Correlating the Macrostructural Variations of an Ion Gel with Its Carbon Dioxide Sorption Capacity
Abstract
1. Introduction
2. Materials and Experiments
2.1. Materials
2.2. Sample Preparation
2.3. Characterizations
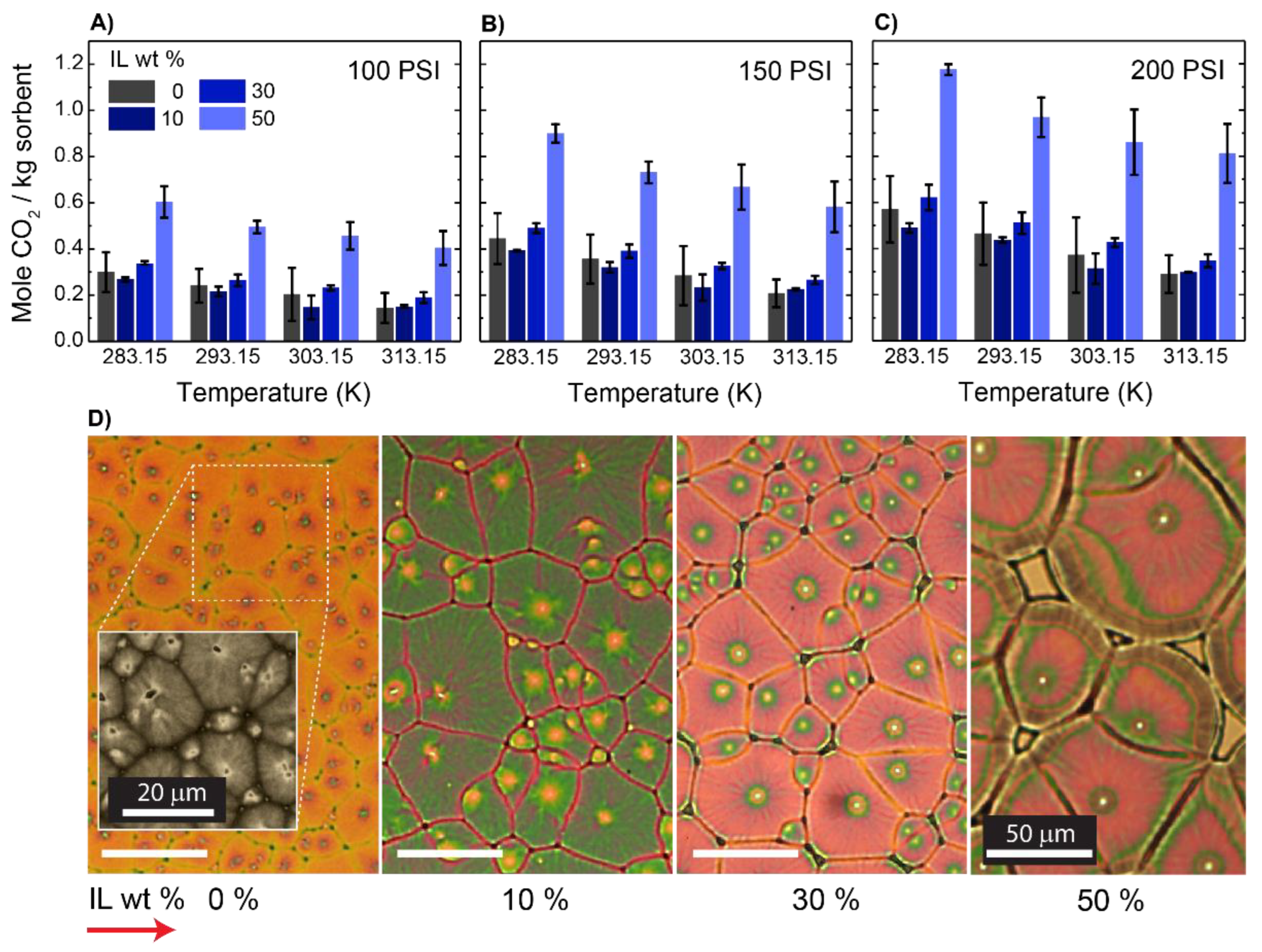
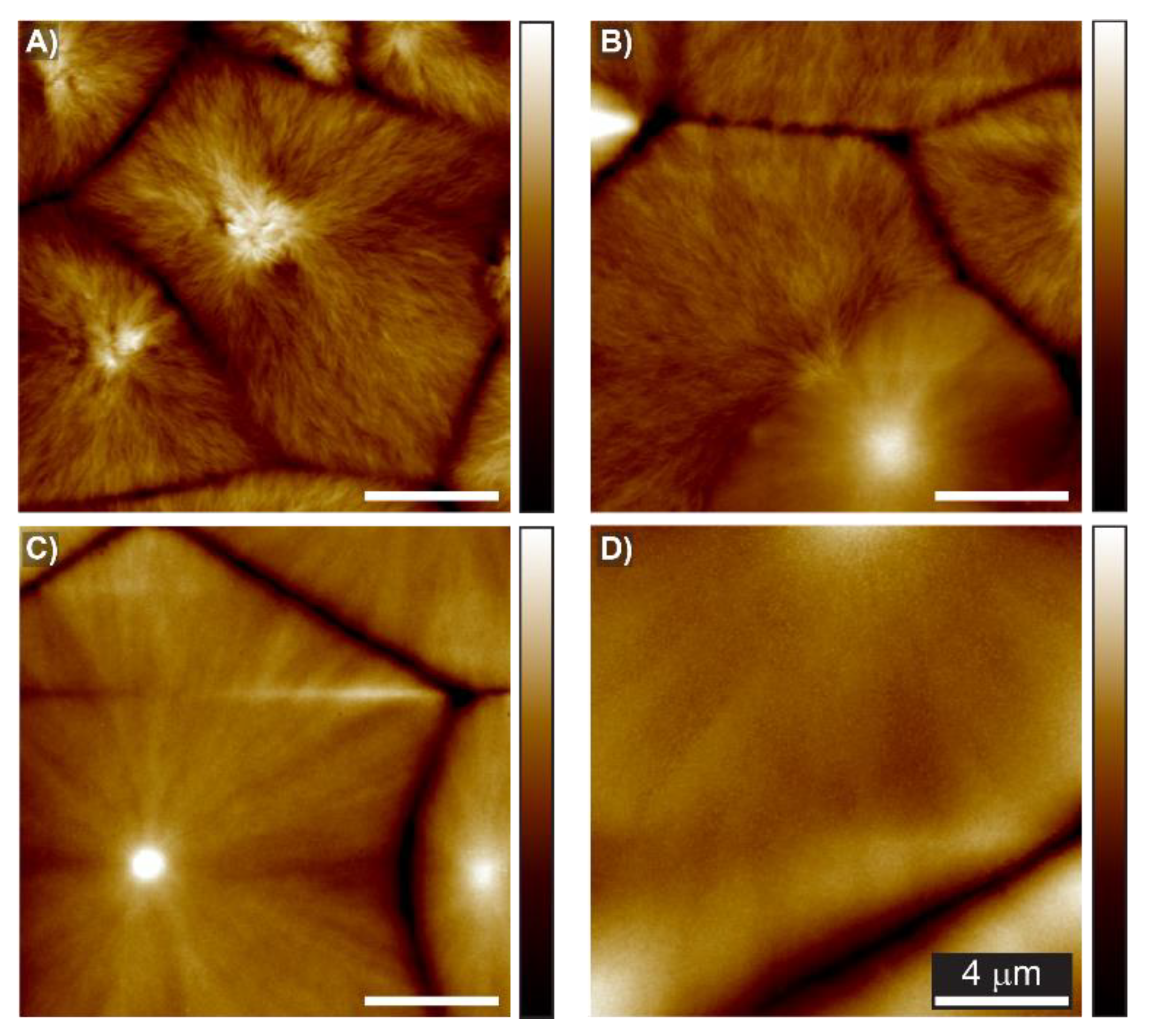
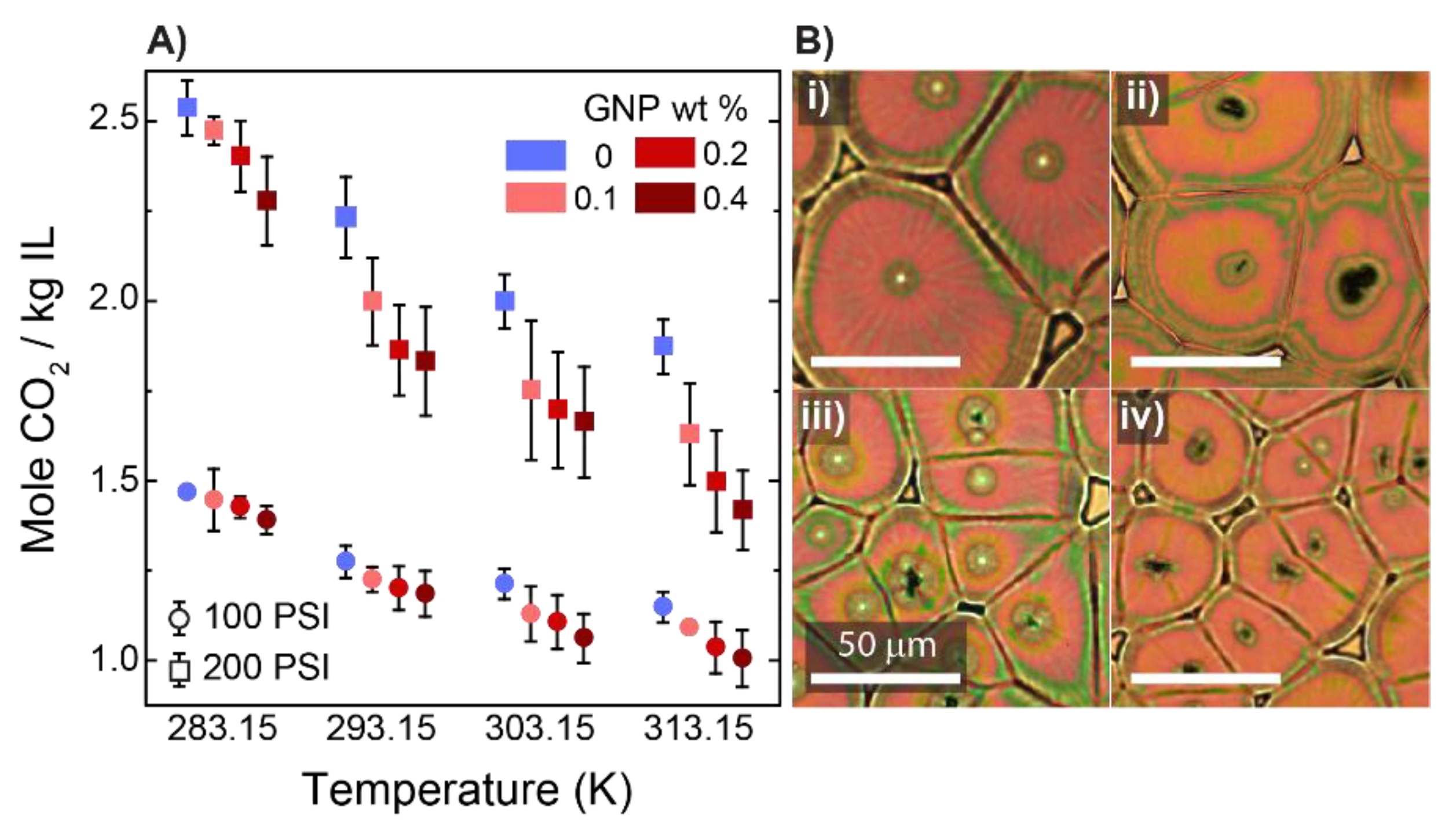
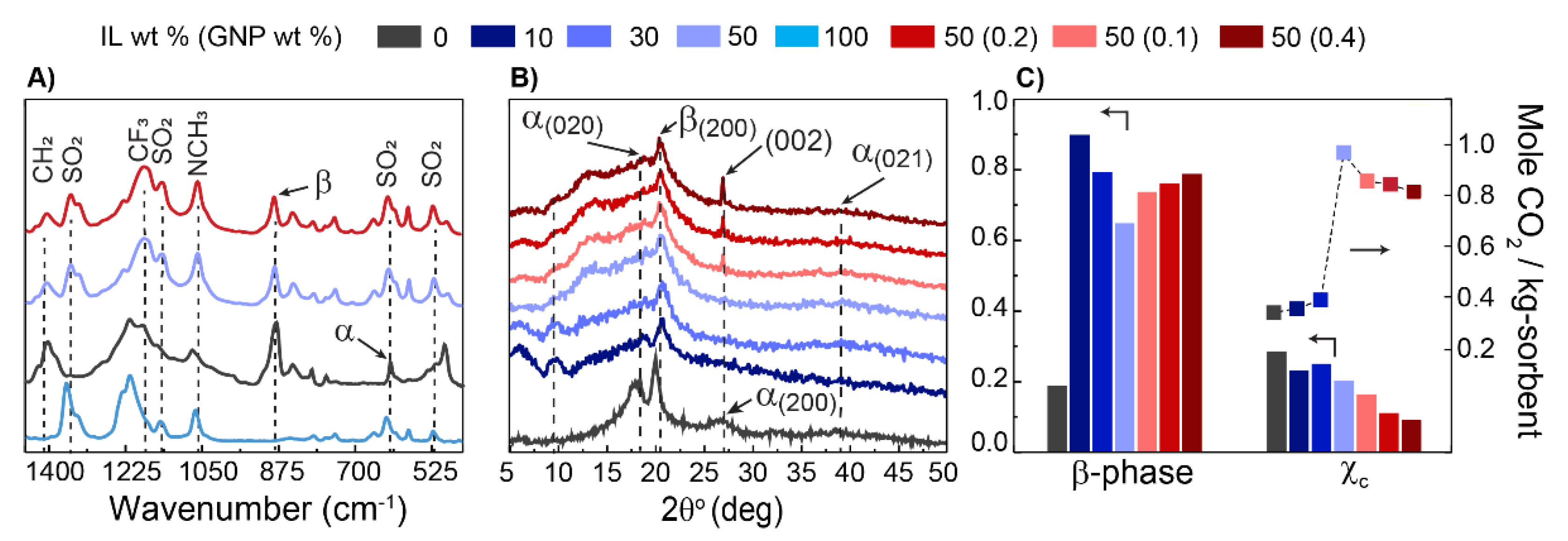
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ueki, T.; Watanabe, M. Macromolecules in Ionic Liquids: Progress, Challenges, and Opportunities. Macromolecules 2008, 41, 3739–3749. [Google Scholar] [CrossRef]
- Noro, A.; Tomita, Y.; Shinohara, Y.; Sageshima, Y.; Walish, J.J.; Matsushita, Y.; Thomas, E.L. Photonic Block Copolymer Films Swollen with an Ionic Liquid. Macromolecules 2014, 47, 4103–4109. [Google Scholar] [CrossRef]
- Aoki, K.; Sugawara-Narutaki, A.; Doi, Y.; Takahashi, R. Structure and Rheology of Poly(Vinylidene Difluoride-Co-Hexafluoropropylene) in an Ionic Liquid: The Solvent Behaves as a Weak Cross-Linker through Ion–Dipole Interaction. Macromolecules 2022, 55, 5591–5600. [Google Scholar] [CrossRef]
- Yang, J.; Pruvost, S.; Livi, S.; Duchet-Rumeau, J. Understanding of Versatile and Tunable Nanostructuration of Ionic Liquids on Fluorinated Copolymer. Macromolecules 2015, 48, 4581–4590. [Google Scholar] [CrossRef]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J.H. State-of-the-Art of CO2 Capture with Ionic Liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Pinto, A.M.; Rodríguez, H.; Arce, A.; Soto, A. Carbon Dioxide Absorption in the Ionic Liquid 1-Ethylpyridinium Ethylsulfate and in Its Mixtures with Another Ionic Liquid. Int. J. Greenh. Gas Control 2013, 18, 296–304. [Google Scholar] [CrossRef]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of Ionic Liquids with Membrane Technology: A New Approach for CO2 Separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Branco, L.C.; Crespo, J.G.; Afonso, C.A.M. Highly Selective Transport of Organic Compounds by Using Supported Liquid Membranes Based on Ionic Liquids. Angew. Chem. Int. Ed. 2002, 41, 2771–2773. [Google Scholar] [CrossRef]
