Multisensory Systems Based on Perfluorosulfonic Acid Membranes Modified with Functionalized CNTs for Determination of Sulfamethoxazole and Trimethoprim in Pharmaceuticals
Abstract
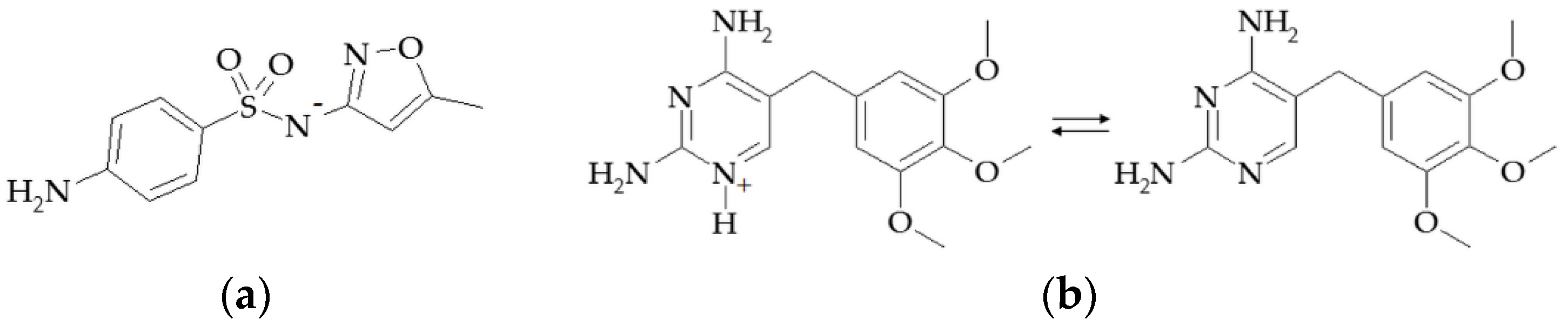
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
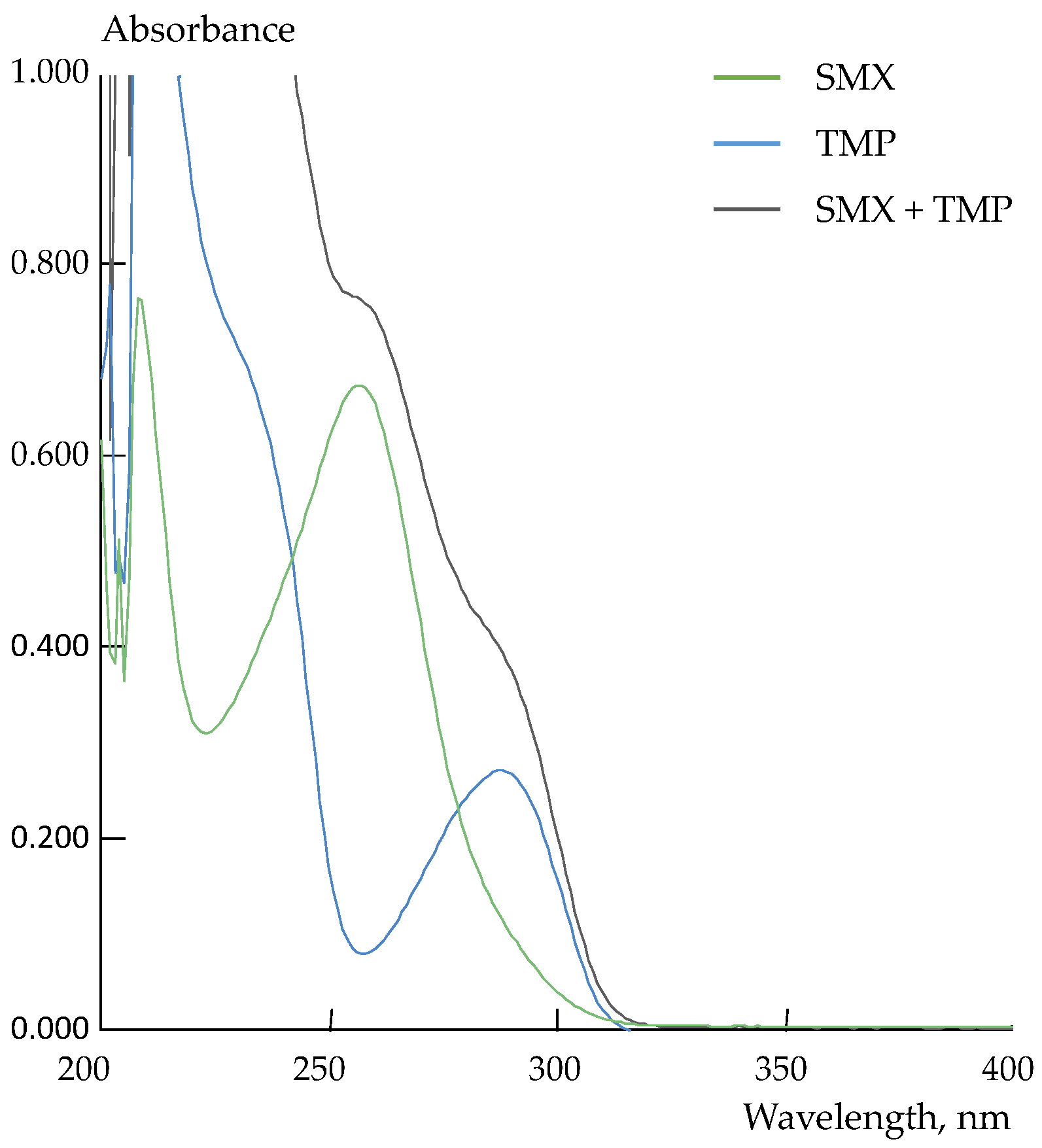
2.2. Preparation of Model Solutions
2.3. Preparation of Pharmaceutical Solutions
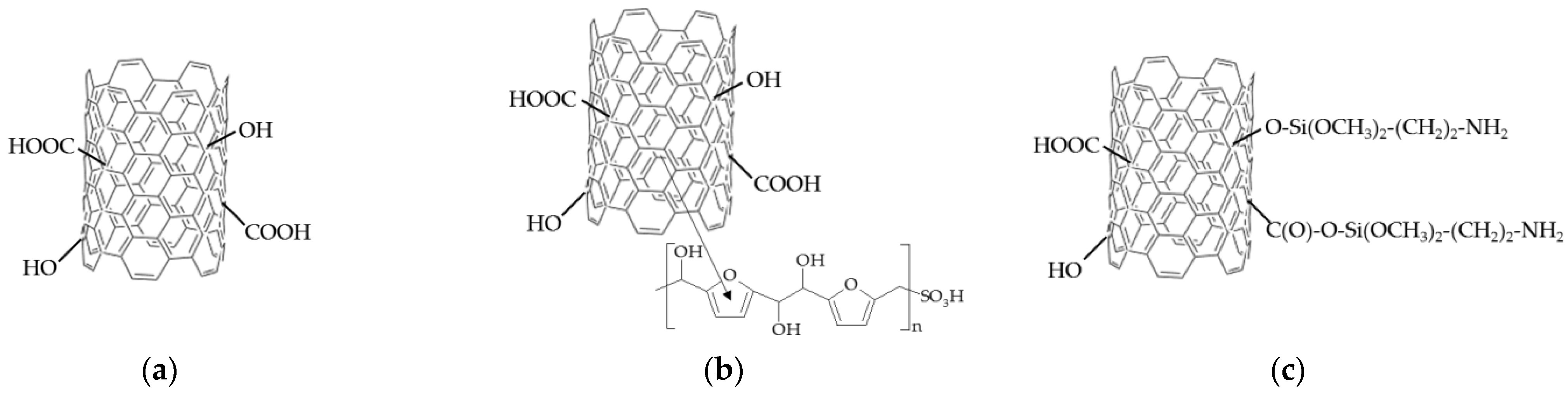
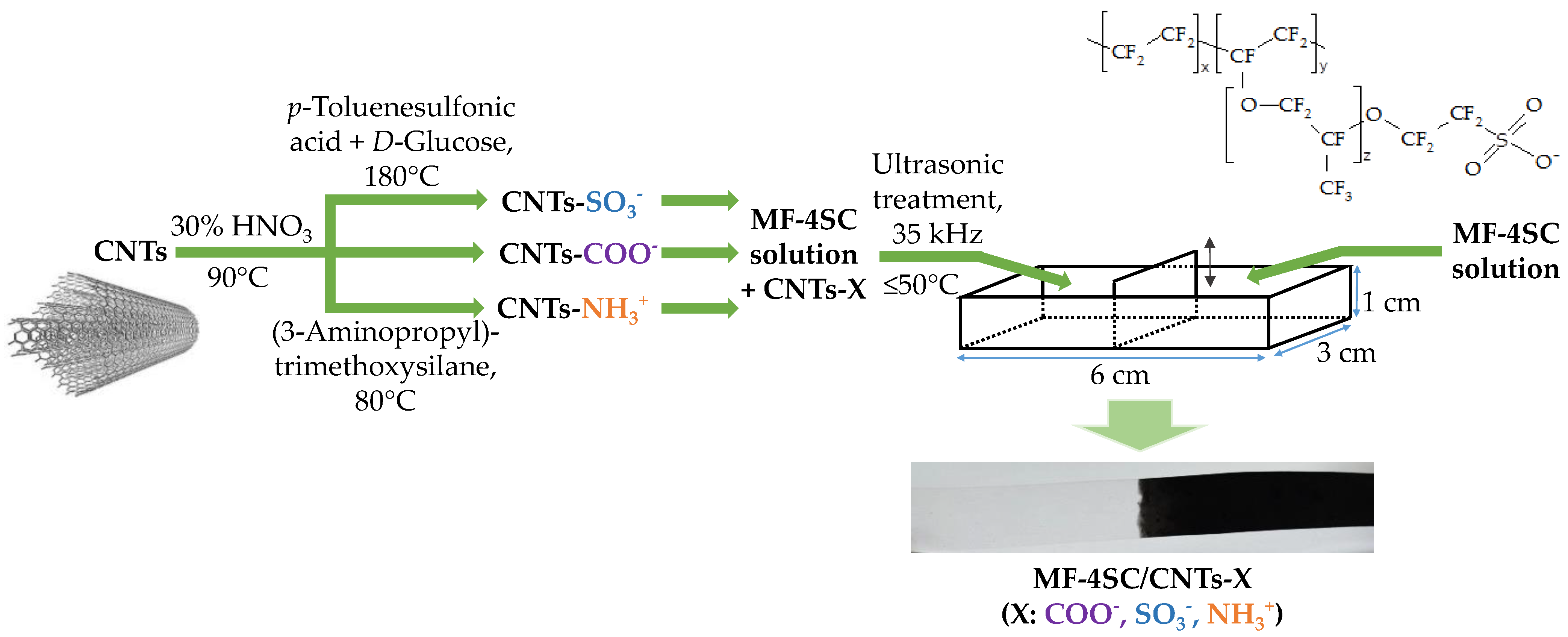
2.4. Functionalization of CNTs
2.5. Membrane Preparation
2.6. Apparatus and Experiment Procedure
2.7. Data Processing Procedure
3. Results and Discussion
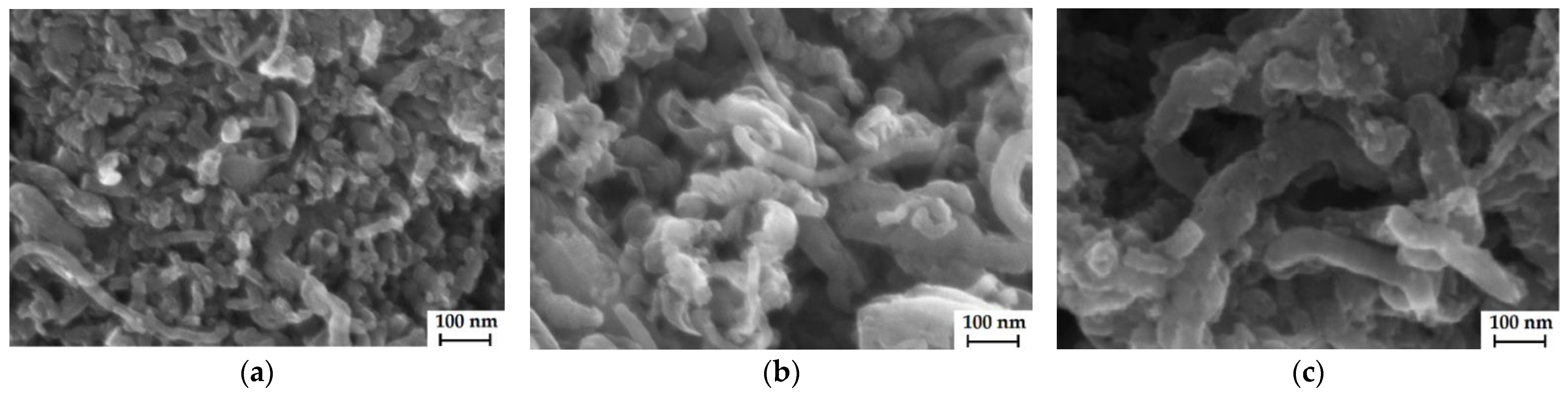
3.1. Properties of CNTs
3.2. Properties of Membranes
3.3. Characteristics of the DP-Sensors
3.3.1. Cross-Sensitivity of the DP-Sensors
3.3.2. Analysis of Model Solutions and Pharmaceuticals
3.3.3. Stability and Reproducibility of the Multisensory System Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mistri, H.N.; Jangid, A.G.; Pudage, A.; Shah, A.; Shrivastav, P.S. Simultaneous Determination of Sulfamethoxazole and Trimethoprim in Microgram Quantities from Low Plasma Volume by Liquid Chromatography–Tandem Mass Spectrometry. Microchem. J. 2010, 94, 130–138. [Google Scholar] [CrossRef]
- Le-Minh, N.; Stuetz, R.M.; Khan, S.J. Determination of Six Sulfonamide Antibiotics, Two Metabolites and Trimethoprim in Wastewater by Isotope Dilution Liquid Chromatography/Tandem Mass Spectrometry. Talanta 2012, 89, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Franklin, A.M.; Andrews, D.M.; Williams, C.F.; Watson, J.E. Simultaneous Extraction of Four Antibiotic Compounds from Soil and Water Matrices. Separations 2022, 9, 200. [Google Scholar] [CrossRef]
- Dinali, L.A.F.; de Oliveira, H.L.; Teixeira, L.S.; da Silva, A.T.M.; D’Oliveira, K.A.; Cuin, A.; Borges, K.B. Efficient Development of a Magnetic Molecularly Imprinted Polymer for Selective Determination of Trimethoprim and Sulfamethoxazole in Milk. Microchem. J. 2020, 154, 104648. [Google Scholar] [CrossRef]
- Cesarino, I.; Cesarino, V.; Lanza, M.R.V. Carbon Nanotubes Modified with Antimony Nanoparticles in a Paraffin Composite Electrode: Simultaneous Determination of Sulfamethoxazole and Trimethoprim. Sens. Actuators B Chem. 2013, 188, 1293–1299. [Google Scholar] [CrossRef]
