Preparation of Polyvinylidene Fluoride Nano-Filtration Membranes Modified with Functionalized Graphene Oxide for Textile Dye Removal
Abstract
:1. Introduction
2. Materials and Methods
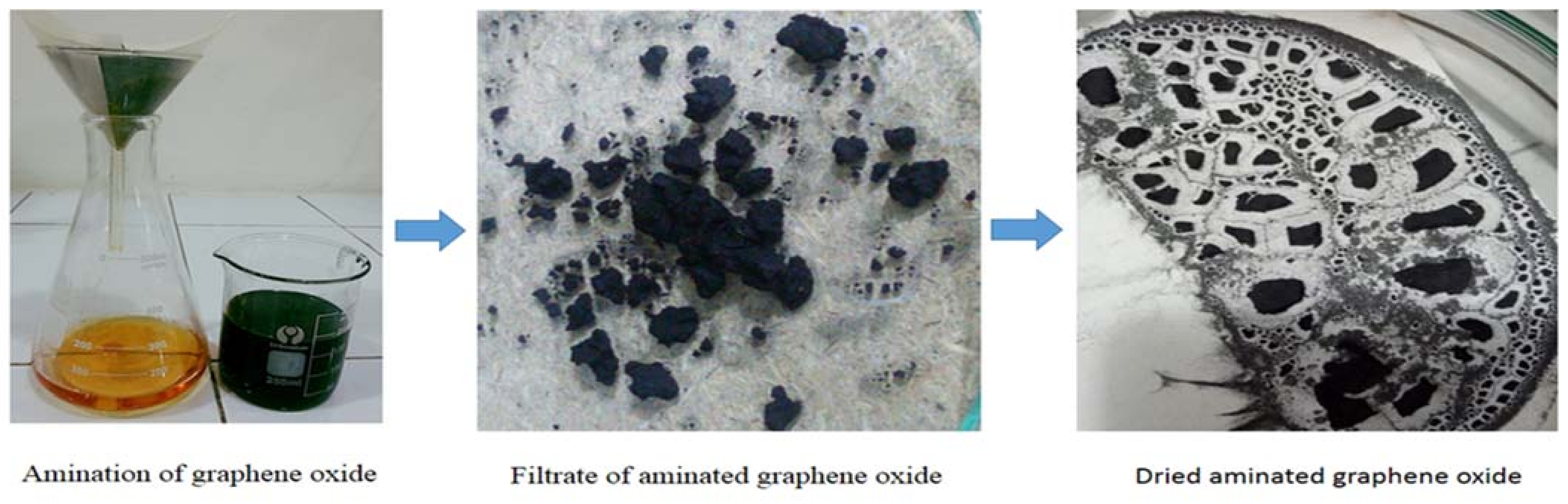
2.1. Reagents, Materials Preparation and Fabrication
2.2. Membrane Performance
2.3. Characterization of Nanocomposite Membranes
3. Result and Discussion
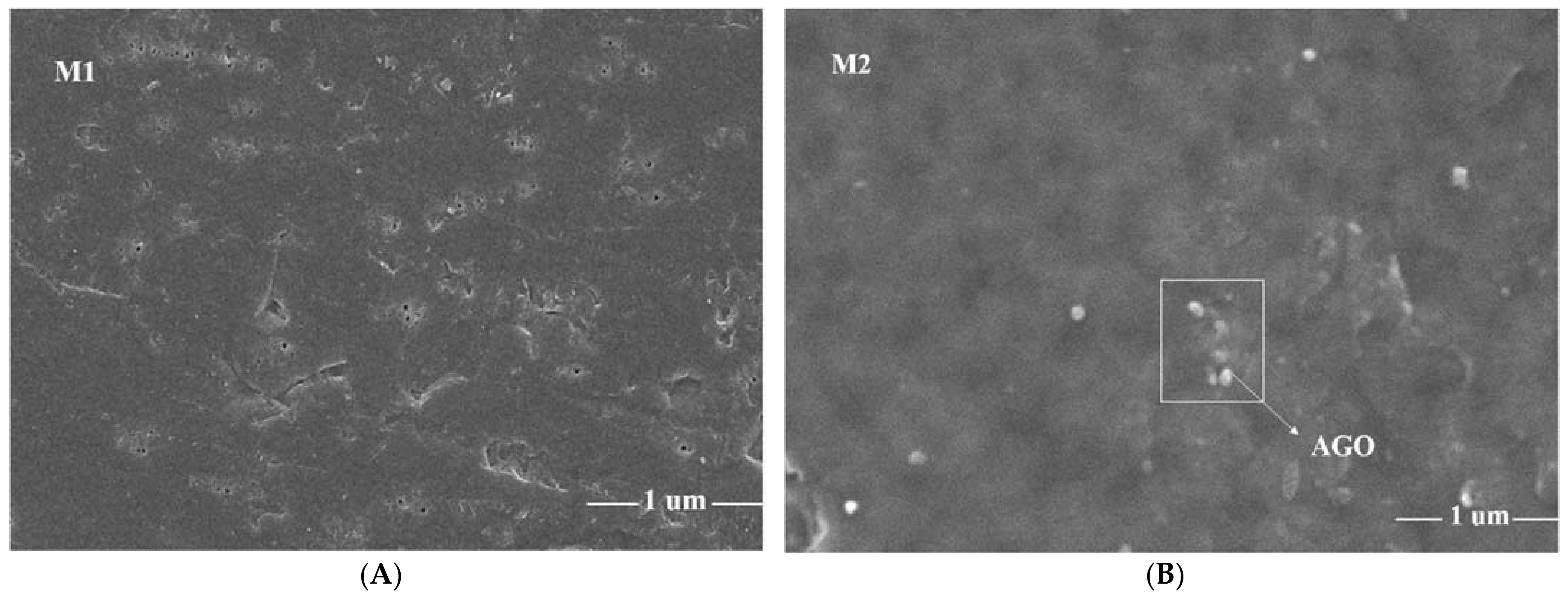
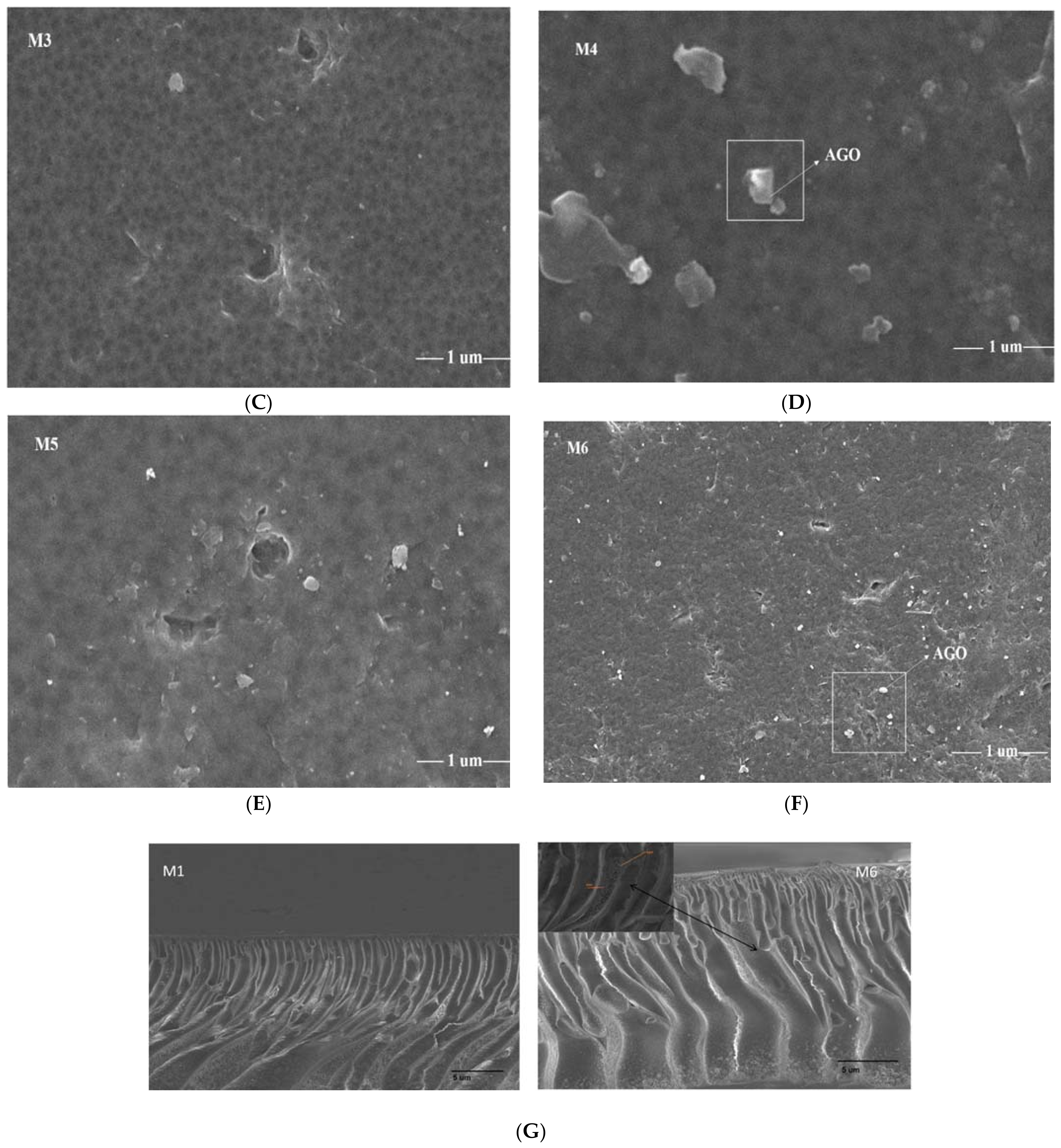
3.1. Scanning Electron Microscopy
3.2. X-ray Diffraction
3.3. Fourier-Transform Infrared Spectroscopy
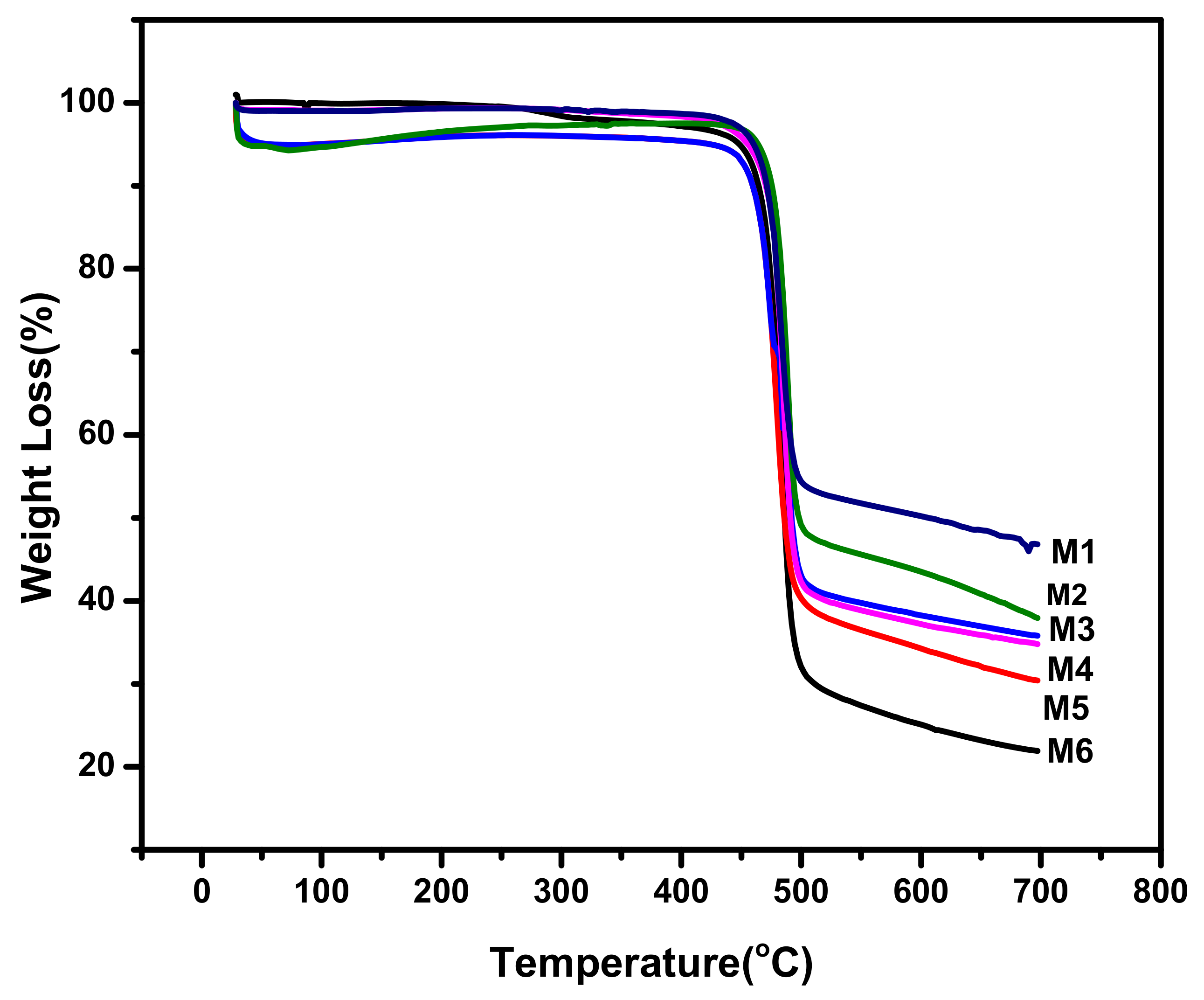
3.4. Thermogravimetric Analysis
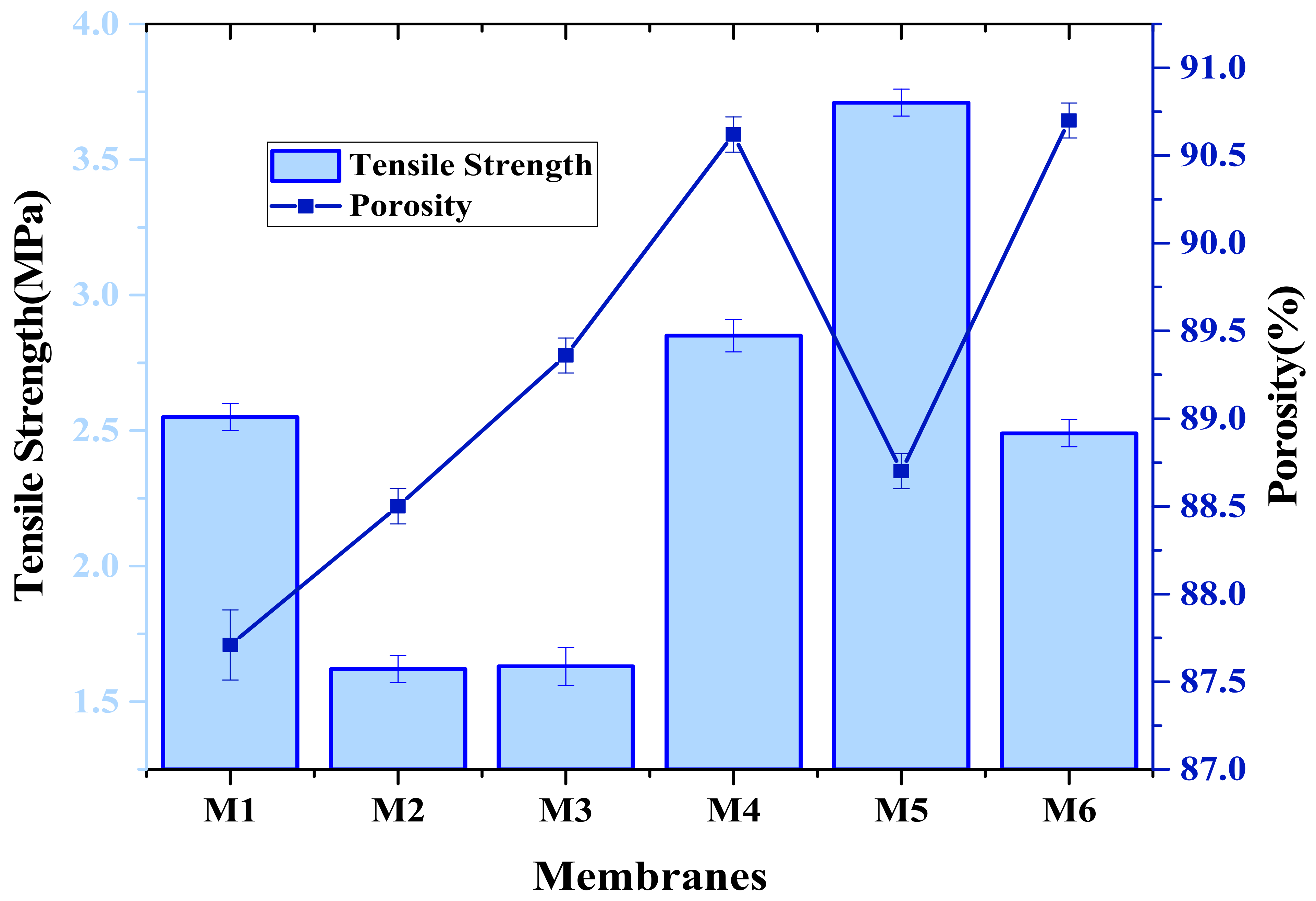
3.5. Tensile Strength
3.6. Membrane Porosity and Contact Angle
3.7. Water Permeability
3.8. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [Green Version]
- Tabasum, A.; Zahid, M.; Bhatti, H.N.; Asghar, A. Fe3O4-GO composite as efficient heterogeneous photo-Fenton’s catalyst to degrade pesticides. Mater. Res. Express 2019, 6, 015608. [Google Scholar]
- Savenije, H.H. Water scarcity indicators; the deception of the numbers. Phys. Chem. Earth Part B Hydrol. Ocean. Atmos. 2000, 25, 199–204. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nanosci. Technol. A Collect. Rev. Nat. J. 2010, 337–346. [Google Scholar] [CrossRef]
- Agenson, K.O.; Oh, J.-I.; Urase, T. Retention of a wide variety of organic pollutants by different nanofiltration/reverse osmosis membranes: Controlling parameters of process. J. Membr. Sci. 2003, 225, 91–103. [Google Scholar] [CrossRef]
- Zahid, M.; Khalid, T.; Rehan, Z.A.; Javed, T.; Akram, S.; Rashid, A.; Mustafa, S.K.; Shabbir, R.; Mora-Poblete, F.; Asad, M.S.; et al. Fabrication and Characterization of Sulfonated Graphene Oxide (SGO) Doped PVDF Nanocomposite Membranes with Improved Anti-Biofouling Performance. Membranes 2021, 11, 749. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Rashid, A.; Akram, S.; Shakir, H.M.; Rehan, Z.A.; Javed, T.; Shabbir, R.; Hessien, M.M. Fabrication and characterization of sulfonated graphene oxide-doped polymeric membranes with improved anti-biofouling behavior. Membranes 2021, 11, 563. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Mänttäri, M.; Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol. 2008, 63, 251–263. [Google Scholar] [CrossRef]
- Zahid, M.; Akram, S.; Rashid, A.; Rehan, Z.A.; Javed, T.; Shabbir, R.; Hessien, M.M.; El-Sayed, M.E. Investigating the antibacterial activity of polymeric membranes fabricated with aminated graphene oxide. Membranes 2021, 11, 510. [Google Scholar] [CrossRef]
- Basile, A.; Gallucci, F. Membranes for Membrane Reactors: Preparation, Optimization and Selection; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Mohammadi, B.; Yousefi, A.A.; Bellah, S.M. Effect of tensile strain rate and elongation on crystalline structure and piezoelectric properties of PVDF thin films. Polym. Test. 2007, 26, 42–50. [Google Scholar] [CrossRef]
- Boom, R.M.; Wienk, I.M.; Van den Boomgaard, T.; Smolders, C.A. Microstructures in phase inversion membranes. Part 2. The role of a polymeric additive. J. Membr. Sci. 1992, 73, 277–292. [Google Scholar] [CrossRef] [Green Version]
- Hendrix, K.; Koeckelberghs, G.; Vankelecom, I.F.J. Study of phase inversion parameters for PEEK-based nanofiltration membranes. J. Membr. Sci. 2014, 452, 241–252. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Schaule, G.J.D. Biofouling on membranes-a microbiological approach. Desalination 1988, 70, 95–119. [Google Scholar] [CrossRef]
- Baker, J.; Dudley, L. Biofouling in membrane systems-a review. Desalination 1998, 118, 81e90. [Google Scholar] [CrossRef]
