Development of Pomegranate Peel Extract and Nano ZnO Co-Reinforced Polylactic Acid Film for Active Food Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLA-Based Composite Films
2.3. Characterization of PLA Based Composite Films
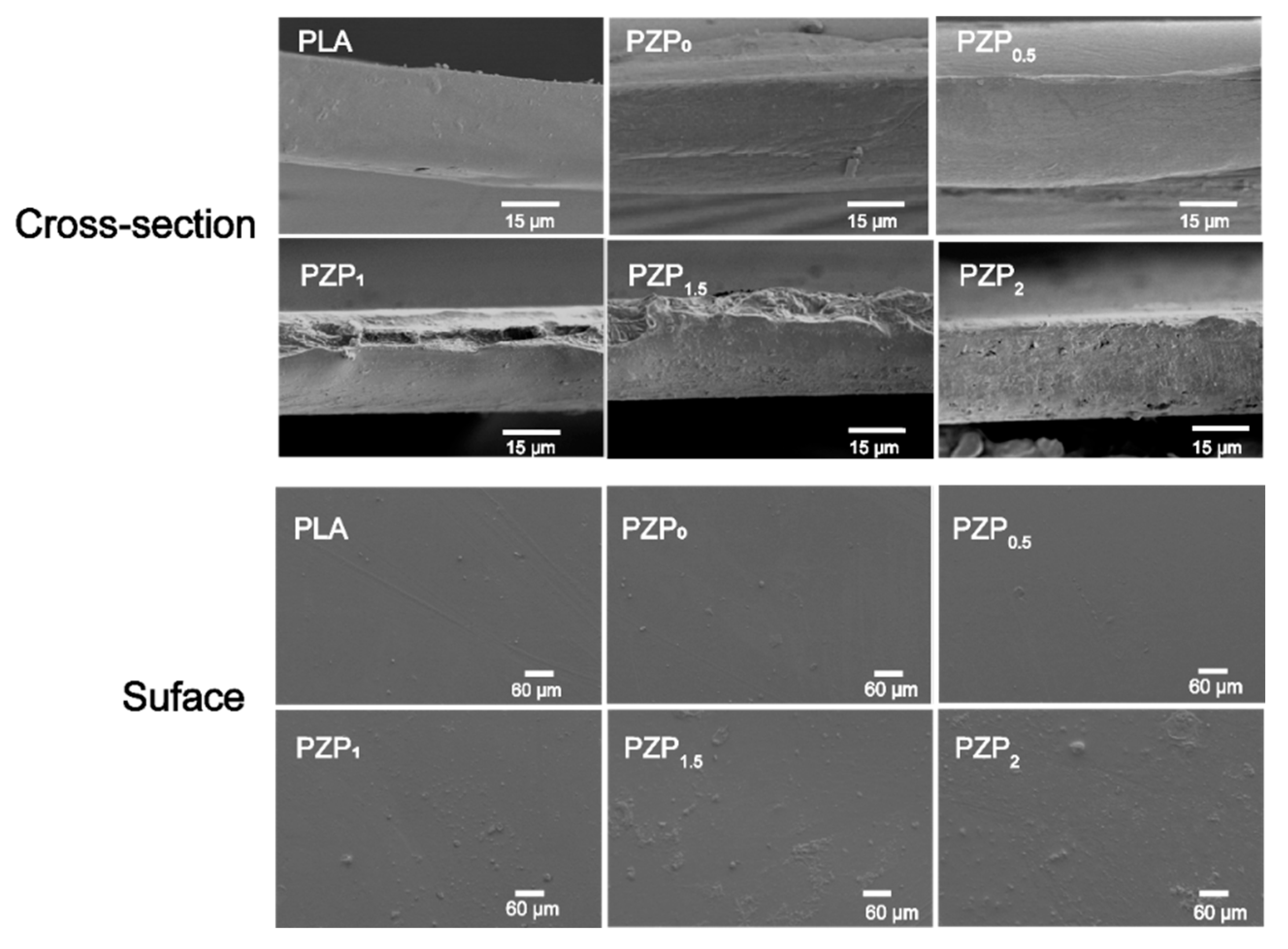
2.3.1. Morphology of Surface and Cross-Section
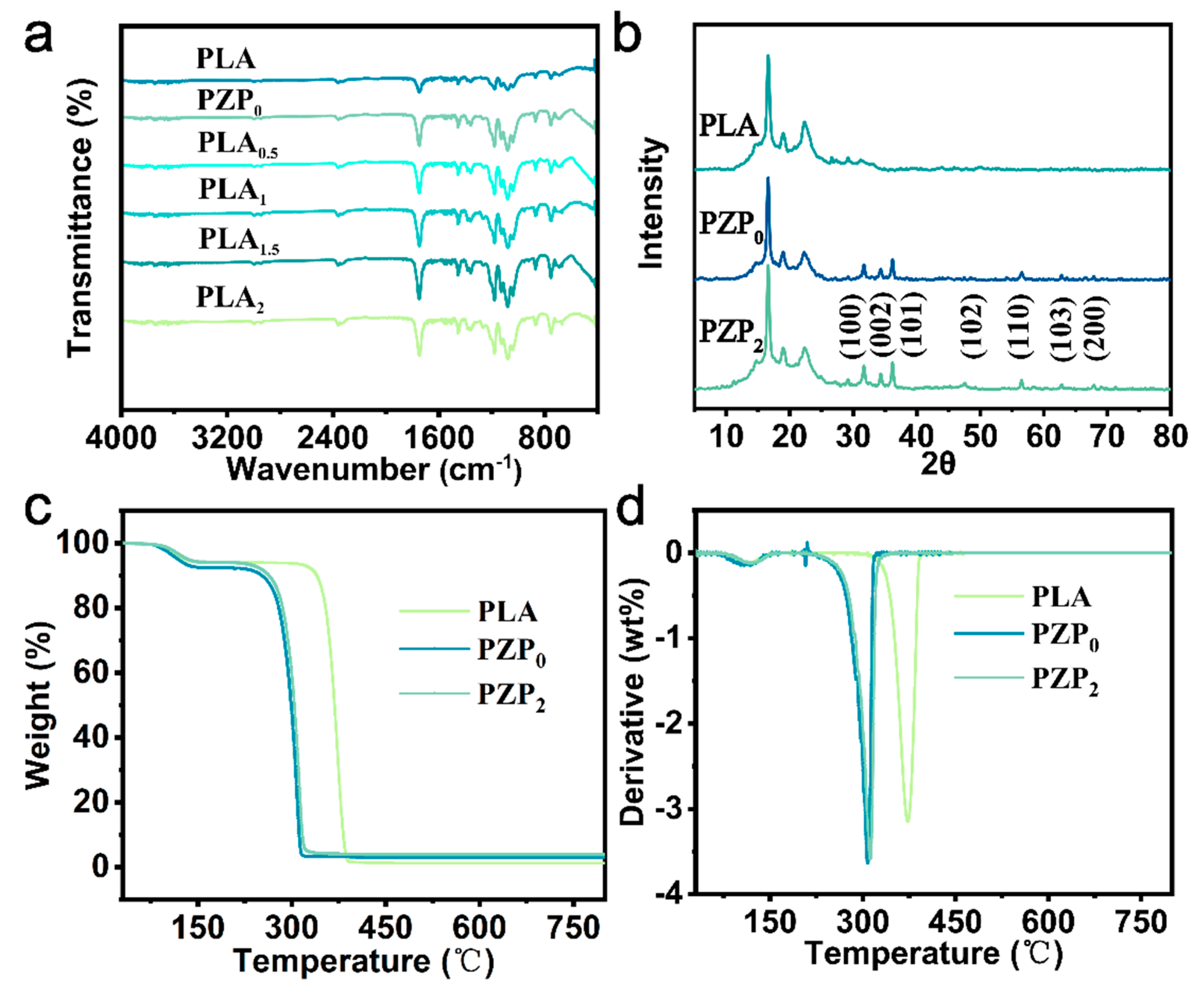
2.3.2. FTIR Spectroscopy and XRD Analysis
2.3.3. Thermal Stability
2.3.4. Optical Property
2.3.5. Mechanical Properties
2.3.6. Moisture Content and Water Solubility
2.3.7. Water Vapor Permeability (WVP) and Water Contact Angle (WCA)
2.4. Antioxidant Activity
2.5. Antibacterial Activity
2.6. Evaluation of Cherry Tomatoes’ Preservation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of PLA-Based Composite Films
3.1.1. Morphology
3.1.2. FT-IR Analysis
3.1.3. XRD Analysis
3.1.4. Thermal Stability of PLA-Based Composite Films
3.1.5. Optical Property of PLA-Based Composite Films
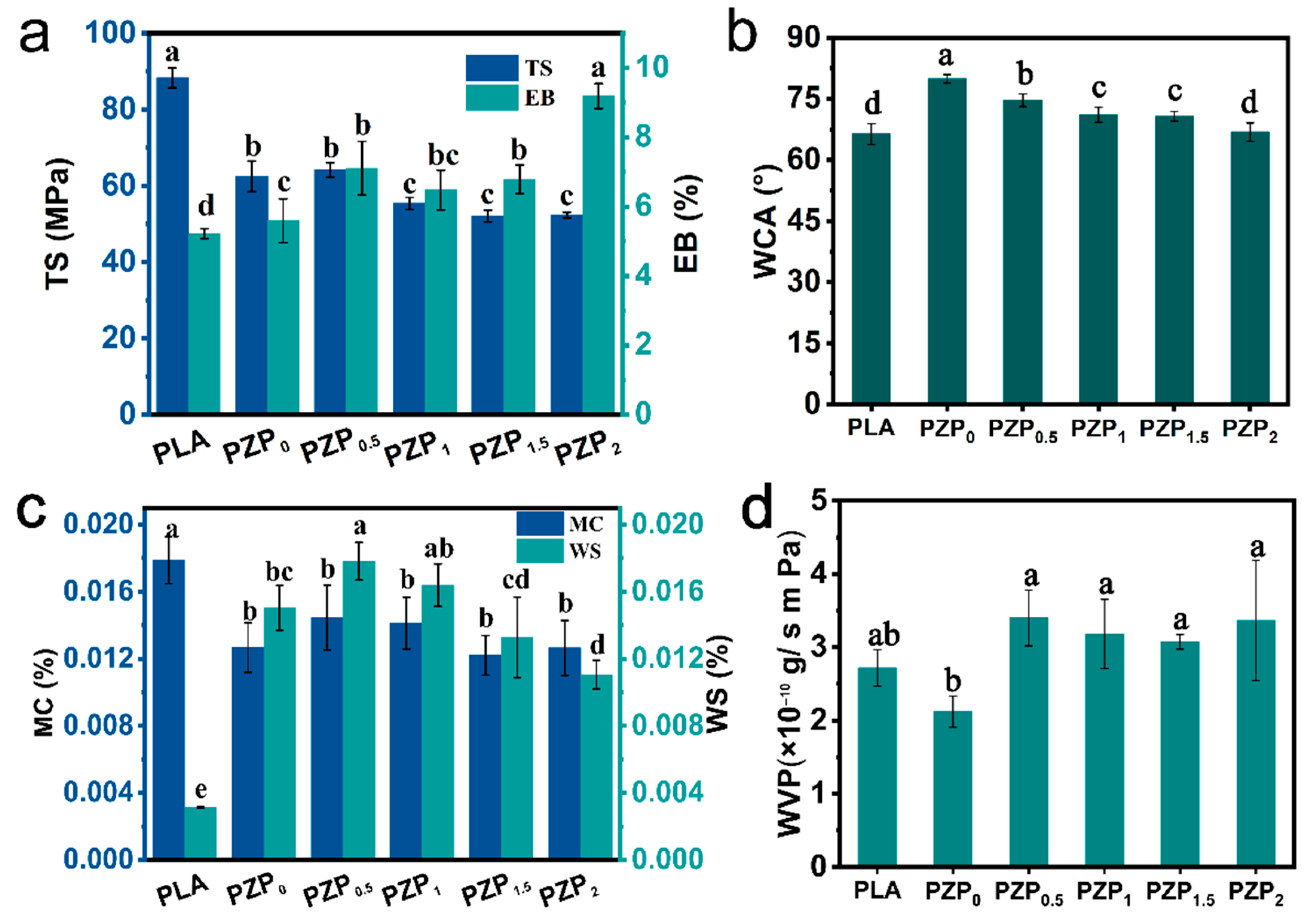
3.1.6. Thickness and Mechanical Properties of PLA-Based Composite Films
3.1.7. Moisture Content and Water Solubility
3.1.8. Water Contact Angle
3.1.9. Water Vapor Permeability
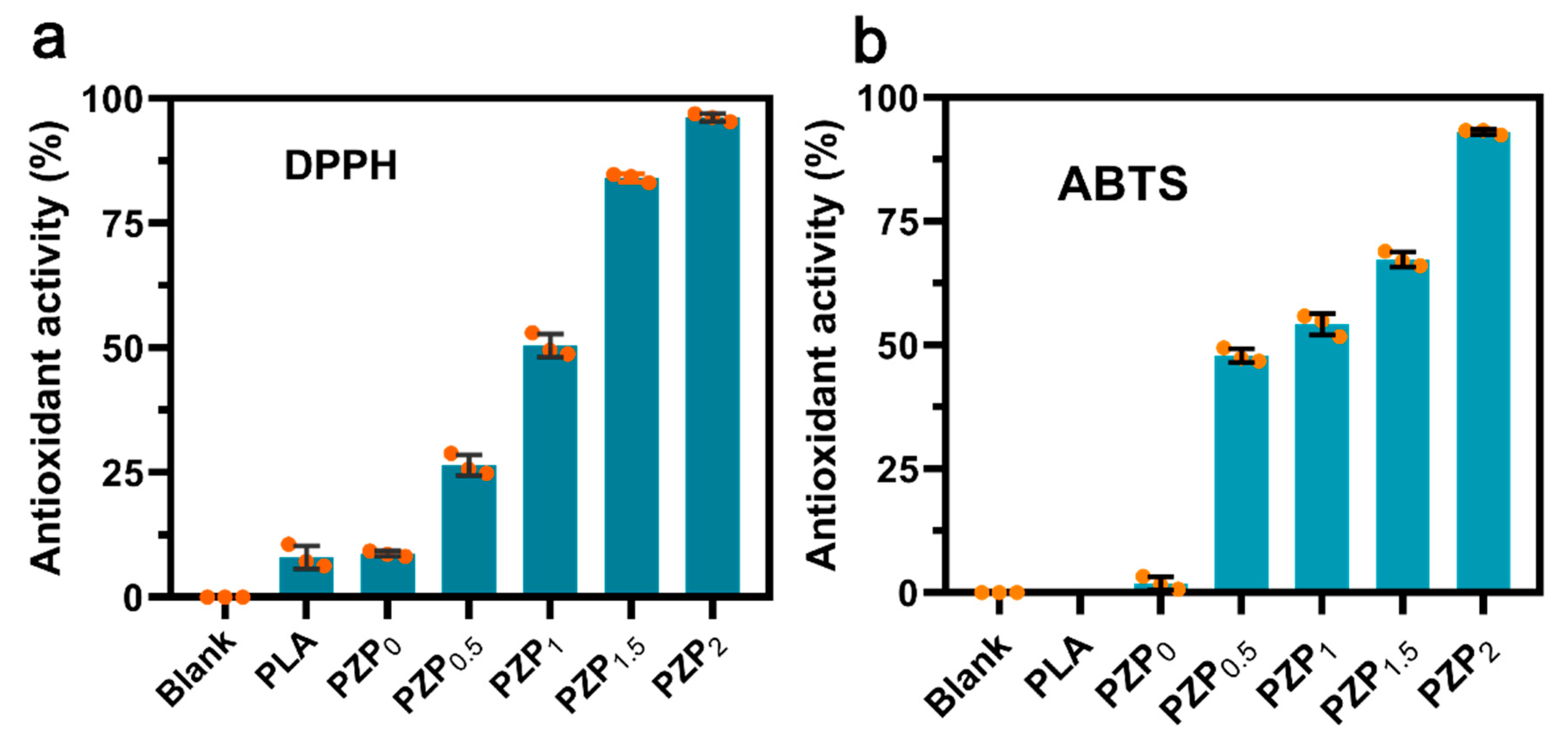
3.2. Antioxidant Activity of PLA Based Composite Films
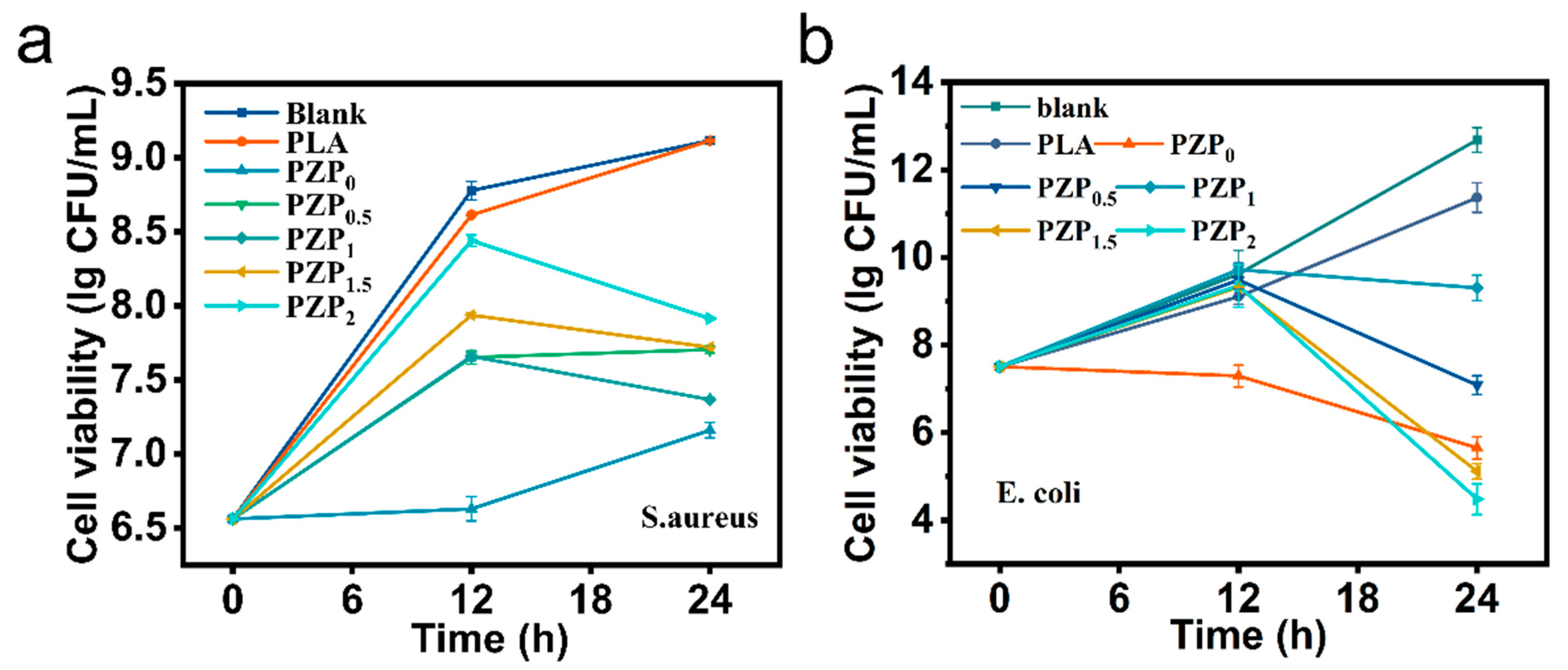
3.3. Antimicrobial Activity of PLA-Based Composite Films
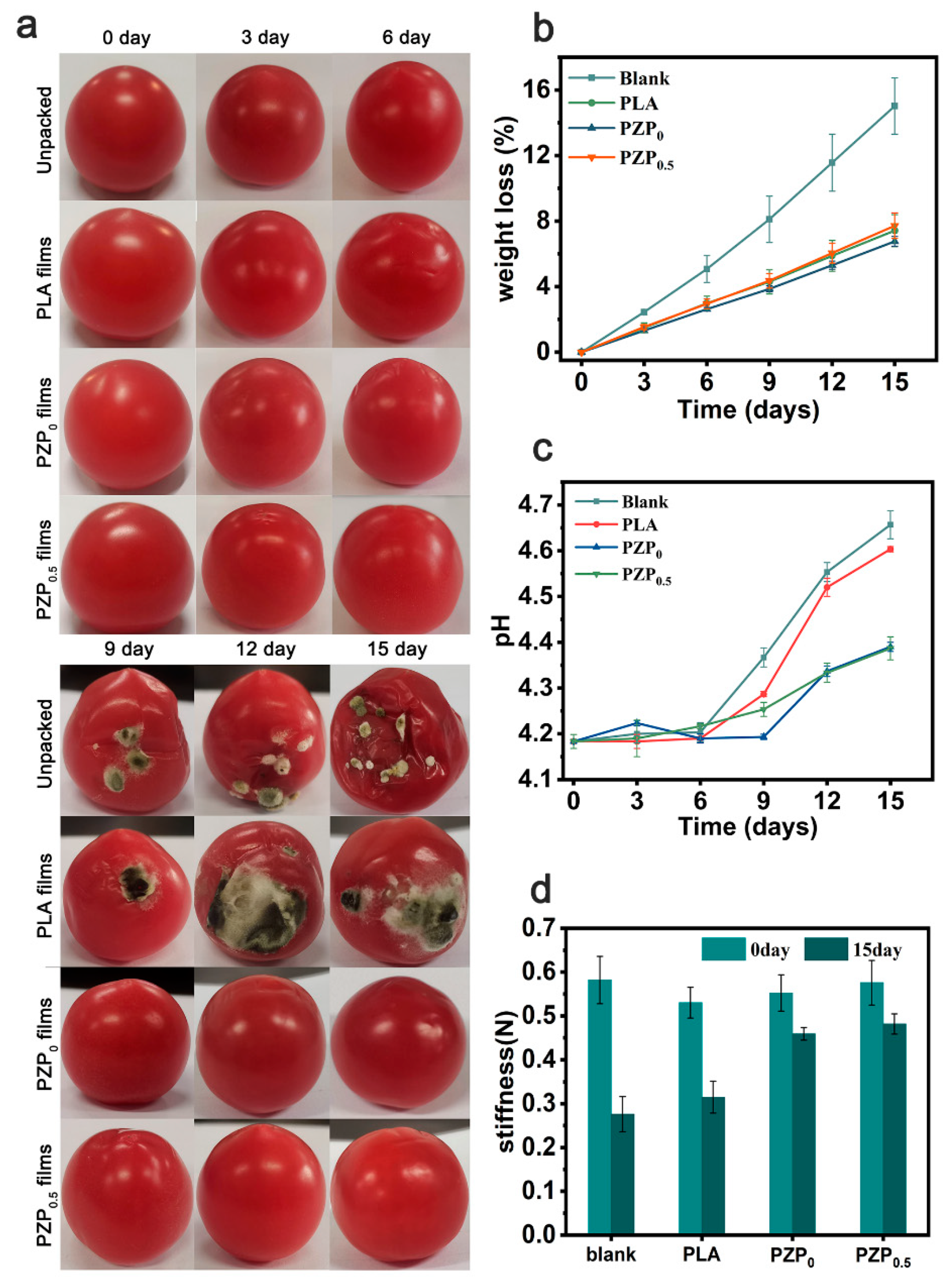
3.4. Evaluation of Cherry Tomatoes Preservation
