Reuse of Water in Laundry Applications with Micro- and Ultrafiltration Ceramic Membrane
Abstract
:1. Introduction
- 445 kg of tents washed/loaded (on average).
- Each machine runs on average 3.58 loads/day.
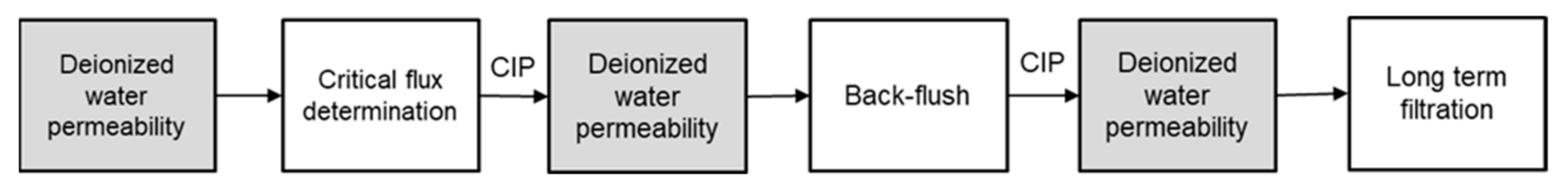
2. Materials and Methods
- Filtration of deionized water mixed with nylon microplastics obtained through cryogenic grinding (or cryogrinding) of red nylon fibers with 500 µm length. This technique consists of freezing materials by pouring over liquid nitrogen (−196 °C) and then reducing it into a small particles size through milling (IKA A11 Basic Analytical mill). The length of MPs particles obtained was 80 µm, standard deviation: ±39 µm.
