhtar, F.H.; Ybyraiymkul, D.; Shahzad, M.W.; Li, Y.; Ng, K.C. A Decentralized Water/Electricity Cogeneration System Integrating Concentrated Photovoltaic/Thermal Collectors and Vacuum Multi-Effect Membrane Distillation. Energy 2021, 230, 120852. [Google Scholar] [CrossRef]
- Bahar, R.; Ng, K.C. Fresh Water Production by Membrane Distillation (MD) Using Marine Engine’s Waste Heat. Sustain. Energy Technol. Assess. 2020, 42, 100860. [Google Scholar] [CrossRef]
- Khayet, M. Treatment of Radioactive Wastewater Solutions by Direct Contact Membrane Distillation Using Surface Modified Membranes. Desalination 2013, 321, 60–66. [Google Scholar] [CrossRef]
- Zakrzewska-Trznadel, G.; Harasimowicz, M.; Chmielewski, A.G. Concentration of Radioactive Components in Liquid Low-Level Radioactive Waste by Membrane Distillation. J. Membr. Sci. 1999, 163, 257–264. [Google Scholar] [CrossRef]
- Fortunato, L.; Elcik, H.; Blankert, B.; Ghaffour, N.; Vrouwenvelder, J. Textile Dye Wastewater Treatment by Direct Contact Membrane Distillation: Membrane Performance and Detailed Fouling Analysis. J. Membr. Sci. 2021, 636, 119552. [Google Scholar] [CrossRef]
- Villalobos García, J.; Dow, N.; Milne, N.; Zhang, J.; Naidoo, L.; Gray, S.; Duke, M. Membrane Distillation Trial on Textile Wastewater Containing Surfactants Using Hydrophobic and Hydrophilic-Coated Polytetrafluoroethylene (PTFE) Membranes. Membranes 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Salmi, M.; Laqbaqbi, M.; Al-Obaidani, S.; Al-Maamari, R.S.; Khayet, M.; Al-Abri, M. Application of Membrane Distillation for the Treatment of Oil Field Produced Water. Desalination 2020, 494, 114678. [Google Scholar] [CrossRef]
