Magnetic Core-Shell Iron Oxides-Based Nanophotocatalysts and Nanoadsorbents for Multifunctional Thin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Methods
2.3. Equipments
3. Results and Discussion
3.1. Morphology and Structure
3.2. Textural Analysis
3.3. Magnetic Properties
3.4. Nanosorption and Photocatalytic Activities
3.4.1. Sorption Kinetics
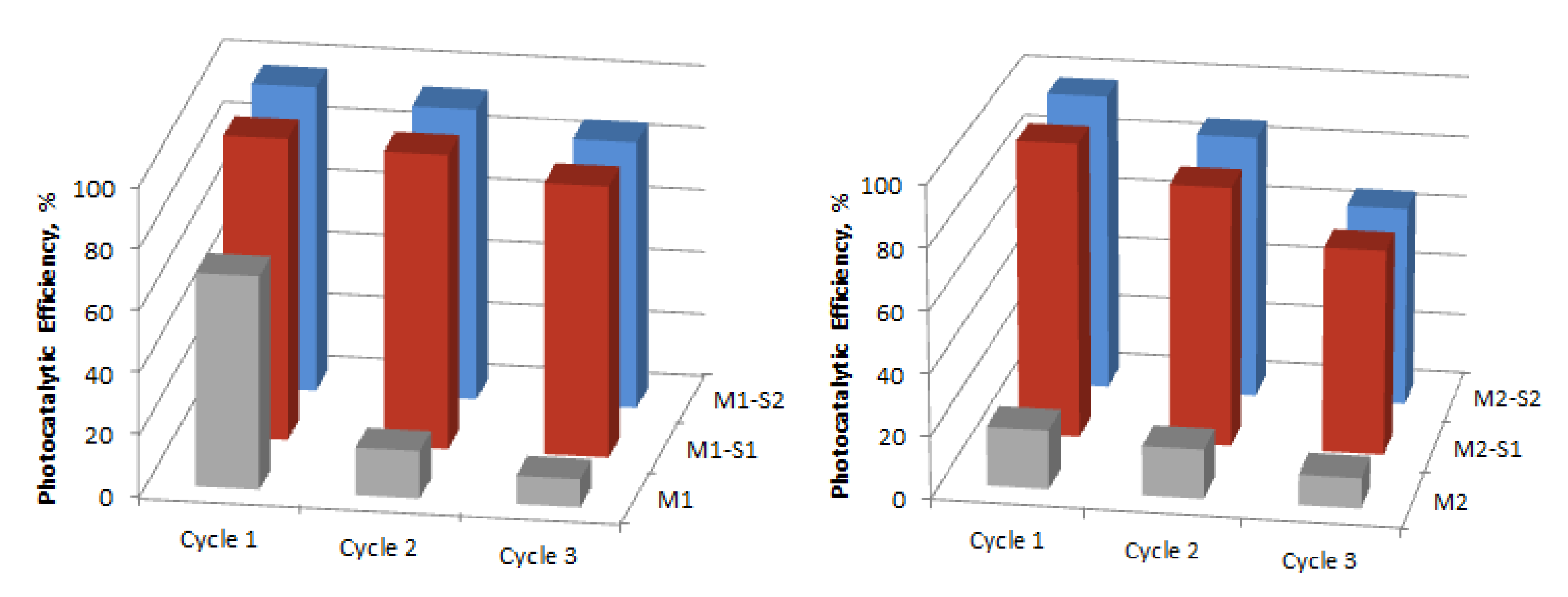
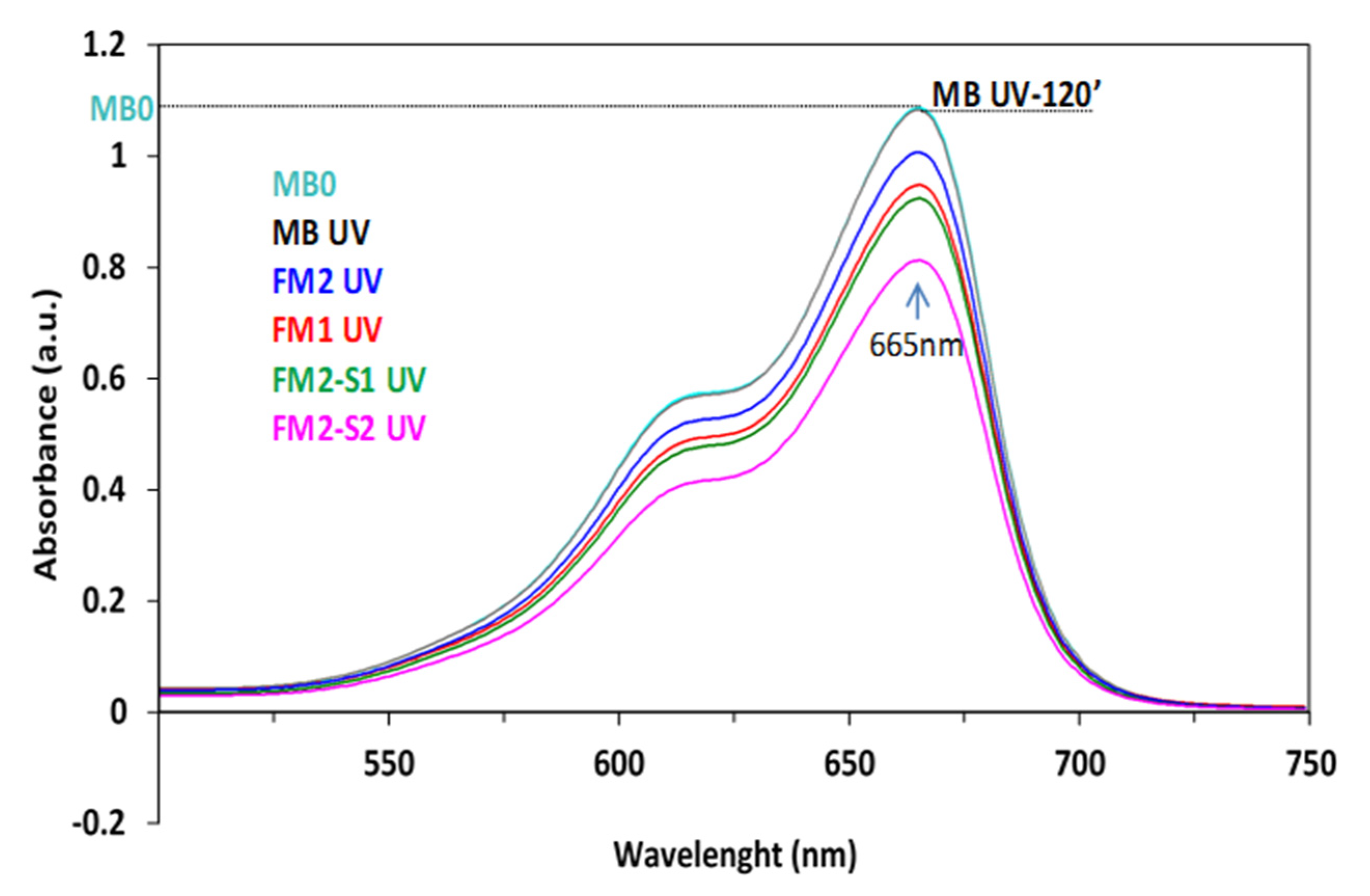
3.4.2. Photocatalytic Activity and Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ajinkya, N.; Yu, X.; Kaithal, P.; Luo, H.; Somani, P.; Ramakrishna, S. Magnetic Iron Oxide Nanoparticle (IONP) Synthesis to Applications: Present and Future. Materials 2020, 13, 4644. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, J.; Wang, Y.; Sheng, J.; Wang, F.; Sun, M. Application of Iron Magnetic Nanoparticles in Protein Immobilization. Molecules 2014, 19, 11465–11486. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Hoque, M.A.; Rahman, G.T.; Gafur, M.T.; Khan, R.A.; Hossain, M.K. Study on the mechanical, electrical and optical properties of metal-oxide nanoparticles dispersed unsaturated polyester resin nanocomposites. Results Phys. 2019, 13, 102264. [Google Scholar] [CrossRef]
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional magnetic iron oxide nanoparticles: Diverse synthetic approaches, surface modifications, cytotoxicity towards biomedical and industrial applications. BMC Mater. 2019, 1, 2. [Google Scholar] [CrossRef]
- Khalaj, M.J.; Ahmadi, H.; Lesankhosh, H.; Khalaj, G. Study of physical and mechanical properties of polypropylene nanocomposites for food packaging application: Nano-clay modified with iron nanoparticles. Trends Food. Sci. Technol. 2016, 51, 41–48. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Y.; Yan, M.; Wang, Q.; Xu, H.; Wang, X. Fabrication of iron oxide@MOF-808 as a sorbent for magnetic solid phase extraction of benzoylurea insecticides in tea beverages and juice samples. J. Chromatogr. A 2020, 1615, 460766. [Google Scholar] [CrossRef]
- Zhang, Z.; Lou, Y.; Guo, C.; Jia, Q.; Song, Y.; Tian, J.-Y.; Zhang, S.; Wang, M.; He, L.; Du, M. Metal–organic frameworks (MOFs) based chemosensors/biosensors for analysis of food contaminants. Trends Food. Sci. Technol. 2021, 118, 569–588. [Google Scholar] [CrossRef]
- Liu, J.F.; Zhao, Z.S.; Jiang, G.B. Coating Fe3O4 Magnetic Nanoparticles with Humic Acid for High Efficient Removal of Heavy Metals in Water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef]
