Efficacy of Two Stabilizers in Nanoemulsions with Whey Proteins and Thyme Essential Oil as Edible Coatings for Zucchini
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Zucchini
2.1.2. Coating Materials
2.2. Methods
2.2.1. Preparation of the Coating Nanoemulsion
2.2.2. Characterization of Coating Nanoemulsion
Rheological Measurements
Color
2.2.3. Characterization of Edible Coating Applied to Zucchinis
SEM
Untargeted Fingerprinting by SMPE Gas Chromatography-Mass Spectrometry (GC-MS)
2.2.4. Zucchini Coating
2.2.5. Storage of the Coated Zucchinis
2.2.6. Physicochemical Analysis of Coated Zucchinis
Weight Loss, Water Activity, pH, °Brix of Coated Zucchinis
Texture Evaluation
Enzyme Extraction
Gas Chromatography for Volatile Fingerprinting in Coated Zucchinis
Color Changes of the Coated Zucchinis
Sensory Evaluation of Coated Zucchinis
2.2.7. Microscopic Evaluation of Coatings Applied to Zucchinis
2.2.8. Statistical Analysis
3. Results and Discussions
3.1. Characterization of Coating Nanoemulsion
3.1.1. Rheometric Measurements
3.1.2. Color Estimation
3.2. Characterization of Membranes with T and S Nanoemulsions
3.2.1. SEM Coating Evaluation
3.2.2. GC Fingerprint
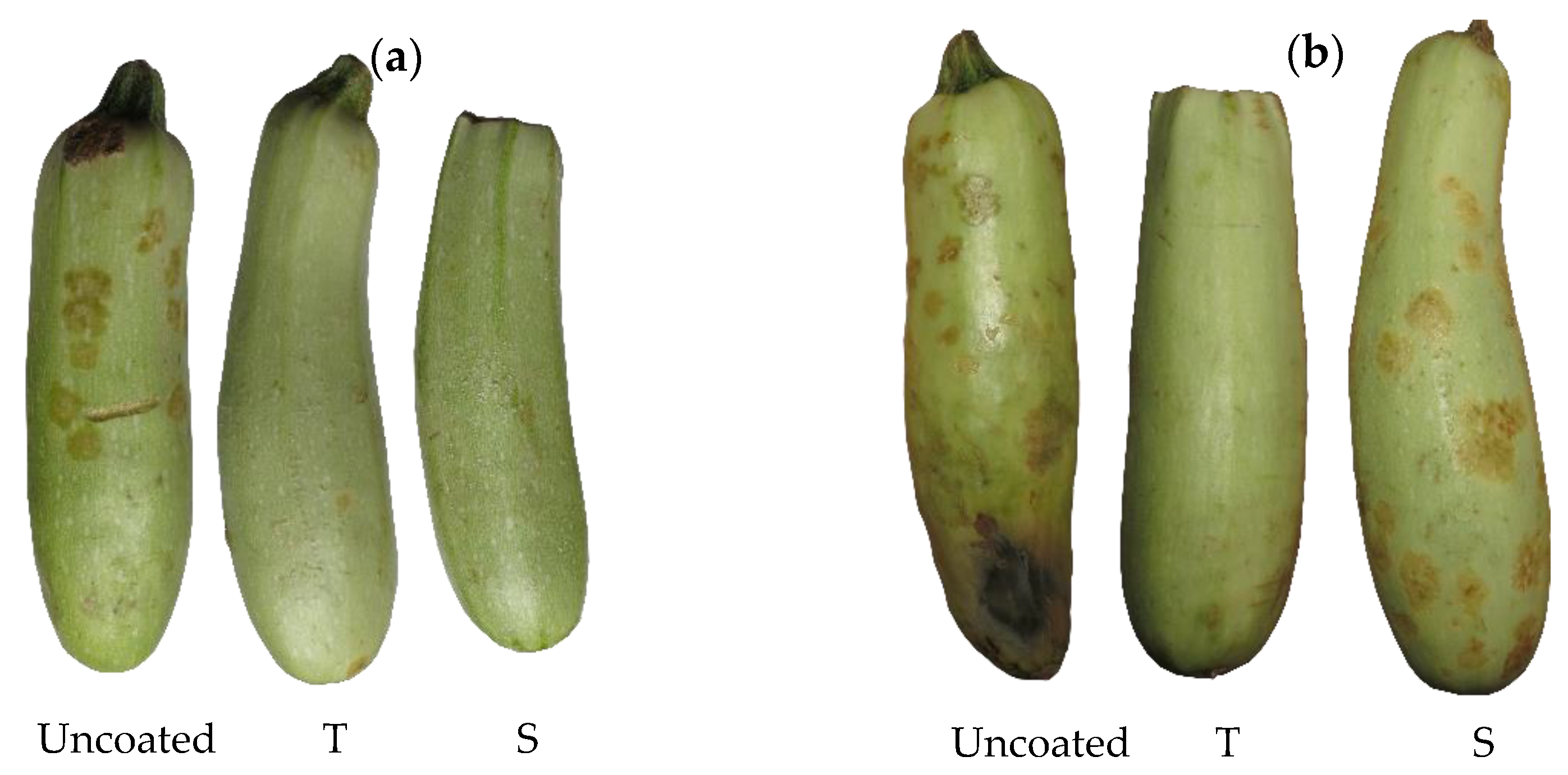
3.3. Physicochemical Analysis of the Coated Zucchinis
3.3.1. Weight Loss, Water Activity, pH, Brix
3.3.2. Texture Analysis
3.3.3. Enzymatic Activity
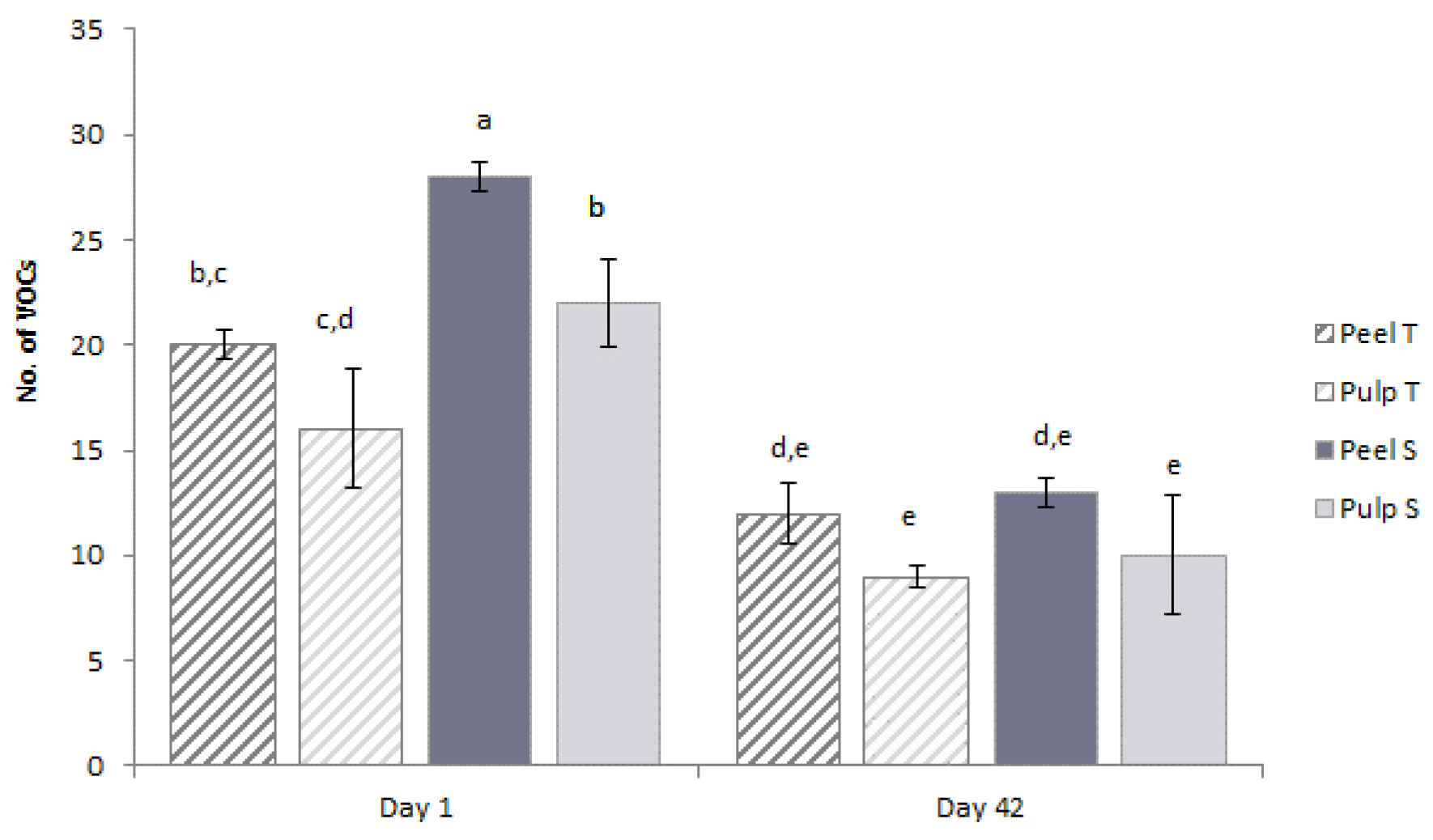
3.3.4. VOCs Profile Changes in Stored Coated Zucchinis Assessed by SPME GC-MS
3.3.5. Color Changes of the Coated Zucchinis during Storage
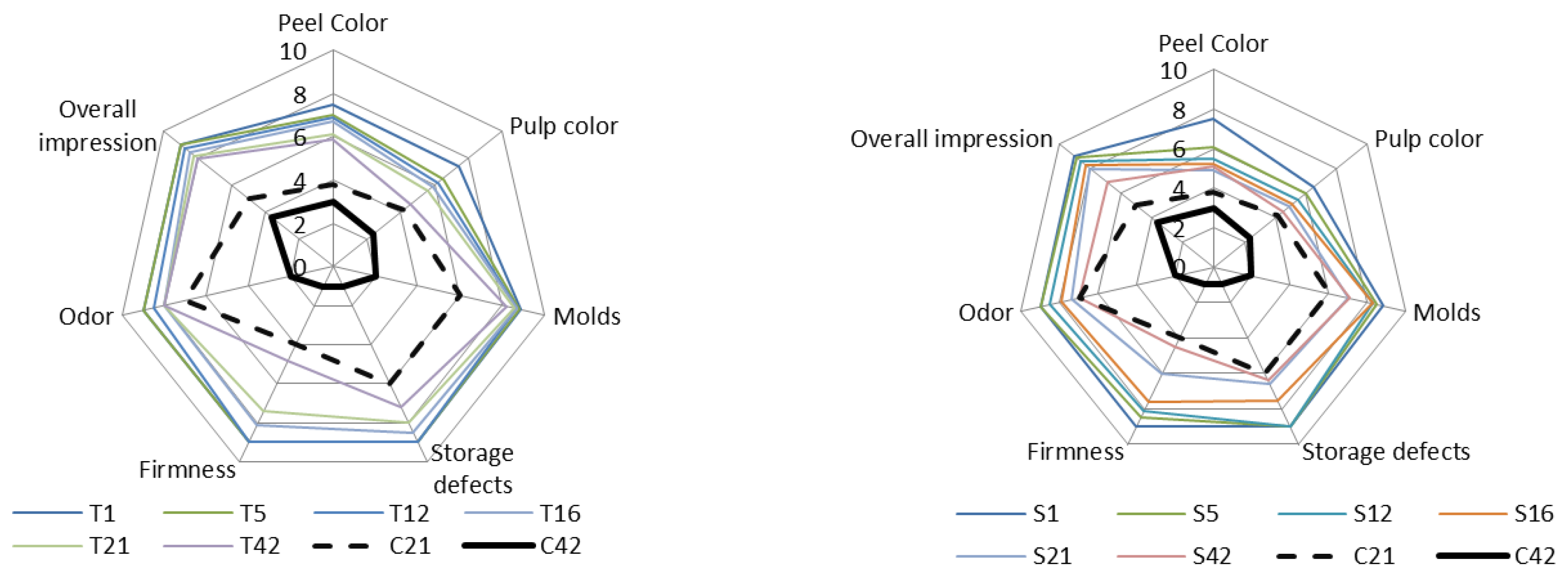
3.3.6. Sensory Evaluation of Coated Zucchinis
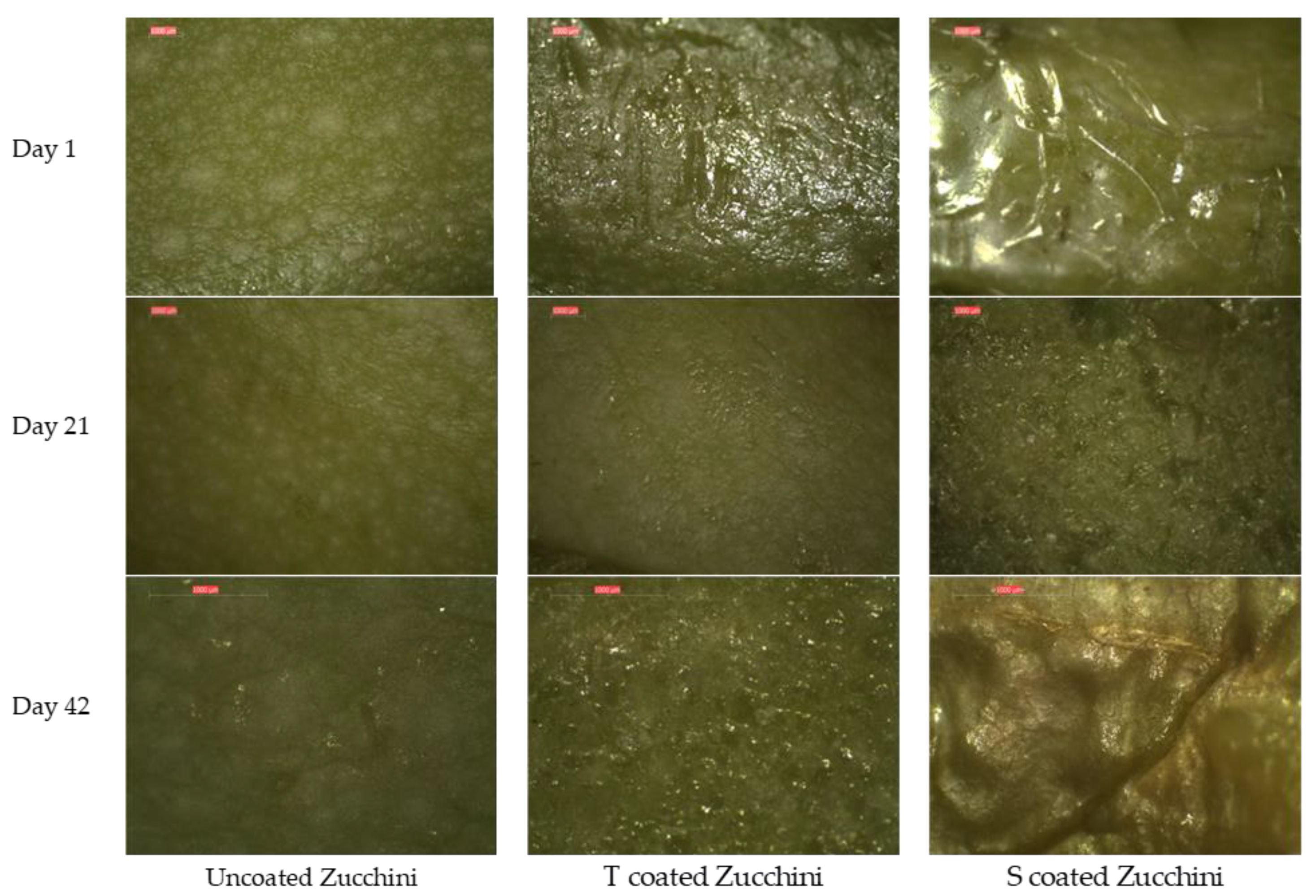
3.3.7. Stereomicroscopic Evaluation of Coating Applied to Zucchinis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucera, A.; Costa, C.; Mastromatteo, M.; Conte, A.; Del Nobile, M. Influence of different packaging systems on fresh-cut zucchini (Cucurbita pepo). Innov. Food Sci. Emerg. Technol. 2010, 11, 361–368. [Google Scholar] [CrossRef]
- Desai, U.T.; Musmade, A.M. Pumpkins, squashes, and gourds. In Handbook of Vegetable Science and Technology: Production, Compostion, Storage, and Processing; Salunkhe, D.K., Kadam, S.S., Eds.; CRC Press: New York, NY, USA, 1998; pp. 273–280. [Google Scholar]
- Zuo, X.; Cao, S.; Zhang, M.; Cheng, Z.; Cao, T.; Jin, P.; Zheng, Y. High relative humidity (HRH) storage alleviates chilling injury of zucchini fruit by promoting the accumulation of proline and ABA. Postharvest Biol. Technol. 2021, 171, 111344. [Google Scholar] [CrossRef]
- Rodov, V.; Paris, H.S.; Friedman, H.; Mihiret, M.; Vinokur, Y.; Fennec, A. Chilling sensitivity of four near-isogenic fruit-color genotypes of summer squash (Cucurbita pepo, Cucurbitaceae) and its association with tocopherol content. Postharvest Biol. Technol. 2020, 168, 111279. [Google Scholar] [CrossRef]