- Scovazzo, P.; Visser, A.E.; Davis, J.H.; Rogers, R.D.; Koval, C.A.; DuBois, D.L.; Noble, R.D. Supported Ionic Liquid Membranes and Facilitated Ionic Liquid Membranes. In Ionic Liquids; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2002; Volume 818, pp. 69–87. [Google Scholar] [CrossRef]
- Susan, M.A.B.H.; Kaneko, T.; Noda, A.; Watanabe, M. Ion Gels Prepared by in Situ Radical Polymerization of Vinyl Monomers in an Ionic Liquid and Their Characterization as Polymer Electrolytes. J. Am. Chem. Soc. 2005, 127, 4976–4983. [Google Scholar] [CrossRef]
- Gómez, P.; Daviou, M.C.; Ibáñez, R.; Eliceche, A.M.; Ortiz, I. Comparative Behaviour of Hydrophilic Membranes in the Pervaporative Dehydration of Cyclohexane. J. Membr. Sci. 2006, 279, 635–644. [Google Scholar] [CrossRef]
- Wang, J.; Ding, H.; Cao, B.; Huang, X. Effect of Ionic Liquid Confinement on CO2 Solubility and Permeability Characteristics. Greenh. Gases Sci. Technol. 2017, 7, 474–485. [Google Scholar] [CrossRef]
- Bandegi, A.; Garcia, M.M.; Bañuelos, J.L.; Firestone, M.A.; Foudazi, R. Soft Nanoconfinement of Ionic Liquids in Lyotropic Liquid Crystals. Soft Matter 2021, 17, 8118–8129. [Google Scholar] [CrossRef] [PubMed]
- Pohorille, A.; Pratt, L.R. Cavities in Molecular Liquids and the Theory of Hydrophobic Solubilities. J. Am. Chem. Soc. 1990, 112, 5066–5074. [Google Scholar] [CrossRef] [PubMed]
- Vericella, J.J.; Baker, S.E.; Stolaroff, J.K.; Duoss, E.B.; Hardin, J.O.; Lewicki, J.; Glogowski, E.; Floyd, W.C.; Valdez, C.A.; Smith, W.L.; et al. Encapsulated Liquid Sorbents for Carbon Dioxide Capture. Nat. Commun. 2015, 6, 6124. [Google Scholar] [CrossRef] [PubMed]
- Banu, L.A.; Wang, D.; Baltus, R.E. Effect of Ionic Liquid Confinement on Gas Separation Characteristics. Energy Fuels 2013, 27, 4161–4166. [Google Scholar] [CrossRef]