- Gabelica, I.; Curkovic, L.; Mandic, V.; Panžic, I.; Ljubas, D.; Zadro, K. Rapid Microwave-Assisted Synthesis of Fe3O4/SiO2/TiO2 Core-2-Layer-Shell Nanocomposite for Photocatalytic Degradation of Ciprofloxacin. Catalysts 2021, 11, 1136. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Konstantopoulos, C.; Poelman, H.; Marin, G.B. Fe-Based Nano-Materials in Catalysis. Materials 2018, 11, 831. [Google Scholar] [CrossRef]
- Tao, Q.; Bi, J.; Huang, X. Fabrication, application, optimization and working mechanism of Fe2O3 and its composites for contaminants elimination from wastewater. Chemosphere 2021, 263, 127889. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sharma, K.; Hasija, V.; Sharma, V.; Sharma, S.; Raizada, P.; Singh, M.; Sainiad, A.K.; Hosseini-Bandegharaei, A.; Thakur, V.K. Systematic review on applicability of magnetic iron oxides–integrated photocatalysts for degradation of organic pollutants in water. Mater. Today Chem. 2019, 14, 100186. [Google Scholar] [CrossRef]
- Bharti, J.J.S.; Kumar, S.S.; Kumar, V.; Kumar, D. A review on the capability of zinc oxide and iron oxides nanomaterials, as a water decontaminating agent: Adsorption and photocatalysis. Appl. Water Sci. 2022, 12, 46. [Google Scholar] [CrossRef]
- Saber, O.; Kotb, H.M.; Osama, M.; Khater, H.A. An Effective Photocatalytic Degradation of Industrial Pollutants through Converting Titanium Oxide to Magnetic Nanotubes and Hollow Nanorods by Kirkendall Effect. Nanomaterials 2022, 12, 440. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Ta, H.K.T.; Park, S.; Phan, T.B.; Pham, N.K. Resistive switching effect and magnetic properties of iron oxide nanoparticles embedded-polyvinyl alcohol film. RSC Adv. 2020, 10, 12900–12907. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Schneider, M.G.M.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef]
- Tran, H.-V.; Ngo, N.M.; Medhi, R.; Srinoi, P.; Liu, T.; Rittikulsittichai, S.; Lee, T.R. Multifunctional Iron Oxide MagneticNanoparticles for Biomedical Applications: A Review. Materials 2022, 15, 503. [Google Scholar] [CrossRef]
- Levdansky, V.V.; Šolcová, O.; Izák, P. Size effects in physicochemical processes in nanoparticles and nanopores. Mater. Chem. Phys. 2018, 211, 117–122. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y.K. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Filip, M.; Petcu, G.; Anghel, E.M.; Petrescu, S.; Trica, B.; Osiceanu, P.; Stanica, N.; Atkinson, I.; Munteanu, M.; Mureseanu, M.; et al. FeTi-SBA-15 magnetic nanocomposites with photocatalytic properties. Catal. Today 2021, 366, 10–19. [Google Scholar] [CrossRef]