- Werber, J.R.; Bull, S.K.; Elimelech, M. Acyl-chloride quenching following interfacial polymerization to modulate the water permeability, selectivity, and surface charge of desalination membranes. J. Membr. Sci. 2017, 535, 357–364. [Google Scholar] [CrossRef]
- Kim, J.; Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-H.; Yang, S.Y.; Wang, J.Y.; Tien, H.W.; Hsiao, S.T.; Wang, Y.S.; Li, S.M.; Ma, C.C.M.; Wu, Y.F. Effect of molecular chain length on the mechanical and thermal properties of amine-functionalized graphene oxide/polyimide composite films prepared by in situ polymerization. ACS Appl. Mater. Interfaces 2013, 5, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-H.; Lin, Y.Y.; Lin, C.H.; Chan, C.C.; Huang, Y.C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2014, 5, 535–550. [Google Scholar] [CrossRef]
- Yang, L.; Hu, J.; Wu, W.; Tang, J.; Ding, K.; Li, J. In situ NH2-functionalized graphene oxide/SiO2 composites to improve Cu (II) removal from ammoniacal solutions. Chem. Eng. J. 2016, 306, 77–85. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Lai, L.; Chen, L.; Zhan, D.; Sun, L.; Liu, J.; Lim, S.H.; Poh, C.K.; Shen, Z.; Lin, J. One-step synthesis of NH2-graphene from in situ graphene-oxide reduction and its improved electrochemical properties. Carbon. 2011, 49, 3250–3257. [Google Scholar] [CrossRef]
- Gzara, L.; Rehan, Z.A.; Khan, S.B.; Alamry, K.A.; Albeirutty, M.H.; El-Shahawi, M.S.; Asiri, A.M. Preparation and characteriza-tion of PES-cobalt nanocomposite membranes with enhanced anti-fouling properties and performances. J. Tai. Instit. Chem. Engin. 2016, 65, 405–419. [Google Scholar] [CrossRef]
- Akbari, A.; Remigy, J.C.; Aptel, P. Treatment of textile dye effluent using a polyamide-based nanofiltration membrane. Chem. Eng. Processing Process Intensif. 2002, 41, 601–609. [Google Scholar] [CrossRef]
- Khajouei, M.; Jahanshahi, M.; Peyravi, M.; Hoseinpour, H.; Rad, A.S. Anti-bacterial assay of doped membrane by zero valent Fe nanoparticle via in-situ and ex-situ aspect. Chem. Eng. Res. Des. 2017, 117, 287–300. [Google Scholar] [CrossRef]