- Schilderman, A.M.; Raeissi, S.; Peters, C.J. Solubility of Carbon Dioxide in the Ionic Liquid 1-Ethyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide. Fluid Phase Equilibria 2007, 260, 19–22. [Google Scholar] [CrossRef]
- Close, J.J.; Farmer, K.; Moganty, S.S.; Baltus, R.E. CO2/N2 Separations Using Nanoporous Alumina-Supported Ionic Liquid Membranes: Effect of the Support on Separation Performance. J. Membr. Sci. 2012, 390–391, 201–210. [Google Scholar] [CrossRef]
- Moya, C.; Alonso-Morales, N.; Gilarranz, M.A.; Rodriguez, J.J.; Palomar, J. Encapsulated Ionic Liquids for CO2 Capture: Using 1-Butyl-Methylimidazolium Acetate for Quick and Reversible CO2 Chemical Absorption. ChemPhysChem 2016, 17, 3891–3899. [Google Scholar] [CrossRef]
- Moya, C.; Alonso-Morales, N.; de Riva, J.; Morales-Collazo, O.; Brennecke, J.F.; Palomar, J. Encapsulation of Ionic Liquids with an Aprotic Heterocyclic Anion (AHA-IL) for CO2 Capture: Preserving the Favorable Thermodynamics and Enhancing the Kinetics of Absorption. J. Phys. Chem. B 2018, 122, 2616–2626. [Google Scholar] [CrossRef]
- Baltus, R.E.; Culbertson, B.H.; Dai, S.; Luo, H.; DePaoli, D.W. Low-Pressure Solubility of Carbon Dioxide in Room-Temperature Ionic Liquids Measured with a Quartz Crystal Microbalance. J. Phys. Chem. B 2004, 108, 721–727. [Google Scholar] [CrossRef]
- Anthony, J.L.; Maginn, E.J.; Brennecke, J.F. Solubilities and Thermodynamic Properties of Gases in the Ionic Liquid 1-n-Butyl-3-Methylimidazolium Hexafluorophosphate. J. Phys. Chem. B 2002, 106, 7315–7320. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Maginn, E.J. The Solubility of Gases in Ionic Liquids. AIChE J. 2017, 63, 4722–4737. [Google Scholar] [CrossRef]
- Cadena, C.; Anthony, J.L.; Shah, J.K.; Morrow, T.I.; Brennecke, J.F.; Maginn, E.J. Why Is CO2 So Soluble in Imidazolium-Based Ionic Liquids? J. Am. Chem. Soc. 2004, 126, 5300–5308. [Google Scholar] [CrossRef] [PubMed]
- Abbrent, S.; Plestil, J.; Hlavata, D.; Lindgren, J.; Tegenfeldt, J.; Wendsjö, Å. Crystallinity and Morphology of PVdF–HFP-Based Gel Electrolytes. Polymer 2001, 42, 1407–1416. [Google Scholar] [CrossRef]