- Sgobbi, L.F.; Razzino, C.A.; Machado, S.A.S. A Disposable Electrochemical Sensor for Simultaneous Detection of Sulfamethoxazole and Trimethoprim Antibiotics in Urine Based on Multiwalled Nanotubes Decorated with Prussian Blue Nanocubes Modified Screen-Printed Electrode. Electrochim. Acta 2016, 191, 1010–1017. [Google Scholar] [CrossRef]
- Martins, T.S.; Bott-Neto, J.L.; Oliveira, O.N., Jr.; Machado, S.A.S. Paper-Based Electrochemical Sensors with Reduced Graphene Nanoribbons for Simultaneous Detection of Sulfamethoxazole and Trimethoprim in Water Samples. J. Electroanal. Chem. 2021, 882, 114985. [Google Scholar] [CrossRef]
- Yue, X.; Li, Z.; Zhao, S. A New Electrochemical Sensor for Simultaneous Detection of Sulfamethoxazole and Trimethoprim Antibiotics Based on Graphene and ZnO Nanorods Modified Glassy Carbon Electrode. Microchem. J. 2020, 159, 105440. [Google Scholar] [CrossRef]
- Calaça, G.N.; Pessoa, C.A.; Wohnrath, K.; Nagata, N. Simultaneous Determination of Sulfamethoxazole and Trimethoprim in Pharmaceutical Formulations by Square Wave Voltammetry. Int. J. Pharm. Pharm. Sci. 2014, 6, 438–442. [Google Scholar]
- Santos Andrade, L.; Cardozo Rocha-Filho, R.; Bezerra Cass, Q.; Fatibello-Filho, O. A Novel Multicommutation Stopped-Flow System for the Simultaneous Determination of Sulfamethoxazole and Trimethoprim by Differential Pulse Voltammetry on a Boron-Doped Diamond Electrode. Anal. Methods 2010, 2, 402. [Google Scholar] [CrossRef]
- Abrahem, S.A.; Abass, A.M.; Ahmed, A. Trimethoprim Determination with Drug-Selective Electrodes. Asian J. Pharm. Clin. Res. 2019, 12, 83–87. [Google Scholar] [CrossRef]
- Rebelo, T.S.C.R.; Almeida, S.A.A.; Guerreiro, J.R.L.; Montenegro, M.C.B.S.M.; Sales, M.G.F. Trimethoprim-Selective Electrodes with Molecularly Imprinted Polymers Acting as Ionophores and Potentiometric Transduction on Graphite Solid-Contact. Microchem. J. 2011, 98, 21–28. [Google Scholar] [CrossRef]
- Almeida, S.A.A.; Heitor, A.M.; Sá, L.C.; Barbosa, J.; da Conceição, M.; Montenegro, B.S.M.; Sales, M.G.F. Solid Contact PVC Membrane Electrodes Based on Neutral or Charged Carriers for the Selective Reading of Anionic Sulfamethoxazole and Their Application to the Analysis of Aquaculture Water. Int. J. Environ. Anal. Chem. 2012, 92, 479–495. [Google Scholar] [CrossRef][Green Version]
- Arvand, M.; Alirezanejad, F. Sulfamethoxazole-Imprinted Polymeric Receptor as Ionophore for Potentiometric Transduction. Electroanalysis 2011, 23, 1948–1957. [Google Scholar] [CrossRef]
