Current-Voltage Characteristics of Membranes with Different Cation-Exchanger Content in Mineral Salt—Neutral Amino Acid Solutions under Electrodialysis
Abstract
1. Introduction
2. Experimental
2.1. Membranes
2.2. Electromembrane System
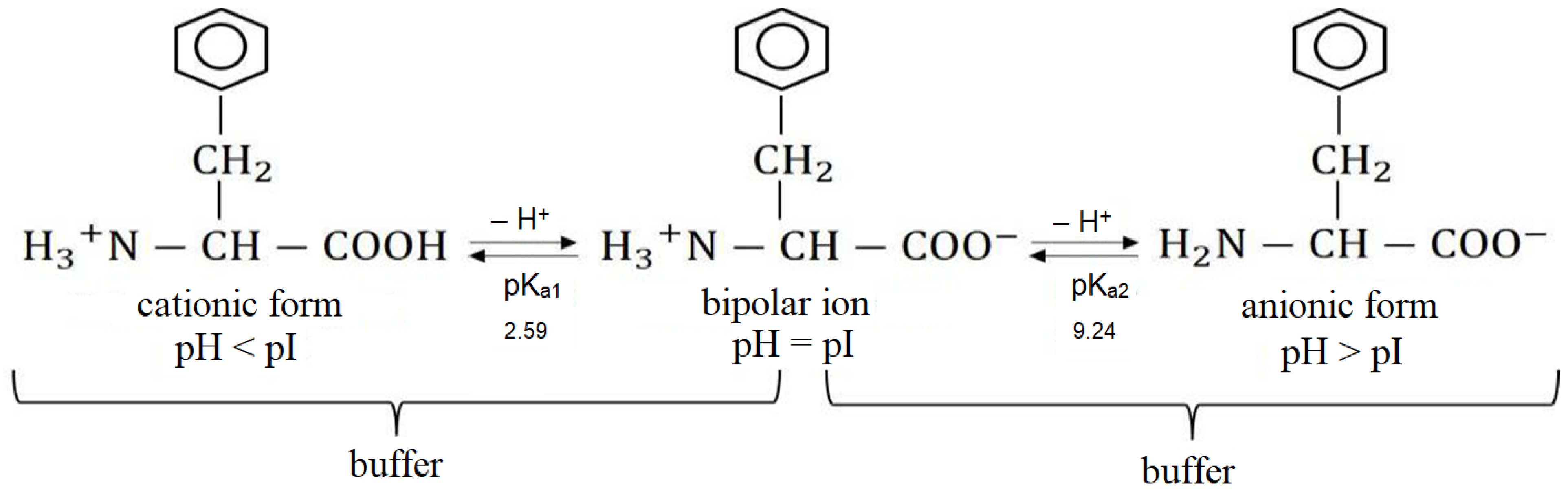
2.3. Chemical Equilibria in the System
2.4. Physical-Chemical Properties
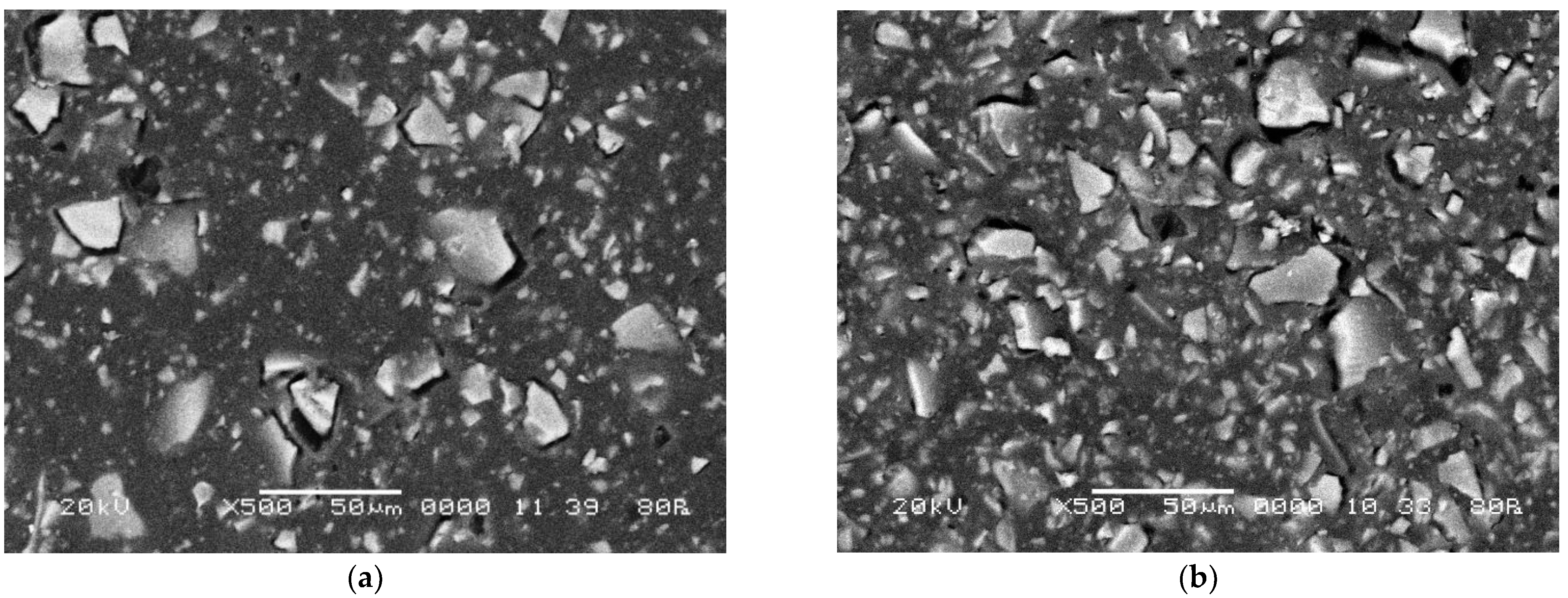
2.5. Scanning Electron Microscopy
3. Results and Discussion
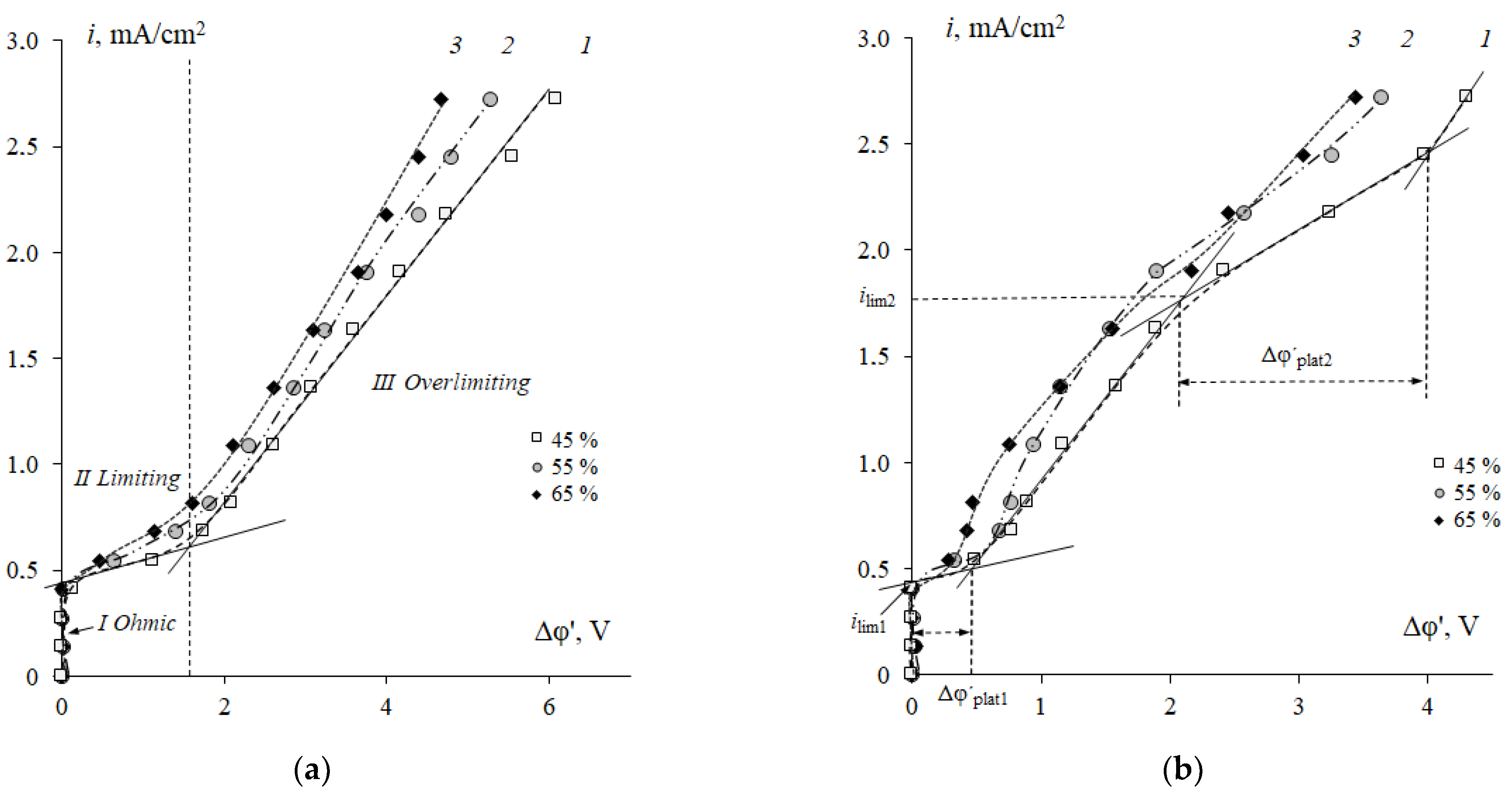
3.1. Influence of Phenylalanine on CVCs of Sulfonated Cation-Exchange Membranes
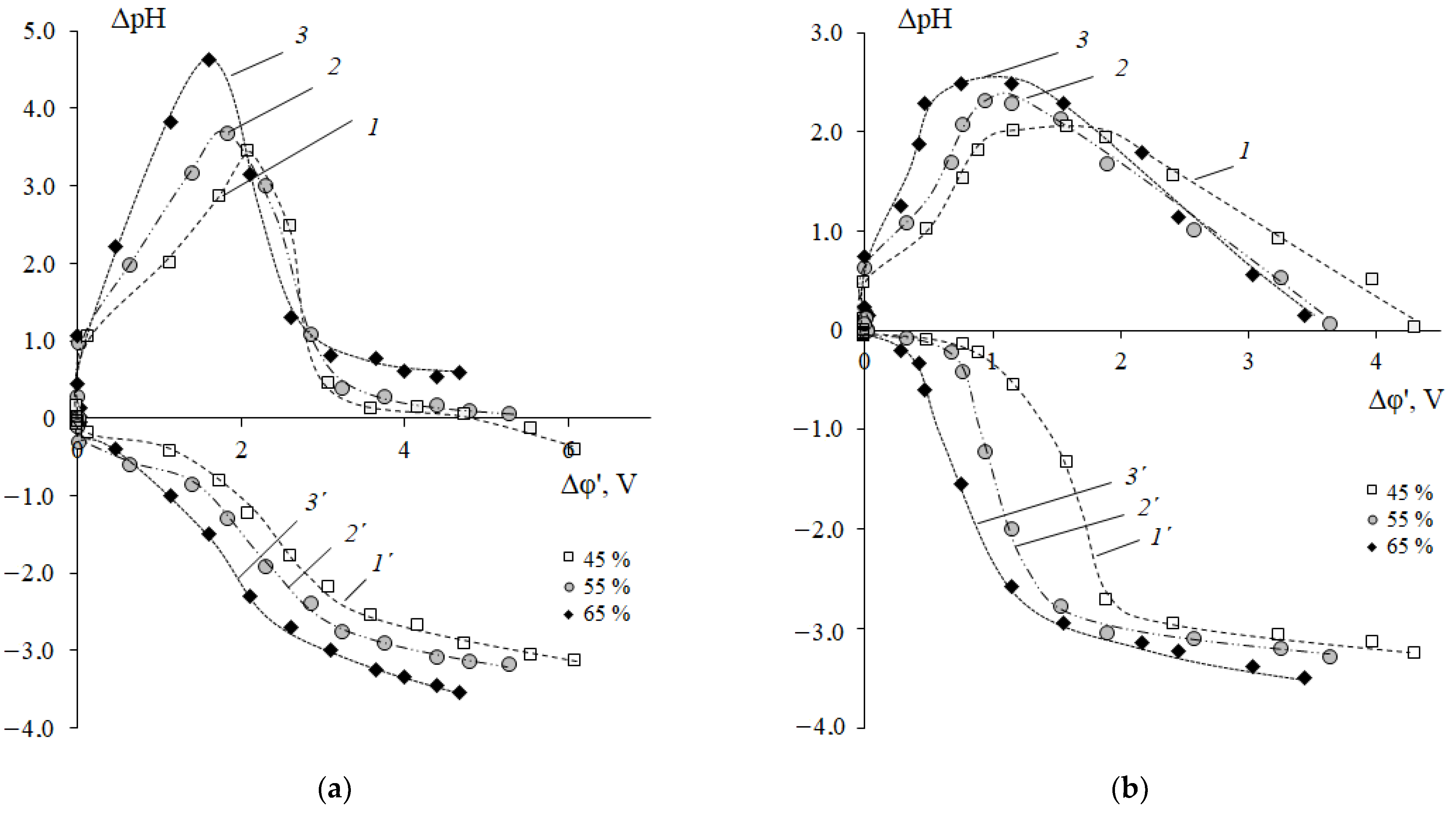
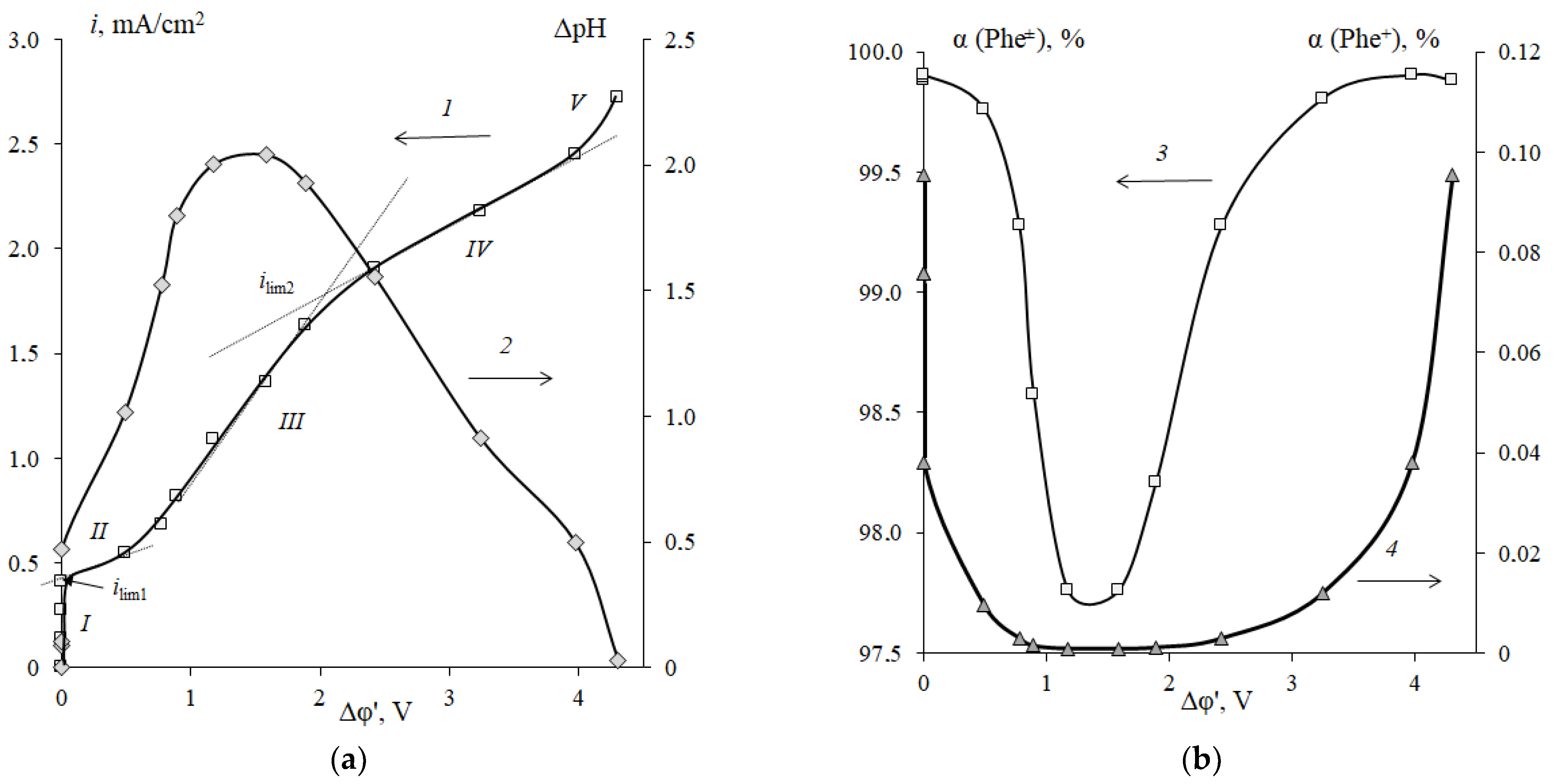
3.2. Features of the H+/OH− Ion Generation in the Electromembrane System with Cation-Exchange Membranes and the Neutral Amino Acid
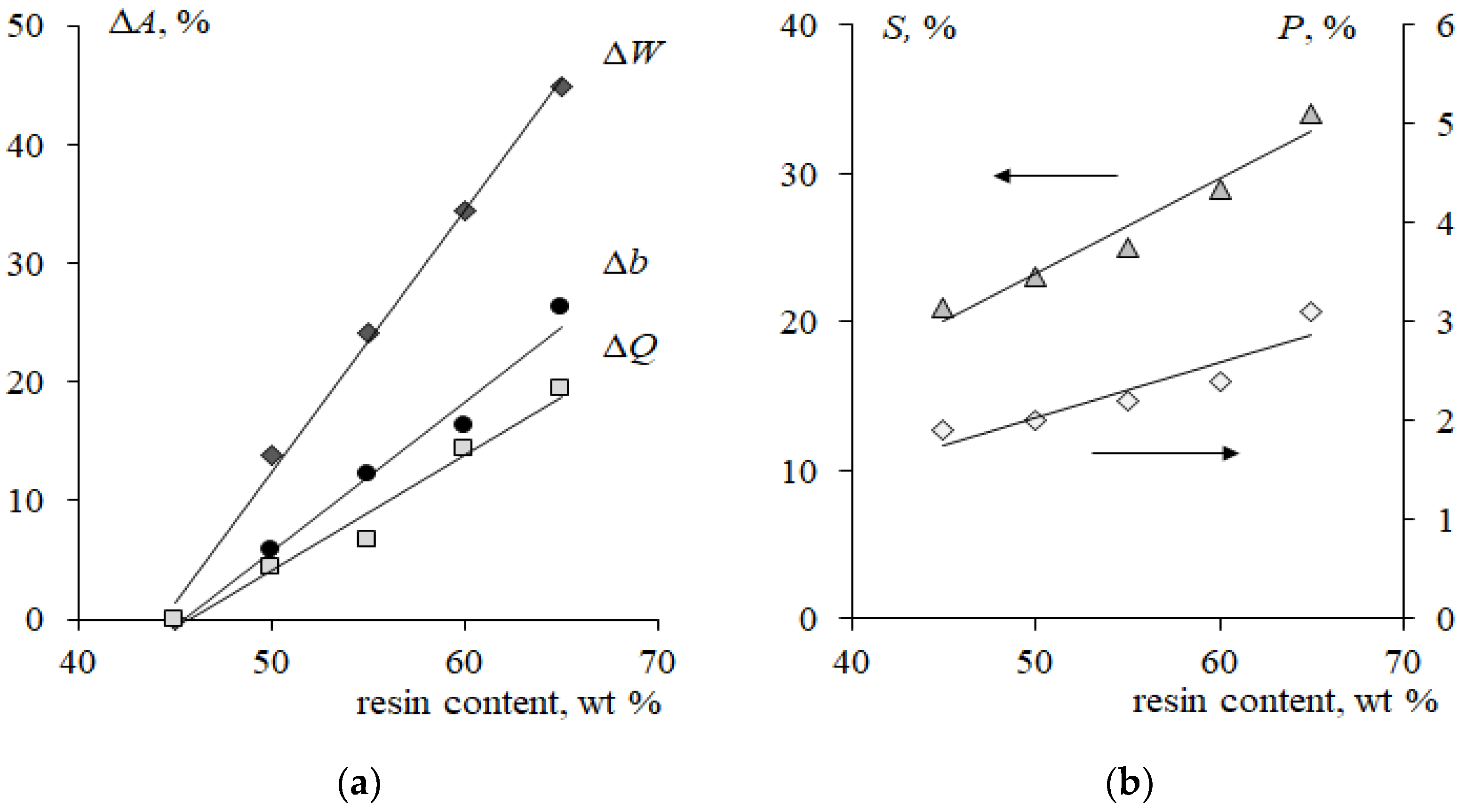
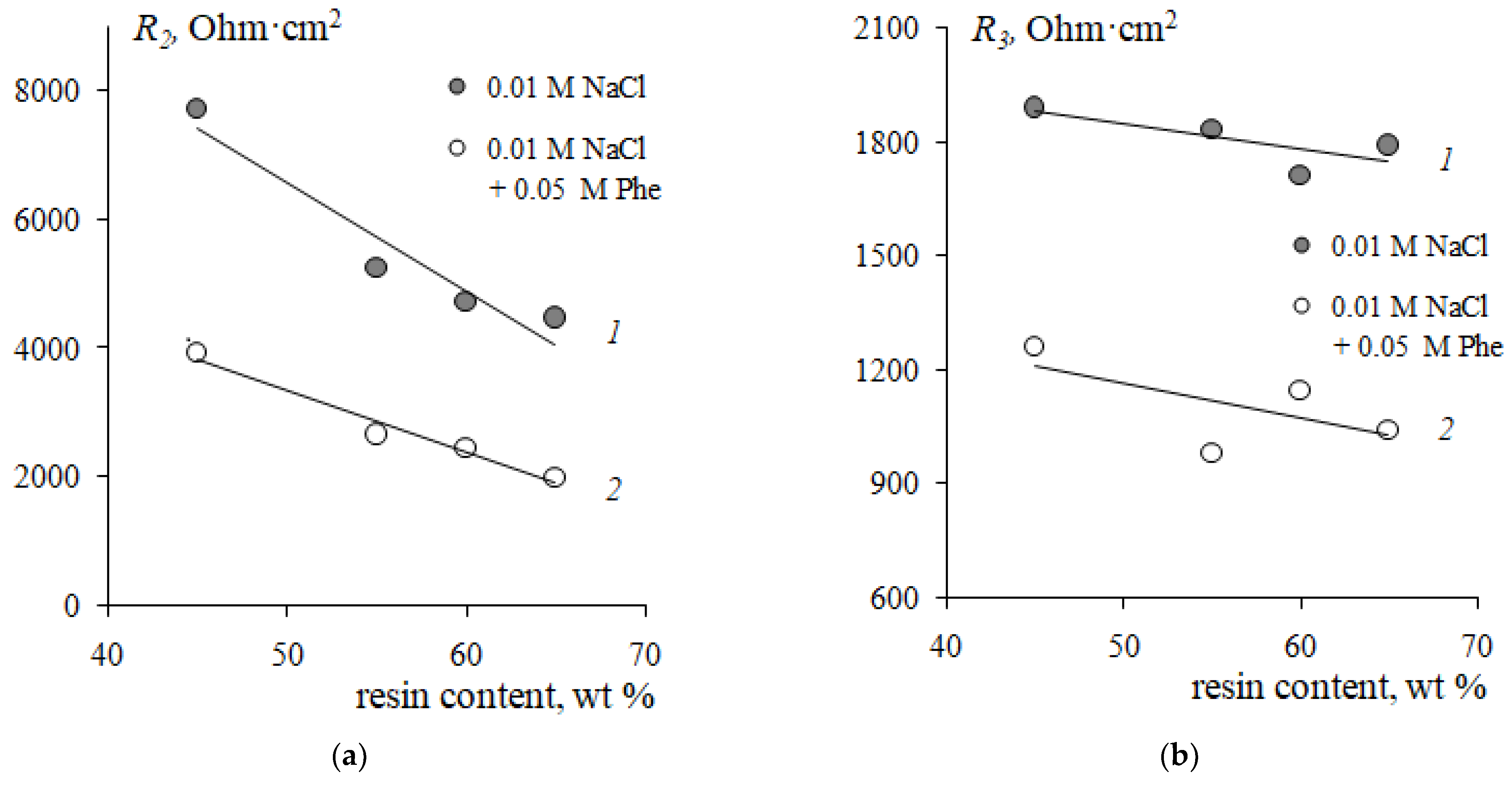
3.3. Influence of Resin Content on CVCs of Cation-Exchange Membranes in Strong Electrolyte and Phenylalanine-Containing Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
References
- Shaposhnik, V.A.; Eliseeva, T.V. Barrier effect during the electrodialysis of ampholytes. J. Membr. Sci. 1999, 161, 223–228. [Google Scholar] [CrossRef]
- Liu, L.-F.; Yang, L.-L.; Jin, K.-Y.; Xu, D.-Q.; Gao, C.-J. Recovery of L-tryptophan from crystallization wastewater by combined membrane process. Sep. Purif. Technol. 2009, 66, 443–449. [Google Scholar] [CrossRef]
- Bukhovets, A.; Eliseeva, T.; Daltrophe, N.; Oren, Y. The influence of current density on the electrochemical properties of anion exchange membranes in electrodialysis of phenylalanine solution. Electrochim. Acta 2011, 56, 10283–10287. [Google Scholar] [CrossRef]