- Gozdz, A.S.; Schmutz, C.N.; Tarascon, J.-M. Rechargeable Lithium Intercalation Battery with Hybrid Polymeric Electrolyte. U.S. Patent US5296318A, 22 March 1994. [Google Scholar]
- Nurkhamidah, S.; Woo, E.M. Mechanisms of Multiple Types of Lamellae and Spherulites in Poly(l-Lactic Acid) Interacting with Poly(4-Vinyl Phenol). Macromol. Chem. Phys. 2013, 214, 2345–2354. [Google Scholar] [CrossRef]
- Chuang, W.-T.; Hong, P.-D.; Chuah, H.H. Effects of Crystallization Behavior on Morphological Change in Poly(Trimethylene Terephthalate) Spherulites. Polymer 2004, 45, 2413–2425. [Google Scholar] [CrossRef]
- Jeon, K.; Krishnamoorti, R. Morphological Behavior of Thin Linear Low-Density Polyethylene Films. Macromolecules 2008, 41, 7131–7140. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Chen, E.-Q. Polymer Crystallization of Ultrathin Films on Solid Substrates. Coord. Chem. Rev. 2010, 254, 1011–1037. [Google Scholar] [CrossRef]
- Fukushima, T.; Kosaka, A.; Ishimura, Y.; Yamamoto, T.; Takigawa, T.; Ishii, N.; Aida, T. Molecular Ordering of Organic Molten Salts Triggered by Single-Walled Carbon Nanotubes. Science 2003, 300, 2072–2074. [Google Scholar] [CrossRef]
- Kou, Y.; Cheng, X.; Macosko, C.W. Polymer/Graphene Composites via Spinodal Decomposition of Miscible Polymer Blends. Macromolecules 2019, 52, 7625–7637. [Google Scholar] [CrossRef]
- Bidsorkhi, H.C.; D’Aloia, A.G.; De Bellis, G.; Proietti, A.; Rinaldi, A.; Fortunato, M.; Ballirano, P.; Bracciale, M.P.; Santarelli, M.L.; Sarto, M.S. Nucleation Effect of Unmodified Graphene Nanoplatelets on PVDF/GNP Film Composites. Mater. Today Commun. 2017, 11, 163–173. [Google Scholar] [CrossRef]
- Izák, P.; Hovorka, Š.; Bartovský, T.; Bartovská, L.; Crespo, J.G. Swelling of Polymeric Membranes in Room Temperature Ionic Liquids. J. Membr. Sci. 2007, 296, 131–138. [Google Scholar] [CrossRef]
