1. Introduction
Track membranes (TMs) are a special type of membrane. A TM is a thin polymer film with many identical through-pores. In general, TMs are a product of radiation technologies. It was found that when microparticles (ions, fission fragments) pass through some polymers, hidden (so-called “latent”) tracks are formed [
1,
2,
3]. Subsequent visualization of these tracks made it possible to study parameters of microparticles. Later, this approach was used to obtain porous films (membranes). For this, particles with known parameters (for example, accelerated heavy ions), which uniformly irradiated the surface of a polymer film, were used. The irradiated film was then subjected to a special treatment—sensitization and etching of latent tracks. This approach made it possible to obtain a thin-film material with through-pores of the same size and controlled quantity per unit area (the so-called pore density). Most often they have a regular cylindrical shape and are directed perpendicular to the surface (respectively, parallel to each other).
The resulting porous membrane material, due to the uniformity of all pores, and the possibility of changing them within wide range, began to be used as filters (so-called nuclear filters or track membranes) [
4]. From the filtration point of view, they are ideal sieves, since they have narrow pore size distribution and, accordingly, high selectivity. For some purposes, it is also important that the filtration takes place on the membrane surface. For about four decades, such materials have found practical application for fine filtration—in medicine, biology, and the food industry.
Another area of using TMs was a method developed in the 1990s, so-called “matrix synthesis” (or “template synthesis”) [
5,
6,
7]. In this case, the pores in the film were used as nano-sized containers, which were filled with the required material. As a result, pore replicas were formed—as a rule, repeating their shape, i.e., in the form of elongated cylinders. These structures with a high aspect ratio (diameters in the tens of nanometers and lengths of several microns) are called nanowires (NWs, nanorods, nanoneedles). With this approach, they are formed inside the pores of TM, as a rule, in the form of an array (ensemble) of parallel oriented structures with a high density of arrangement in space. Since the mid-90 s, matrix synthesis has been actively used by many authors to obtain metallic NWs [
5,
6]. The main questions here are the choice of the template matrix, the metal deposited in its pores and the method of deposition.
Note that other materials can also be used in matrix synthesis. One of the competitors of TMs is porous aluminum oxide (PAO) [
8]. Among TMs there are different types of matrixes. Polycarbonate (PC) track matrices are common in Europe. In Russia, membranes made of Polyethylene terephthalate (PET, terephthalic acid ester) are used more often. The use of each of the listed types of membranes has its own specifics and differs greatly for different tasks.
Metals are the main material loaded into the pores of TM [
9]. In this case, to obtain NWs, a galvanic (electrochemical) method of metal electrodeposition is used. At the first stage, a metal layer is deposited on one side of the matrix to create electrical conductivity. Such a metallized matrix is placed in a galvanic cell with an electrolyte containing ions of the desired metal (where it works as a cathode). When a current is passed through the cell, the metal is deposited into the pores of the TM. The resulting metal replicas (copy) of pore channels are NWs.
The obtained NWs are in a growth polymer matrix, one side of which is coated with a metal base layer. During subsequent operations, the polymer matrix can be removed, thus forming an array of free-standing NWs, fixed on a common base. In another approach, the base layer can be removed. This forms the so-called metal–polymer composite of many isolated NWs in a polymer matrix. Another option can be the removal of both bases and matrices—in this case, separate NWs are formed.
In such NWs, some of their geometric parameters are determined by the initial growth matrix. Thus, the number of NWs (per unit volume) corresponds to the pore density in the matrix. The diameter of the NWs and their shape are determined by the diameter and shape of the pores in the matrix. The slope of the pores in the matrix determines the geometric orientation of NWs.
Other parameters—the composition of the NWs and the type of NWs—are determined by the composition of the electrolyte and the synthesis parameters. Depending on these conditions, it is possible to obtain one-component NWs (from one metal) or multicomponent NWs.
The simplest type of object, are one-component homogeneous NWs consisting of one metal. The work on matrix synthesis began with this type of NWs. For these objects, changes in growth conditions primarily affect the structure. It has been found that uniform filling of pores is also important, with papers devoted to control of this process in PAO matrices, e.g., [
10].
More complex types of NWs are known as multicomponent, consisting of two or more metals. Here two main cases can be distinguished: Homogeneous NWs with a uniform distribution of two (or more) components in the length of the NW, the so-called alloys (alloyed NWs). In this case, growth is carried out in electrolytes containing ions of both (or more) metals, at voltages exceeding the equilibrium voltages for all metals. The second type of structure is heterogeneous: they consist of periodic layers of different elemental composition. This type of structure are called layered NWs. Such NWs can be obtained in two ways, by periodically changing the electrolyte (or transferring the sample to another cell, “bath”), the so-called “two-bath method”. It is also possible to carry out the process in one electrolyte, in one cell, the so-called “one-bath method”. In this case, the electrolyte contains ions of all metals, and a periodic change in the composition of the deposited material is achieved by changing the growth potential. At a lower potential, only one element is deposited—the first—with a lower equilibrium deposition potential (more noble metal). At higher potential, both metals are deposited (but the concentration of the first one could be reduced).
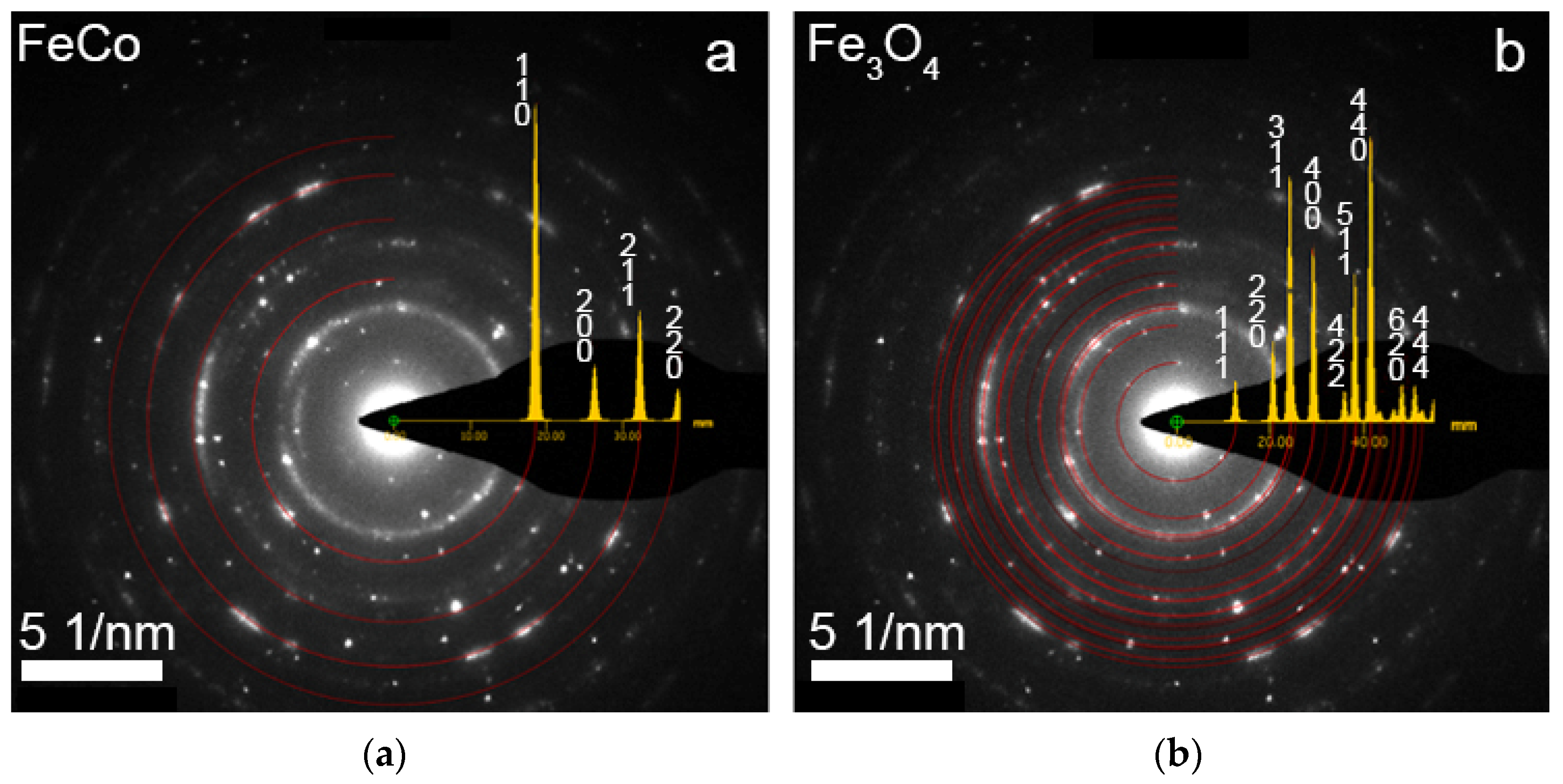
The unique magnetic properties of the samples were detected in the first papers which were devoted to fabrication of Fe-group elements NWs [
11]. At first, the most investigated samples were homogenous one-metal NWs [
12]. A modern review of magnetic NWs is given in [
13].