- Gryta, M. Bilge Water Separation by Membrane Distillation. Sep. Purif. Technol. 2020, 237, 116332. [Google Scholar] [CrossRef]
- Gryta, M. Separation of Saline Oily Wastewater by Membrane Distillation. Chem. Pap. 2020, 74, 2277–2286. [Google Scholar] [CrossRef] [Green Version]
- Tomczak, W.; Gryta, M. Membrane Distillation of Saline Water Contaminated with Oil and Surfactants. Membranes 2021, 11, 988. [Google Scholar] [CrossRef]
- Kalla, S. Use of Membrane Distillation for Oily Wastewater Treatment—A Review. J. Environ. Chem. Eng. 2021, 9, 104641. [Google Scholar] [CrossRef]
- Han, L.; Tan, Y.Z.; Netke, T.; Fane, A.G.; Chew, J.W. Understanding Oily Wastewater Treatment via Membrane Distillation. J. Membr. Sci. 2017, 539, 284–294. [Google Scholar] [CrossRef]
- Velioğlu, S.; Han, L.; Chew, J.W. Understanding Membrane Pore-Wetting in the Membrane Distillation of Oil Emulsions via Molecular Dynamics Simulations. J. Membr. Sci. 2018, 551, 76–84. [Google Scholar] [CrossRef]
- Tibi, F.; Charfi, A.; Cho, J.; Kim, J. Fabrication of Polymeric Membranes for Membrane Distillation Process and Application for Wastewater Treatment: Critical Review. Process Saf. Environ. Prot. 2020, 141, 190–201. [Google Scholar] [CrossRef]
- Yao, M.; Tijing, L.D.; Naidu, G.; Kim, S.-H.; Matsuyama, H.; Fane, A.G.; Shon, H.K. A Review of Membrane Wettability for the Treatment of Saline Water Deploying Membrane Distillation. Desalination 2020, 479, 114312. [Google Scholar] [CrossRef]
- Guan, Y.; Li, J.; Cheng, F.; Zhao, J.; Wang, X. Influence of Salt Concentration on DCMD Performance for Treatment of Highly Concentrated NaCl, KCl, MgCl2 and MgSO4 Solutions. Desalination 2015, 355, 110–117. [Google Scholar] [CrossRef]