- Jiménez-Muñoz, R.; Palma, F.; Carvajal, F.; Castro-Cegrí, A.; Pulido, A.; Jamilena, M.; Romero-Puertas, M.; Garrido, D. Pre-storage nitric oxide treatment enhances chilling tolerance of zucchini fruit (Cucurbita pepo L.) by S-nitrosylation of proteins and modulation of the antioxidant response. Postharvest Biol. Technol. 2021, 171, 111345. [Google Scholar] [CrossRef]
- Occhino, E.; Hernando, I.; Llorca, E.; Neri, L.; Pittia, P. Effect of Vacuum Impregnation Treatments to Improve Quality and Texture of Zucchini (Cucurbita Pepo L.). Procedia Food Sci. 2011, 1, 829–835. [Google Scholar] [CrossRef] [Green Version]
- Palma, F.; Carvajal, F.; Jiménez-Muñoz, R.; Pulido, A.; Jamilena, M.; Garrido, D. Exogenous γ-aminobutyric acid treatment improves the cold tolerance of zucchini fruit during postharvest storage. Plant Physiol. Biochem. 2019, 136, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Wang, S.; Cao, Q.; Li, Y.; Zhou, J.; Zhu, B.-W. Functional food packaging for reducing residual liquid food: Thermo-resistant edible super-hydrophobic coating from coffee and beeswax. J. Colloid Interface Sci. 2019, 533, 742–749. [Google Scholar] [CrossRef]
- Eddin, A.S.; Ibrahim, S.A.; Tahergorabi, R. Egg quality and safety with an overview of edible coating application for egg preservation. Food Chem. 2019, 296, 29–39. [Google Scholar] [CrossRef]
- Fakhouri, F.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Moreira, M.D.R.; Ponce, A.; del Valle, C.E.; Roura, S.I. Edible coatings on fresh squash slices: Effect of film drying temperature on the nutritional and microbiological quality. J. Food Process. Preserv. 2009, 33, 226–236. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Mendoza, M.; Maté, J.I. Whey protein isolate edible films with essential oils incorporated to improve the microbial quality of poultry. J. Sci. Food Agric. 2013, 93, 2986–2994. [Google Scholar] [CrossRef]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A.; Khazaei, N. Effect of quince seed mucilage edible films incorporated with oregano or thyme essential oil on shelf life extension of refrigerated rainbow trout fillets. Int. J. Food Microbiol. 2014, 174, 88–97. [Google Scholar] [CrossRef]
- Jancikova, S.; Dordevic, D.; Tesikova, K.; Antonic, B.; Tremlova, B. Active Edible Films Fortified with Natural Extracts: Case Study with Fresh-Cut Apple Pieces. Membranes 2021, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Saini, C.S. Edible composite bi-layer coating based on whey protein isolate, xanthan gum and clove oil for prolonging shelf life of tomatoes. Meas. Food 2021, 2, 100005. [Google Scholar] [CrossRef]
- Torun, M.; Ozdemir, F. Milk protein and zein coatings over peeled garlic cloves to extend their shelf life. Sci. Hortic. 2022, 291, 110571. [Google Scholar] [CrossRef]