Alloyed NWs FeCo and FeNi are of great interest. In NWs from alloys, due to a change in composition and/or structure, it is possible to obtain both magnetically hard and magnetically soft, hard materials. The first one can be used to create micro-magnets and magnetic memory elements, while the second can be used for production of magnetic circuits and protective screens. In Ref. [
14] FeCo NWs were studied: the saturation magnetization was found to be very high-about 22–24 kG. This makes these wires a potential candidate for deposition of the writer poles in hard disk drives. It was also supposed that the deposition process can be qualitatively divided into two stages. During the initial stage the current decrease with time because of deposition is controlled by planar diffusion due to ion depletion within the pores. Then the diffusion length is decreased (as the pores are filled) which leads to current increase.
In Ref. [
15] FeNi and FeCo alloyed NWs (with approximately equiatomic composition) have been electrodeposited in PAO membrane with pores diameter 70 nm. It was shown that the NWs’ composition does not correspond to the content of metals in the electrolyte. The dependence of composition on deposition potential was found. The anomalous co-deposition was also detected, this effect is known as preferential deposition of less-noble metal, in respect to its counterpart. In this case it was preferential deposition of Fe, which was higher in FeNi NWs than in FeCo NWs. The authors also found that this effect is different for the deposition of alloys on the planar electrode and into the pores of PAO matrix. The difference could be explained by the fact that the convection within the pores is more difficult than at the planar electrode, consequently, transport of the less-concentrated ions is more affected. Authors [
16] also noted enhancement of the anomalous codeposition in the FeNi alloy when deposited in the nanopores. They explained such behavior by the role of hydrogen bubbling inside the pores, which finally leads to controlling of deposition by mass transport.
In Refs. [
17,
18] it is shown that by reducing the diameter of FeNi NWs (from 200 to 30 nm), the coercive force can be increased more than 10-fold. In this case, the NWs with the composition of a typical soft magnetic material—permalloy—turn into a hard magnetic material. The coercive force of FeCo changes greatly with a change in composition, and this dependence is not the same as that of a bulk material.
Fe-Co and Fe-Ni NWs were obtained in the pores of PAO in [
19]. It was shown that by changing the deposition regimes both types of NWs (layered and alloyed) could be electrodeposited using approximately the same growth solutions. Authors found that cobalt structure in layered NWs depends on the thickness of Co layers: it was FCC for thin and HCP for thick layers. The effect of anomalous co-deposition of Fe in these cases was also detected.
Layered NWs are of great interest too. The effect of dependence of resistance of multilayered NW on an external magnetic field (the so-called giant magneto-resistance, GMR) was described in [
20]. The magnetic properties of different types of NW were first reviewed in [
21]. Later the possibilities of tuning of NWs’ magnetic properties were supposed in [
22]. The application of NWs as sensors for different purposes was proposed in [
23].
In Refs. [
24,
25] NWs with alternating magnetic layers (iron or cobalt) and non-magnetic layers (copper) were studied. It was shown that the nature of the interaction and the magnetic properties depended on the thickness of the nonmagnetic interlayer—the spacer. The alternation of metals with different magnetic properties made it possible to change the spin polarization for passing current and to use these NWs as elements of spintronics. In [
26], it was proposed to record bits of information “along the length” of single layered NW in order to increase recording density.
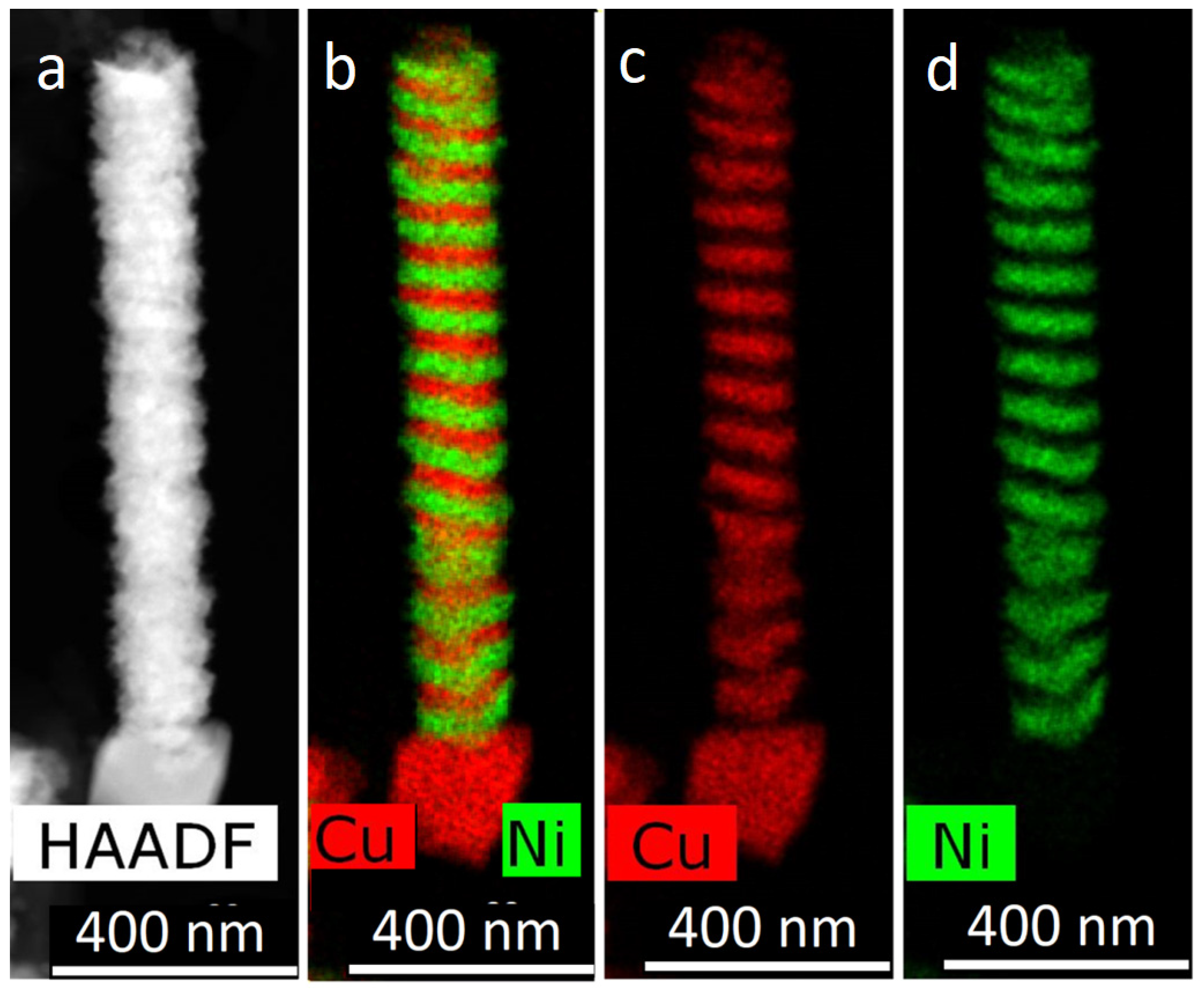
In layered NWs the lengths of the layers and the nature of their alternation can be changed. Such NWs are of interest as elements of electronics, spintronics, sensors, and valves [
27]. In [
24] it was shown that their magnetic properties also depend on the ratio of the thicknesses of the magnetic and non-magnetic layers. By changing this ratio, the orientation of the easy magnetization axis can be changed. Additionally, in multilayered NWs all segments must have the same compositional and geometrical parameters. In some cases, the NWs with very thin layers—less than the spin relaxation length for electrons—are needed. Moreover, some magnetic transport effect could be obtained only in NWs with definite borders between layers (the so-called interfaces), and these borders must be thin and flat.