- Laqbaqbi, M.; García-Payo, M.C.; Khayet, M.; El Kharraz, J.; Chaouch, M. Application of Direct Contact Membrane Distillation for Textile Wastewater Treatment and Fouling Study. Sep. Purif. Technol. 2019, 209, 815–825. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, W.; Zhang, X.; Liu, Q.; Li, J.; Ma, J.; Zhang, T. Membrane Scaling and Wetting in Membrane Distillation: Mitigation Roles Played by Humic Substances. Environ. Sci. Technol. 2022, 56, 328–3266. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Bopape, M.F.; Mahlangu, O.T.; Mamba, B.B.; Van der Bruggen, B.; Quist-Jensen, C.A.; Richards, H. Fouling, Performance and Cost Analysis of Membrane-Based Water Desalination Technologies: A Critical Review. J. Environ. Manag. 2022, 301, 113922. [Google Scholar] [CrossRef]
- Yang, S.; Abdalkareem Jasim, S.; Bokov, D.; Chupradit, S.; Nakhjiri, A.T.; El-Shafay, A.S. Membrane Distillation Technology for Molecular Separation: A Review on the Fouling, Wetting and Transport Phenomena. J. Mol. Liq. 2022, 349, 118115. [Google Scholar] [CrossRef]
- Chamani, H.; Woloszyn, J.; Matsuura, T.; Rana, D.; Lan, C.Q. Pore Wetting in Membrane Distillation: A Comprehensive Review. Prog. Mater. Sci. 2021, 122, 100843. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, Z.; Liu, Y.; Li, X.; Yin, H.; Volkov, A.; He, T. Understanding the Fouling/Scaling Resistance of Superhydrophobic/Omniphobic Membranes in Membrane Distillation. Desalination 2021, 499, 114864. [Google Scholar] [CrossRef]
- Horseman, T.; Yin, Y.; Christie, K.S.; Wang, Z.; Tong, T.; Lin, S. Wetting, Scaling, and Fouling in Membrane Distillation: State-of-the-Art Insights on Fundamental Mechanisms and Mitigation Strategies. ACS EST Eng. 2021, 1, 117–140. [Google Scholar] [CrossRef]