- Marquez, G.R.; Di Pierro, P.; Mariniello, L.; Esposito, M.; Giosafatto, C.V.L.; Porta, R. Fresh-cut fruit and vegetable coatings by transglutaminase-crosslinked whey protein/pectin edible films. LWT 2017, 75, 124–130. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible coatings and antimicrobial nanoemulsions for enhancing shelf life and reducing foodborne pathogens of fruits and vegetables: A review. Sustain. Mater. Technol. 2020, 26, e00215. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Nafchi, A.M.; Salehabadi, A.; Oladzad-Abbasabadi, N.; Jafari, S.M. Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv. Colloid Interface Sci. 2021, 291, 102405. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Paramasivam, N.; Vadivel, V. Essential oil based nanoemulsions to improve the microbial quality of minimally processed fruits and vegetables: A review. Food Res. Int. 2018, 111, 509–523. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Nastasi, J.R.; Kontogiorgos, V.; Daygon, V.D.; Fitzgerald, M.A. Pectin-based films and coatings with plant extracts as natural preservatives: A systematic review. Trends Food Sci. Technol. 2022, 120, 193–211. [Google Scholar] [CrossRef]
- Bleoancă, I.; Enachi, E.; Borda, D. Thyme Antimicrobial Effect in Edible Films with High Pressure Thermally Treated Whey Protein Concentrate. Foods 2020, 9, 855. [Google Scholar] [CrossRef]
- Goyeneche, R.; Agüero, M.V.; Roura, S.; Di Scala, K. Application of citric acid and mild heat shock to minimally processed sliced radish: Color evaluation. Postharvest Biol. Technol. 2014, 93, 106–113. [Google Scholar] [CrossRef]
- ASTM. Standard practice for conditioning plastics for testing, D618-00. In Annual Book of ASTM Standards; ASTM: West Conshohocken, PA, USA, 2000. [Google Scholar]
- Alegre, I.; Viñas, I.; Usall, J.; Anguera, M.; Abadias, M. Microbiological and physicochemical quality of fresh-cut apple enriched with the probiotic strain Lactobacillus rhamnosus GG. Food Microbiol. 2011, 28, 59–66. [Google Scholar] [CrossRef]
- Waran, P.; Mallikarjunan, K. Physical measurements. Monitoring and measuring techniques for quality and safety. In Handbook of Frozen Food Processing and Packaging; Sun, D.-W., Ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 556. [Google Scholar]
- Palma, F.; Carvajal, F.; Jamilena, M.; Garrido, D. Putrescine treatment increases the antioxidant response and carbohydrate content in zucchini fruit stored at low temperature. Postharvest Biol. Technol. 2016, 118, 68–70. [Google Scholar] [CrossRef]
- Lago, A.M.T.; Neves, I.C.O.; Oliveira, N.L.; Botrel, D.A.; Minim, L.A.; de Resende, J.V. Ultrasound-assisted oil-in-water nanoemulsion produced from Pereskia aculeata Miller mucilage. Ultrason. Sonochem. 2019, 50, 339–353. [Google Scholar] [CrossRef]
- Farshi, P.; Tabibiazar, M.; Ghorbani, M.; Mohammadifar, M.A.; Amirkhiz, M.B.; Hamishehkar, H. Whey protein isolate-guar gum stabilized cumin seed oil nanoemulsion. Food Biosci. 2019, 28, 49–56. [Google Scholar] [CrossRef]
- Mezger, T.G. Applied Rheology; Anton Paar GmbH: Graz, Austria, 2015. [Google Scholar]