- Kim, K.-M.; Woo, S.H.; Lee, J.S.; Park, H.S.; Park, J.; Min, B.R. Improved permeate flux of PVDF ultrafiltration membrane containing PVDF-g-PHEA synthesized via ATRP. Appl. Sci. 2015, 5, 1992–2008. [Google Scholar] [CrossRef] [Green Version]
- Simone, S.; Figoli, A.; Criscuoli, A.; Carnevale, M.C.; Alfadul, S.M.; Al-Romaih, H.S.; al Shabouna, F.S.; Al-Harbi, O.A.; Drioli, E. Effect of selected spinning parameters on PVDF hollow fiber morphology for potential application in desalination by VMD. Desalination 2014, 344, 28–35. [Google Scholar] [CrossRef]
- Figoli, A.; Simone, S.; Criscuoli, A.; AL-Jlil, S.A.; Al Shabouna, F.S.; Al-Romaih, H.S.; Di Nicolò, E.; Al-Harbi, O.A.; Drioli, E. Hollow fibers for seawater desalination from blends of PVDF with different molecular weights: Morphology, properties and VMD performance. Polymer 2014, 55, 1296–1306. [Google Scholar] [CrossRef]
- Gzara, L.; Rehan, Z.A.; Simone, S.; Galiano, F.; Hassankiadeh, N.T.; Al-Sharif, S.F.; Figoli, A.; Drioli, E. Tailoring PES membrane morphology and properties via selected preparation parameters. J. Polym. Eng. 2017, 37, 69–81. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Simone, S.; Macedonio, F.; Al-Jlil, S.A.; Al Shabonah, F.S.; Al-Romaih, H.S.; Al-Harbi, O.; Figoli, A.; Criscuoli, A. Novel PVDF hollow fiber membranes for vacuum and direct contact membrane distillation applications. Sep. Purif. Technol. 2013, 115, 27–38. [Google Scholar] [CrossRef]
- Rehan, Z.A.; Gzara, L.; Khan, S.B.; Alamry, K.A.; El-Shahawi, M.S.; Albeirutty, M.H.; Figoli, A.; Drioli, E.; Asiri, M.A. Synthesis and Characterization of Silver Nanoparticles-Filled Polyethersulfone Membranes for Antibacterial and Anti-Biofouling Application. Recent Pat. Nanotechnol. 2016, 10, 231–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, W.; Yu, Y.; Deng, B.; Li, J.; Jin, J. Sol–gel preparation of PAA-g-PVDF/TiO2 nanocomposite hollow fiber membranes with extremely high-water flux and improved antifouling property. J. Membr. Sci. 2013, 432, 25–32. [Google Scholar] [CrossRef]