- Subianto, S.; Mistry, M.K.; Choudhury, N.R.; Dutta, N.K.; Knott, R. Composite Polymer Electrolyte Containing Ionic Liquid and Functionalized Polyhedral Oligomeric Silsesquioxanes for Anhydrous PEM Applications. ACS Appl. Mater. Interfaces 2009, 1, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Xu, H.; Yu, J.; Wen, L.; Zhang, J.; He, J. Plasticization of [C12MIM][PF6] Ionic Liquid on Foaming Performance of Poly(Methyl Methacrylate) in Supercritical CO2. Ind. Eng. Chem. Res. 2012, 51, 12329–12336. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, D.-D.; Gao, X.; Han, C.C.; Jin, X.-G.; Li, L.; Wang, Y.; Chan, C.-M. Lamellar Branching of Poly(Bisphenol A-Co-Decane) Spherulites at Different Temperatures Studied by High-Temperature AFM. Macromolecules 2003, 36, 3652–3655. [Google Scholar] [CrossRef]
- Waddon, A.J.; Petrovic, Z.S. Spherulite Crystallization in Poly(Ethylene Oxide)–Silica Nanocomposites. Retardation of Growth Rates through Reduced Molecular Mobility. Polym. J. 2002, 34, 876–881. [Google Scholar] [CrossRef]
- Zheng, X.; Sauer, B.B.; Van Alsten, J.G.; Schwarz, S.A.; Rafailovich, M.H.; Sokolov, J.; Rubinstein, M. Reptation Dynamics of a Polymer Melt near an Attractive Solid Interface. Phys. Rev. Lett. 1995, 74, 407–410. [Google Scholar] [CrossRef]
- Wu, Y.; Hsu, S.L.; Honeker, C.; Bravet, D.J.; Williams, D.S. The Role of Surface Charge of Nucleation Agents on the Crystallization Behavior of Poly(Vinylidene Fluoride). J. Phys. Chem. B 2012, 116, 7379–7388. [Google Scholar] [CrossRef]
- Lopes, A.C.; Costa, C.M.; Tavares, C.J.; Neves, I.C.; Lanceros-Mendez, S. Nucleation of the Electroactive γ Phase and Enhancement of the Optical Transparency in Low Filler Content Poly(Vinylidene)/Clay Nanocomposites. J. Phys. Chem. C 2011, 115, 18076–18082. [Google Scholar] [CrossRef]
- Sownthari, K.; Austin Suthanthiraraj, S. Preparation and Properties of a Gel Polymer Electrolyte System Based on Poly-ε-Caprolactone Containing 1-Ethyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide. J. Phys. Chem. Solids 2014, 75, 746–751. [Google Scholar] [CrossRef]