- Lee, W.J.; Ng, Z.C.; Hubadillah, S.K.; Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F.; Hilal, N. Fouling Mitigation in Forward Osmosis and Membrane Distillation for Desalination. Desalination 2020, 480, 114338. [Google Scholar] [CrossRef]
- Choudhury, M.R.; Anwar, N.; Jassby, D.; Rahaman, M.S. Fouling and Wetting in the Membrane Distillation Driven Wastewater Reclamation Process—A Review. Adv. Colloid Interface Sci. 2019, 269, 370–399. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Lienhard, V.J.H.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting Phenomena in Membrane Distillation: Mechanisms, Reversal, and Prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Vigneswaran, S.; Hwang, T.-M.; Choi, Y.-J.; Kim, S.-H. A Review on Fouling of Membrane Distillation. Desalin. Water Treat. 2016, 57, 10052–10076. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard, V.J.H. Scaling and Fouling in Membrane Distillation for Desalination Applications: A Review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Fouling and Its Control in Membrane Distillation—A Review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Gryta, M. Resistance of Polypropylene Membrane to Oil Fouling during Membrane Distillation. Membranes 2021, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.M.A.; Kargari, A. A Review on Applications of Membrane Distillation (MD) Process for Wastewater Treatment. J. Membr. Sci. Res. 2015, 1, 101–112. [Google Scholar] [CrossRef]
- Macedonio, F.; Ali, A.; Poerio, T.; El-Sayed, E.; Drioli, E.; Abdel-Jawad, M. Direct Contact Membrane Distillation for Treatment of Oilfield Produced Water. Sep. Purif. Technol. 2014, 126, 69–81. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane Distillation: A Comprehensive Review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Anvari, A.; Azimi Yancheshme, A.; Kekre, K.M.; Ronen, A. State-of-the-Art Methods for Overcoming Temperature Polarization in Membrane Distillation Process: A Review. J. Membr. Sci. 2020, 616, 118413. [Google Scholar] [CrossRef]
- Ge, J.; Peng, Y.; Li, Z.; Chen, P.; Wang, S. Membrane Fouling and Wetting in a DCMD Process for RO Brine Concentration. Desalination 2014, 344, 97–107. [Google Scholar] [CrossRef]
- Julian, H.; Yaohanny, F.; Devina, A.; Purwadi, R.; Wenten, I.G. Apple Juice Concentration Using Submerged Direct Contact Membrane Distillation (SDCMD). J. Food Eng. 2020, 272, 109807. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J. Treatment of Radioactive Wastewater Using Direct Contact Membrane Distillation. J. Hazard. Mater. 2013, 261, 307–315. [Google Scholar] [CrossRef]
- Sparenberg, M.-C.; Hanot, B.; Molina-Fernández, C.; Luis, P. Experimental Mass Transfer Comparison between Vacuum and Direct Contact Membrane Distillation for the Concentration of Carbonate Solutions. Sep. Purif. Technol. 2021, 275, 119193. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Ameen, N.A.M. Desalination of Highly Saline Water Using Direct Contact Membrane Distillation (DCMD). Al-Khwarizmi Eng. J. 2019, 14, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Boubakri, A.; Hafiane, A.; Al Tahar Bouguecha, S. Nitrate Removal from Aqueous Solution by Direct Contact Membrane Distillation Using Two Different Commercial Membranes. Desalin. Water Treat. 2015, 56, 2723–2730. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, S.; Zhu, Y.; Shen, Y.; Gao, X.; Shi, W.; Hwa Tay, J. Adsorption Mechanisms of Crude Oil onto Polytetrafluoroethylene Membrane: Kinetics and Isotherm, and Strategies for Adsorption Fouling Control. Sep. Purif. Technol. 