- Wang, B.; Wei, Q.; Qu, S. Synthesis and characterization of uniform and crystalline magnetite nanoparticles via oxidation-precipitation and modified co-precipitation methods. Int. J. Electrochem. Sci. 2013, 8, 3786–3793. [Google Scholar]
- Zhang, X.; Jia, X.; Li, M.; Shi, Z.; Xu, R.; Zhao, J.; Niu, Y. Surface modification, adsorption behavior, and optical properties of α-Fe2O3@SiO2/Au core-shell ellipsoids. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126888. [Google Scholar] [CrossRef]
- Nistico, R. A synthetic guide toward the tailored production of magnetic iron oxide nanoparticles. Bol. Soc. Esp. Ceram. Vidr. 2021, 60, 29–40. [Google Scholar] [CrossRef]
- Poddar, M.K.; Arjmand, M.; Sundararaj, U.; Moholkar, V.S. Ultrasound-assisted synthesis and characterization of magnetite nanoparticles and poly(methyl methacrylate)/magnetite nanocomposites. Ultrason. Sonochem. 2018, 43, 38–51. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.-I.; Crăciun, L.N.; Ficai, D.; Ficai, A.; Andronescu, E. Magnetite-Silica Core/Shell Nanostructures: From Surface Functionalization towards Biomedical Applications—A Review. Appl. Sci. 2021, 11, 11075. [Google Scholar] [CrossRef]
- Kang, H.J.H.; Ali, R.F.; Paul, M.T.Y.; Radford, M.J.; Andreu, I.; Leea, A.W.H.; Gates, B.D. Tunable functionalization of silica coated iron oxide nanoparticles achieved through a silanol–alcohol condensation reaction. Chem. Commun. 2019, 55, 10452–10455. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Wang, P.; Tang, Z.; Niu, H.; Cai, Y.; Wu, F.; Wang, H.; Meng, W.; Giesy, J.P. Surfactant-modified flowerlike layered double hydroxide-coated magnetic nanoparticles for preconcentration of phthalate esters from environmental water samples. J. Chromatogr. A 2015, 1414, 22–30. [Google Scholar] [CrossRef]
- Abbas, M. Fe3O4/SiO2 Core/Shell Nanocubes: Novel Coating Approach with Tunable Silica Thickness and Enhancement in Stability and Biocompatibility. J. Nanomed. Nanotechnol. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Chishti, A.N.; Ni, L.; Guo, F.; Lin, X.; Liu, Y.; Wu, H.; Chen, M.; Diao, G.W. Magnetite-Silica core-shell nanocomposites decorated with silver nanoparticles for enhanced catalytic reduction of 4-nitrophenol and degradation of methylene blue dye in the water. J. Environ. Chem. Eng. 2021, 9, 104948. [Google Scholar] [CrossRef]