Weight Loss, Hardness, and pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Le Tien, C.; Letendre, M.; Ispas-Szabo, P.; Mateescu, M.; Delmas-Patterson, G.; Yu, H.-L.; Lacroix, M. Development of biodegradable films from whey proteins by cross-linking and entrapment in cellulose. J. Agric. Food Chem. 2000, 48, 5566–5575. [Google Scholar] [CrossRef] [PubMed]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Viswanathan, K.; Kasi, G.; Sadeghi, K.; Thanakkasaranee, S.; Seo, J. Poly (lactic acid)/ZnO bionanocomposite films with positively charged ZnO as potential antimicrobial food packaging materials. Polymers 2019, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Kian, L.; Saba, N.; Jawaid, M.; Sultan, M. A review on processing techniques of bast fibers nanocellulose and its polylactic acid (PLA) nanocomposites. Int. J. Biol. Macromol. 2019, 121, 1314–1328. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of active packaging film made from poly (lactic acid) incorporated essential oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Chi, H.; Li, L.; Lan, T.; Han, P.; Chen, H.; Qin, Y. Development of antimicrobial packaging film made from poly (lactic acid) incorporating titanium dioxide and silver nanoparticles. Molecules 2017, 22, 1170. [Google Scholar] [CrossRef]
- Swaroop, C.; Shukla, M. Nano-magnesium oxide reinforced polylactic acid biofilms for food packaging applications. Int. J. Biol. Macromol. 2018, 113, 729–736. [Google Scholar] [CrossRef]