- Almeida, S.A.A.; Truta, L.A.A.N.A.; Queirós, R.B.; Montenegro, M.C.B.S.M.; Cunha, A.L.; Sales, M.G.F. Optimizing Potentiometric Ionophore and Electrode Design for Environmental On-Site Control of Antibiotic Drugs: Application to Sulfamethoxazole. Biosens. Bioelectron. 2012, 35, 319–326. [Google Scholar] [CrossRef][Green Version]
- Almeida, S.A.A.; Moreira, F.T.C.; Heitor, A.M.; Montenegro, M.C.B.S.M.; Aguilar, G.G.; Sales, M.G.F. Sulphonamide-Imprinted Sol–Gel Materials as Ionophores in Potentiometric Transduction. Mater. Sci. Eng. C 2011, 31, 1784–1790. [Google Scholar] [CrossRef]
- Almeida, S.A.A.; Arasa, E.; Puyol, M.; Martinez-Cisneros, C.S.; Alonso-Chamarro, J.; Montenegro, M.C.B.S.M.; Sales, M.G.F. Novel LTCC-Potentiometric Microfluidic Device for Biparametric Analysis of Organic Compounds Carrying Plastic Antibodies as Ionophores: Application to Sulfamethoxazole and Trimethoprim. Biosens. Bioelectron. 2011, 30, 197–203. [Google Scholar] [CrossRef][Green Version]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, M.; Bhandari, B.; Adhikari, B. Application of Electronic Tongue for Fresh Foods Quality Evaluation: A Review. Food Rev. Int. 2018, 34, 746–769. [Google Scholar] [CrossRef]
- Kirsanov, D.; Correa, D.; Gaal, G.; Riul, A.; Braunger, M.; Shimizu, F.; Oliveira, O.; Liang, T.; Wan, H.; Wang, P.; et al. Electronic Tongues for Inedible Media. Sensors 2019, 19, 5113. [Google Scholar] [CrossRef]
- Ashina, J.; Babain, V.; Kirsanov, D.; Legin, A. A Novel Multi-Ionophore Approach for Potentiometric Analysis of Lanthanide Mixtures. Chemosensors 2021, 9, 23. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Reut, J.; Öpik, A.; Furchner, A.; Syritski, V. Hybrid Molecularly Imprinted Polymer for Amoxicillin Detection. Biosens. Bioelectron. 2018, 118, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Safronova, E.; Parshina, A.; Kolganova, T.; Bobreshova, O.; Pourcelly, G.; Yaroslavtsev, A. Potentiometric Sensors Arrays Based on Perfluorinated Membranes and Silica Nanoparticles with Surface Modified by Proton-Acceptor Groups, for the Determination of Aspartic and Glutamic Amino Acids Anions and Potassium Cations. J. Electroanal. Chem. 2018, 816, 21–29. [Google Scholar] [CrossRef]