- Dagher, S.; Soliman, A.; Ziout, A.; Tit, N.; Hilal-Alnaqbi, A.; Khashan, S.; Alnaimat, F.; Qudeiri, J.A. Photocatalytic removal of methylene blue using titania- and silicacoated magnetic nanoparticles. Mater. Res. Express 2018, 5, 065518. [Google Scholar] [CrossRef]
- Sun, Z.; Li, H.; Cui, G.; Tian, Y.; Yan, S. Multifunctional magnetic core-shell dendritic mesoporous silica nanospheres decorated with tiny Ag nanoparticles as a highly active heterogeneous catalyst. Appl. Surf. Sci. 2016, 360, 252–262. [Google Scholar] [CrossRef]
- Sadighian, S.; Sharifan, K.; Khanmohammadi, A.; Rohani, M.K. A Facile Synthesis of Fe3O4@SiO2@ZnO for Curcumin Delivery. Biointerface Res. Appl. Chem. 2022, 12, 7994–8002. [Google Scholar] [CrossRef]
- Zhang, T. Heterogeneous Catalytic Process for Wastewater Treatment. In Advanced Oxidation Processes; Bustillo-Lecompte, C., Ed.; IntechOpen: London, UK, 2020; pp. 1–30. [Google Scholar] [CrossRef]
- Duta, A.; Covei, M.; Bogatu, C.; Perniu, D. 23-TiO2-Copper Zinc Tin Sulfide (CZTS) Photocatalytic Thin Films for Up-Scalable Wastewater Treatment in Materials Science in Photocatalysis; Lopez, E.G., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 371–383. [Google Scholar] [CrossRef]
- Yepseu, A.P.; Isac, L.; Nyamen, L.D.; Cleymand, F.; Duta, A.; Ndifon, P.T. Optical and photocatalytic properties of CuxS/ZnO composite thin films deposited by robotic spray pyrolysis deposition. J. Nanomater. 2021, 2021, 9975600. [Google Scholar] [CrossRef]
- Chen, Y.H.; Tu, K.J. Thickness dependent of photocatalytic activity of hematite thin films. Int. J. Photoenergy 2012, 2012, 980595. [Google Scholar] [CrossRef]
- Schultz, A.M.; Salvador, P.A.; Rohrer, G.S. Enhanced photochemical activity of α-Fe2O3 films supported on SrTiO3 substrates under visible light illumination. Chem. Comm. 2012, 48, 2012–2014. [Google Scholar] [CrossRef][Green Version]
- Kiziltaş, H.; Tekina, T.; Tekin, D. Synthesis, characterization of Fe3O4@SiO2@ZnO composite with a coreshell structure and evaluation of its photocatalytic activity. J. Environ. Chem. Eng. 2020, 8, 104160. [Google Scholar] [CrossRef]
- Crintea, L.C.; Muşat, V.; Poloşan, S.; Cantaragiu, A.; Başliu, V.; Dediu, A.B.; Dinică, R. Synthesis and characterization of magnetic oxide nanoparticles and corresponding thin films for wastewaters treatment. Ovidius Univ. Ann. Chem. 2020, 31, 122–131. [Google Scholar] [CrossRef]