- Tsukahara, S.; Nanzai, B.; Igawa, M. Selective transport of amino acids across a double membrane system composed of a cation- and an anion-exchange membrane. J. Membr. Sci. 2013, 448, 300–307. [Google Scholar] [CrossRef]
- Melnikova, E.D.; Pismenskaya, N.D.; Bazinet, L.; Mikhaylin, S.; Nikonenko, V.V. Effect of ampholyte nature on current-voltage characteristic of anion-exchange membrane. Electrochim. Acta 2018, 285, 185–191. [Google Scholar]
- Sato, K. Effects of the stripping solution concentrations on the separation degree in Donnan dialysis for binary systems of amino acids. J. Membr. Sci. 2008, 309, 175–181. [Google Scholar] [CrossRef]
- Ueno, K.; Doi, T.; Nanzai, B.; Igawa, M. Selective transport of neutral amino acids across a double-membrane system comprising cation and anion exchange membranes. J. Membr. Sci. 2017, 537, 344–352. [Google Scholar] [CrossRef]
- Kozmai, A.; Goleva, E.; Vasil’eva, V.; Nikonenko, V.; Pismenskaya, N. Neutralization dialysis for phenylalanine and mineral salt separation. Simple theory and experiment. Membranes 2019, 9, 171. [Google Scholar] [CrossRef]
- Vasil’eva, V.; Goleva, E.; Pismenskaya, N.; Kozmai, A.; Nikonenko, V. Effect of surface profiling of a cation-exchange membrane on the phenylalanine and NaCl separation performances in diffusion dialysis. Sep. Purif. Technol. 2019, 210, 48–59. [Google Scholar] [CrossRef]
- Wang, G.; Tanabe, H.; Igawa, M. Transport of glycine by neutralization dialysis. J. Membr. Sci. 1995, 106, 207–211. [Google Scholar] [CrossRef]
- Aristov, I.V.; Bobreshova, O.V.; Kulintsov, P.I.; Zagorodnykh, L.A. Transfer of amino acids through a membrane/solution interface in the presence of heterogeneous chemical protonation reaction. Russ. J. Electrochem. 2001, 37, 218–221. [Google Scholar] [CrossRef]