- Kumar, S.S.; Venkateswarlu, P.; Rao, V.R.; Rao, G.N. Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett. 2013, 3, 30. [Google Scholar] [CrossRef]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, characterization, and spectroscopic properties of ZnO nanoparticles. Int. Sch. Res. Not. 2012, 2012, 372505. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.d.F.F.; Coimbra, J.S.d.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Carboxymethyl cellulose-based antioxidant and antimicrobial active packaging film incorporated with curcumin and zinc oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Beltran, A.; Fortunati, E.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled release of thymol from poly (lactic acid)-based silver nanocomposite films with antibacterial and antioxidant activity. Antioxidants 2020, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Marangoni Júnior, L.; Gonçalves, S.d.Á.; Silva, R.G.d.; Martins, J.T.; Vicente, A.A.; Alves, R.M.V.; Vieira, R.P. Effect of green propolis extract on functional properties of active pectin-based films. Food Hydrocoll. 2022, 131, 107746. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Amjadi, S.; Mohammadi, M.; Lorenzo, J.M.; Hamishehkar, H. Active gelatin/cress seed gum-based films reinforced with chitosan nanoparticles encapsulating pomegranate peel extract: Preparation and characterization. Food Hydrocoll. 2022, 129, 107620. [Google Scholar] [CrossRef]