- Dudric, R.; Souca, G.; Szatmári, A.; Szilárd, T.; Nitica, S.; Iacovita, C.; Moldovan, A.I.; Stiufiuc, R.; Tetean, R.; Burzo, E. Magnetite Nanoparticles for Medical Applications. AIP Conf. Proc. 2020, 2218, 030014. [Google Scholar] [CrossRef]
- Mikhaylova, A.B.; Sirotinkin, V.P.; Fedotov, M.A.; Korneyev, V.P.; Shamray, B.F.; Kovalenko, L.V. Quantitative Determination of Content of Magnetite and Maghemite in Their Mixtures by X-Ray Diffraction Methods. Inorg. Mater. Appl. Res. 2016, 7, 130–136. [Google Scholar] [CrossRef]
- Cuenca, J.A.; Bugler, K.; Taylor, S.; Morgan, D.; Williams, P.; Bauer, J.; Porch, A. Study of the magnetite to maghemite transition using microwave permittivity and permeability measurements. J. Phys. Condens. Matter 2016, 28, 106002. [Google Scholar] [CrossRef] [PubMed]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (IUPAC Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Petrova, D.A.; Ivantsova, R.D.; Zharkova, S.M.; Velikanova, D.A.; Molokeeva, M.S.; Linc, C.-R.; Tsoc, C.T.; Hsuc, S.H.; Tsengc, Y.-T.; Linc, E.-S.; et al. Magnetic and magneto-optical properties of Fe3O4 nanoparticles modified with Ag. J. Mag. Magn. Mater. 2010, 493, 165692. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Hochella, M.F.; Madden, A.S. Size-dependent structural transformations of hematite nanoparticles. 1. Phase transition. Phys. Chem. Chem. Phys. 2007, 9, 1736–1750. [Google Scholar] [CrossRef]
- Machala, L.; Tucek, J.; Zboril, R. Polymorphous Transformations of Nanometric Iron (III) Oxide: A Review. Chem. Mater. 2011, 23, 3255–3327. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman study of magnetite (Fe3O4): Laser-induced thermal effects and oxidation. J. Raman Spectrosc. 2003, 34, 845–852. [Google Scholar] [CrossRef]
- Sartoratto, P.P.C.; Caiado, K.L.; Pedroza, R.C.; Silva, S.W.; Morais, P.C. The thermal stability of maghemite-silica nanocomposites: An investigation using X-ray diffraction and Raman spectroscopy. J. Alloy. Compds. 2007, 434–435, 650–654. [Google Scholar] [CrossRef]
- Galeener, F.L.; Mikkelsen, J.C. Vibrational dynamics in 18O-substituted vitreous SiO2. Phys. Rev. B 1981, 23, 5527–5530. [Google Scholar] [CrossRef]
- Cheng, H.-M.; Lin, K.-F.; Hsu, H.-C.; Hsieh, W.F. Size dependence of photoluminescence and resonant Raman scattering from ZnO quantum dots. Appl. Phys. Lett. 2006, 88, 261909. [Google Scholar] [CrossRef]