- Amjadi, S.; Nazari, M.; Alizadeh, S.A.; Hamishehkar, H. Multifunctional betanin nanoliposomes-incorporated gelatin/chitosan nanofiber/ZnO nanoparticles nanocomposite film for fresh beef preservation. Meat Sci. 2020, 167, 108161. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, S.; Almasi, H.; Ghadertaj, A.; Mehryar, L. Whey protein isolate-based films incorporated with nanoemulsions of orange peel (Citrus sinensis) essential oil: Preparation and characterization. J. Food Process. Preserv. 2021, 45. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Critzer, F.; Davidson, P.M.; Zivanovic, S.; Zhong, Q. Physical, mechanical, and antimicrobial properties of chitosan films with microemulsions of cinnamon bark oil and soybean oil. Food Hydrocoll. 2016, 52, 533–542. [Google Scholar] [CrossRef]
- Ahmadi, O.; Jafarizadeh-Malmiri, H. Intensification process in thyme essential oil nanoemulsion preparation based on subcritical water as green solvent and six different emulsifiers. Green Process. Synth. 2021, 10, 430–439. [Google Scholar] [CrossRef]
- Oymaci, P.; Altinkaya, S.A. Improvement of barrier and mechanical properties of whey protein isolate based food packaging films by incorporation of zein nanoparticles as a novel bionanocomposite. Food Hydrocoll. 2016, 54, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Mirabzadeh, S.; Shahvalizadeh, R.; Hamishehkar, H. Development of novel active packaging films based on whey protein isolate incorporated with chitosan nanofiber and nano-formulated cinnamon oil. Int. J. Biol. Macromol. 2020, 149, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Agudelo-Cuartas, C.; Granda-Restrepo, D.; Sobral, P.J.; Hernandez, H.; Castro, W. Characterization of whey protein-based films incorporated with natamycin and nanoemulsion of α-tocopherol. Heliyon 2020, 6, e03809. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of Micro- and Nanoemulsions as Vehicles for Essential Oils: Formulation, Preparation and Stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, M.P.; Carneiro-da-Cunha, M.G. Nanotechnology in edible packaging. In Edible Food Packaging: Materials and Processing Technologies; Cerqueira, M.Â.P.R., Pereira, R.N.C., Ramos, O.L.S., Teixeira, J.A.C., Vicente, A.A., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 288–317. [Google Scholar]
- Bleoanca, I.; Saje, K.; Mihalcea, L.; Oniciuc, E.-A.; Smole-Mozina, S.; Nicolau, A.I.; Borda, D. Contribution of high pressure and thyme extract to control Listeria monocytogenes in fresh cheese—A hurdle approach. Innov. Food Sci. Emerg. Technol. 2016, 38, 7–14. [Google Scholar] [CrossRef]
- Ali, I.B.E.; Zaouali, Y.; Bejaoui, A.; Boussaid, M. Variation of the Chemical Composition of Essential Oils in Tunisian Populations of Thymus algeriensis Boiss. et Reut. (Lamiaceae) and Implication for Conservation. Chem. Biodivers. 2010, 7, 1276–1289. [Google Scholar] [CrossRef]
- Chizzola, R.; Michitsch, H.; Franz, C. Antioxidative Properties of Thymus vulgaris Leaves: Comparison of Different Extracts and Essential Oil Chemotypes. J. Agric. Food Chem. 2008, 56, 6897–6904. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Aguado, E.; Cebrián, G.; Iglesias, J.; Romero, J.; Martínez, C.; Garrido, D.; Rebolloso, M.; Valenzuela, J.; Jamilena, M. Effect of Ethylene-Insensitive Mutation etr2b on Postharvest Chilling Injury in Zucchini Fruit. Agriculture 2020, 10, 532. [Google Scholar] [CrossRef]