- Filtration of wastewater from a single washing cycle of a PVC tent in an industrial washing machine.
3. Results
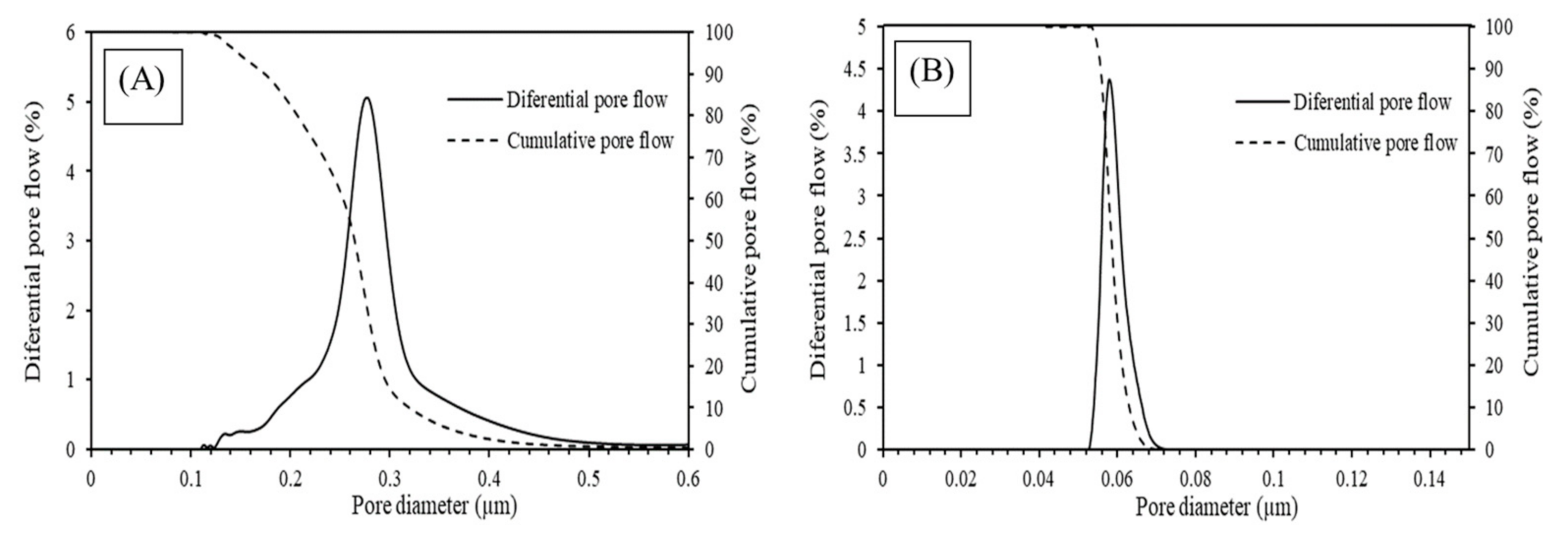
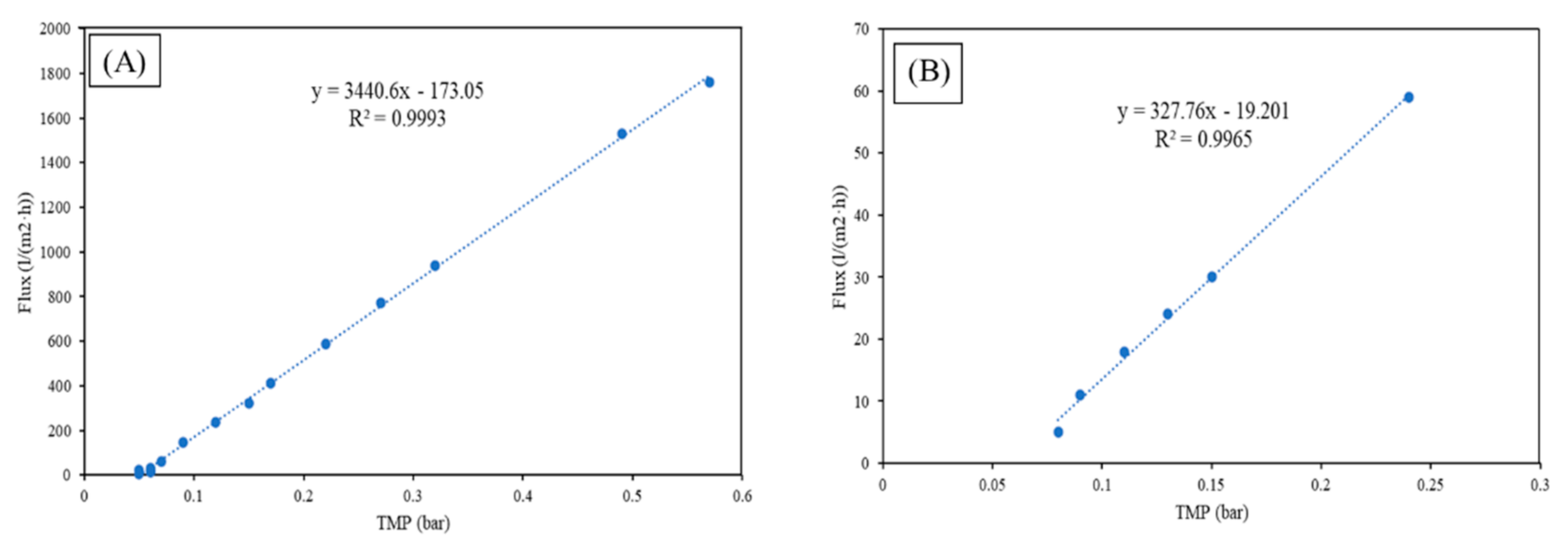
3.1. Membrane Characterization
3.2. Synthetic Feed
3.3. Wastewater from Washing Machine of Industrial Tent Laundering
3.3.1. Filtration Experiments
3.3.2. Physical-Chemical Analysis of Water after Filtration
4. Conclusions
- From the membrane characterization, it was found that both membranes had a defect-free and homogeneous surface. Furthermore, the membrane made by SiC was in the microfiltration range, with d90 of ~302 nm, whereas the membrane made using ZrO2 was in the ultrafiltration range, with a d90 of ~52 nm.