- Hess, C. New advances in using Raman spectroscopy for the characterization of catalysts and catalytic reactions. Chem. Soc. Rev. 2021, 50, 3519. [Google Scholar] [CrossRef] [PubMed]
- Stoicescu, C.S.; Culita, D.; Stanica, N.; Papa, F.; State, R.N.; Munteanu, G. Temperature programmed reduction of a core-shell synthetic magnetite: Dependence on the heating rate of the reduction mechanism. Thermochim. Acta 2022, 709, 179146. [Google Scholar] [CrossRef]
- Lu, J.; Jiao, X.; Chen, D.; Li, W. Solvothermal Synthesis and Characterization of Fe3O4 and γ-Fe2O3 Nanoplates. J. Phys. Chem. C 2009, 113, 4012–4017. [Google Scholar] [CrossRef]
- Cao, D.; Li, H.; Pan, L.; Li, J.; Wang, X.; Jing, P.; Cheng, X.; Wang, W.; Wang, J.; Liu, Q. High saturation magnetization of γ-Fe2O3 nano-particles by a facile one-step synthesis approach. Sci. Rept. 2016, 6, 32360. [Google Scholar] [CrossRef] [PubMed]
- Coduri, M.; Masala, P.; Del Bianco, L.; Spizzo, F.; Ceresoli, D.; Castellano, C.; Cappelli, S.; Oliva, C.; Checchia, S.; Allieta, M.; et al. Local Structure and Magnetism of Fe2O3 Maghemite. Nanocrystals: The Role of Crystal Dimension. Nanomaterials 2020, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hsu, H.S.; Huang, Y.H. Spin-dependent optical charge transfer in magnetite from transmitting optical magnetic circular dichroism. Phys. Rev. B 2018, 98, 085141. [Google Scholar] [CrossRef]
- Fontijn, W.F.J.; Zaag, P.J.; Devillers, M.A.C.; Brabers, V.A.M.; Metselaar, R. Optical and magneto-optical polar Kerr spectra of Fe3O4 and Mg2+- or Al3+-substituted Fe3O4. Phys. Rev. B 1997, 56, 5432. [Google Scholar] [CrossRef]
- Edelman, I.; Ivanova, O.; Ivantsov, R.; Velikanov, D.; Zabluda, V.; Zubavichus, Y.; Veligzhanin, A.; Zaikovskiy, V.; Stepanov, S.; Artemenko, A.; et al. Magnetic nanoparticles formed in glasses co-doped with iron and larger radius elements. J. Appl. Phys. 2012, 112, 084331. [Google Scholar] [CrossRef]
- He, Y.P.; Miao, Y.M.; Li, C.R.; Wang, S.Q.; Cao, L.; Xie, S.S.; Yang, G.Z.; Zou, B.S. Size and structure effect on optical transitions of iron oxide nanocrystals. Phys. Rev. B 2005, 71, 125411. [Google Scholar] [CrossRef]
- Markovin, P.A.; Kalashnikova, A.M.; Pisarev, R.V.; Rasing, T. Optical Study of the Electronic Structure and Magnetic Ordering in a Weak Ferromagnet FeBO3. JETP Lett. 2007, 86, 712–717. [Google Scholar] [CrossRef][Green Version]
- Zhang, Q.; Yang, X.; Guan, J. Applications of Magnetic Nanomaterials in Heterogeneous Catalysis. ACS Appl. Nano Mater. 2019, 2, 4681–4697. [Google Scholar] [CrossRef]