- Pandey, G.P.; Agrawal, R.C.; Hashmi, S.A. Magnesium Ion-Conducting Gel Polymer Electrolytes Dispersed with Nanosized Magnesium Oxide. J. Power Sources 2009, 190, 563–572. [Google Scholar] [CrossRef]
- Shih, C.-J.; Vijayaraghavan, A.; Krishnan, R.; Sharma, R.; Han, J.-H.; Ham, M.-H.; Jin, Z.; Lin, S.; Paulus, G.L.C.; Reuel, N.F.; et al. Bi- and Trilayer Graphene Solutions. Nat. Nanotech 2011, 6, 439–445. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Parvathy, C.; Balachandran, N.; Bhuvaneswari, S.; Vijayalakshmi, K.P.; George, B.K. PVDF-Ionic Liquid Modified Clay Nanocomposites: Phase Changes and Shish-Kebab Structure. Polymer 2017, 115, 70–76. [Google Scholar] [CrossRef]
- Dias, J.C.; Correia, D.M.; Costa, C.M.; Ribeiro, C.; Maceiras, A.; Vilas, J.L.; Botelho, G.; de Zea Bermudez, V.; Lanceros-Mendez, S. Improved Response of Ionic Liquid-Based Bending Actuators by Tailored Interaction with the Polar Fluorinated Polymer Matrix. Electrochim. Acta 2019, 296, 598–607. [Google Scholar] [CrossRef]
- Shalu; Kumar Singh, V.; Kumar Singh, R. Development of Ion Conducting Polymer Gel Electrolyte Membranes Based on Polymer PVdF-HFP, BMIMTFSI Ionic Liquid and the Li-Salt with Improved Electrical, Thermal and Structural Properties. J. Mater. Chem. C 2015, 3, 7305–7318. [Google Scholar] [CrossRef]
- Prosa, T.J.; Winokur, M.J.; Moulton, J.; Smith, P.; Heeger, A.J. X-Ray Structural Studies of Poly(3-Alkylthiophenes): An Example of an Inverse Comb. Macromolecules 1992, 25, 4364–4372. [Google Scholar] [CrossRef]
- Lopes, A.C.; Caparros, C.; Ferdov, S.; Lanceros-Mendez, S. Influence of Zeolite Structure and Chemistry on the Electrical Response and Crystallization Phase of Poly(Vinylidene Fluoride). J. Mater. Sci. 2013, 48, 2199–2206. [Google Scholar] [CrossRef]
- Martins, P.; Caparros, C.; Gonçalves, R.; Martins, P.M.; Benelmekki, M.; Botelho, G.; Lanceros-Mendez, S. Role of Nanoparticle Surface Charge on the Nucleation of the Electroactive β-Poly(Vinylidene Fluoride) Nanocomposites for Sensor and Actuator Applications. J. Phys. Chem. C 2012, 116, 15790–15794. [Google Scholar] [CrossRef]