To summarize these reviewed papers, it could be concluded that there are many published data, but they are often contradictory. The main point here is the features of electrodeposition into narrow pore channels. Systematic data on the relationship between structure and growth conditions is needed. In the case of magnetic metals, the composition and the structure of the obtained nanowires will obviously determine their unique magnetic properties. Therefore, the aim of this work is to compare different ways of fabricating NWs and to find new approaches to managing their structure.
2. Materials and Methods
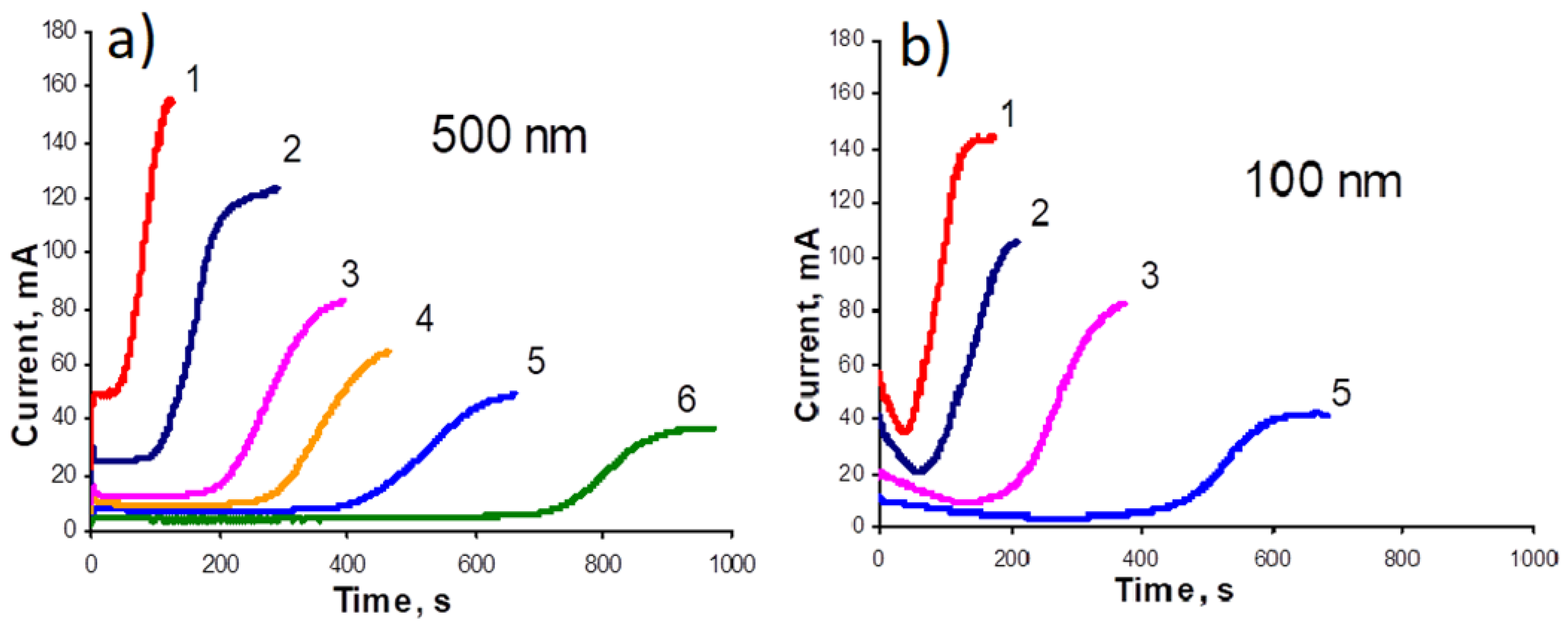
Track-etched membranes (fabricated in JINR, Dubna) with cylindrical through-pores were used as templates. The membrane parameters were as follows: film thickness was about 10 μm, the pore diameter in different membranes was 70–500 nm, and the pore density was 108–109 pores per.cm2. To carry out electrodeposition, one of membrane sides was coated with a 2–10-μm-thick continuous conducting copper layer. The last one was deposited by a two-step technique: thermal evaporation of copper (formation of thin Cu layer) was followed by galvanic deposition of copper (formation of thick layer).
Electrodeposition of metals into the pores was carried out in a special home-made galvanic cell. This cell provided vertical fixation of a prepared matrix with a conductive layer and its electrical connection to conducting wires. Different types of anodes were used (see below). The anodes were immersed vertically in a galvanic cell with electrolyte. Deposition was performed in the two-electrode geometry on a sample area of 2.5 cm2. An Elins P-2X potentiostat–galvanostat was used as a current source. The process was carried out in the galvanostatic mode at the constant potentials of different values (see below), at room temperature. During the electrodeposition, the time dependence of current was recorded, due to which the process could be controlled.
The compositions of electrolytes differed depending on the experiment. In general, as a metal-ion source, the corresponding metal salts were used, usually sulfate and chloride salts. These salts are highly soluble, and their concentration could be varied in wide range.
For example: the solution for producing NW from FeNi alloys contained NiSO4 ∙ 7H2O (16 g/L), NiCl2 ∙ 6H2O (40 g/L), and FeSO4 ∙ 7H2O (from 4 to 32 g/L). Electrolytes for formation of FeCo NWs contained CoSO4 · 7H2O (16 g/L) and CoCl2 · 6H2O (40 g/L). The concentration of FeSO4 · 7H2O was varied from 4 to 72 g/L, which correspond to a change in the iron-to-nickel ionic ratio from 6% to 53%. To increase the relative iron ion concentration more significantly, the cobalt salt concentrations were reduced as follows: 12, 8, 4, and 2 g/L for CoSO4 ·7H2O and 32, 24, 16, 8, and 4 g/L for CoCl2·6H2O. This technique made it possible to change the relative iron ion concentration from 59% to 91%.