- Safronova, E.; Parshina, A.; Kolganova, T.; Yelnikova, A.; Bobreshova, O.; Pourcelly, G.; Yaroslavtsev, A. Potentiometric Multisensory System Based on Perfluorosulfonic Acid Membranes and Carbon Nanotubes for Sulfacetamide Determination in Pharmaceuticals. J. Electroanal. Chem. 2020, 873, 114435. [Google Scholar] [CrossRef]
- Parshina, A.; Yelnikova, A.; Titova, T.; Kolganova, T.; Yurova, P.; Stenina, I.; Bobreshova, O.; Yaroslavtsev, A. Multisensory Systems Based on Perfluorosulfonic Acid Membranes Modified with Polyaniline and PEDOT for Multicomponent Analysis of Sulfacetamide Pharmaceuticals. Polymers 2022, 14, 2545. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Ionic Mobility in Ion-Exchange Membranes. Membranes 2021, 11, 198. [Google Scholar] [CrossRef]
- Apel, P.Y.; Velizarov, S.; Volkov, A.V.; Eliseeva, T.V.; Nikonenko, V.V.; Parshina, A.V.; Pismenskaya, N.D.; Popov, K.I.; Yaroslavtsev, A.B. Fouling and Membrane Degradation in Electromembrane and Baromembrane Processes. Membr. Membr. Technol. 2022, 4, 69–92. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Shamagsumova, R.; Hianik, T. Advances in Electrochemical Aptasensors Based on Carbon Nanomaterials. Chemosensors 2020, 8, 96. [Google Scholar] [CrossRef]
- Koteshwara Reddy, K.; Satyanarayana, M.; Yugender Goud, K.; Vengatajalabathy Gobi, K.; Kim, H. Carbon Nanotube Ensembled Hybrid Nanocomposite Electrode for Direct Electrochemical Detection of Epinephrine in Pharmaceutical Tablets and Urine. Mater. Sci. Eng. C 2017, 79, 93–99. [Google Scholar] [CrossRef]
- Cho, G.; Azzouzi, S.; Zucchi, G.; Lebental, B. Electrical and Electrochemical Sensors Based on Carbon Nanotubes for the Monitoring of Chemicals in Water—A Review. Sensors 2021, 22, 218. [Google Scholar] [CrossRef]
- Abellán-Llobregat, A.; González-Gaitán, C.; Vidal, L.; Canals, A.; Morallón, E. Portable Electrochemical Sensor Based on 4-Aminobenzoic Acid-Functionalized Herringbone Carbon Nanotubes for the Determination of Ascorbic Acid and Uric Acid in Human Fluids. Biosens. Bioelectron. 2018, 109, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.U.; Deen, M.J. Bisphenol A Electrochemical Sensor Using Graphene Oxide and β-Cyclodextrin-Functionalized Multi-Walled Carbon Nanotubes. Anal. Chem. 2020, 92, 5532–5539. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yang, X.; Mo, Z.; Guo, R.; Liu, N.; Zhao, P.; Liu, Z.; Ouyang, M. Voltammetric Enantiomeric Differentiation of Tryptophan by Using Multiwalled Carbon Nanotubes Functionalized with Ferrocene and β-Cyclodextrin. Electrochim. Acta 2019, 297, 650–659. [Google Scholar] [CrossRef]