- Andrade, J.D.; Smith, L.M.; Gregonis, D.E. The contact angle and interface energetics. In Surface and Interfacial Aspects of Biomedical Polymers; Springer: Berlin/Heidelberg, Germany, 1985; pp. 249–292. [Google Scholar]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloid and Surface Chemistry; Dekker, M., Ed.; CRC Press: New York, NY, USA, 1986; Volume 188. [Google Scholar]
- Chin, A.; Temkin, H.; Roedel, R. Transmission cathodoluminescence: A new SEM technique to study defects in bulk semiconductor samples. Appl. Phys. Lett. 1979, 34, 476–478. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F.; Li, F.; Xia, Y.; Li, Y. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Tabasum, A.; Bhatti, I.A.; Nadeem, N.; Zahid, M.; Rehan, Z.A.; Hussain, T.; Jilani, A. Degradation of acetamiprid using graphene-oxide-based metal (Mn and Ni) ferrites as Fenton-like photocatalysts. Water Sci. Technol. 2020, 81, 178–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Chai, Y.; Yuan, R.; Luo, J. Square wave anodic stripping voltammetry determination of lead based on the Hg (II) immobilized graphene oxide composite fifilm as an enhanced sensing platform. Sens. Actuators B. 2013, 178, 379–384. [Google Scholar] [CrossRef]
- Hafizovic, J.; Bjørgen, M.; Olsbye, U.; Dietzel, P.D.; Bordiga, S.; Prestipino, C.; Lamberti, C.; Lillerud, K.P. The inconsistency in adsorption properties and powder XRD data of MOF-5 is rationalized by framework interpenetration and the presence of organic and inorganic species in the nanocavities. J. Am. Chem. Soc. 2007, 129, 3612–3620. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, B.; Bi, Q.; Qu, B. An experimental study on the effect of igneous intrusions on chemical structure and combustion characteristics of coal in Daxing Mine, China. Fuel 2018, 226, 307–315. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.-J. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Gul, S.; Khan, S.A.; Rehan, Z.A.; Akhtar, K.; Khan, M.A.; Rashid, M.I.; Asiri, A.M.; Khan, S.B. Antibacterial CuO-PES-CA nancomposite membranes supported Cu0 nanoparticles for water permeability and reduction of organic pollutants. J. Mater. Sci. Mater. Electron. 2019, 30, 10835–10847. [Google Scholar] [CrossRef]
- Park, G.D.; Kim, J.H.; Park, S.K.; Kang, Y.C. MoSe2 embedded CNT-reduced graphene oxide composite microsphere with superior sodium ion storage and electrocatalytic hydrogen evolution performances. ACS Appl. Mater. Interfaces 2017, 9, 10673–10683. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Enhanced adsorption of ammonia on metal-organic framework/graphite oxide composites: Analysis of surface interactions. Adv. Funct. Mater. 2010, 20, 111–118. [Google Scholar] [CrossRef]
- Yang, J.-P.; Chen, Z.K.; Yang, G.; Fu, S.Y.; Ye, L. Simultaneous improvements in the cryogenic tensile strength, ductility and impact strength of epoxy resins by a hyperbranched polymer. Polymer. 2008, 49, 3168–3175. [Google Scholar] [CrossRef]
- Hudaib, B.; Gomes, V.; Shi, J.; Zhou, C.; Liu, Z. Poly (vinylidene fluoride)/polyaniline/MWCNT nanocomposite ultrafiltration membrane for natural organic matter removal. Sep. Purif. Technol. 2018, 190, 143–155. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Borovanska, I.; Todorov, P.; Ivanov, E.; Menseidov, D.; Chakraborty, S.; Bhattacharjee, C. Tensile and surface mechanical properties of polyethersulphone (pes) and polyvinylidene fluoride (PVDF) membranes. J. Theor. Appl. Mech. 2018, 48, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Sile-Yuksel, M.; Tas, B.; Koseoglu-Imer, D.Y.; Koyuncu, I. Effect of silver nanoparticle (AgNP) location in nanocomposite membrane matrix fabricated with different polymer type on antibacterial mechanism. Desalination 2014, 347, 120–130. [Google Scholar] [CrossRef]
- Ayyaru, S.; Ahn, Y.-H. Application of sulfonic acid group functionalized graphene oxide to improve hydrophilicity, permeability, and antifouling of PVDF nanocomposite ultrafiltration membranes. J. Membr. Sci. 2017, 525, 210–219. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.B.; Alamry, K.A.; Bifari, E.N.; Asiri, A.M.; Yasir, M.; Gzara, L.; Ahmad, R.Z. Assessment of antibacterial cellulose nanocomposites for water permeability and salt rejection. J. Ind. Eng. Chem. 2015, 24, 266–275. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Zhang, Y.; Tian, M.; He, T.; Liu, J.; Chen, V. Polymeric antimicrobial membranes enabled by nanomaterials for water treatment. J. Membr. Sci. 2018, 550, 173–197. [Google Scholar] [CrossRef]
- Maziya, K.; Dlamini, B.C.; Malinga, S.P. Hyperbranched polymer nanofibrous membrane grafted with silver nanoparticles for dual antifouling and antibacterial properties against Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa. React. Funct. Polym. 2020, 148, 104494. [Google Scholar] [CrossRef]
- Valentini, F.; Calcaterra, A.; Ruggiero, V.; Pichichero, E.; Martino, A.; Iosi, F.; Bertuccini, L.; Antonaroli, S.; Mardente, S.; Zicari, A.; et al. Functionalized Graphene Derivatives: Antibacterial Properties and Cytotoxicity. J. Nanomater. 2019, 2019, 2752539. [Google Scholar] [CrossRef] [Green Version]









| Membrane | PVDF (wt.%) | DMAc (wt.%) | AGO (wt.%) |
|---|---|---|---|
| M1 | 18 | 82 | 0 |
| M2 | 18 | 81.8 | 0.2 |
| M3 | 18 | 81.6 | 0.4 |
| M4 | 18 | 81.4 | 0.6 |
| M5 | 18 | 81.2 | 0.8 |
| M6 | 18 | 81.0 | 1.0 |