- Sargent, S.; Maynard, D. Cucurbits. In Crop Post-Harvest: Science and Technology, Volume 3: Perishables; Rees, D., Farrell, G., Orchard, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 297. [Google Scholar]
- Nguyen, L.T.; Tay, A.; Balasubramaniam, V.; Legan, J.; Turek, E.J.; Gupta, R. Evaluating the impact of thermal and pressure treatment in preserving textural quality of selected foods. LWT 2010, 43, 525–534. [Google Scholar] [CrossRef]
- Pugliese, M.A.; Goitia, M.T.; Yossen, M.; Cifone, N.; Agulló, E.; Andreucetti, N. Improved postharvest quality in patagonian squash (Cucurbita moschata) coated with radiation depolymerized chitosan. Radiat. Phys. Chem. 2011, 80, 1406–1413. [Google Scholar] [CrossRef]
- Mishra, V.K.; Gamage, T.V. Postharvest physiology of fruit and vegetables. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Zhang, M.; Liu, W.; Li, C.; Shao, T.; Jiang, X.; Zhao, H.; Ai, W. Postharvest hot water dipping and hot water forced convection treatments alleviate chilling injury for zucchini fruit during cold storage. Sci. Hortic. 2019, 249, 219–227. [Google Scholar] [CrossRef]
- Gualanduzzi, S.; Baraldi, E.; Braschi, I.; Carnevali, F.; Gessa, C.E.; De Santis, A. Respiration, hydrogen peroxide levels and antioxidant enzyme activities during cold storage of zucchini squash fruit. Postharvest Biol. Technol. 2009, 52, 16–23. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Rafudeen, M.S.; Ganie, S.A.; Hossain, M.S.; Gomaa, A.M. Seed priming with cypress leaf extract enhances photosynthesis and antioxidative defense in zucchini seedlings under salt stress. Sci. Hortic. 2021, 293, 110707. [Google Scholar] [CrossRef]
- Hiraga, S.; Sasaki, K.; Ito, H.; Ohashi, Y.; Matsui, H. A Large Family of Class III Plant Peroxidases. Plant Cell Physiol. 2001, 42, 462–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C. Temperature preconditioning affects ascorbate antioxidant system in chilled zucchini squash. Postharvest Biol. Technol. 1996, 8, 29–36. [Google Scholar] [CrossRef]
- Zheng, Y.; Fung, R.W.; Wang, S.Y.; Wang, C.Y. Transcript levels of antioxidative genes and oxygen radical scavenging enzyme activities in chilled zucchini squash in response to superatmospheric oxygen. Postharvest Biol. Technol. 2008, 47, 151–158. [Google Scholar] [CrossRef]
- Wang, C.Y. Effect of temperature preconditioning on catalase, peroxidase, and superoxide dismutase in chilled zucchini squash. Postharvest Biol. Technol. 1995, 5, 67–76. [Google Scholar] [CrossRef]
- Ilic, J.; Tomasevic, I.; Djekic, I. Purple eggplant and zucchini color, mechanical properties, mastication, and sensory perception influenced by boiling and grilling. J. Texture Stud. 2021. [Google Scholar] [CrossRef]
- Wilson, C. (Ed.) Intelligent and Active Packaging for Fruits and Vegetables; CRC Press: Boca Raton, FL, USA, 2007; p. 99. [Google Scholar]