- Mao, A.; Li, H.; Yu, L.; Hu, X. Electrochemical Sensor Based on Multi-Walled Carbon Nanotubes and Chitosan-Nickel Complex for Sensitive Determination of Metronidazole. J. Electroanal. Chem. 2017, 799, 257–262. [Google Scholar] [CrossRef]
- Arvand, M.; Zamani, M.; Sayyar Ardaki, M. Rapid Electrochemical Synthesis of Molecularly Imprinted Polymers on Functionalized Multi-Walled Carbon Nanotubes for Selective Recognition of Sunset Yellow in Food Samples. Sens. Actuators B Chem. 2017, 243, 927–939. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Athira, V.S.; Chithra Sekhar, V. Electrochemical Sensing and Nano Molar Level Detection of Bisphenol-A with Molecularly Imprinted Polymer Tailored on Multiwalled Carbon Nanotubes. Polymer 2018, 146, 312–320. [Google Scholar] [CrossRef]
- Kulapina, E.G.; Kulapina, O.I.; Ankina, V.D. Screen-Printed Potentiometric Sensors Based on Carbon Materials for Determining Cefotaxime and Cefuroxime. J. Anal. Chem. 2020, 75, 231–237. [Google Scholar] [CrossRef]
- Parrilla, M.; Cuartero, M.; Crespo, G.A. Wearable Potentiometric Ion Sensors. TrAC Trends Anal. Chem. 2019, 110, 303–320. [Google Scholar] [CrossRef]
- Yin, T.; Qin, W. Applications of Nanomaterials in Potentiometric Sensors. TrAC Trends Anal. Chem. 2013, 51, 79–86. [Google Scholar] [CrossRef]
- Gupta, S.; Murthy, C.N.; Prabha, C.R. Recent Advances in Carbon Nanotube Based Electrochemical Biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Prykhodko, Y.; Fatyeyeva, K.; Hespel, L.; Marais, S. Progress in Hybrid Composite Nafion®-Based Membranes for Proton Exchange Fuel Cell Application. Chem. Eng. J. 2021, 409, 127329. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Ryu, S.K.; Yoo, D.J. Structurally Modulated and Functionalized Carbon Nanotubes as Potential Filler for Nafion Matrix toward Improved Power Output and Durability in Proton Exchange Membrane Fuel Cells Operating at Reduced Relative Humidity. J. Membr. Sci. 2022, 649, 120393. [Google Scholar] [CrossRef]
- Asgari, M.S.; Nikazar, M.; Molla-abbasi, P.; Hasani-Sadrabadi, M.M. Nafion®/Histidine Functionalized Carbon Nanotube: High-Performance Fuel Cell Membranes. Int. J. Hydrog. Energy 2013, 38, 5894–5902. [Google Scholar] [CrossRef]