- Bertolo, M.R.; Martins, V.C.; Horn, M.M.; Brenelli, L.B.; Plepis, A.M. Rheological and antioxidant properties of chitosan/gelatin-based materials functionalized by pomegranate peel extract. Carbohydr. Polym. 2020, 228, 115386. [Google Scholar] [CrossRef]
- Xiang, Q.; Li, M.; Wen, J.; Ren, F.; Yang, Z.; Jiang, X.; Chen, Y. The bioactivity and applications of pomegranate peel extract: A review. J. Food Biochem. 2022, 46, e14105. [Google Scholar] [CrossRef]
- He, L.; Lan, W.; Ahmed, S.; Qin, W.; Liu, Y. Electrospun polyvinyl alcohol film containing pomegranate peel extract and sodium dehydroacetate for use as food packaging. Food Packag. Shelf Life 2019, 22, 100390. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha; Trajkovska Petkoska, A.; Khojah, E.; Sami, R.; Al-Mushhin, A.A.M. Chitosan Edible Films Enhanced with Pomegranate Peel Extract: Study on Physical, Biological, Thermal, and Barrier Properties. Materials 2021, 14, 3305. [Google Scholar] [CrossRef]
- Barbosa, M.H.R.; Gonçalves, S.d.Á.; Marangoni Júnior, L.; Alves, R.M.V.; Vieira, R.P. Physicochemical properties of chitosan-based films incorporated with limonene. J. Food Meas. Charact. 2022, 16, 2011–2023. [Google Scholar] [CrossRef]