- Shalaby, S.M.; Madkour, F.F.; El-Kassas, H.Y.; Mohamed, A.A.; Elgarahy, A.M. Green synthesis of recyclable iron oxide nanoparticles using Spirulina platensis microalgae for adsorptive removal of cationic and anionic dyes. Environ. Sci. Pollut. Res. 2021, 28, 65549–65572. [Google Scholar] [CrossRef] [PubMed]
- Petit, M.; Michez, L.; Raimundo, J.M.; Malinowski, T.; Dumas, P. An introduction to photocatalysis through methylene blue photodegradation. Eur. J. Phys. 2016, 37, 065808. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Tarcea, C.I.; Pantilimon, C.M.; Matei, E.; Predescu, A.M.; Berbecaru, A.C.; Rapa, M.; Turcanu, A.; Predescu, C. Photocatalytic Degradation of Methylene Blue Dye Using TiO2 and Fe3O4/SiO2/TiO2 as Photocatalysts. IOP Conf. Ser. Mater. Sci. Eng. 2020, 877, 012008. [Google Scholar] [CrossRef]
- Raheb, I.; Manlla, M.S. Kinetic and thermodynamic studies of the degradation of methylene blue by photo-Fenton reaction. Heliyon 2021, 7, e07427. [Google Scholar] [CrossRef]
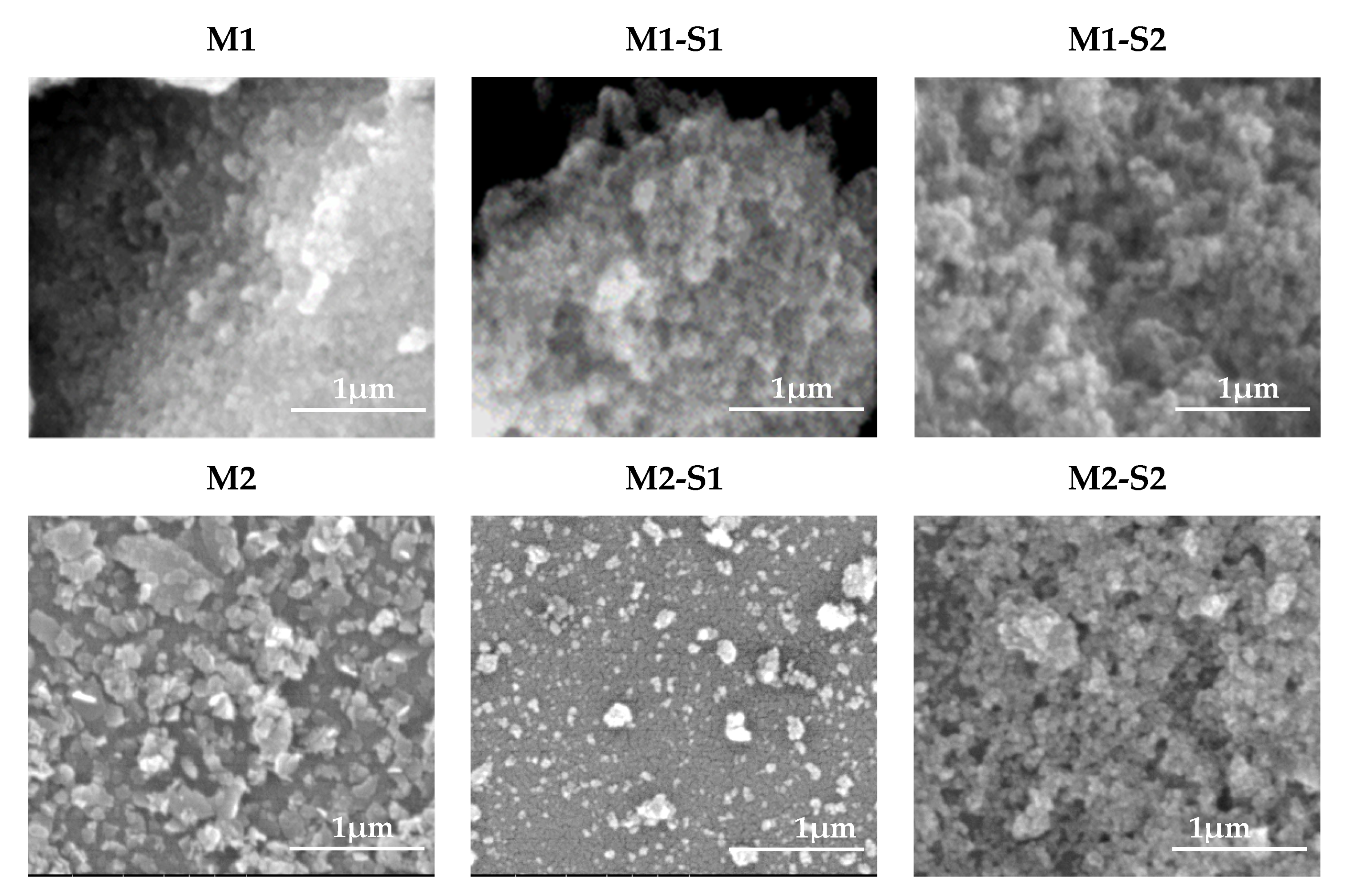
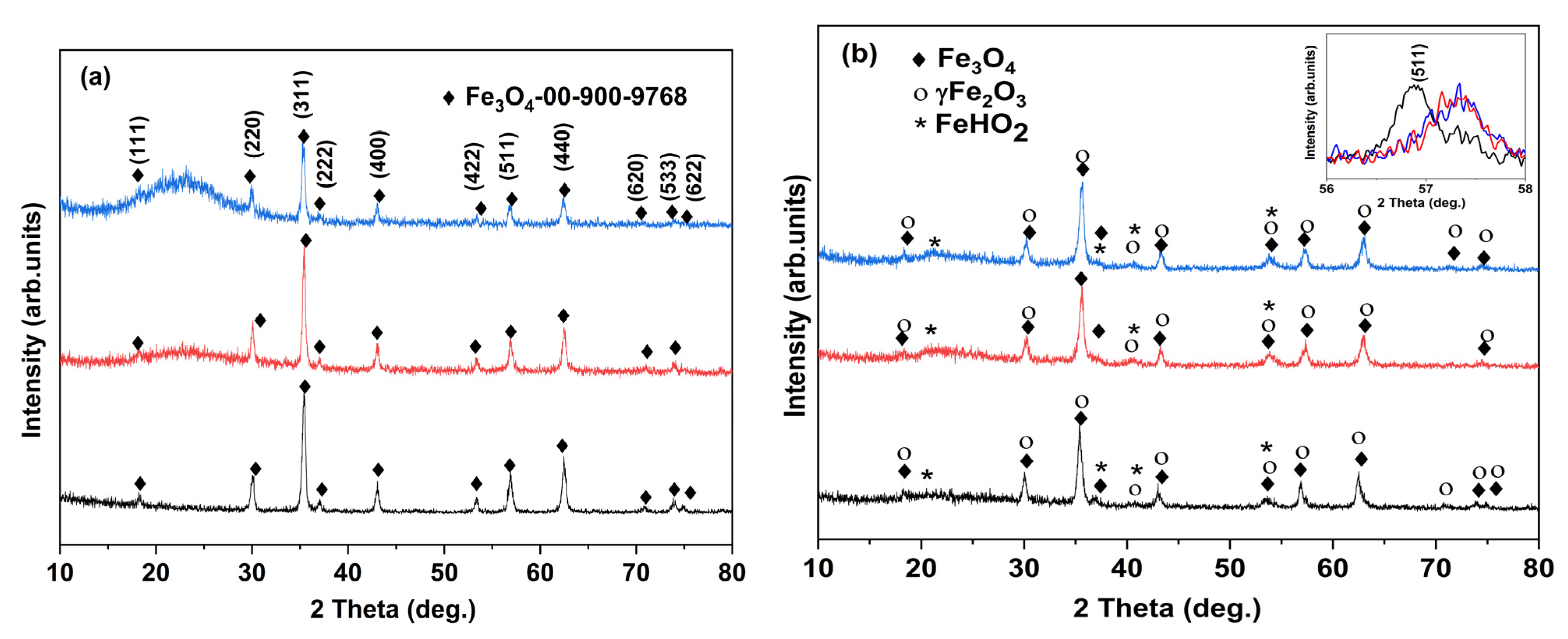
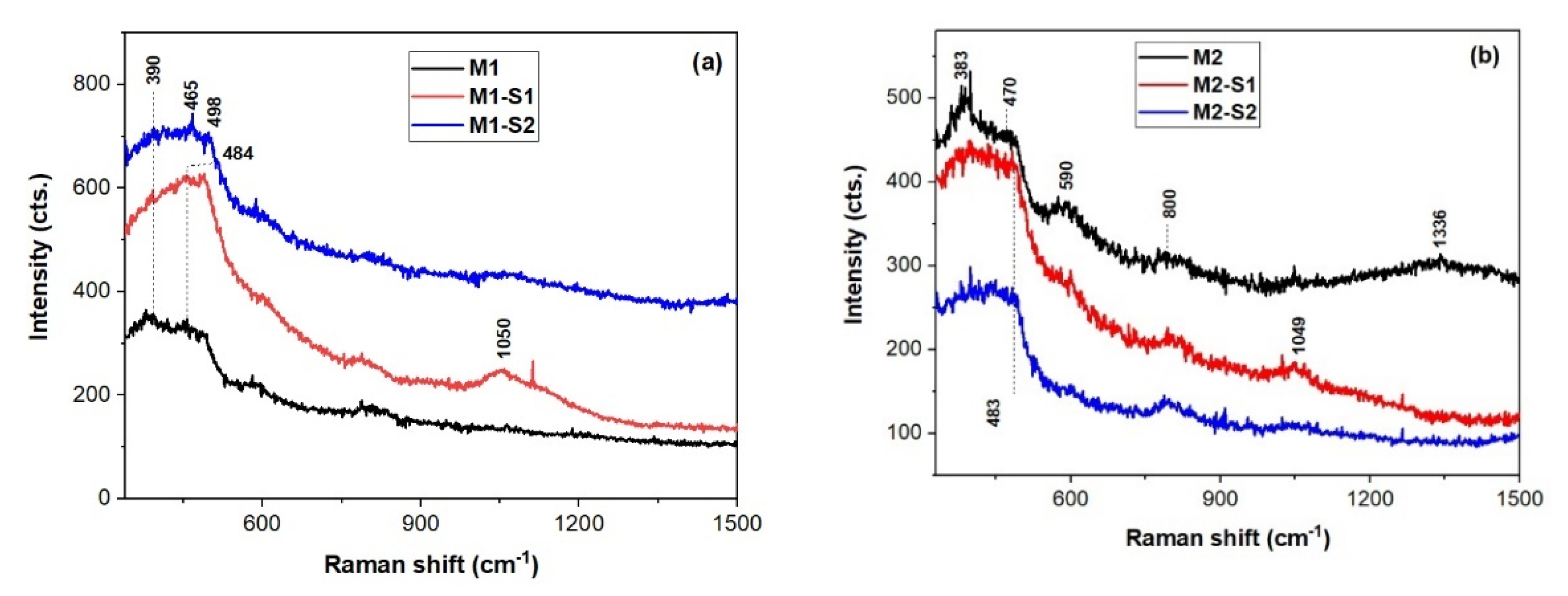

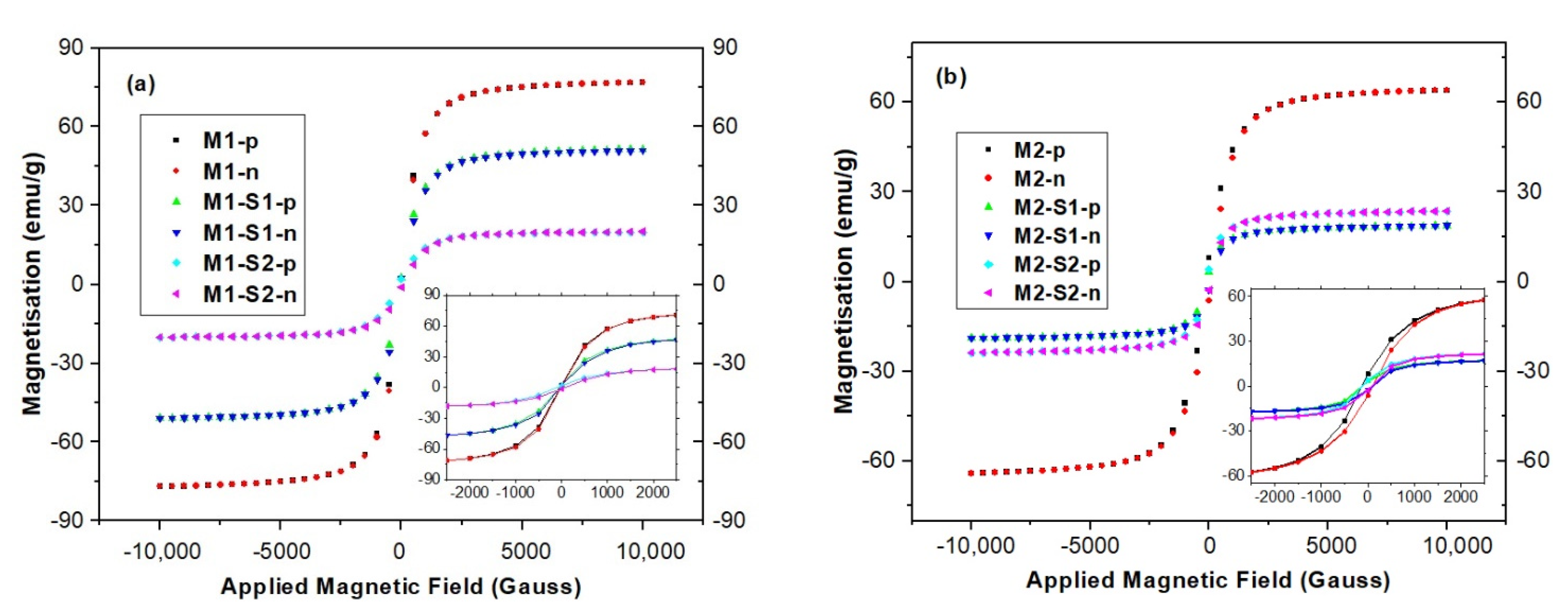
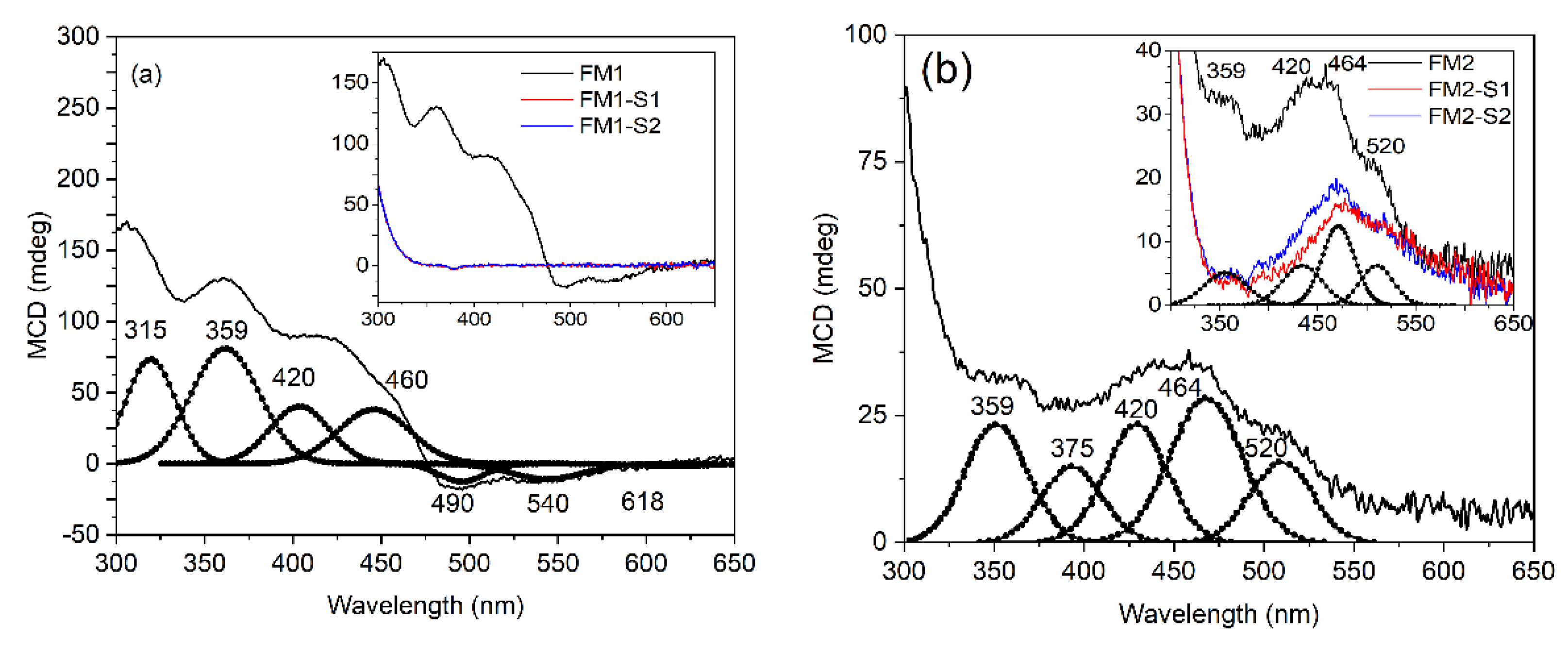
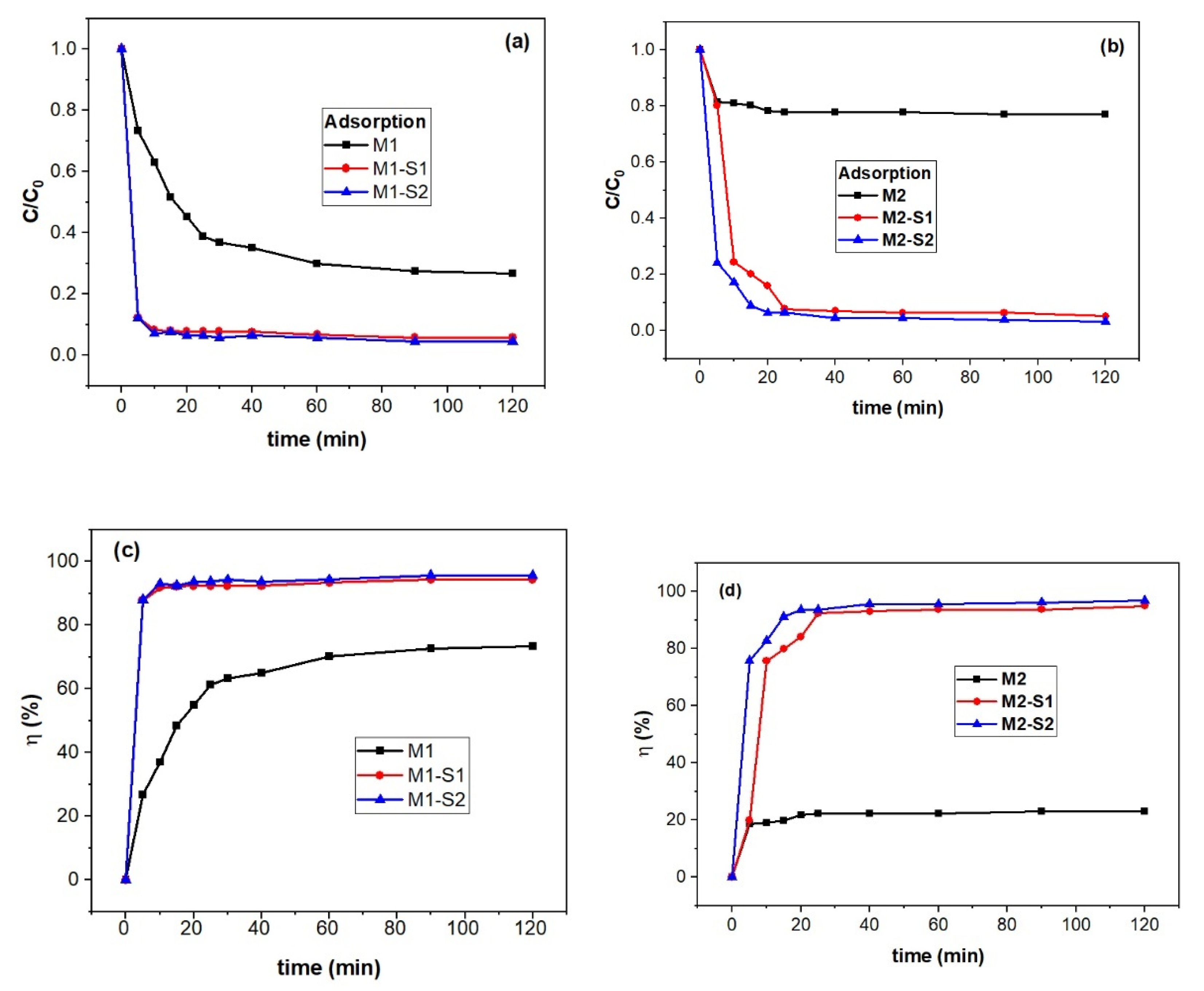
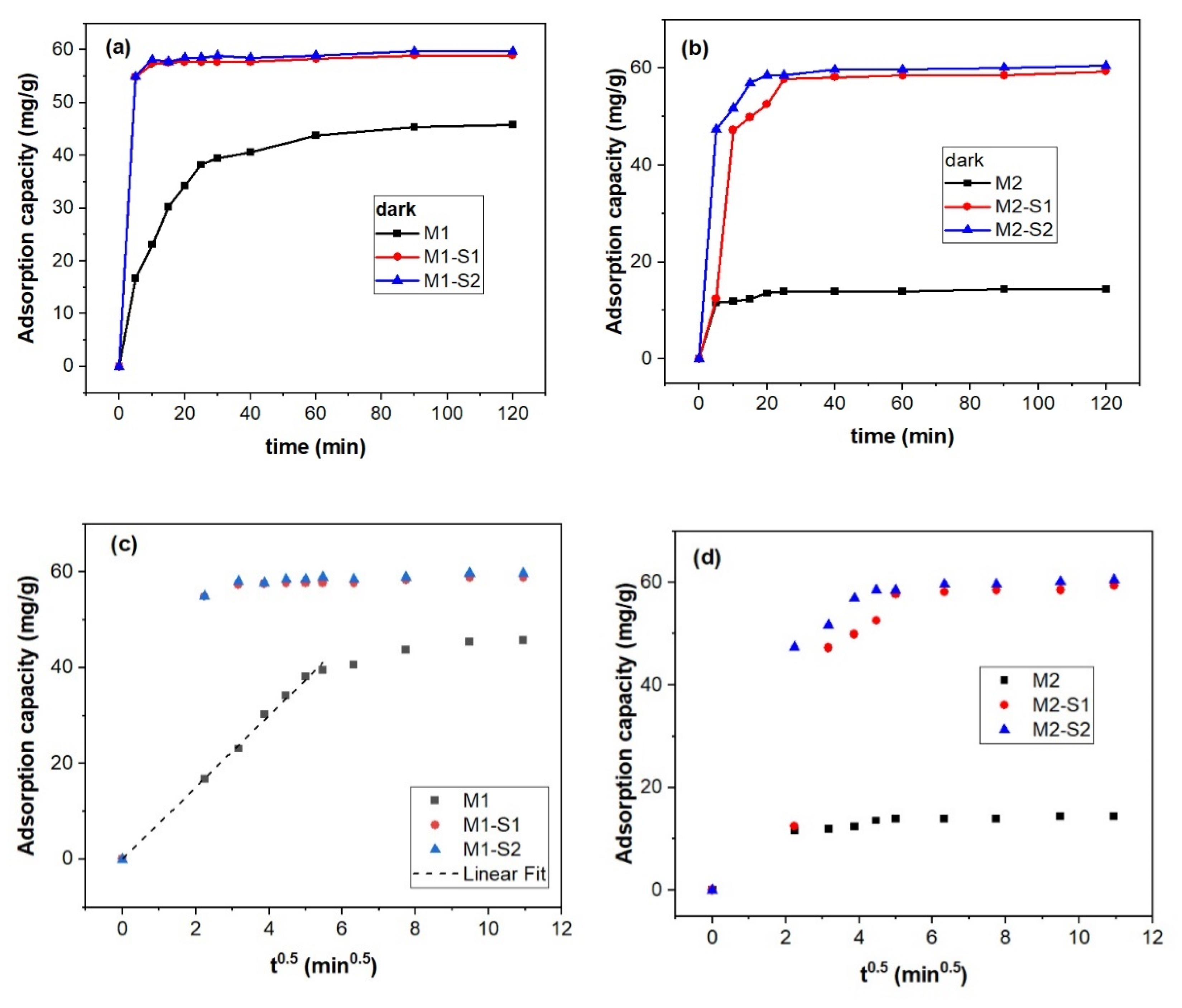
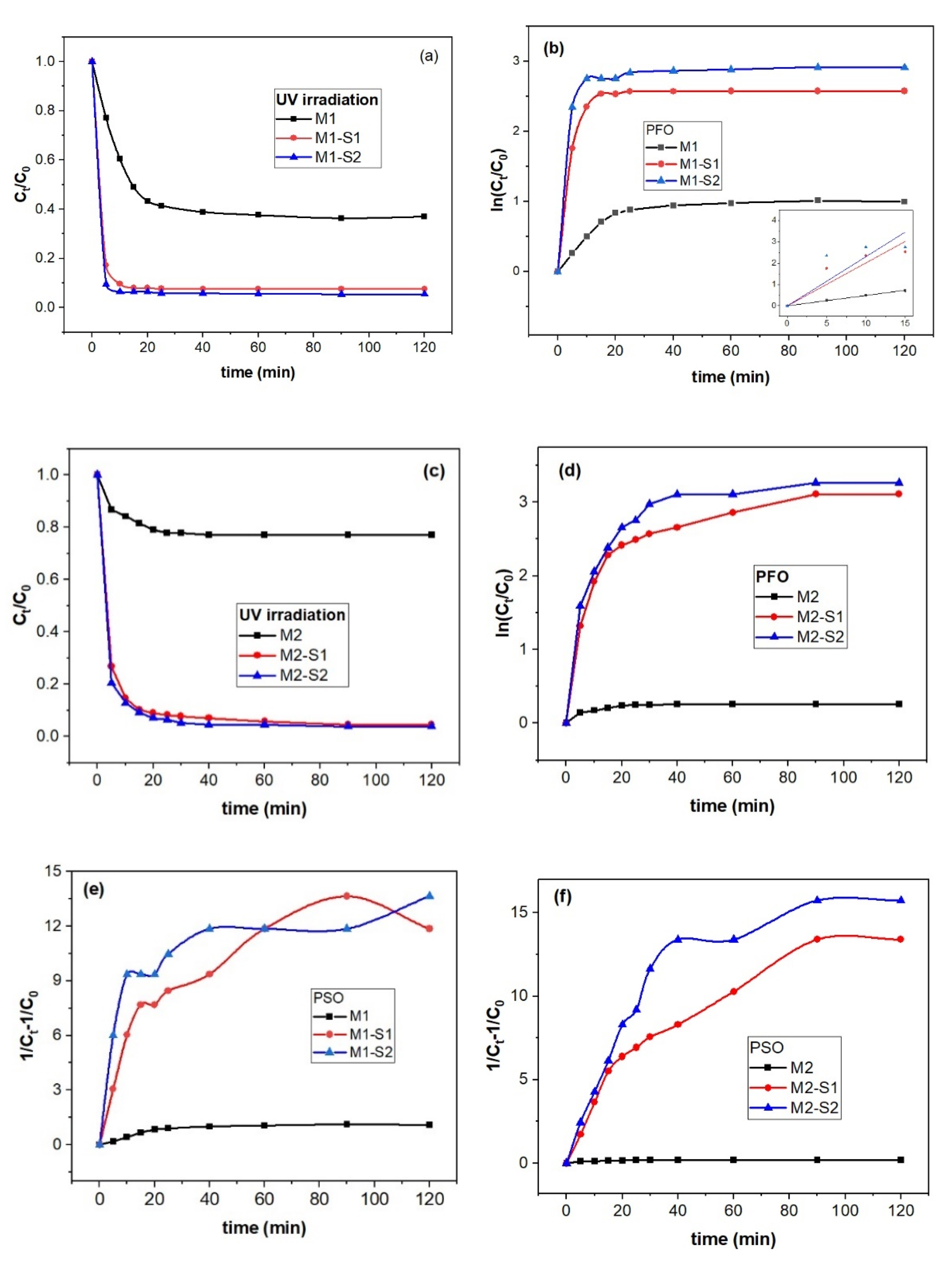

| Sample | XRD Results/Structural Parameters | TexturalParameters | Magnetic Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phases | wt% | 2θ (°) (311) | FWHM (°) | Lattice Parameter a = b = c (Å) | Cryst Size (Å) | SBET (m2g−1) | Pore Volume (cm3g−1) | Ms [emu/g] | Quality Factor | |
| M1 | Fe3O4 | 78 | 35.431 | 0.391 | 8.409(6) | 22(1) | 63.3 | 0.393 | 70.12 | 4.6 × 10−5 |
| γ-Fe2O3 | 22 | — | — | — | — | |||||
| M1-S1 | Fe3O4 | 33 | 35.426 | 0.301 | 8.413(4) | 29(2) | 334.7 | 0.498 | 34.39 | 5.7 × 10−4 |
| γ-Fe2O3 | 67 | — | — | — | — | |||||
| M1-S2 | Fe3O4 | 24.3 | 35.283 | 0.374 | 8.414(3) | 23(1) | 338.0 | 0.473 | 31.41 | 4.5 × 10−5 |
| γ-Fe2O3 | 75.7 | — | — | — | — | |||||
| M2 | Fe3O4 | 37.08 | 35.363 | 0.504 | 8.4107(9) | 17(2) | 70.6 | 0.322 | 66.82 | 6.0 × 10−4 |
| γ-Fe2O3 | 61.16 | |||||||||
| Fe(HO)2 | 1.76 | |||||||||
| Fe3O4 | 11.32 | 8.3517(7) | ||||||||
| M2-S1 | γ-Fe2O3 | 70.84 | 35.595 | 0.487 | 18(2) | 364.8 | 0.387 | 19.5 | 9.5 × 10−4 | |
| Fe(HO)2 | 17.84 | |||||||||
| M2-S2 | Fe3O4 | 15.28 | 35.582 | 0.478 | 8.3554(4) | 18(2) | 396.7 | 0.391 | 24.02 | 7.5 × 10−4 |