Some additives were used in all types of electrolytes: Lauryl sylfate, for wetting and Boric acid, for pH stabilization (25 g/L). In iron-containing electrolyte ascorbic acid (1 g/L) was used in order to prevent Fe2+ ions oxidating to Fe3+.
The typical regimes for electrodeposition of alloyed NWs were: voltages changing from 1 to 2 V, depending on the experiment; Fe was used as anode; and the duration of pulses was selected from the chronoampere dependences.
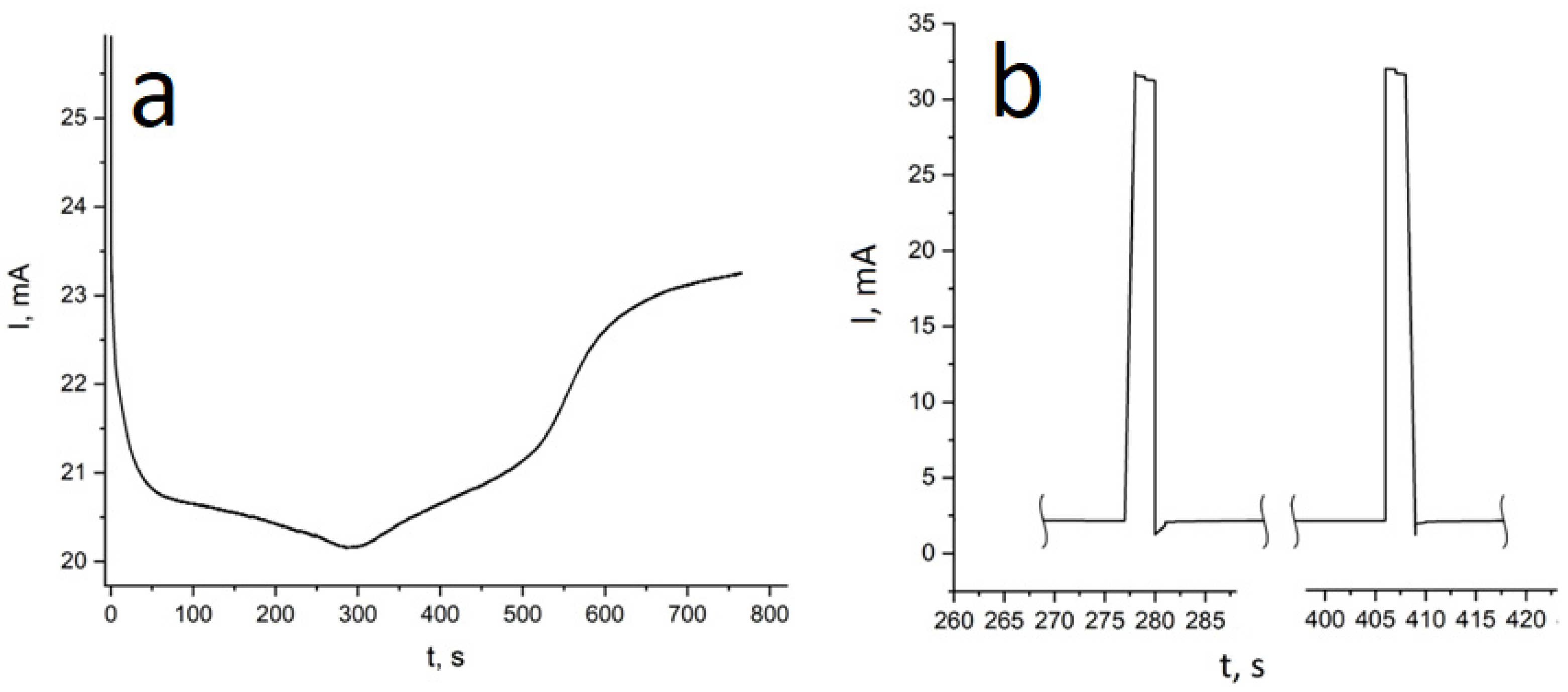
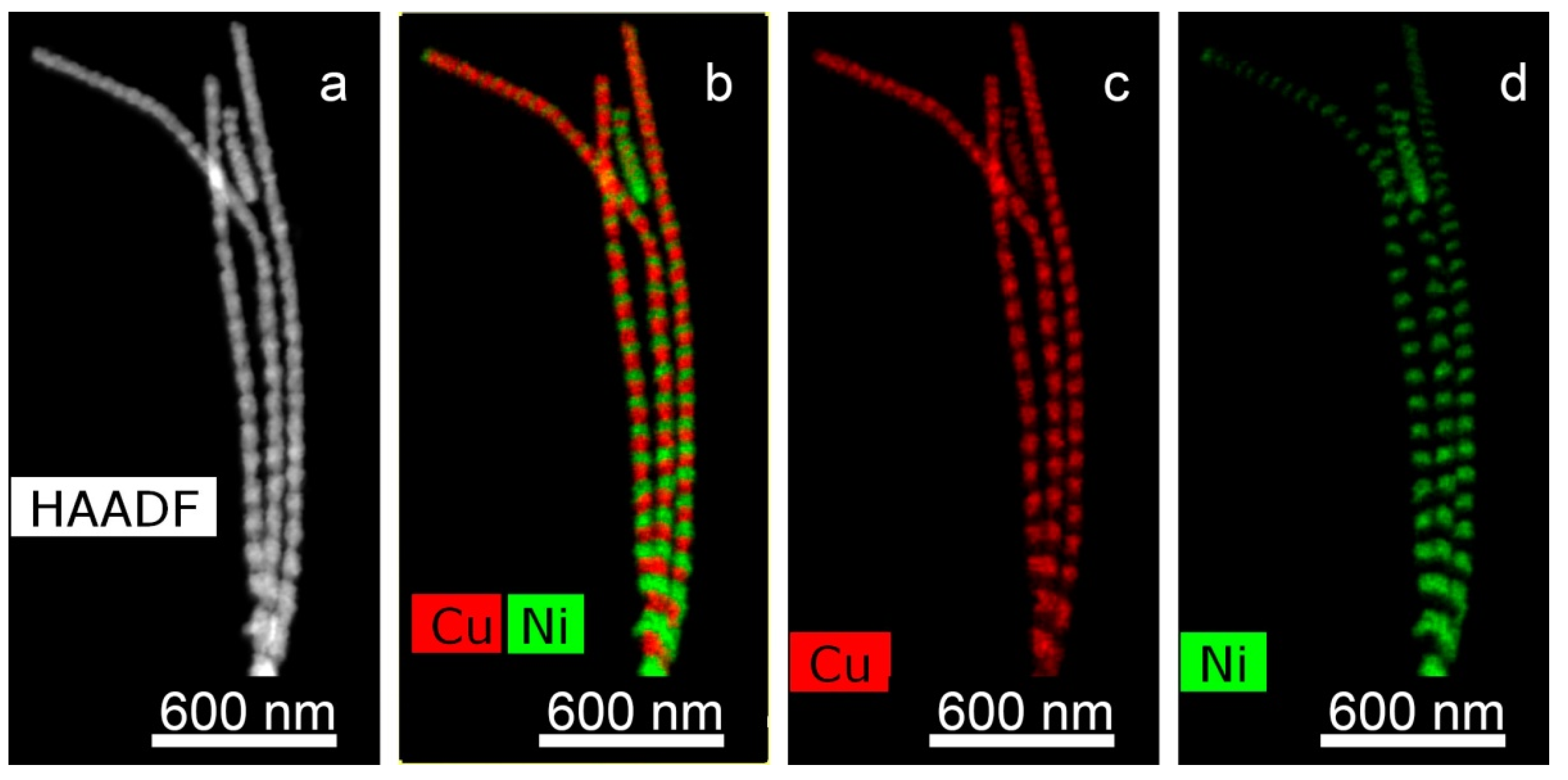
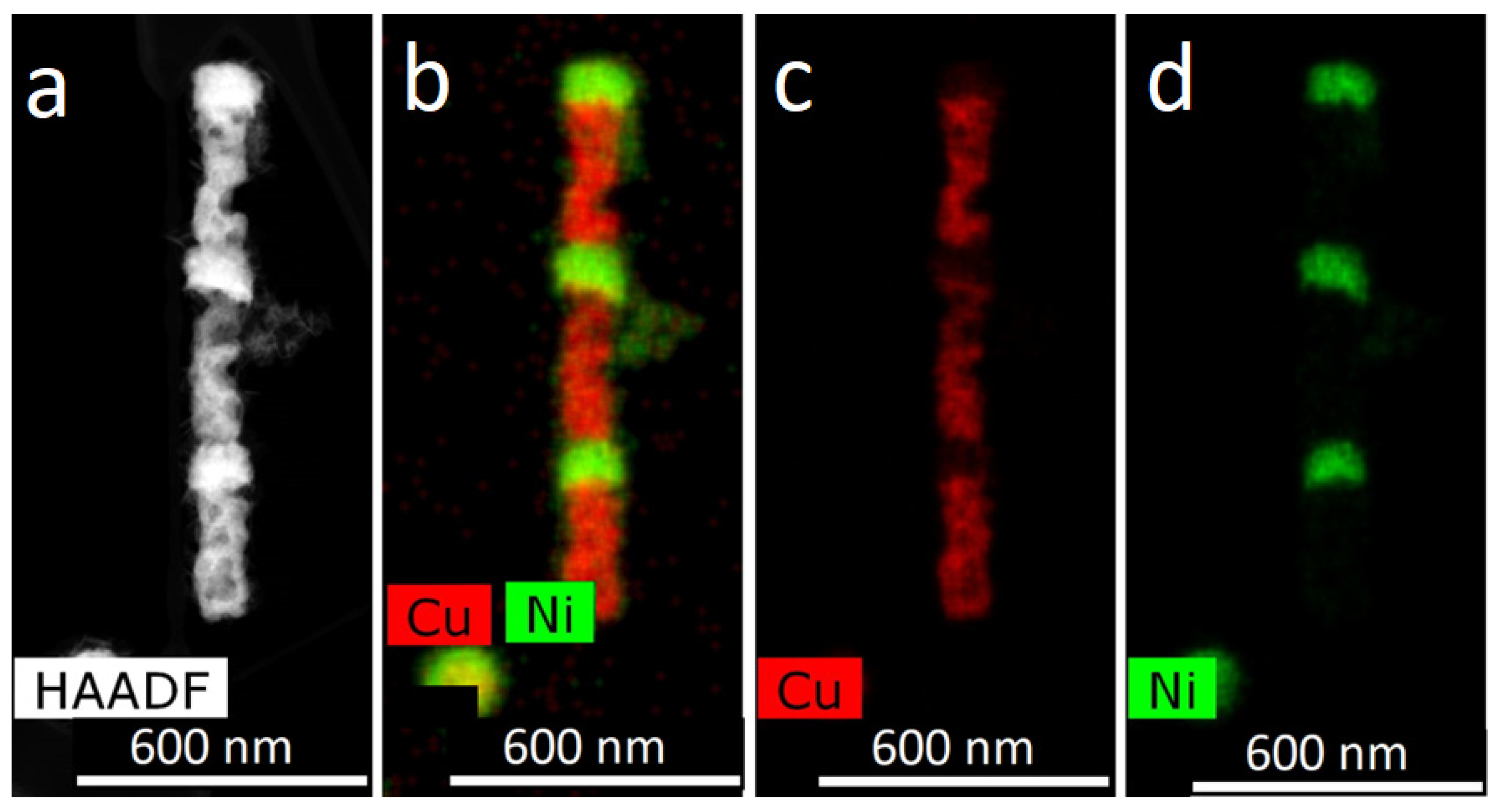