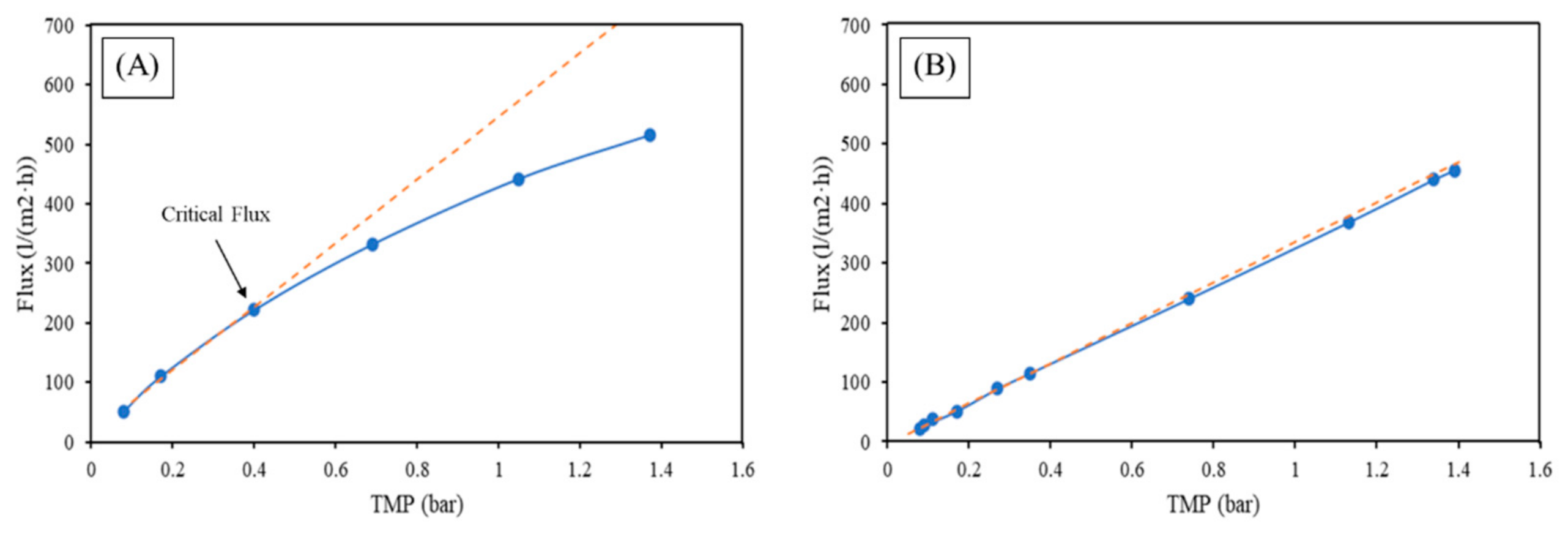
- The filtration of the synthetic feed with nylon fibers of 80 µm showed a critical flux value, in the case of MF, of 200 L/(m2·h). This demonstrates an effect of MPs in terms, most probably, of pore blocking. With the capabilities of the unit, it was not possible to obtain a critical flux for the UF, because no reduction of flux was observed along with the increasing TMP cycles. There is a clear indication that the fouling occurs earlier in MF compared to UF. In both cases, a 100% rate of removal of the fibers was achieved.