2020, 235, 116212. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, S. Membrane Fouling and Wetting in Membrane Distillation and Their Mitigation by Novel Membranes with Special Wettability. Water Res. 2017, 112, 38–47. [Google Scholar] [CrossRef]
- Tang, Y.P.; Chan, J.X.; Chung, T.S.; Weber, M.; Staudt, C.; Maletzko, C. Simultaneously Covalent and Ionic Bridging towards Antifouling of GO-Imbedded Nanocomposite Hollow Fiber Membranes. J. Mater. Chem. A 2015, 3, 10573–10584. [Google Scholar] [CrossRef]
- Gryta, M. Mitigation of Membrane Wetting by Applying a Low Temperature Membrane Distillation. Membranes 2020, 10, 158. [Google Scholar] [CrossRef]
- Gryta, M. Polyphosphates Used for Membrane Scaling Inhibition during Water Desalination by Membrane Distillation. Desalination 2012, 285, 170–176. [Google Scholar] [CrossRef]
- Gryta, M. Calcium Sulphate Scaling in Membrane Distillation Process. Chem. Pap. 2009, 63, 146–151. [Google Scholar] [CrossRef]
- Zhong, W.; Li, H.; Ye, Y.; Chen, V. Evaluation of Silica Fouling for Coal Seam Gas Produced Water in a Submerged Vacuum Membrane Distillation System. Desalination 2016, 393, 52–64. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Z.; Guo, H.; Liu, Y.; Wang, Y.; Yin, H.; Li, X.; Song, J.; Nghiem, L.D.; He, T. Scaling Mitigation in Membrane Distillation: From Superhydrophobic to Slippery. Desalination 2019, 466, 36–43. [Google Scholar] [CrossRef]
- Yin, Y.; Jeong, N.; Tong, T. The Effects of Membrane Surface Wettability on Pore Wetting and Scaling Reversibility Associated with Mineral Scaling in Membrane Distillation. J. Membr. Sci. 2020, 614, 118503. [Google Scholar] [CrossRef]