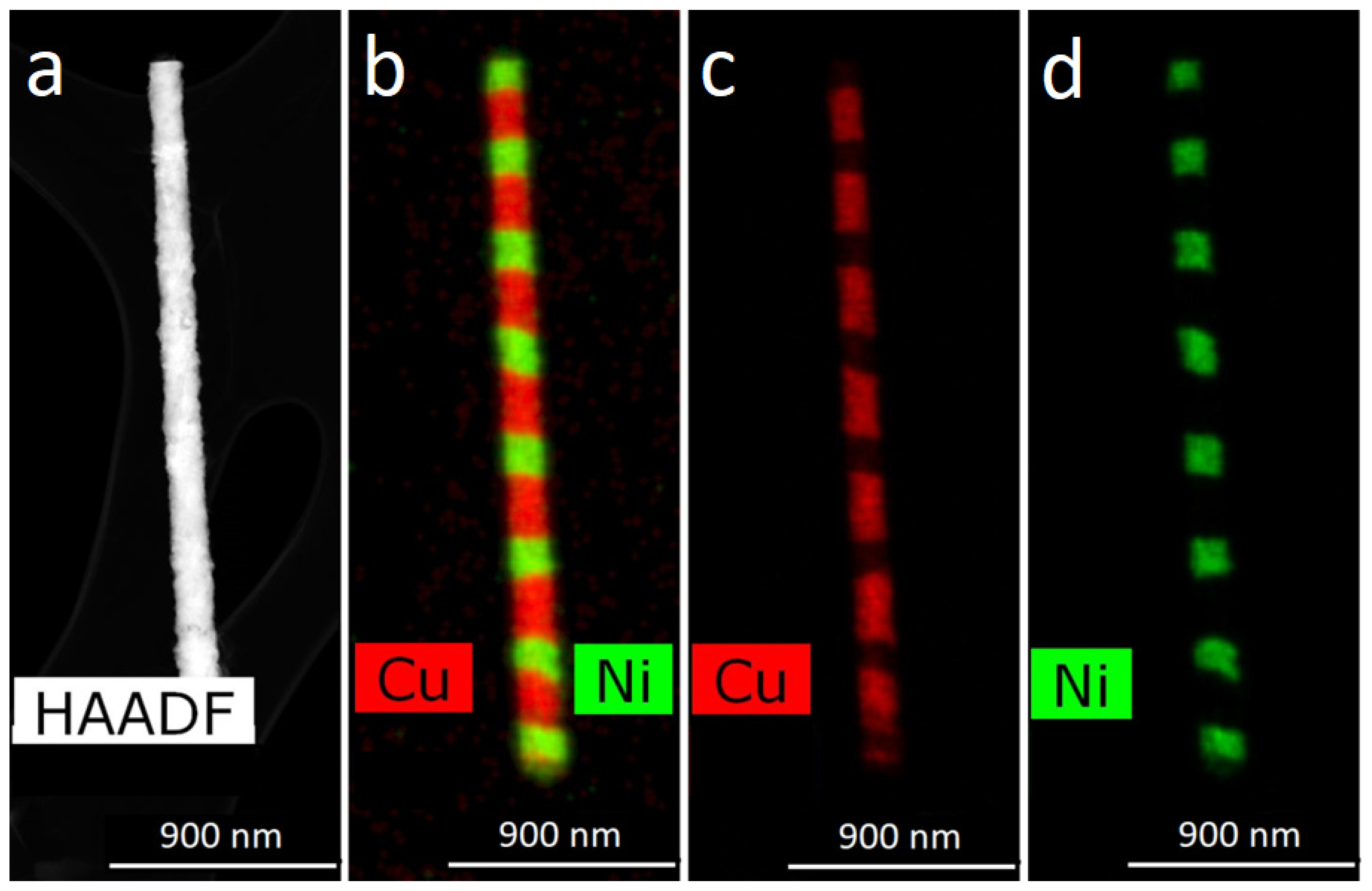
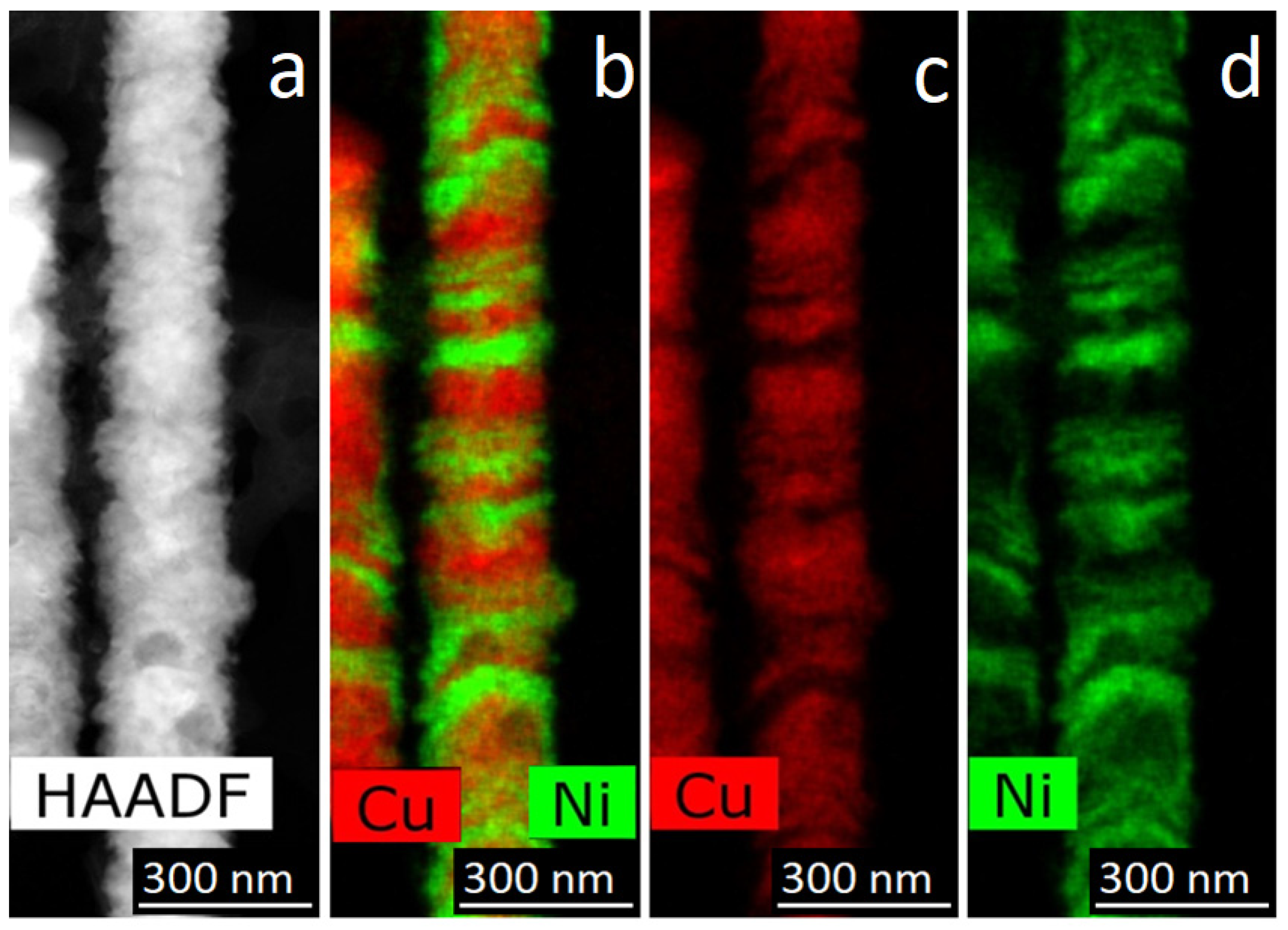
For obtaining of layered NWs: voltage for Cu deposition −0, 5 V, and 1.5–1.8 V for deposition of Ni (or Co). In the case of time-control, the deposition time was calculated from the thickness of the layer to be deposited. In the case of charge control, for example, a charge flow of 271 mC is required for Cu layers and 287 mC for magnetic layers.
For further investigations, the NWs were removed from the polymer. In order to release NWs from the host membrane, the last one was dissolved in alkali solution (5N NaOH, t = 50 °C, 2 h). Chemical removal of the polymer was followed by washing of the NW sample in distilled water.
Scanning electron microscopy (SEM) with energy-dispersive X-ray analysis (EDXA) was performed on a JEOL JSM 6000 plus microscope (JEOL, Japan) in the secondary-electron mode at an accelerating voltage of 15 kV. X-Ray diffraction (XRD) analysis was carried out on a RIGAKU MiniFlex 600 diffractometer (Rigaku, Japan) using CuKα radiation, at 2θ angles in the range 40°–80° with a scan step of 0.01° and a rate of 1 deg/min.