- Brunauer, S. Chapter VIII: The Heat of Adsorption II. In The Adsorption of Gases and Vapors; H. Milford, Oxford University Press: Oxford, UK, 1943; Volume 1, pp. 218–270. [Google Scholar]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Adsorption Thermodynamics and Isosteric Heat of Adsorption of Toxic Metal Ions onto Bagasse Fly Ash (BFA) and Rice Husk Ash (RHA). Chem. Eng. J. 2007, 132, 267–278. [Google Scholar] [CrossRef]
- Manna, S.; Batabyal, S.K.; Nandi, A.K. Preparation and Characterization of Silver−Poly(Vinylidene Fluoride) Nanocomposites: Formation of Piezoelectric Polymorph of Poly(Vinylidene Fluoride). J. Phys. Chem. B 2006, 110, 12318–12326. [Google Scholar] [CrossRef] [PubMed]
- Höfft, O.; Bahr, S.; Kempter, V. Investigations with Infrared Spectroscopy on Films of the Ionic Liquid [EMIM]Tf2N. Langmuir 2008, 24, 11562–11566. [Google Scholar] [CrossRef] [PubMed]
- Mirarab, M.; Sharifi, M.; Ghayyem, M.A.; Mirarab, F. Prediction of Solubility of CO2 in Ethanol–[EMIM][Tf2N] Ionic Liquid Mixtures Using Artificial Neural Networks Based on Genetic Algorithm. Fluid Phase Equilibria 2014, 371, 6–14. [Google Scholar] [CrossRef]
- Carvalho, P.J.; Álvarez, V.H.; Machado, J.J.B.; Pauly, J.; Daridon, J.-L.; Marrucho, I.M.; Aznar, M.; Coutinho, J.A.P. High Pressure Phase Behavior of Carbon Dioxide in 1-Alkyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide Ionic Liquids. J. Supercrit. Fluids 2009, 48, 99–107. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Sebastian Seung, H. Trainable Weka Segmentation: A Machine Learning Tool for Microscopy Pixel Classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Alam, M.M.; Mandal, D. The in Situ Formation of Platinum Nanoparticles and Their Catalytic Role in Electroactive Phase Formation in Poly(Vinylidene Fluoride): A Simple Preparation of Multifunctional Poly(Vinylidene Fluoride) Films Doped with Platinum Nanoparticles. RSC Adv. 2014, 4, 41886–41894. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Yoon, S.; Kim, K.J.; Park, C. Preferential Formation of Electroactive Crystalline Phases in Poly(Vinylidene Fluoride)/Organically Modified Silicate Nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 2173–2187. [Google Scholar] [CrossRef]