| γ-Fe2O3 | 72.55 | |||||||||
| Fe(HO)2 | 12.17 | |||||||||
| Reference (JCPDS no. 01-076-1849) | 35.41 | — | 8.400 | — | ||||||
| Sample | M1 | M1-S1 | M1-S2 | M2 | M2-S1 | M2-S2 |
|---|---|---|---|---|---|---|
| Intraparticle diffusion (ID) qt = kID t0.5 | ||||||
| kid (mg/g h) | 7.46676 ± 0.22619 | 14.3152 ± 1.55213 | 14.46265 ± 1.54519 | 3.22096 ± 0.27047 | 11.91919 ± 0.91285 | 13.92478 ± 1.11899 |
| R2 | 0.99543 | 0.94448 | 0.94601 | 0.96594 | 0.97151 | 0.96872 |
| Sample | k1 (min−1) | R2 | k2 (mg dm−3 min−1) | R2 |
|---|---|---|---|---|
| M1 | 0.04861 ± 8.9805 × 10−4 | 0.9989 | 0.03945 ± 0.00146 | 0.99323 |
| M1-S1 | 0.20105 ± 0.02947 | 0.9939 | 0.40462 ± 0.03925 | 0.95508 |
| M1-S2 | 0.23024 ± 0.045 | 0.8971 | 0.5188 ± 0.07531 | 0.90468 |
| M2 | 0.01203 ± 0.00136 | 0.9398 | 0.0086 ± 8.9857 × 10−4 | 0.94820 |
| M2-S1 | 0.12413 ± 0.0148 | 0.9336 | 0.31748 ± 0.0163 | 0.98690 |
| M2-S2 | 0.13549 ± 0.01641 | 0.9314 | 0.39957 ± 0.00884 | 0.99707 |
| Equation [9] | ln(Ct/C0) = k1t | (1/Ct−1/C0) = k2t |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muşat, V.; Stănică, N.; Anghel, E.M.; Atkinson, I.; Culiţă, D.C.; Poloşan, S.; Crintea, L.; Cantaragiu Ceoromila, A.; Buruiană, C.-T.; Carp, O. Magnetic Core-Shell Iron Oxides-Based Nanophotocatalysts and Nanoadsorbents for Multifunctional Thin Films. Membranes 2022, 12, 466. https://doi.org/10.3390/membranes12050466
Muşat V, Stănică N, Anghel EM, Atkinson I, Culiţă DC, Poloşan S, Crintea L, Cantaragiu Ceoromila A, Buruiană C-T, Carp O. Magnetic Core-Shell Iron Oxides-Based Nanophotocatalysts and Nanoadsorbents for Multifunctional Thin Films. Membranes. 2022; 12(5):466. https://doi.org/10.3390/membranes12050466
Chicago/Turabian StyleMuşat, Viorica, Nicolae Stănică, Elena Maria Anghel, Irina Atkinson, Daniela Cristina Culiţă, Silviu Poloşan, Lenuţa Crintea (Căpăţână), Alina Cantaragiu Ceoromila, Cristian-Teodor Buruiană, and Oana Carp. 2022. "Magnetic Core-Shell Iron Oxides-Based Nanophotocatalysts and Nanoadsorbents for Multifunctional Thin Films" Membranes 12, no. 5: 466. https://doi.org/10.3390/membranes12050466
APA StyleMuşat, V., Stănică, N., Anghel, E. M., Atkinson, I., Culiţă, D. C., Poloşan, S., Crintea, L., Cantaragiu Ceoromila, A., Buruiană, C.-T., & Carp, O. (2022). Magnetic Core-Shell Iron Oxides-Based Nanophotocatalysts and Nanoadsorbents for Multifunctional Thin Films. Membranes, 12(5), 466. https://doi.org/10.3390/membranes12050466