- Bertotto, C.; Bilck, A.P.; Yamashita, F.; Anjos, O.; Bakar Siddique, M.A.; Harrison, S.M.; Brunton, N.P.; Carpes, S.T. Development of a biodegradable plastic film extruded with the addition of a Brazilian propolis by-product. LWT 2022, 157, 113124. [Google Scholar] [CrossRef]
- Ni, Y.; Sun, J.; Wang, J. Enhanced antimicrobial activity of konjac glucomannan nanocomposite films for food packaging. Carbohydr. Polym. 2021, 267, 118215. [Google Scholar] [CrossRef] [PubMed]
- Assifaoui, A.; Loupiac, C.; Chambin, O.; Cayot, P. Structure of calcium and zinc pectinate films investigated by FTIR spectroscopy. Carbohydr. Res. 2010, 345, 929–933. [Google Scholar] [CrossRef]
- Chae, B.; Yang, Y.; Lee, S.; Jang, M.; Lee, S.; Kim, S.; Baek, W.; Kwon, S. Comparative analysis for the crystalline and ferroelectric properties of Pb (Zr, Ti) O3 thin films deposited on metallic LaNiO3 and Pt electrodes. Thin Solid Film. 2002, 410, 107–113. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar]
- Rhim, J.-W.; Hong, S.-I.; Park, H.-M.; Ng, P.K. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef]
- Yu, F.; Fei, X.; He, Y.; Li, H. Poly (lactic acid)-based composite film reinforced with acetylated cellulose nanocrystals and ZnO nanoparticles for active food packaging. Int. J. Biol. Macromol. 2021, 186, 770–779. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of bioactive functional poly (lactic acid)/curcumin composite film for food packaging application. Int. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar] [CrossRef]