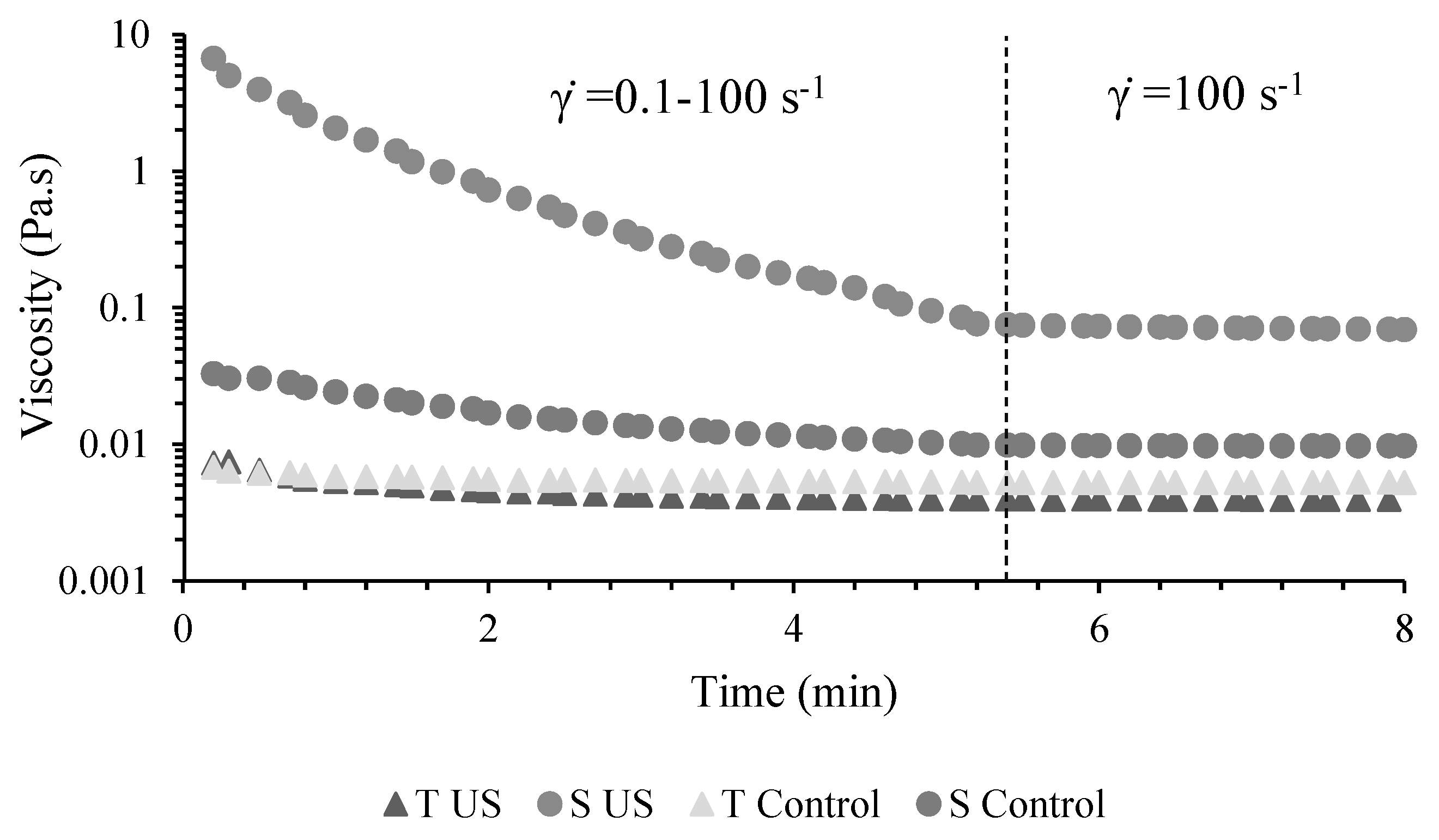

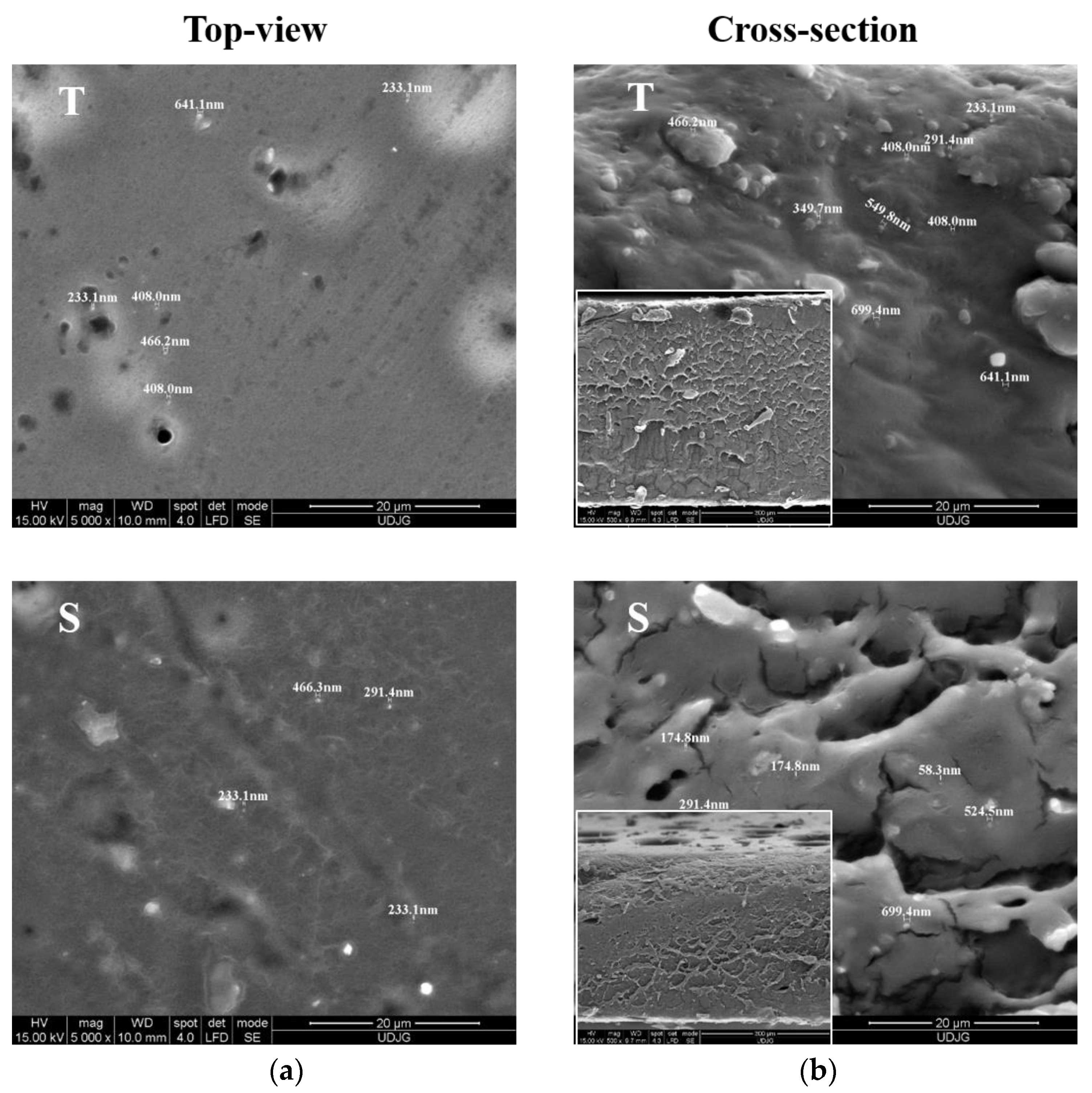

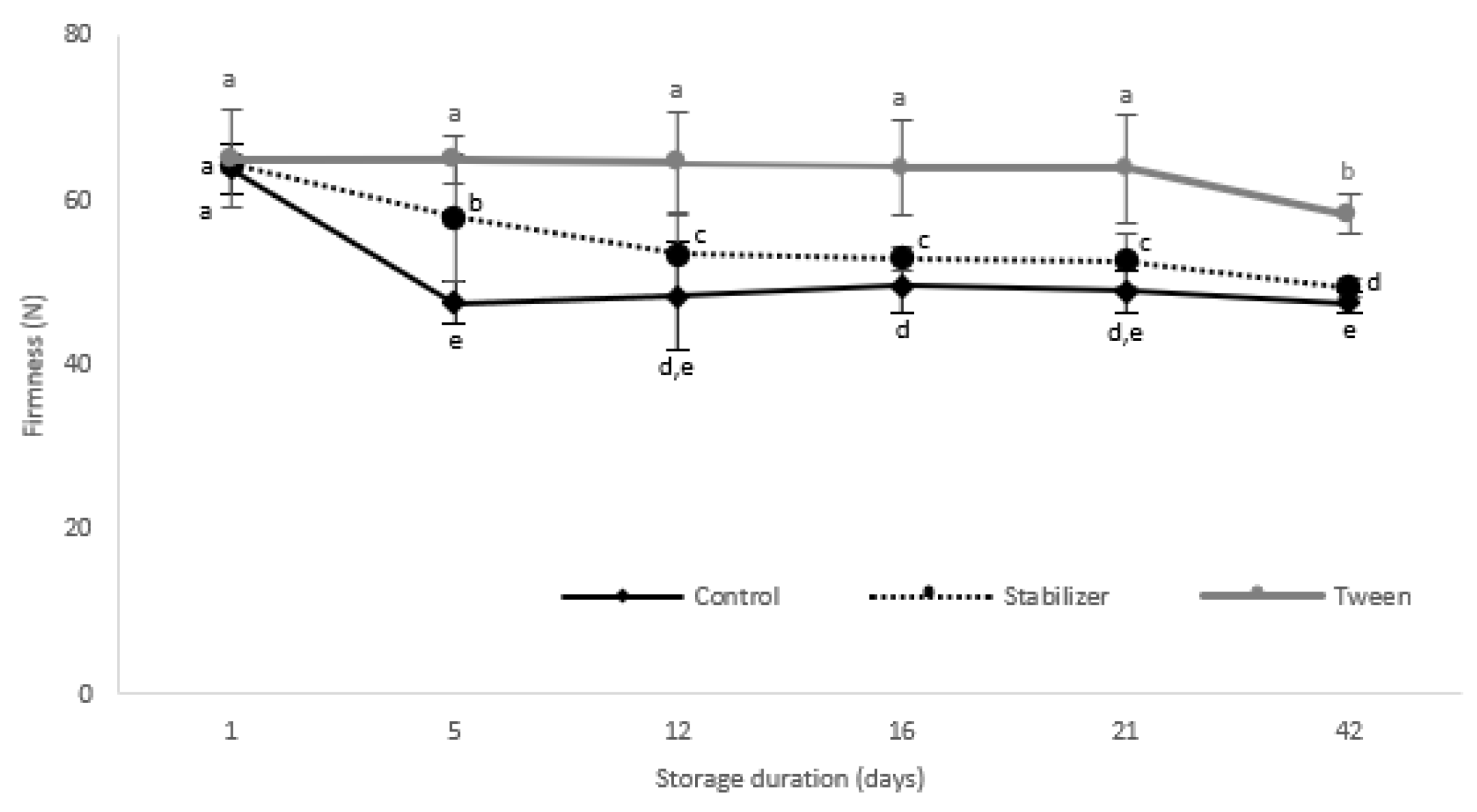
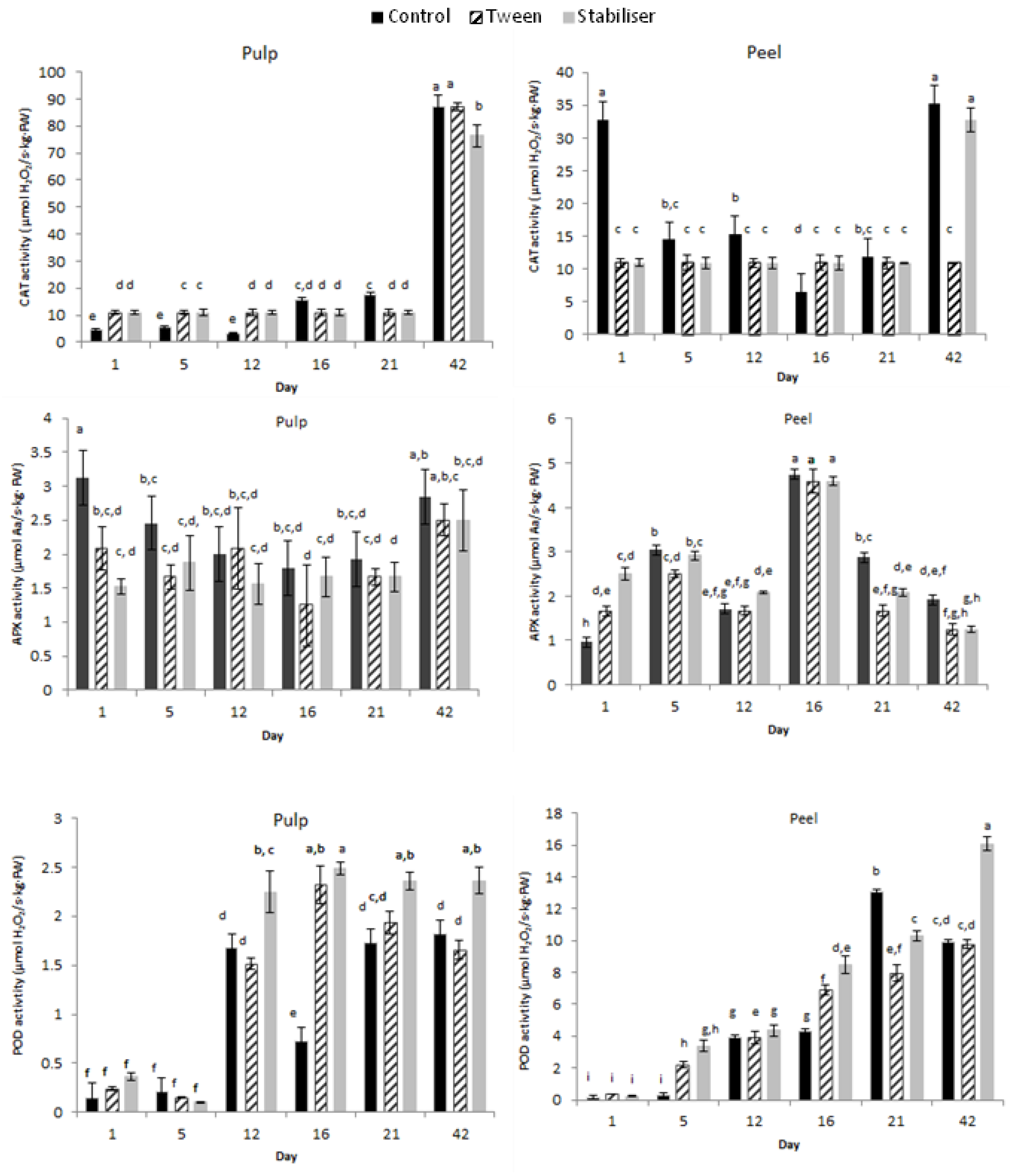
| Parameters | L* | a* | b* | ΔE* | |
|---|---|---|---|---|---|
| Sample | |||||
| T Control | 73.23 ± 0.07 **A | −1.42 ± 0.01 A | 4.37 ± 0.02 A | ||
| T | 71.06 ± 0.32 B | −1.07 ± 0.31 A | 5.15 ± 0.66 B | 2.33 ± 0.40 A | |
| S Control | 71.75 ± 0.07 C | −1.48 ± 0.01 A,B | 5.06 ± 0.00 B | ||
| S | 70.29 ± 0.19 D | −1.88 ± 0.14 B | 7.05 ± 0.24 B | 2.45 ± 0.28 A | |
| No | KI | Ions | Compound | Formulae | Thyme EO (%) | T (%) | S (%) | Heatmap Area (S-T) |
|---|---|---|---|---|---|---|---|---|
| 1 | 843 | 91;93;77;51;121 | 3-Thujene *,a | C10H16 | 1.21 ± 0.05 a,E,F | 0.21 ± 0.05 c,E,F | 0.54 ± 0.02 b,D | |
| 2 | 890 | 81;107;135;150;53 | Tricyclene | C10H16 | 1.63 ± 0.08 a,E,F | 0.14 ± 0.01 c,F | 0.86 ± 0.06 b,D | |
| 3 | 922 | 91;93;77;51;121 | ç-Terpinene | C10H16 | 0.59 ± 0.04 a,E,F | 0.28 ± 0.02 c,E,F | 0.42 ± 0.02 b,D | |
| 4 | 966 | 91;93;77;65;105 | α-Phellandrene | C10H16 | 0.44 ± 0.03 aF | 0.08 ± 0.03 c,F | 0.25 ± 0.02 b,D | |
| 5 | 986 | 119;91;115;135;134 | 1,3,8-Menthatriene | C10H14 | 1.25 ± 0.12 b,E,F | 0.51 ± 0.02 c,E,F | 1.63 ± 0.13 a,D | |
| 6 | 992 | 91;93;77;121;136 | 2-Bornene | C6H10 | 0.86 ± 0.07 a,E,F | 0.21 ± 0.02 c,E,F | 0.60 ± 0.05 b,D | |
| 7 | 1024 | 67;93;91;108;65 | Camphenone | C5H8 | 0.03 ± 0.00 c,F | 0.37 ± 0.02 a,E,F | 0.24 ± 0.02 b,D | |
| 9 | 1082 | 91;115;117;132;77 | 1H-Indene | C10H12 | 0.03 ± 0.00 a,F | 0.02 ± 0.00 b,F | 0.03 ± 0.00 a,D | |
| 10 | 1096 | 91;93;77;121;111 | α-Pinene | C10H16 | 2.79 ± 0.03 a,E | 1.72 ± 0.12 b,E,F | 1.11 ± 0.01 c,D | |
| 11 | 1107 | 105;119;91;120;77 | Aromadendrene | C15H24 | 0.01 ± 0.00 a,F | 0.04 ± 0.00 b,F | 0.01 ± 0.00 a,D | |
| 12 | 1119 | 95;67;108;93;81 | Limonene | C10H16 | 1.15 ± 0.01 c,E,F | 0.34 ± 0.02 a,E,F | 0.23 ± 0.01 b,D | |
| 13 | 1127 | 91;77;93;67;121 | Camphene | C10H16 | 1.91 ± 0.12 a,E,F | 1.63 ± 0.15 a,E,F | 1.21 ± 0.11 b,D | |
| 14 | 1132 | 91;77;93;121;107 | Santolina triene | C10H16 | 0.62 ± 0.02 a,E,F | 0.43 ± 0.03 b,E,F | 0.56 ± 0.05 a,D | |
| 15 | 1143 | 93;91;95;67;121 | 1,3,6-Heptatriene | C7H10 | 0.98± 0.05 a,E,F | 0.64 ± 0.05 b,E,F | 0.96 ± 0.06 a,D | |
| 16 | 1150 | 91;105;77;119;133 | Caryophyllene | C15H24 | 22.03 ± 1.58 a,B | 9.46 ± 1.02 c,C | 14.52 ±0.15 b,B | |
| 17 | 1176 | 91;77;93;105;119 | Seychellene | C15H24 | 0.48 ± 0.05 a,F | 0.48 ± 0.02 a,E,F | 0.40 ± 0.01 b,D | |
| 18 | 1188 | 91;93;67;77;121 | Ocimene | C10H16 | 0.24 ± 0.00 b,F | 0.23 ± 0.00 a,F | 0.24 ± 0.00 b,D | |
| 19 | 1189 | 105;161;91;119;133 | Germacrene | C15H24 | 0.36 ± 0.03 a,F | 0.11 ± 0.01 c,F | 0.24 ± 0.01 b,D | |
| 20 | 1193 | 91;93;77;79;67 | cis-α-Bisabolene | C15H24 | 0.41 ± 0.03 a,F | 0.40 ± 0.03 a,E,F | 0.46 ± 0.03 a,D | |
| 21 | 1221 | 93;91;121;67;136 | 4-Carene | C10H16 | 30.77 ± 3.21 b,A | 40.03 ± 2.75 a,A | 41.42 ± 3.59 a,A | |