- Prikhno, I.A.; Safronova, E.Y.; Yaroslavtsev, A.B. Hybrid Materials Based on Perfluorosulfonic Acid Membrane and Functionalized Carbon Nanotubes: Synthesis, Investigation and Transport Properties. Int. J. Hydrog. Energy 2016, 41, 15585–15592. [Google Scholar] [CrossRef]
- Prikhno, I.A.; Safronova, E.Y.; Il’in, A.B.; Yaroslavtsev, A.B. MF-4SC Hybrid Membranes Doped with Carbon Nanotubes Functionalized with Proton-Acceptor Groups. Nanotechnol. Russ. 2017, 12, 236–242. [Google Scholar] [CrossRef]
- Parshina, A.V.; Safronova, E.Y.; Kolganova, T.S.; Habtemariam, G.Z.; Bobreshova, O.V. Perfluorosulfonic Acid Membranes with Functionalized Carbon Nanotubes in Potentiometric Sensors for the Analysis of Nicotinic Acid Pharmaceuticals. J. Anal. Chem. 2022, 77, 195–205. [Google Scholar] [CrossRef]
- Fu, Q.; Gao, B.; Dou, H.; Hao, L.; Lu, X.; Sun, K.; Jiang, J.; Zhang, X. Novel Non-Covalent Sulfonated Multiwalled Carbon Nanotubes from p-Toluenesulfonic Acid/Glucose Doped Polypyrrole for Electrochemical Capacitors. Synth. Met. 2011, 161, 373–378. [Google Scholar] [CrossRef]
- Ansaloni, L.; Zhao, Y.; Jung, B.T.; Ramasubramanian, K.; Baschetti, M.G.; Ho, W.S.W. Facilitated Transport Membranes Containing Amino-Functionalized Multi-Walled Carbon Nanotubes for High-Pressure CO2 Separations. J. Membr. Sci. 2015, 490, 18–28. [Google Scholar] [CrossRef]
- Konwar, L.J.; Mäki-Arvela, P.; Mikkola, J.-P. SO 3 H-Containing Functional Carbon Materials: Synthesis, Structure, and Acid Catalysis. Chem. Rev. 2019, 119, 11576–11630. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Pourcelly, G.; Yaroslavtsev, A.B. The Transformation and Degradation of Nafion® Solutions under Ultrasonic Treatment. The Effect on Transport and Mechanical Properties of the Resultant Membranes. Polym. Degrad. Stab. 2020, 178, 109229. [Google Scholar] [CrossRef]
- Mikheev, A.G.; Safronova, E.Y.; Yurkov, G.Y.; Yaroslavtsev, A.B. Hybrid Materials Based on MF-4SC Perfluorinated Sulfo Cation-Exchange Membranes and Silica with Proton-Acceptor Properties. Mendeleev Commun. 2013, 23, 66–68. [Google Scholar] [CrossRef]
- Sarapulova, V.V.; Klevtsova, A.V.; Pismenskaya, N.D. Electrostatic Interactions of Ion-Exchange Materials with Anthocyanins in the Processes of Their Sorption and Electrodialysis Extraction from Liquid Media. Membr. Membr. Technol. 2020, 2, 272–282. [Google Scholar] [CrossRef]
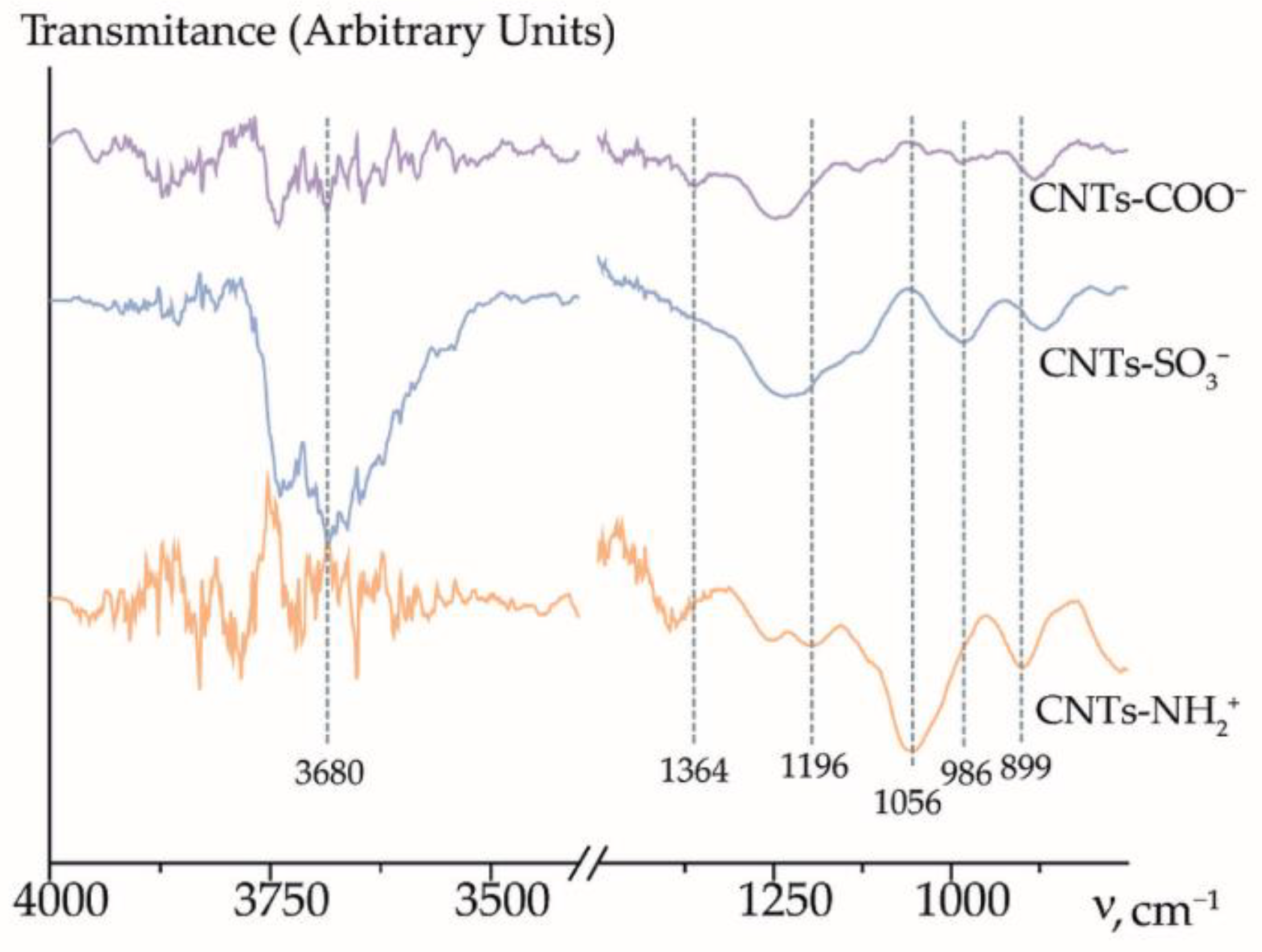
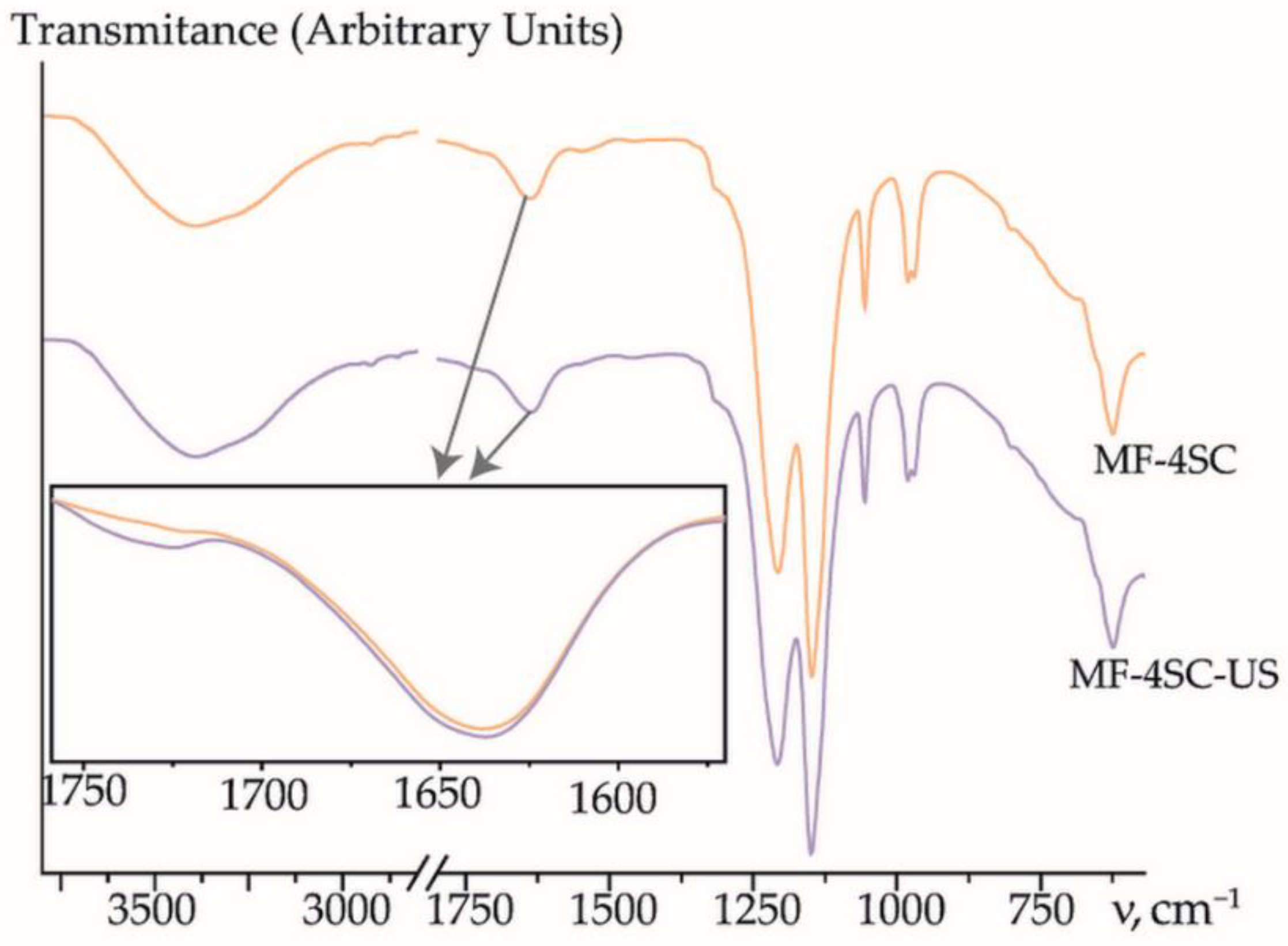