- Chu, Z.; Zhao, T.; Li, L.; Fan, J.; Qin, Y. Characterization of antimicrobial poly (lactic acid)/nano-composite films with silver and zinc oxide nanoparticles. Materials 2017, 10, 659. [Google Scholar] [CrossRef]
- Lai, W.-C. Thermal Behavior and Crystal Structure of Poly (l-lactic acid) with 1,3:2,4-Dibenzylidene-d-sorbitol. J. Phys. Chem. B 2011, 115, 11029–11037. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Gupta, S.; Zafeiropoulos, N.E.; Oertel, U.; Häßler, R.; Stamm, M. Nano-Level Mixing of ZnO into Poly (methyl methacrylate). Macromol. Chem. Phys. 2010, 211, 1925–1932. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. Antioxidant and antibacterial chitosan film with tea polyphenols-mediated green synthesis silver nanoparticle via a novel one-pot method. Int. J. Biol. Macromol. 2020, 155, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Mehrotra, G.; Bhartiya, P.; Singh, A.; Dutta, P. Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, C.; Wang, K.; Shi, D.; Liu, Z.; Yang, M. Super-Toughed PLA Blown Film with Enhanced Gas Barrier Property Available for Packaging and Agricultural Applications. Materials 2019, 12, 1663. [Google Scholar] [CrossRef]
- Pirsa, S.; Karimi Sani, I.; Pirouzifard, M.K.; Erfani, A. Smart film based on chitosan/Melissa officinalis essences/pomegranate peel extract to detect cream cheeses spoilage. Food Addit. Contam. Part A 2020, 37, 634–648. [Google Scholar] [CrossRef]