| 22 | 1228 | 91;77;93;119;65 | Cis pinen 3-ol | C10H16O | 5.32 ± 0.22 b,D | 5.20 ± 0.34 b,D | 6.74 ± 0.58 a,C | |
| 23 | 1299 | 91;93;79;67;121 | 3-Carene | C10H16 | 0.72 ± 0.01 a,E,F | 0.84 ± 0.06 a,E,F | 0.84 ± 0.07 a,D | |
| 24 | 1313 | 91;93;67;121;123 | cis-Geraniol | C10H18O | 10.64 ± 1.58 b,C | 17.05 ± 1.33 a,B | 13.58 ± 1.12 b,B | |
| 25 | 1330 | 91;77;119;134;51 | cis-Cyclooctene | C8H14 | 0.50 ± 0.01 c,E,F | 1.97 ± 0.15 a,F | 1.65 ± 0.15 b,D | |
| 26 | 1334 | 91;93;67;121;65 | α-Ocimene | C10H16 | 0.25 ± 0.01 c,F | 0.38 ± 0.01 a,E,F | 0.34 ± 0.02 b,D | |
| 27 | 1346 | 91;77;119;65;51 | trans-Cyclooctene | C8H14 | 0.06 ± 0.00 a,F | 0.09 ± 0.01 a,E | 0.08 ± 0.04 a,D | |
| 28 | 1382 | 91;77;105;107;67 | Lanceol-cis | C15H24O | 0.02 ± 0.00 a,F | 0.05 ± 0.00 b,F | 0.02 ± 0.00 a,D | |
| 29 | 1387 | 91;79;77;105;57 | Ledene oxide | C15H24O | 0.29 ± 0.01 c,F | 0.62 ± 0.03 a,E,F | 0.38 ± 0.02 b,D | |
| 30 | 1411 | 91;105;107;67;119 | Azulene | C10H8 | 0.01 ± 0.00 a,F | 0.07 ± 0.00 b,F | 0.03 ± 0.00 c,D | |
| 31 | 1415 | 79;77;67;91;93 | Myrtenol | C10H16O | 0.02 ± 0.00 a,F | 0.08 ± 0.00 c,F | 0.05 ± 0.00 b,D | |
| 32 | 1439 | 91;105;119;79;161 | α-Guaiene | C15H24 | 0.01 ± 0.00 b,F | 0.02 ± 0.00 a,F | 0.02 ± 0.00 a,D | |
| 33 | 1455 | 91;105;119;77;131 | Spathulenol | C15H24O | 0.01 ± 0.00 a,F | 0.14 ± 0.01 b,F | 0.03 ± 0.00 b,D | |
| 34 | 1473 | 135;115;91;150;79 | Thymol | C10H14O | 0.04 ± 0.01 b,F | 0.11 ± 0.05 a,F | 0.08 ± 0.02 a,D | |
| 35 | 1485 | 91;119;77;136;67 | 2,5-Octadecadienoic acid | C19H34O2 | 0.02 ± 0.00 c,F | 0.1 ± 0.01 a,F | 0.06 ± 0.01 b,D |
| Storage | Sample | L* | a* | b* | ΔE* |
|---|---|---|---|---|---|
| Day 1 | Uncoated | 62.55 ± 0.23 B,C,D,E,F | −7.28 ± 0.07 E | 27.39 ± 0.33 C,D,E,F,G | |
| T | 58.44 ± 2.44 C,D,E,F,G,H | −7.24 ± 0.27 E | 29.51 ± 1.58 A,B,C,D,E,F,G | 4.62 ± 2.88 B,C | |
| S | 51.30 ± 0.26 H | −7.15 ± 0.02 D,E | 28.11 ± 2.98 C,D,E,F,G | 11.28 ± 0.48 A,B,C | |
| Day 5 | Uncoated | 63.70 ± 0.10 B,C,D,E | −6.97 ± 0.17 C,D,E | 26.58 ± 0.43 D,E,F,G | |
| T | 52.67 ± 7.23 F,G,H | −5.23 ± 0.88 B,C | 24.57 ± 2.68 F,G | 11.34 ± 5.86 A,B,C | |
| S | 57.91 ± 1.21 C,D,E,F,G,H | −6.09 ± 0.86 C,D,E | 30.39 ± 0.18 A,B,C,D,E,F | 6.99 ± 1.00 A,B,C | |
| Day 12 | Uncoated | 64.43 ± 0.08 B,C,D,E | −7.07 ± 0.07 D,E | 32.96 ± 0.20 A,B,C | |
| T | 58.66 ± 0.01 B,C,D,E,F,G,H | −5.90 ± 0.34 C,D,E | 27.27 ± 1.46 C,D,E,F,G | 8.19 ± 0.44 A,B,C | |
| S | 51.90 ± 4.31 G,H | −5.47 ± 0.44 C,D | 23.80 ± 3.57 G | 15.61 ± 5.59 A | |
| Day 16 | Uncoated | 61.90 ± 0.16 * B,C,D,E,F,G | −7.03 ± 0.03 D,E | 29.61 ± 0.12 A,B,C,D,E,F,G | |
| T | 63.42 ± 1.68 B,C,D,E | −5.63 ± 0.96 C,D,E | 30.25 ± 2.75 A,B,C,D,E,F | 2.16 ± 1.06 C | |
| S | 55.27 ± 2.15 D,E,F,G,H | −5.65 ± 0.04 C,D,E | 25.93 ± 1.57 E,F,G | 7.71 ± 2.59 A,B,C | |
| Day 21 | Uncoated | 66.72 ± 0.10 B,C | −6.37 ± 0.08 C,D,E | 32.29 ± 0.11 A,B,C,D | |
| T | 64.97 ± 3.12 B,C,D | −5.68 ± 0.40 C,D,E | 30.98 ± 1.29 A,B,C,D,E | 2.29 ± 1.32 C | |
| S | 54.57 ± 1.94 E,F,G,H | −5.33 ± 0.70 C | 25.19 ± 2.12 E,F,G | 14.11 ± 2.78 A,B,C | |