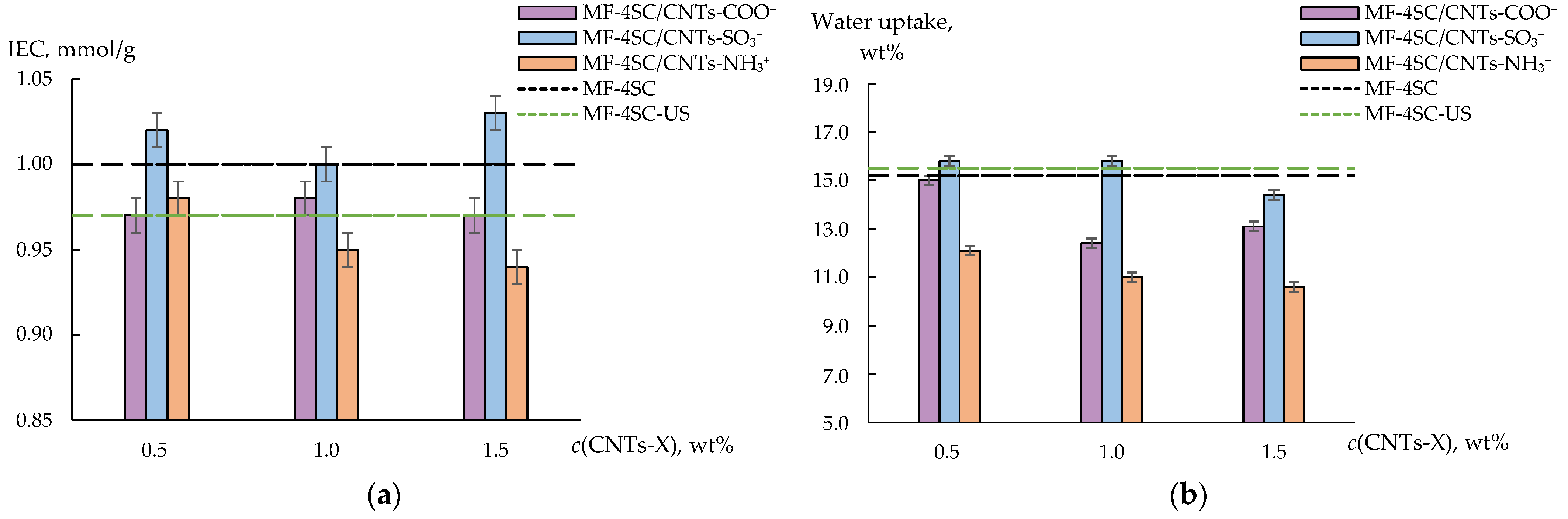
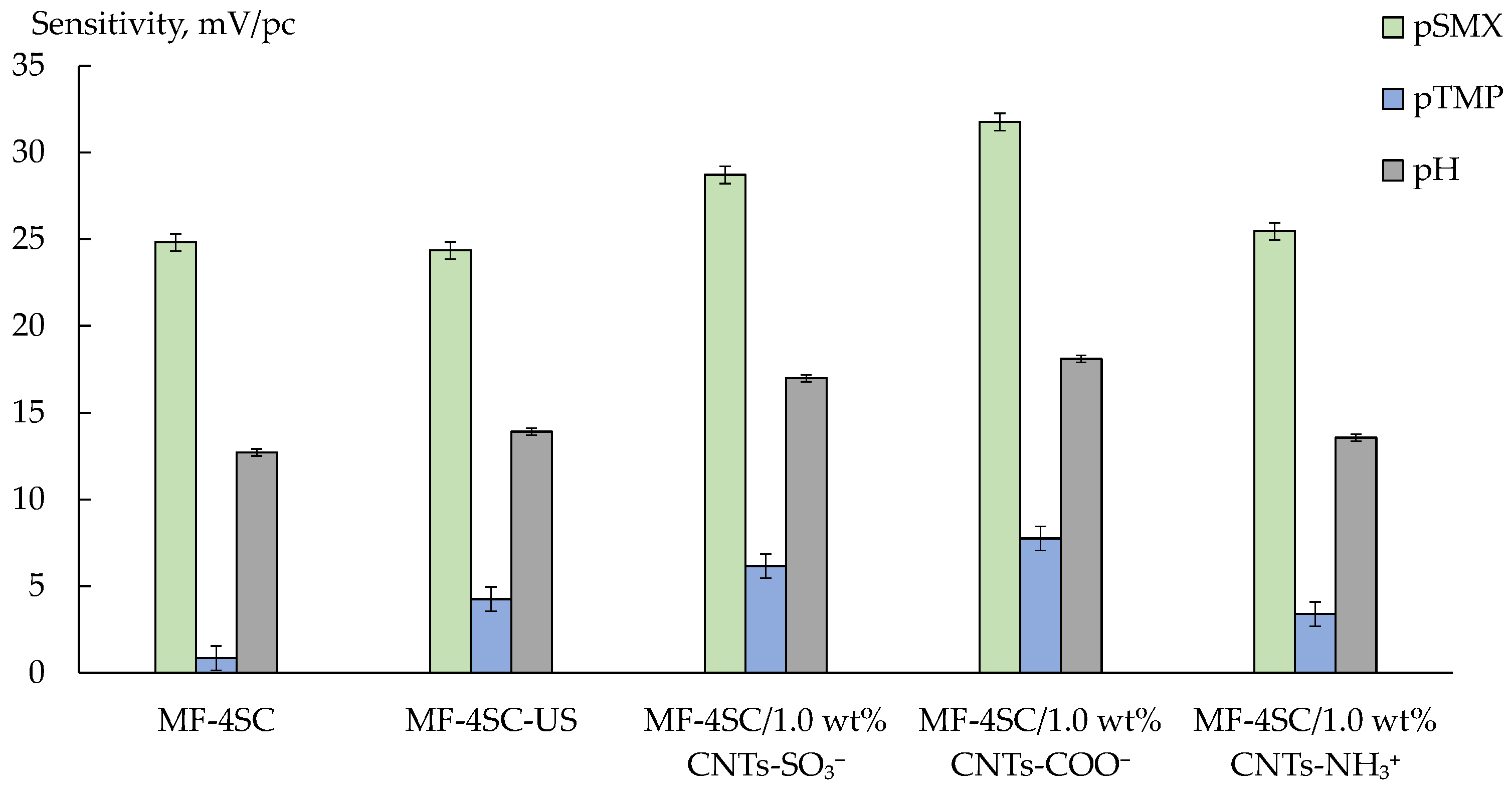
| Analysis Object | Method; Sensor Composition | Linear Range, M; LOD, M | Relative Error, %; RSD, % | Comments | Ref. |
|---|---|---|---|---|---|
| Natural water | DPV; Paste electrode: paraffin/MWCNTs/SbNPs | (0.1–0.7)×10−6/(0.1–0.7)×10−6; 2.4×10−8/3.1×10−8 | 0.9–4.8/1.6–2.8; -/- | A pH correction to 7.0; polishing and thorough washing before every measurement was required | [5] |
| Urine model solution | DPV; SPE: polyester/MWCNTs/PBNCs | (1.0–10.0)×10−6/(0.1–10.0)×10−6; 3.8×10−8/6.0×10−8 | 0.6–7.7/1.2–8.7; 3.5–4/2.1–3.3 | A pH correction to 7.0; stability up to 3 weeks | [6] |
| Tap water | DPV; SPE: parchment paper/rGNRs | 1.0–10.0)×10−6/(1.0–10.0)×10−6; 0.9×10−7/0.4×10−7 | 1.51–2.17/0.14–0.48; 1.06–3.54/1.96–3.81 | A pH correction to 6.0 | [7] |
| Tap water | DPV; GCE/ GR-ZnO nanorods | 1.0×10−6–2.2×10−4/1.0×10−6–1.8×10−4; 0.4×10−6/0.3×10−6 | 3.8–3/4.7–1.0; -/- | A pH correction to 7.0; stability up to 3 weeks | [8] |
| Lake water | 0.9–5/2.3–6.5; -/- | ||||
| Urine | 1.2–6/2.3–5.2; -/- | ||||
| Serum | 3.5–5.4/3.2–4.5; -/- | ||||
| Tablets | SWV; GCE | 5.5×10−5–3.95×10−4/1.05×10−5–1.04×10−4; 8.52×10−6/9.31×10−7 | 0.53–2.41/1.89–3.20; -/- | A pH correction to 6.0; rapid deactivation of the surface due to adsorption of oxidation and reduction products | [9] |
| Oral suspension | 0.46–7.35/0–4.05; -/- | ||||
| Injections | 0.97–1.88/−1.00–2.10; -/- | ||||
| Bactrim® tablets | DPV; Diamond electrode dopped with boron | (0.39–3.2)×10−5/(0.69–5.5)×10−6; 0.651×10−7/0.630×10−7 | −9.4/–4.5; -/- | A pH correction to 7.0 | [10] |
| Bactrim F® tablets | −2.7/–3.5; -/- | ||||
| Aquaculture waters | Potentiometry; PVC-membrane with MIP | 5×10−5–1×10−3/1×10−6–5×10−5; 1.7×10−5/3.0×10−7 | 0.4–5.4/0.3–3.1; -/- | A pH correction to 5.0; the sensor worked in flow injection mode | [17] |
| Membrane | Dopant Concentration, wt% | σ, mS/cm | P·108, cm2/s |
|---|---|---|---|
| MF-4SC | - | 3.8 | 7.94 |
| MF-4SC-US | - | 5.7 | 15.6 |
| MF-4SC/CNTs-COO− | 0.5 | 4.3 | 13.9 |
| 1.0 | 4.7 | 16.8 | |
| 1.5 | 4.5 | 15.3 | |
| MF-4SC/CNTs-SO3− | 0.5 | 4.6 | 9.83 |
| 1.0 | 5.2 | 12.6 | |
| 1.5 | 6.8 | 18.0 | |
| MF-4SC/CNTs-NH3+ | 0.5 | 5.0 | 48.9 |
| 1.0 | 5.1 | 56.5 | |
| 1.5 | 4.7 | 28.5 |
| Analyte | LOD, M | Relative Error, % | RSD,% (n = 4, p = 0.95) |
|---|---|---|---|
| SMX− | 3.5 × 10−7 | 1.4–15 | 4–18 |