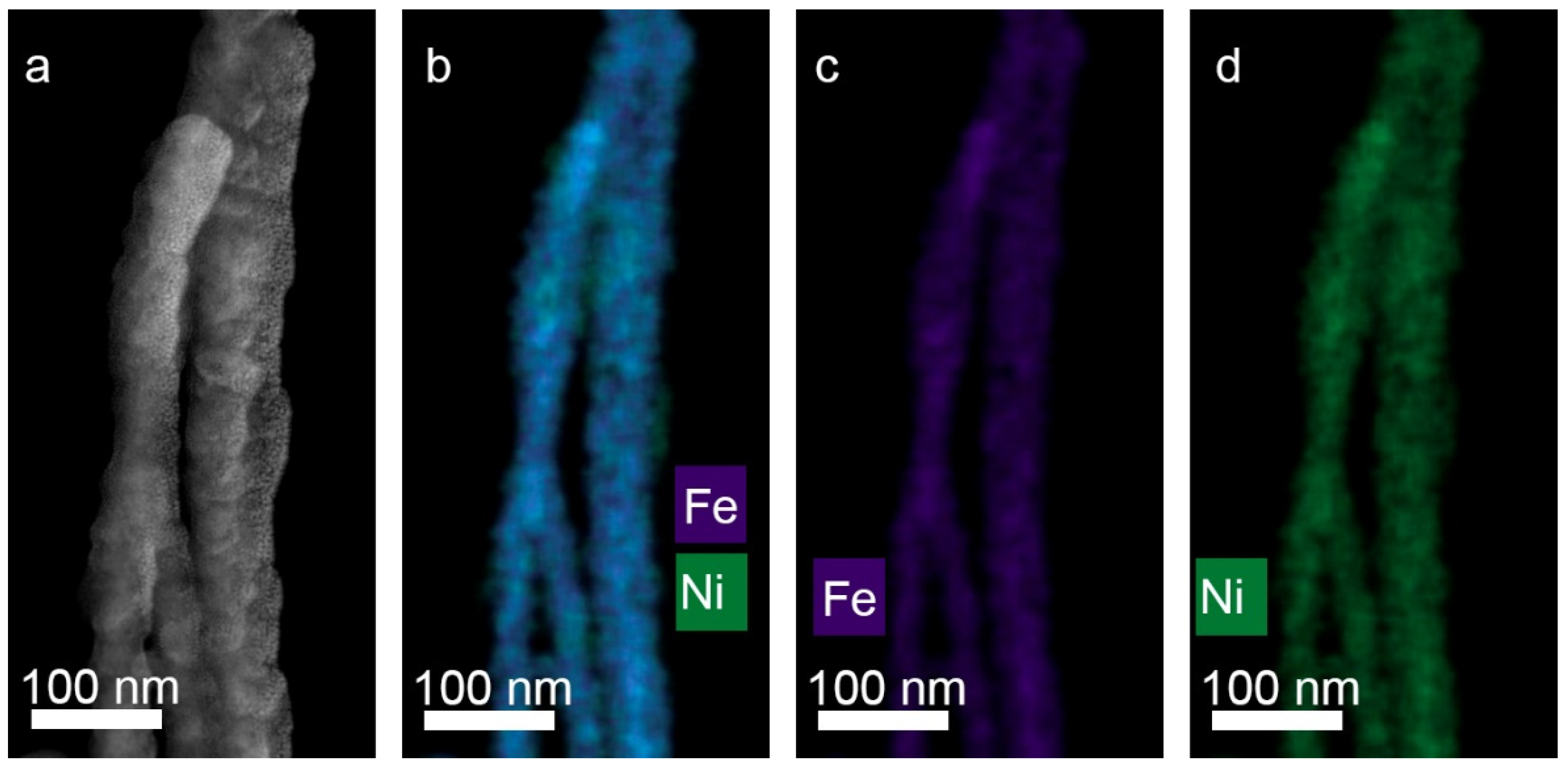
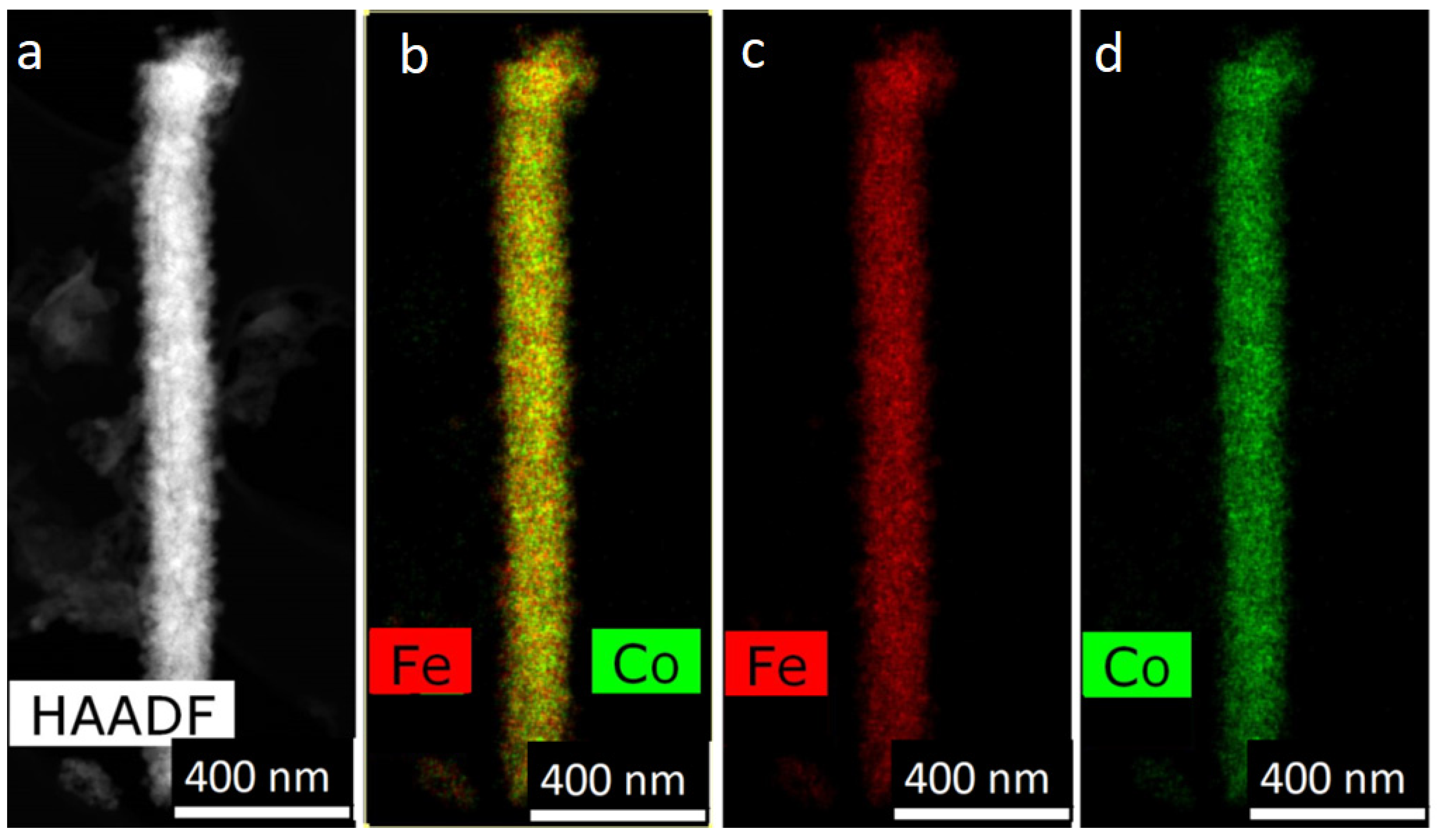
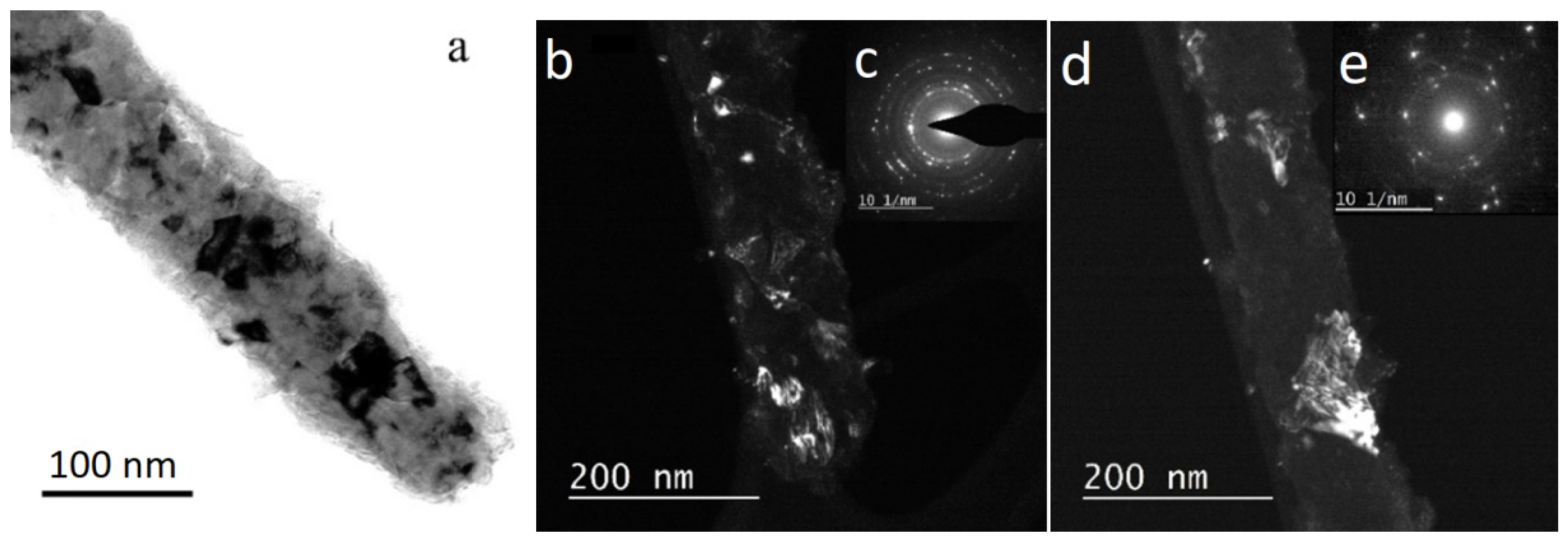
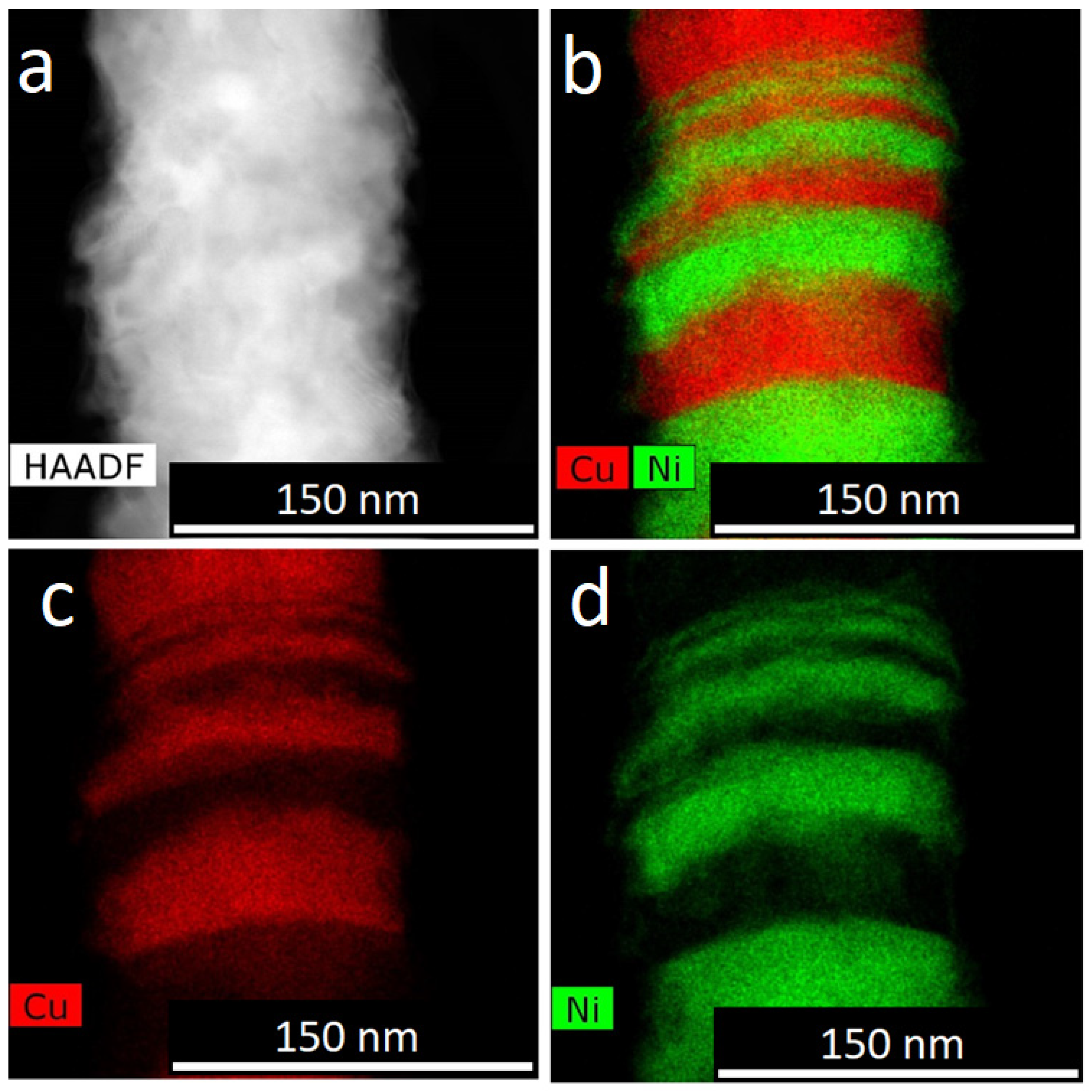
The structural studies of individual NWs were performed by transmission electron microscopy (TEM), high-resolution TEM (HRTEM), scanning TEM (STEM), with a high-angle annular dark field (HAADF) detector and electron diffraction using an Osiris microscope (Termo Fisher Scientific, USA) at an accelerating voltage of 200 kV. The elemental analysis and distribution maps of chemical elements were obtained using a special EDXA system SuperX equipped with four Si detectors. The images and electron diffraction patterns obtained in the electron microscope were processed and analyzed using the DigitalMicrograph, Esprit, TIA, and JEMS software.
4. Conclusions
In this work, we investigated the features of the process of obtaining one-component and two-component nanowires by electrodeposition into the pores of track membranes. The work was carried out for metals of the iron group, the nanoscale structures of which can have unique magnetic properties.
Using one-component NWs as an example, the origins for the nonlinearity of the galvanic process are revealed: the competition between the processes of depletion of the electrolyte due to the slowing down of the supply of ions and the change in the thickness of the electrolyte layer in the pores. It was shown that, at a high pore density, the areas of diffusion supply of ions from neighboring pores overlap, which leads to the appearance of a rather thick continuous diffusion layer above the membrane surface. The presence of this thick layer leads to the stabilization of the process of electrodeposition. For cobalt NWs, it was shown that an increase in the acidity of the electrolyte leads to a transition from a cubic to a hexagonal structure.