| Day 42 | Uncoated | 70.35 ± 0.18 A,B | −5.63 ± 0.05 C,D,E | 34.13 ± 0.28 A,B | |
| T | 79.22 ± 6.75 A | −2.10 ± 1.44 A | 35.23 ± 3.46 A | 9.61 ± 5.62 A,B,C | |
| S | 57.05 ± 5.97 C,D,E,F,G,H | −3.53 ± 0.41 A,B | 28.95 ± 2.21 B,C,D,E,F,G | 14.43 ± 6.20 A,B |
| Storage | Sample | L* | a* | b* | ΔE* |
|---|---|---|---|---|---|
| Day 1 | Uncoated | 82.09 ± 0.10 *B | −1.26 ± 0.04 A,B,C | 29.52 ± 0.06 E,F,G | |
| T | 79.35 ± 2.62 B,C,D | −3.52 ± 0.80 E | 31.21 ± 1.58 C,D,E,F | 3.94 ± 2.76 B,C | |
| S | 80.31 ± 0.94 B,C | −2.91 ± 0.65 D,E | 30.46 ± 1.26 D,E,F,G | 2.60 ± 0.64 B,C | |
| Day 5 | Uncoated | 80.88 ± 0.25 B,C | −1.32 ± 0.04 A,B,C | 30.91 ± 0.30 C,D,E,F,G | |
| T | 80.60 ± 0.02 B,C | −2.13 ± 0.06 C,D,E | 29.26 ± 0.91 E,F,G | 1.85 ± 0.76 B,C | |
| S | 77.80 ± 1.96 D | −1.82 ± 1.48 B,C,D | 35.18 ± 0.10 B | 5.73 ± 1.42 B | |
| Day 12 | Uncoated | 81.05 ± 0.25 B,C | −1.11 ± 0.05 A,B,C | 30.77 ± 0.09 C,D,E,F,G | |
| T | 81.07 ± 0.87 B,C | −1.18 ± 0.27 A,B,C | 29.03 ± 0.28 F,G | 1.74 ± 0.28 B,C | |
| S | 79.53 ± 1.27 B,C,D | −0.80 ± 0.15 A,B,C | 33.02 ± 2.45 B,C,D | 2.74 ± 2.56 B,C | |
| Day 16 | Uncoated | 80.35 ± 0.25 B,C | −0.84 ± 0.08 A,B,C | 31.46 ± 0.10 C,D,E,F | |
| T | 80.09 ± 1.24 B,C,D | −1.31 ± 0.54 A,B,C | 30.00 ± 2.03 D,E,F,G | 1.61 ± 1.27 B,C | |
| S | 78.80 ± 0.06 C,D | −0.84 ± 0.50 A,B,C | 33.97 ± 0.51 B,C | 2.95 ± 0.39 B,C | |
| Day 21 | Uncoated | 80.73 ± 0.04 B,C | −0.35 ± 0.02 A,B | 30.22 ± 0.09 D,E,F,G | |
| T | 81.99 ± 0.41 B | −0.84 ± 0.34 A,B,C | 27.74 ± 0.71 G | 2.83 ± 0.85 B,C | |
| S | 81.15 ± 0.63 B,C | −1.02 ± 0.04 A,B,C | 30.00 ±1.75 D,E,F,G | 0.82 ± 0.77 C | |
| Day 42 | Uncoated | 86.64 ± 0.05 A | −0.10 ± 0.03 A | 30.86 ± 0.07 C,D,E,F,G | |
| T | 85.45 ± 0.21 A | −0.49 ± 0.24 A,B | 32.37 ± 0.11 B,C,D,E | 1.96 ± 0.01 B,C | |
| S | 79.77 ± 1.12 B,C,D | −1.50 ± 0.05 A,B,C,D | 39.48 ± 1.28 A | 11.11 ± 1.67 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bleoanca, I.; Lanciu, A.; Patrașcu, L.; Ceoromila, A.; Borda, D. Efficacy of Two Stabilizers in Nanoemulsions with Whey Proteins and Thyme Essential Oil as Edible Coatings for Zucchini. Membranes 2022, 12, 326. https://doi.org/10.3390/membranes12030326
Bleoanca I, Lanciu A, Patrașcu L, Ceoromila A, Borda D. Efficacy of Two Stabilizers in Nanoemulsions with Whey Proteins and Thyme Essential Oil as Edible Coatings for Zucchini. Membranes. 2022; 12(3):326. https://doi.org/10.3390/membranes12030326
Chicago/Turabian StyleBleoanca, Iulia, Andreea Lanciu, Livia Patrașcu, Alina Ceoromila, and Daniela Borda. 2022. "Efficacy of Two Stabilizers in Nanoemulsions with Whey Proteins and Thyme Essential Oil as Edible Coatings for Zucchini" Membranes 12, no. 3: 326. https://doi.org/10.3390/membranes12030326
APA StyleBleoanca, I., Lanciu, A., Patrașcu, L., Ceoromila, A., & Borda, D. (2022). Efficacy of Two Stabilizers in Nanoemulsions with Whey Proteins and Thyme Essential Oil as Edible Coatings for Zucchini. Membranes, 12(3), 326. https://doi.org/10.3390/membranes12030326