- Zagorodnykh, L.A.; Bobreshova, O.V.; Kulintsov, P.I.; Aristov, I.V. Kinetics of the electrical mass transfer of sodium and glycine cations with allowance for the protonation of zwitterions in conditions of limiting concentration polarization of electromembrane systems with cation-exchange membranes. Russ. J. Electrochem. 2005, 41, 275–279. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Igritskaya, K.; Belova, E.; Nikonenko, V.; Pourcelly, G. Transport properties of ion-exchange membrane systems in LysHCl solutions. Desalination 2006, 200, 149–151. [Google Scholar] [CrossRef]
- Eliseeva, T.V.; Kharina, A.Y. Voltammetric and transport characteristics of anion-exchange membranes during electrodialysis of solutions containing alkylaromatic amino acid and a mineral salt. Russ. J. Electrochem. 2015, 51, 63–69. [Google Scholar] [CrossRef]
- Kattan Readi, O.M.; Kuenen, H.J.; Zwijnenberg, H.J.; Nijmeijer, K. Novel membrane concept for internal pH control in electrodialysis of amino acids using a segmented bipolar membrane (sBPM). J. Membr. Sci. 2013, 443, 219–226. [Google Scholar] [CrossRef]
- Kattan Readi, O.M.; Mengers, H.J.; Wiratha, W.; Wessling, M.; Nijmeijer, K. On the isolation of single acidic amino acids for biorefinery applications using electrodialysis. J. Membr. Sci. 2011, 384, 166–175. [Google Scholar] [CrossRef]
- Zhil’tsova, A.V. Diffusion Boundary Layers and Electroconvective Instability at the Ion-Exchange Membrane—Solution Interface under Intense Current Regimes. Ph.D. Thesis, Voronezh State University, Voronezh, Russia, 31 October 2013. (In Russian). [Google Scholar]