| TMP+/TMP | 1.3 × 10−7 | 4–12 | 3–18 |
| Method | The DP-Sensor Array | Spectrophotometry | |
|---|---|---|---|
| c, M (pharmaceutical solution) | SMX− | (0.38 ± 0.02) × 10−4 | (0.387 ± 0.007) × 10−4 |
| TMP+/TMP | (0.66 ± 0.05) × 10−5 | (0.671 ± 0.012) × 10−5 | |
| c, mg (preparation) | SMX | 96 ± 6 | 98.1 ± 1.7 |
| TMP | 19.0 ± 1.3 | 19.5 ± 0.3 | |
| RSD, % (n = 6, p = 0.95) | SMX | 6 | 2 |
| TMP | 7 | 2 | |
| Relative error *, % | SMX | 4 | 1.9 |
| TMP | 5 | 3 | |
| Membrane | b0, mV | b1, mV/pSMX | b2, mV/pTMP | b3, mV/pH | t-Test, f = 11, p = 0.95 | F-Test, f1 = 4, f2 = 7, p = 0.95 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| t | F | t | F | t | F | t | F | |||
| MF-4SC/1.0 wt% CNTs-SO3− | 1.03 | 0.82 | 0.91 | 0.84 | 0.91 | 0.74 | 0.96 | 0.87 | 2.20 | 4.12 |
| MF-4SC/1.0 wt% CNTs-NH3+ | 1.95 | 1.04 | 0.55 | 1.01 | 0.95 | 0.97 | 1.40 | 1.09 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parshina, A.; Yelnikova, A.; Safronova, E.; Kolganova, T.; Kuleshova, V.; Bobreshova, O.; Yaroslavtsev, A. Multisensory Systems Based on Perfluorosulfonic Acid Membranes Modified with Functionalized CNTs for Determination of Sulfamethoxazole and Trimethoprim in Pharmaceuticals. Membranes 2022, 12, 1091. https://doi.org/10.3390/membranes12111091
Parshina A, Yelnikova A, Safronova E, Kolganova T, Kuleshova V, Bobreshova O, Yaroslavtsev A. Multisensory Systems Based on Perfluorosulfonic Acid Membranes Modified with Functionalized CNTs for Determination of Sulfamethoxazole and Trimethoprim in Pharmaceuticals. Membranes. 2022; 12(11):1091. https://doi.org/10.3390/membranes12111091
Chicago/Turabian StyleParshina, Anna, Anastasia Yelnikova, Ekaterina Safronova, Tatyana Kolganova, Victoria Kuleshova, Olga Bobreshova, and Andrey Yaroslavtsev. 2022. "Multisensory Systems Based on Perfluorosulfonic Acid Membranes Modified with Functionalized CNTs for Determination of Sulfamethoxazole and Trimethoprim in Pharmaceuticals" Membranes 12, no. 11: 1091. https://doi.org/10.3390/membranes12111091
APA StyleParshina, A., Yelnikova, A., Safronova, E., Kolganova, T., Kuleshova, V., Bobreshova, O., & Yaroslavtsev, A. (2022). Multisensory Systems Based on Perfluorosulfonic Acid Membranes Modified with Functionalized CNTs for Determination of Sulfamethoxazole and Trimethoprim in Pharmaceuticals. Membranes, 12(11), 1091. https://doi.org/10.3390/membranes12111091