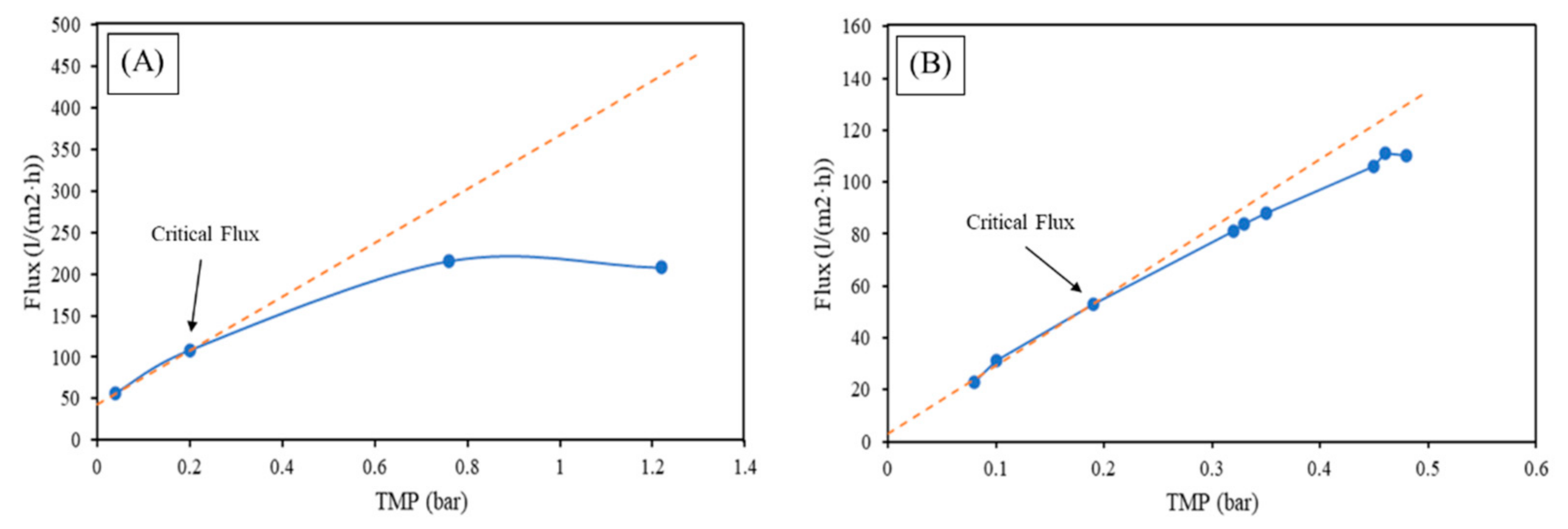
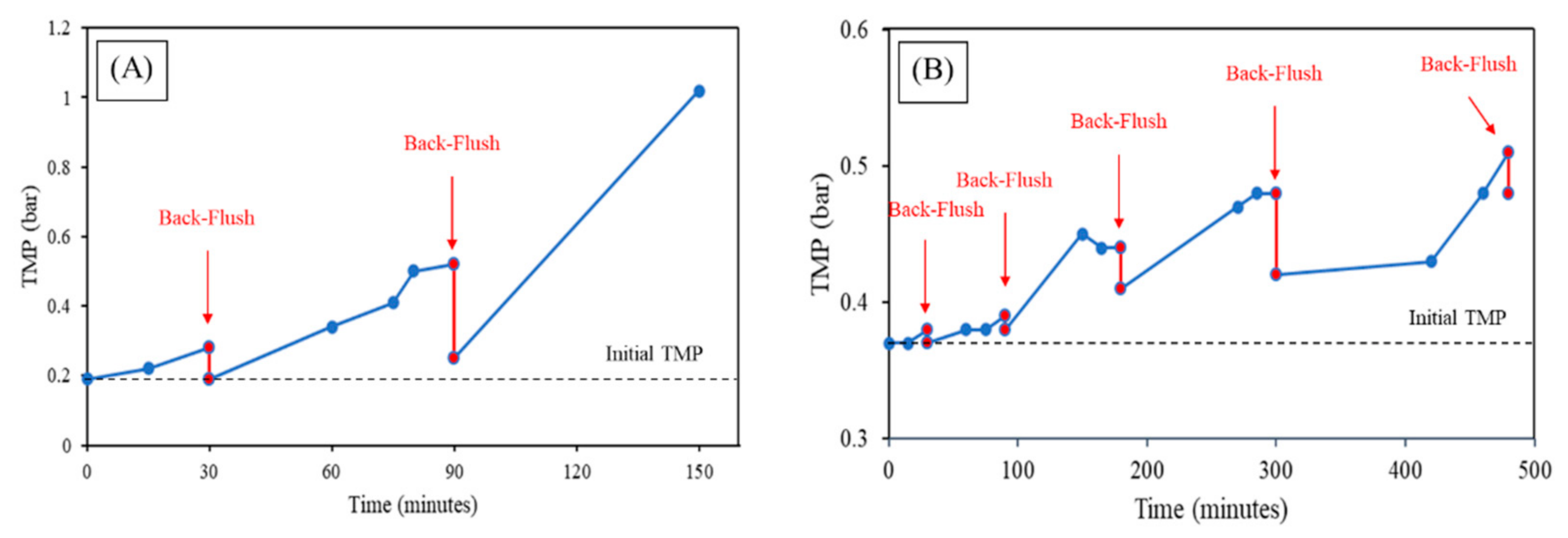
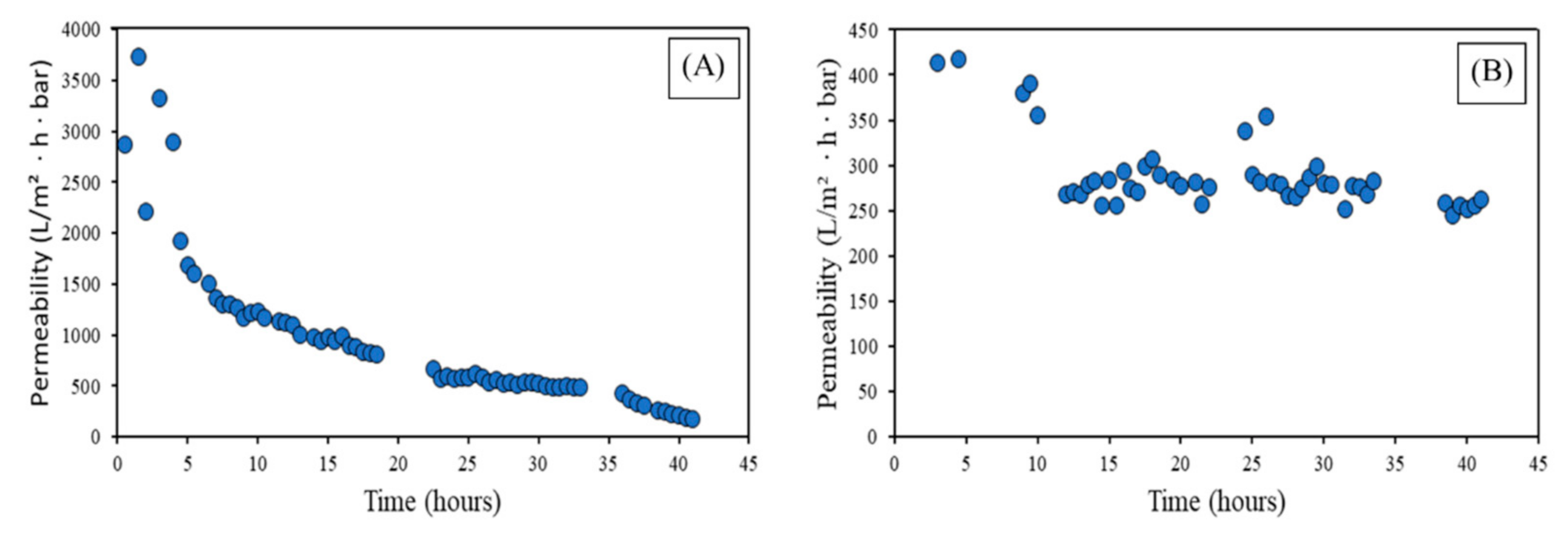
- With the filtration of the real wastewater from the tent laundry outlet, the critical flux value and backflush period for the MF was 90 L/(m2·h) with a 20 min period and 50 L/(m2·h) and 60 min period for the UF. After 4 days of constant filtration, there was a considerable decrease in the permeability of MF (~95%), while much smaller in the case of UF (~37%). Therefore, the better performance of UF in real applications can be established, with a lower necessity of CIP and longer operation periods.