- Eliseeva, T.V.; Kharina, A.Y. Cation-exchange membrane MK-40 characteristics in electrodialysis of mixed solutions of mineral salt and amino acid. Sorpt. Chromatogr. Process. 2017, 17, 148–155. [Google Scholar]
- Pismenskaya, N.; Nikonenko, B.V.; Auclair, G. Pourcelly, Transport of weak-electrolyte anions through anion exchange membranes: Current-voltage characteristics. J. Membr. Sci. 2001, 189, 129–140. [Google Scholar] [CrossRef]
- Belashova, E.D.; Pismenskaya, N.D.; Nikonenko, V.V.; Sistat, P.; Pourcelly, G. Current-voltage characteristic of anion-exchange membrane in monosodium phosphate solution. Modelling and experiment. J. Membr. Sci. 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B.; Pundik, T. Ion-exchange funneling in thin-film coating modification of heterogeneous electrodialysis membranes. Phys. Rev. E 2002, 65, 041507. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, S.H.; Moon, S.-H. Heterogeneity of ion-exchange membranes: The effects of membrane heterogeneity on transport properties. J. Colloid Interface Sci. 2001, 241, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Nikonenko, V.V.; Pismenskaya, N.D.; Belova, E.I.; Sistat, P.; Huguet, P.; Pourcelly, G.; Larchet, C. Intensive current transfer in membrane systems: Modelling, mechanisms and application in electrodialysis. Adv. Colloid Interface Sci. 2010, 160, 101–123. [Google Scholar] [CrossRef]
- Martí-Calatayud, M.C.; Buzzi, D.C.; García-Gabaldón, M.; Bernardes, A.M.; Tenório, J.A.S.; Pérez-Herranz, V. Ion transport through homogeneous and heterogeneous ion-exchange membranes in single salt and multicomponent electrolyte solutions. J. Membr. Sci. 2014, 466, 45–57. [Google Scholar] [CrossRef]