- Qin, Y.-Y.; Zhang, Z.-H.; Li, L.; Yuan, M.-L.; Fan, J.; Zhao, T.-R. Physio-mechanical properties of an active chitosan film incorporated with montmorillonite and natural antioxidants extracted from pomegranate rind. J. Food Sci. Technol. 2015, 52, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, Z.H.; Qin, Y.Y.; Zhao, T.R.; Cheng, C.S. Characterization of antioxidant chitosan film incorporated with pomegranate peel extract. Adv. Mater. Res. 2013, 706, 24–27. [Google Scholar] [CrossRef]
- Slman, A.A. Antibacterial activity of Zno nanoparticle on some gram-positive and gram-negative bacteria. Iraqi J. Phys. 2012, 10, 5–10. [Google Scholar]
- Min, T.; Sun, X.; Yuan, Z.; Zhou, L.; Jiao, X.; Zha, J.; Zhu, Z.; Wen, Y. Novel antimicrobial packaging film based on porous poly (lactic acid) nanofiber and polymeric coating for humidity-controlled release of thyme essential oil. LWT 2021, 135, 110034. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Ni, Y.; Yang, C.; Jin, X.; Wang, Y.; Jin, Y.; Sun, J.; Wang, J. ZnO/C-mediated k-carrageenan based pseudo-pasteurization films for kumquat preservation. Food Hydrocoll. 2022, 128, 107582. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Lee, J.Y.; Lee, Y.S. Development of polyvinyl alcohol and apple pomace bio-composite film with antioxidant properties for active food packaging application. Journal of food science and technology 2016, 53, 1608–1619. [Google Scholar] [CrossRef] [PubMed]