For two-component structures, the processes of obtaining nanowires from FeNi and FeCo alloys have been studied in detail. For nickel compositions, the effect of predominant deposition of iron (anomalous co-deposition of Fe) was found. The latter is significantly enhanced in pores of small diameter and can be associated with the lower mobility of nickel ions. Methods for obtaining layered nanowires with thin layers were considered. A number of techniques were investigated, including electrolyte dilution and the use of additives. It was shown that the most effective methods are the use of a three-electrode cell and the use of a control of the passed charge mode. At the same time, it has been shown that a number of techniques that are successfully used for the growth of bulk materials (e.g., using a pause between current pulses) are inefficient when carrying out the process in narrow pore channels.
The obtained results allow one to vary the geometry of NWs (obtained in the pores of track membrane), their composition and structure and, consequently, their magnetic properties. The possibily of obtaining narrow NWs and/or NWs with precise control of composition enables NWs with high coercive force (Hc). For alloyed NWs, the effect of anomalous codeposition of iron and the factors leading to it were investigated. This effect (which is higher for FeNi NWs) should be taken into consideration when alloyed NWs are produced. Layered NWs with rather thin layers have high magneto-resistance properties. Controllable changing of the layer thickness allows control of the magnetic properties.
All results make it possible to have better control over the structure of the obtained nanowires, which determines their magnetic properties.