- Davidson, S.M.; Wessling, M.; Mani, A. On the dynamical regimes of pattern-accelerated electroconvection. Sci. Rep. 2016, 6, 22505. [Google Scholar] [CrossRef] [PubMed]
- Zabolotsky, V.I.; Novak, L.; Kovalenko, A.V.; Nikonenko, V.V.; Urtenov, M.H.; Lebedev, K.A.; But, A.Y. Electroconvection in systems with heterogeneous ion-exchange membranes. Pet. Chem. 2017, 57, 779–789. [Google Scholar] [CrossRef]
- Nebavskaya, K.A.; Butylskii, D.Y.; Moroz, I.A.; Nebavsky, A.V.; Pismenskaya, N.D.; Nikonenko, V.V. Enhancement of mass transfer through a homogeneous anion-exchange membrane in limiting and overlimiting current regimes by screening part of its surface with nonconductive strips. Pet. Chem. 2018, 58, 780–789. [Google Scholar] [CrossRef]
- Roghmans, F.; Evdochenko, E.; Stockmeier, F.; Schneider, S.; Smailji, A.; Wessling, M. 2D patterned ion-exchange membranes induce electroconvection. Adv. Mater. Interfaces 2019, 6, 1801309. [Google Scholar] [CrossRef]
- Resbeut, S.; Pourcelly, G.; Sandeaux, R.; Gavach, C. Electromembrane processes for waste stream treatment: Electrodialysis applied to the demineralization of phenylalanine solutions. Desalination 1998, 120, 235–245. [Google Scholar] [CrossRef]
- Grib, H.; Belhocine, D.; Lounici, H.; Pauss, A.; Mameri, N. Desalting of phenylalanine solutions by electrodialysis with ion-exchange membranes. J. Appl. Electrochem. 2000, 30, 259–262. [Google Scholar] [CrossRef]
- Montiel, V.; García-García, V.; González-García, J.; Carmona, F.; Aldaz, A. Recovery by means of electrodialysis of an aromatic amino acid from a solution with a high concentration of sulphates and phosphates. J. Membr. Sci. 1998, 140, 243–250. [Google Scholar] [CrossRef][Green Version]