- Based on the water analysis of the feed and permeate during the long-term filtration of both membranes, we can conclude that the UF results in better water quality in the permeate compared to the MF, with almost a 100% rate of removal in all studied parameters. The results obtained in this study pave the way for future wastewater treatment systems for industrial laundries, where the UF ceramic membranes can be used as the polishing step to remove MPs before being reused or discharged into the municipal wastewater stream.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boucher, J.; Friot, D. InternatIonal UnIon for ConservatIon of NatUre a Global Evaluation of Sources Primary Microplastics in the Oceans. 2017. Available online: https://portals.iucn.org/library/sites/library/files/documents/2017-002-En.pdf (accessed on 19 October 2021).
- Cai, Y.; Yang, T.; Mitrano, D.M.; Heuberger, M.; Hufenus, R.; Nowack, B. Systematic Study of Microplastic Fiber Release from 12 Different Polyester Textiles during Washing. Environ. Sci. Technol. 2020, 54, 4847–4855. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Thompson, R.C. Release of Synthetic Microplastic Plastic Fibres from Domestic Washing Machines: Effects of Fabric Type and Washing Conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoinkis, J.; Panten, V. Wastewater Recycling in Laundries-From Pilot to Large-Scale Plant. Chem. Eng. Process. Process Intensif. 2008, 47, 1159–1164. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The Fate of Microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; di Pace, E.; Cocca, M.; Avella, M. The Contribution of Washing Processes of Synthetic Clothes to Microplastic Pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef] [PubMed]
- Pirc, U.; Vidmar, M.; Mozer, A.; Kržan, A. Emissions of Microplastic Fibers from Microfiber Fleece during Domestic Washing. Environ. Sci. Pollut. Res. 2016, 23, 22206–22211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Falco, F.; Gullo, M.P.; Gentile, G.; di Pace, E.; Cocca, M.; Gelabert, L.; Brouta-Agnésa, M.; Rovira, A.; Escudero, R.; Villalba, R.; et al. Evaluation of Microplastic Release Caused by Textile Washing Processes of Synthetic Fabrics. Environ. Pollut. 2018, 236, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Setälä, O.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater?—A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatidou, G.; Arvaniti, O.S.; Stasinakis, A.S. Review on the Occurrence and Fate of Microplastics in Sewage Treatment Plants. J. Hazard. Mater. 2019, 367, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of Microplastics in Wastewater Treatment Plants and Their Environmental Dispersion with Effluent and Sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Kaegi, R.; Voegelin, A.; Ort, C.; Sinnet, B.; Thalmann, B.; Krismer, J.; Hagendorfer, H.; Elumelu, M.; Mueller, E. Fate and Transformation of Silver Nanoparticles in Urban Wastewater Systems. Water Res. 2013, 47, 3866–3877. [Google Scholar] [CrossRef] [PubMed]