- Gryta, M.; Tomaszewska, M.; Karakulski, K. Wastewater Treatment by Membrane Distillation. Desalination 2006, 198, 67–73. [Google Scholar] [CrossRef]





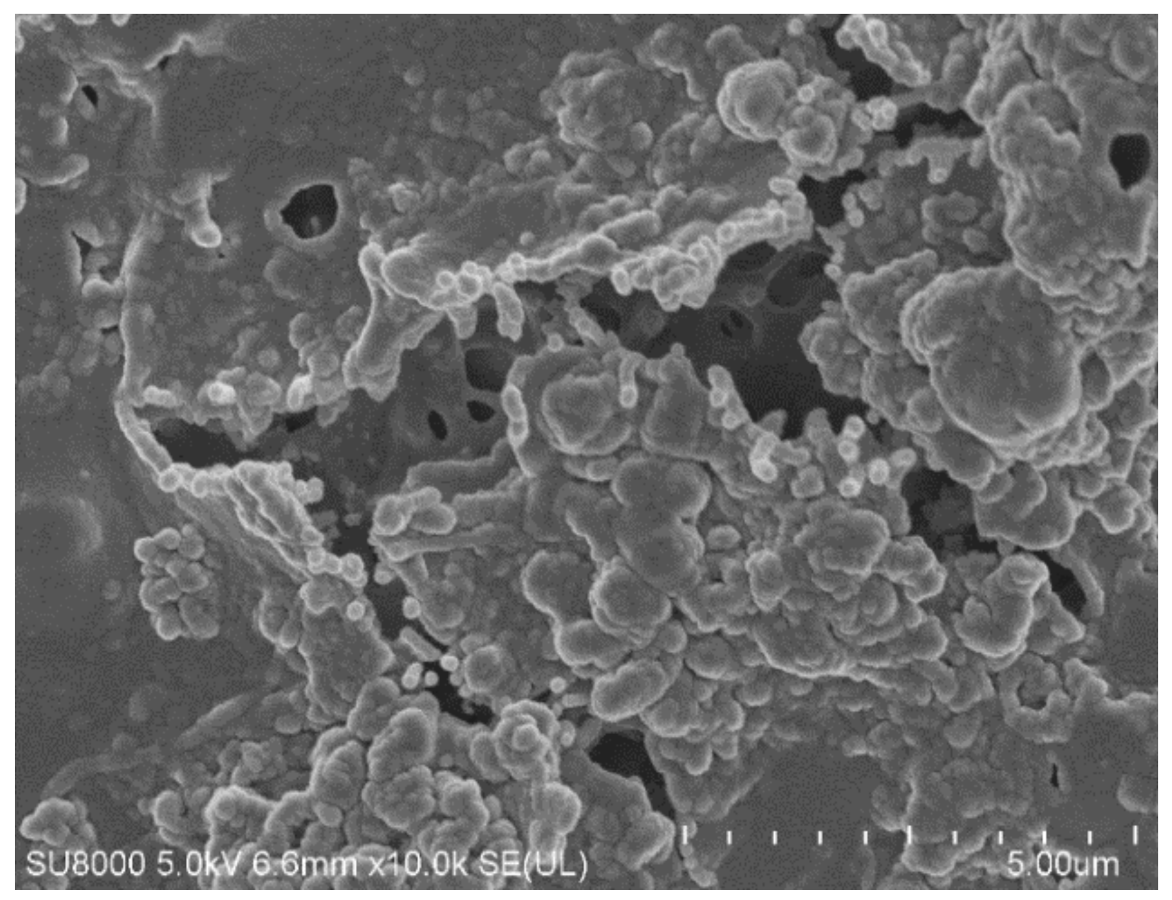



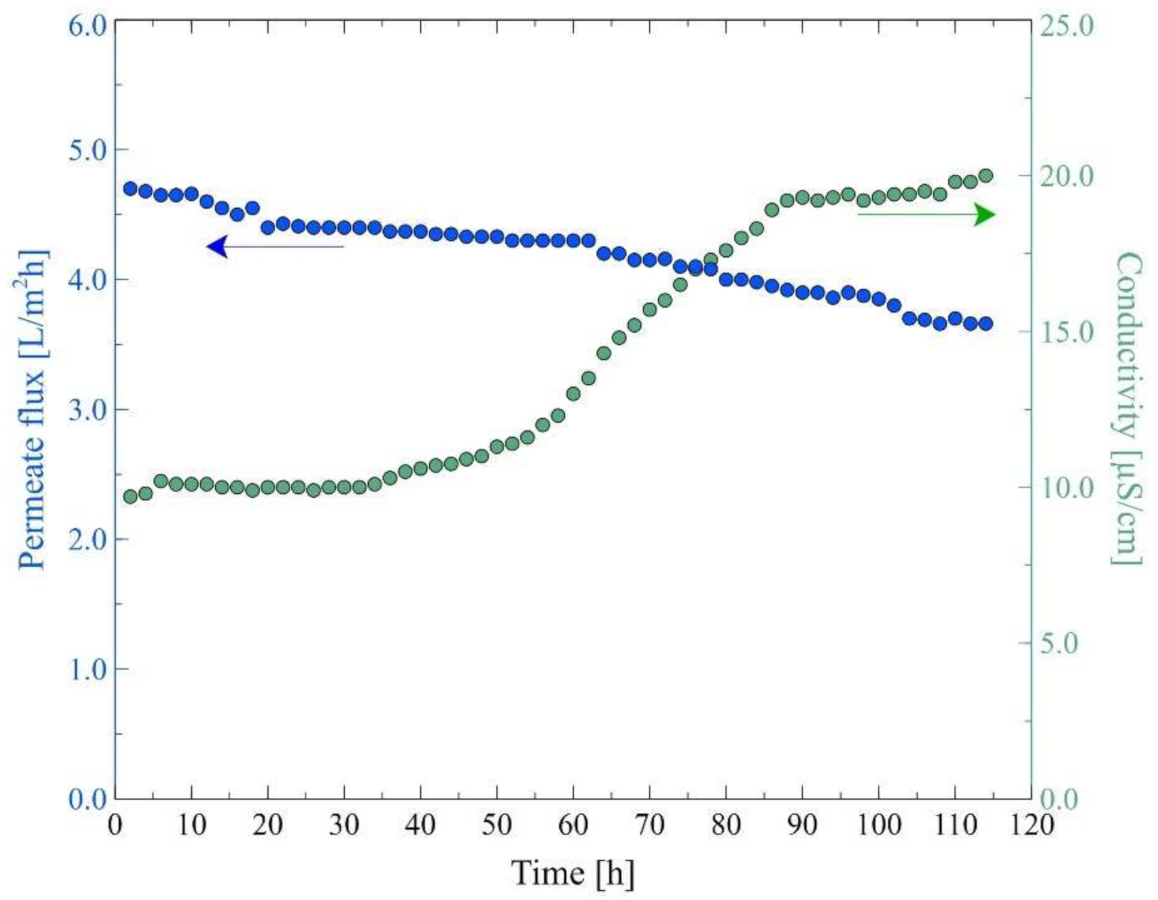
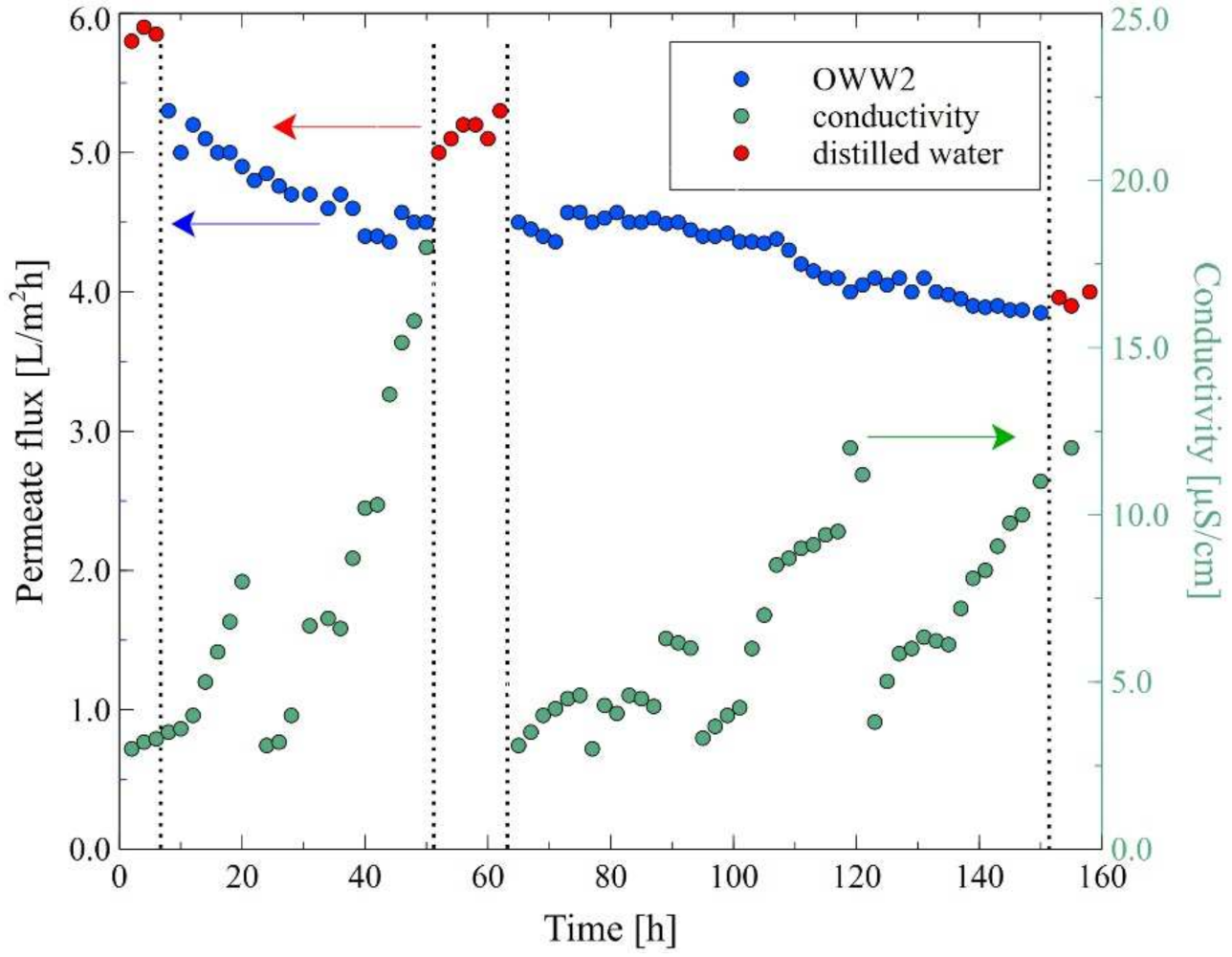

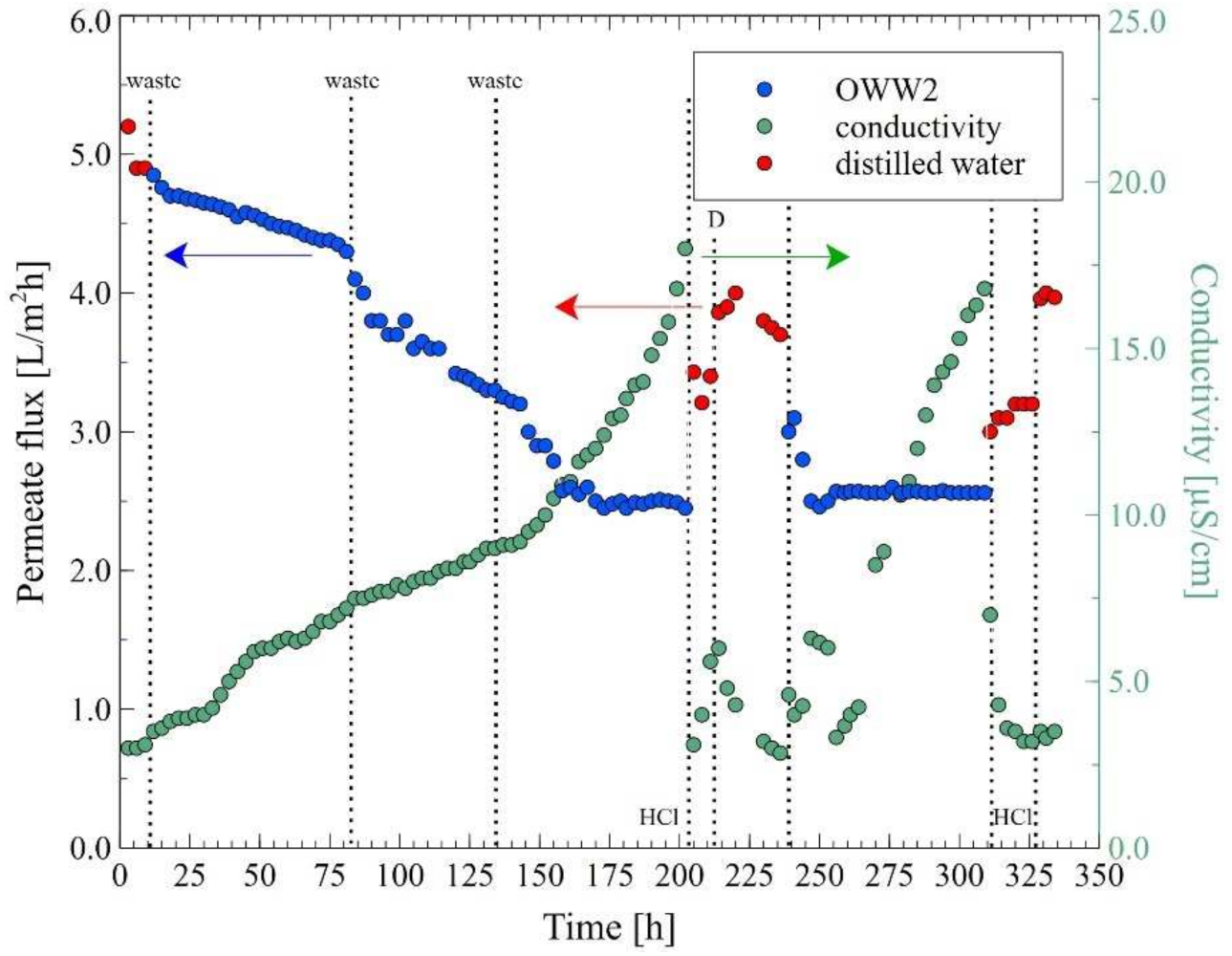

| Ions (mg/L) | Na+ | Cl− | Mg2+ | Ca2+ | K+ | NO3− | SO42− | Oil (mg/L) |
|---|---|---|---|---|---|---|---|---|
| OWW1 | 2159 | 3254 | 318 | 465 | 79 | 110 | 688 | 25 |
| OWW2 | 2775 | 4338 | 415 | 765 | 116 | 138 | 793 | 42 |
| Baltic Seawater | 2297 | 3189 | 258 | 154 | 83 | 5.5 | 478 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, W.; Gryta, M. The Impact of Operational Parameters on Polypropylene Membrane Performance during the Separation of Oily Saline Wastewaters by the Membrane Distillation Process. Membranes 2022, 12, 351. https://doi.org/10.3390/membranes12040351
Tomczak W, Gryta M. The Impact of Operational Parameters on Polypropylene Membrane Performance during the Separation of Oily Saline Wastewaters by the Membrane Distillation Process. Membranes. 2022; 12(4):351. https://doi.org/10.3390/membranes12040351
Chicago/Turabian StyleTomczak, Wirginia, and Marek Gryta. 2022. "The Impact of Operational Parameters on Polypropylene Membrane Performance during the Separation of Oily Saline Wastewaters by the Membrane Distillation Process" Membranes 12, no. 4: 351. https://doi.org/10.3390/membranes12040351
APA StyleTomczak, W., & Gryta, M. (2022). The Impact of Operational Parameters on Polypropylene Membrane Performance during the Separation of Oily Saline Wastewaters by the Membrane Distillation Process. Membranes, 12(4), 351. https://doi.org/10.3390/membranes12040351