- Nakamura, T.; Syukunobe, Y.; Tomizawa, A.; Shigematsu, A.; Koutake, M. Demineralization of cow’s milk casein hydrolysates by electrodialysis. Nippon. Shokuhin Kogyo Gakkaishi 1993, 40, 545–551. [Google Scholar] [CrossRef][Green Version]
- Lin, X.; Pan, J.; Zhou, M.; Xu, Y.; Lin, J.; Shen, J.; Gao, C.; Van der Bruggen, B. Extraction of amphoteric amino acid by bipolar membrane electrodialysis: Methionine acid as a case study. Ind. Eng. Chem. Res. 2016, 55, 2813–2820. [Google Scholar] [CrossRef]
- Eliseeva, T.; Kharina, A. Desalination of neutral amino acid solutions in an electromembrane system. Membranes 2022, 12, 665. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, A.T.; Lightfoot, E.N. Ion fractionation by permselective membranes. Factors affecting relative transfer of glycine and chloride ions. Ind. Eng. Chem. 1958, 50, 691–696. [Google Scholar] [CrossRef]
- Minagawa, M.; Tanioka, A.; Ramirez, P.; Mafe, S. Amino acid transport through cation exchange membranes: Effects of pH on interfacial transport. J. Colloid Interface Sci. 1997, 188, 176–182. [Google Scholar] [CrossRef]
- Choi, J.-H.; Oh, S.-J.; Moon, S.-H. Structural effects of ion-exchange membrane on the separation of L-phenylalanine (L-Phe) from fermentation broth using electrodialysis. J. Chem. Technol. Biotechnol. 2002, 77, 785–792. [Google Scholar] [CrossRef]
- Aghajanyan, A.E.; Hambardzumyan, A.A.; Vardanyan, A.A.; Saghiyan, A.S. Desalting of neutral amino acids fermentative solutions by electrodialysis with ion-exchange membranes. Desalination 2008, 228, 237–244. [Google Scholar] [CrossRef]
- Monopolar Membranes Ltd. Innovative Enterprise “Shchekinoazot”. 2022. Available online: http://www.azotom.com/monopolyarnye-membrany/ (accessed on 26 September 2022).