- Kamali, M.; Suhas, D.P.; Costa, M.E.; Capela, I.; Aminabhavi, T.M. Sustainability Considerations in Membrane-Based Technologies for Industrial Effluents Treatment. Chem. Eng. J. 2019, 368, 474–494. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A Review on Inorganic Membranes for Desalination and Wastewater Treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of Ceramic and Polymeric Membrane Permeability and Fouling Using Surface Water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Eray, E.; Boffa, V.; Jørgensen, M.K.; Magnacca, G.; Candelario, V.M. Enhanced Fabrication of Silicon Carbide Membranes for Wastewater Treatment: From Laboratory to Industrial Scale. J. Membr. Sci. 2020, 606, 118080. [Google Scholar] [CrossRef]
- Bortot Coelho, F.E.; Kaiser, N.N.; Magnacca, G.; Candelario, V.M. Corrosion Resistant ZrO2/SiC Ultrafiltration Membranes for Wastewater Treatment and Operation in Harsh Environments. J. Eur. Ceram. Soc. 2021, 41, 7792–7806. [Google Scholar] [CrossRef]
- Eray, E.; Candelario, V.M.; Boffa, V.; Safafar, H.; Østedgaard-Munck, D.N.; Zahrtmann, N.; Kadrispahic, H.; Jørgensen, M.K. A Roadmap for the Development and Applications of Silicon Carbide Membranes for Liquid Filtration: Recent Advancements, Challenges, and Perspectives. Chem. Eng. J. 2021, 414, 128826. [Google Scholar] [CrossRef]
- Cancino, B.; Ulloa, L.; Astudillo, C. Osmotic Pressure of Salt and Sugar Solutions: Its Influence on Reverse Osmosis in the Food Industry. Inf. Tecnol. 2009, 20, 55–64. [Google Scholar] [CrossRef]

| Parameter | Unit | Instrument/Method |
|---|---|---|
| pH | - | pH probe HQ40D (Hach, Loveland, CO, USA) |
| Turbidity | NTU | Turbidimeter TN-100 (Thermo Scientific Eu, Bufalo, NY, USA) |
| Conductivity | uS/cm | Conductivity meter EC400 model ExStik |
| TDS | mg/L | Weighting and drying filter at 105 °C |
| TSS | mg/L | Weighting and drying water sample at 105 °C |
| VSS | mg/L | Weighting and drying water sample at 500 °C |
| TAL | mg/L | Titration with sulfuric acid |
| COD | mg/L | Cuvette test for COD, 15–150 mg/L O2 |
| Membrane | Maximum Pore Size (nm) | d90 (nm) | d50 (nm) |
|---|---|---|---|
| MF | 604 | 302 | 247 |
| UF | 74 | 63 | 58 |
| Removal Efficiency (%) | ||
|---|---|---|
| Parameter | MF | UF |
| Turbidity | 95 | 99.5 |
| TSS | 76.3 | 95.9 |
| VSS | 79 | 100 |
| COD | 80 | 83.8 |
| microplastics | 98.5 | 99.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luogo, B.D.P.; Salim, T.; Zhang, W.; Hartmann, N.B.; Malpei, F.; Candelario, V.M. Reuse of Water in Laundry Applications with Micro- and Ultrafiltration Ceramic Membrane. Membranes 2022, 12, 223. https://doi.org/10.3390/membranes12020223
Luogo BDP, Salim T, Zhang W, Hartmann NB, Malpei F, Candelario VM. Reuse of Water in Laundry Applications with Micro- and Ultrafiltration Ceramic Membrane. Membranes. 2022; 12(2):223. https://doi.org/10.3390/membranes12020223
Chicago/Turabian StyleLuogo, Beatrice Dal Pio, Toufic Salim, Wenjing Zhang, Nanna B. Hartmann, Francesca Malpei, and Victor M. Candelario. 2022. "Reuse of Water in Laundry Applications with Micro- and Ultrafiltration Ceramic Membrane" Membranes 12, no. 2: 223. https://doi.org/10.3390/membranes12020223
APA StyleLuogo, B. D. P., Salim, T., Zhang, W., Hartmann, N. B., Malpei, F., & Candelario, V. M. (2022). Reuse of Water in Laundry Applications with Micro- and Ultrafiltration Ceramic Membrane. Membranes, 12(2), 223. https://doi.org/10.3390/membranes12020223