| Film Samples | Thickness (mm) |
|---|---|
| PLA | 0.0325 ± 0.00173 d |
| PLA/ZnONPs | 0.0400 ± 0.00115 c |
| PLA/ZnONPs/PEE0.5 | 0.0433 ± 0.00171 b |
| PLA/ZnONPs/PEE1 | 0.0445 ± 0.00191 b |
| PLA/ZnONPs/PEE1.5 | 0.0463 ± 0.00096 ab |
| PLA/ZnONPs/PEE2 | 0.0480 ± 0.00082 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Li, R.; Liang, Y.; Liu, Y.; Zhang, W.; Shi, S. Development of Pomegranate Peel Extract and Nano ZnO Co-Reinforced Polylactic Acid Film for Active Food Packaging. Membranes 2022, 12, 1108. https://doi.org/10.3390/membranes12111108
Dai L, Li R, Liang Y, Liu Y, Zhang W, Shi S. Development of Pomegranate Peel Extract and Nano ZnO Co-Reinforced Polylactic Acid Film for Active Food Packaging. Membranes. 2022; 12(11):1108. https://doi.org/10.3390/membranes12111108
Chicago/Turabian StyleDai, Lu, Runli Li, Yanmin Liang, Yingsha Liu, Wentao Zhang, and Shuo Shi. 2022. "Development of Pomegranate Peel Extract and Nano ZnO Co-Reinforced Polylactic Acid Film for Active Food Packaging" Membranes 12, no. 11: 1108. https://doi.org/10.3390/membranes12111108
APA StyleDai, L., Li, R., Liang, Y., Liu, Y., Zhang, W., & Shi, S. (2022). Development of Pomegranate Peel Extract and Nano ZnO Co-Reinforced Polylactic Acid Film for Active Food Packaging. Membranes, 12(11), 1108. https://doi.org/10.3390/membranes12111108