- Maletzki, F.; Rosler, H.-W.; Staude, E.J. Ion transport across electrodialysis membranes in the overlimiting current range: Stationary voltage current noise power spectra under different conditions of free convection. J. Membr. Sci. 1992, 71, 105–115. [Google Scholar] [CrossRef]
- Pis’menskaya, N.D.; Nikonenko, V.V.; Belova, E.I.; Lopatkova, G.Y.; Sistat, P.; Pourcelly, G.; Larshe, K. Coupled convection of solution near the surface of ion-exchange membranes in intensive current regimes. Russ. J. Electrochem. 2007, 43, 307–327. [Google Scholar] [CrossRef]
- Akberova, E.M.; Vasil’eva, V.I.; Zabolotsky, V.I.; Novak, L. Effect of the sulfocation-exchanger dispersity on the surface morphology, microrelief of heterogeneous membranes and development of electroconvection in intense current modes. J. Membr. Sci. 2018, 566, 317–328. [Google Scholar] [CrossRef]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of ion-exchange membrane materials: Properties vs. structure. Adv. Colloid Interface Sci. 2008, 139, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Akberova, E.M.; Vasil’eva, V.I.; Zabolotsky, V.I.; Novak, L. A study of ralex membrane morphology by SEM. Membranes 2019, 9, 169. [Google Scholar] [CrossRef]
- Krol, J.J.; Wessling, M.; Strathmann, H. Concentration polarization with monopolarion exchange membranes: Current-voltage curves and water dissociation. J. Membr. Sci. 1999, 162, 145–154. [Google Scholar] [CrossRef]
- Nikonenko, V.; Kovalenko, A.; Urtenov, M.; Pismenskaya, N.; Han, J.; Sistat, P.; Pourcelly, G. Desalination at overlimiting currents: State-of-the-art and perspectives. Desalination 2014, 342, 85–106. [Google Scholar] [CrossRef]
- Bazinet, L.; Georoy, T.R. Electrodialytic processes: Market overview, membrane phenomena, recent developments and sustainable strategies. Membranes 2020, 10, 221. [Google Scholar] [CrossRef]
- Shaposhnik, V.A.; Vasil’eva, V.I.; Reshetnikova, E.V. Concentration polarization of ion-exchange membranes in electrodialysis: An interferometric study. Russ. J. Electrochem. 2000, 36, 773–777. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Mareev, S.A.; Pis’menskaya, N.D.; Uzdenova, A.M.; Kovalenko, A.V.; Urtenov, M.K.; Pourcelly, G. Effect of electroconvection and its use in intensifying the mass transfer in electrodialysis (Review). Russ. J. Electrochem. 2017, 53, 1122–1144. [Google Scholar] [CrossRef]
- Vasil’eva, V.I.; Akberova, E.M.; Zabolotskii, V.I. Electroconvection in systems with heterogeneous ion-exchange membranes after thermal modification. Russ. J. Electrochem. 2017, 53, 398–410. [Google Scholar] [CrossRef]
- Vasil’eva, V.; Shaposhnik, V.; Zhiltsova, A.; Grigorchuk, O.; Zabolotsky, V. The oscillation of concentration field at the membrane-solution interface and transport mechanisms under overlimiting current density. Desalin. Water Treat. 2010, 14, 214–219. [Google Scholar] [CrossRef]
- Simons, R. Strong electric field effects on proton transfer between membrane-bound amines and water. Nature 1979, 280, 824–826. [Google Scholar] [CrossRef]
- Simons, R. Electric field effects on proton transfer between ionizable groups and water in ion exchange membranes. Electrochim. Acta 1984, 29, 151–158. [Google Scholar] [CrossRef]
- Mishchuk, N.; Dukhin, S.S. Electro-osmotic mechanism of the emergence of the overlimiting current. Khimiya Tekhnologiya Vody 1991, 13, 963–971. [Google Scholar]
- Vasil’eva, V.I.; Goleva, E.A.; Smagin, M.A. Effect of phenylalanine on the physicochemical, structural, and transport characteristics of a profiled MK-40 sulfoacid cation exchange membrane. Russ. J. Phys. Chem. 2019, 93, 1365–1374. [Google Scholar] [CrossRef]
- Belashova, E.D.; Melnik, N.A.; Pismenskaya, N.D.; Shevtsova, K.A.; Nebavsky, A.V.; Lebedev, K.A.; Nikonenko, V.V. Overlimiting mass transfer through cation-exchange membranes modified by Nafion film and carbon nanotubes. Electrochim. Acta 2012, 59, 412–423. [Google Scholar] [CrossRef]
- Zhiltsova, A.V.; Vasil’eva, V.I.; Malykhin, M.D.; Pismenskaya, N.D.; Melnik, N.A. Influence of hydrophobicity of the surface of sulfonated cation exchange membranes on the development of electroconvective instability in stratified systems. Vestnik. VGU Seriya. Khimiya Biol. Farmatsiya 2013, 2, 35–38. [Google Scholar]