| Membrane Samples | Water Permeability (J/L.h−1.m−2) | BSA Rejection (%) | Dye Rejection (Methyl Orange) (%) | Dye Rejection (Methylene Blue) (%) |
|---|---|---|---|---|
| M1 | 102.4 | 88.2 | 86.6 | 86.6 |
| M2 | 115.5 | 92.2 | 90.2 | 88.5 |
| M3 | 126.6 | 95.5 | 92.5 | 84.2 |
| M4 | 138.9 | 97.8 | 94.5 | 81.2 |
| M5 | 152.4 | 98.2 | 96.6 | 80.2 |
| M6 | 170.2 | 96.6 | 95.2 | 78.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, H.; Zahid, M.; Rehan, Z.A.; Rashid, A.; Akram, S.; Aljohani, M.M.H.; Mustafa, S.K.; Khalid, T.; Abdelsalam, N.R.; Ghareeb, R.Y.; et al. Preparation of Polyvinylidene Fluoride Nano-Filtration Membranes Modified with Functionalized Graphene Oxide for Textile Dye Removal. Membranes 2022, 12, 224. https://doi.org/10.3390/membranes12020224
Ahmad H, Zahid M, Rehan ZA, Rashid A, Akram S, Aljohani MMH, Mustafa SK, Khalid T, Abdelsalam NR, Ghareeb RY, et al. Preparation of Polyvinylidene Fluoride Nano-Filtration Membranes Modified with Functionalized Graphene Oxide for Textile Dye Removal. Membranes. 2022; 12(2):224. https://doi.org/10.3390/membranes12020224
Chicago/Turabian StyleAhmad, Hirra, Muhammad Zahid, Zulfiqar Ahmad Rehan, Anum Rashid, Saba Akram, Meshari M. H. Aljohani, Syed Khalid Mustafa, Tayyaba Khalid, Nader R. Abdelsalam, Rehab Y. Ghareeb, and et al. 2022. "Preparation of Polyvinylidene Fluoride Nano-Filtration Membranes Modified with Functionalized Graphene Oxide for Textile Dye Removal" Membranes 12, no. 2: 224. https://doi.org/10.3390/membranes12020224
APA StyleAhmad, H., Zahid, M., Rehan, Z. A., Rashid, A., Akram, S., Aljohani, M. M. H., Mustafa, S. K., Khalid, T., Abdelsalam, N. R., Ghareeb, R. Y., & AL-Harbi, M. S. (2022). Preparation of Polyvinylidene Fluoride Nano-Filtration Membranes Modified with Functionalized Graphene Oxide for Textile Dye Removal. Membranes, 12(2), 224. https://doi.org/10.3390/membranes12020224