- Nebavskaya, K.A.; Sarapulova, V.V.; Sabbatovskiy, K.G.; Sobolev, V.D.; Pismenskaya, N.D.; Sistat, P.; Cretin, M.; Nikonenko, V.V. Impact of ion exchange membrane surface charge and hydrophobicity on electroconvection at underlimiting and overlimiting currents. J. Membr. Sci. 2017, 523, 36–44. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Shel’deshov, N.V.; Gnusin, N.P. Dissociation of water molecules in systems with ion-exchange membranes. Uspekhi Khimii 1988, 57, 1403–1414. [Google Scholar] [CrossRef]
- Zabolotskiy, V.I.; But, A.Y.; Vasil’eva, V.I.; Akberova, E.M.; Melnikov, S.S. Ion transport and electrochemical stability of strongly basic anion-exchange membranes under high current electrodialysis conditions. J. Membr. Sci. 2017, 526, 60–72. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Bugakov, V.V.; Sharafan, M.V.; Chermit, R.K. Transfer of electrolyte ions and water dissociation in anion-exchange membranes under intense current conditions. Russ. J. Electrochem. 2012, 48, 650–659. [Google Scholar] [CrossRef]
- Helfferich, F.G. Ion Exchange; McGraw-Hill: New York, NY, USA, 1962. [Google Scholar]
- Rybalkina, O.A.; Sharafan, M.V.; Nikonenko, V.V.; Pismenskaya, N.D. Two mechanisms of H+/OH− ion generation in anion-exchange membrane systems with polybasic acid salt solutions. J. Membr. Sci. 2022, 651, 120449. [Google Scholar] [CrossRef]
- Volodina, E.; Pismenskaya, N.D.; Nikonenko, V.V.; Larchet, C.; Pourcelly, G. Ion transfer across ion-exchange membranes with homogeneous and heterogeneous surfaces. J. Colloid Interface Sci. 2005, 285, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Vasil’eva, V.I.; Akberova, E.M.; Zabolotsky, V.I.; Novak, L.; Kostylev, D.V. Effect of dispersity of a sulfonated cation-exchanger on the current–voltage characteristics of heterogeneous membranes Ralex CM Pes. Pet. Chem. 2018, 58, 1133–1143. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B. Electro-osmotically induced convection at a permselective membrane. Phys. Rev. E 2002, 62, 2238–2251. [Google Scholar] [CrossRef]
- Ibanez, R.; Stamatialis, D.F.; Wessling, M. Role of membrane surface in concentration polarization at cation exchange membranes. J. Membr. Sci. 2004, 239, 119–128. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, H.-J.; Moon, S.-H. Effects of electrolytes on the transport phenomena in a cation-exchange membrane. J. Colloid Interface Sci. 2001, 238, 188–195. [Google Scholar] [CrossRef]
- Gil, V.V.; Andreeva, M.A.; Pismenskaya, N.D.; Nikonenko, V.V.; Larchet, C.; Dammak, L. Effect of counterion hydration numbers on the development of electroconvection at the surface of heterogeneous cation-exchange membrane modified with an MF-4SK film. Pet. Chem. 2016, 56, 440–449. [Google Scholar] [CrossRef]




| NaCl | NaCl + Phe | |||||||
|---|---|---|---|---|---|---|---|---|
| ilim1, mA/cm2 | Δφ´plat1, V | R2, Ohm∙cm2 | R3, Ohm∙cm2 | ilim1, mA/cm2 | ilim2, mA/cm2 | Δφ´plat1, V | R2, Ohm∙cm2 | R3, Ohm∙cm2 |
| 0.39 | 1.71 | 7730 | 1890 | 0.40 | 1.76 | 0.69 | 3930 | 1260 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasil’eva, V.I.; Akberova, E.M.; Saud, A.M.; Zabolotsky, V.I. Current-Voltage Characteristics of Membranes with Different Cation-Exchanger Content in Mineral Salt—Neutral Amino Acid Solutions under Electrodialysis. Membranes 2022, 12, 1092. https://doi.org/10.3390/membranes12111092
Vasil’eva VI, Akberova EM, Saud AM, Zabolotsky VI. Current-Voltage Characteristics of Membranes with Different Cation-Exchanger Content in Mineral Salt—Neutral Amino Acid Solutions under Electrodialysis. Membranes. 2022; 12(11):1092. https://doi.org/10.3390/membranes12111092
Chicago/Turabian StyleVasil’eva, Vera I., Elmara M. Akberova, Ali M. Saud, and Victor I. Zabolotsky. 2022. "Current-Voltage Characteristics of Membranes with Different Cation-Exchanger Content in Mineral Salt—Neutral Amino Acid Solutions under Electrodialysis" Membranes 12, no. 11: 1092. https://doi.org/10.3390/membranes12111092
APA StyleVasil’eva, V. I., Akberova, E. M., Saud, A. M., & Zabolotsky, V. I. (2022). Current-Voltage Characteristics of Membranes with Different Cation-Exchanger Content in Mineral Salt—Neutral Amino Acid Solutions under Electrodialysis. Membranes, 12(11), 1092. https://doi.org/10.3390/membranes12111092