- Gregorio, R., Jr.; Cestari, M. Effect of Crystallization Temperature on the Crystalline Phase Content and Morphology of Poly(Vinylidene Fluoride). J. Polym. Sci. Part B Polym. Phys. 1994, 32, 859–870. [Google Scholar] [CrossRef]
- Peng, G.; Zhao, X.; Zhan, Z.; Ci, S.; Wang, Q.; Liang, Y.; Zhao, M. New Crystal Structure and Discharge Efficiency of Poly(Vinylidene Fluoride-Hexafluoropropylene)/Poly(Methyl Methacrylate) Blend Films. RSC Adv. 2014, 4, 16849–16854. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; He, J.; Gao, L. The Graphene Oxide Polymer Composites with High Breakdown Field Strength and Energy Storage Ability. In The Graphene Oxide Polymer Composites with High Breakdown Field Strength and Energy Storage Ability | SpringerLink; Springer: Cham, Switzerland, 2013; Volume 1, pp. 431–438. [Google Scholar]
- Feng, Y.; Li, W.L.; Hou, Y.F.; Yu, Y.; Cao, W.P.; Zhang, T.D.; Fei, W.D. Enhanced Dielectric Properties of PVDF-HFP/BaTiO3-Nanowire Composites Induced by Interfacial Polarization and Wire-Shape. J. Mater. Chem. C 2015, 3, 1250–1260. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. Analysis Method: FTIR Studies of β-Phase Crystal Formation in Stretched PVDF Films. Polym. Test. 2003, 22, 699–704. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Li, S. Thermal Stability and Flame Retardancy of an Epoxy Resin Modified with Phosphoric Triamide and Glycidyl POSS. High Perform. Polym. 2019, 31, 1217–1225. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, P.; Gui, H.; Yang, S.; Ding, Y. Effect of Graphene Modified by a Long Alkyl Chain Ionic Liquid on Crystallization Kinetics Behavior of Poly(Vinylidene Fluoride). RSC Adv. 2015, 5, 92418–92427. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Xue, F.; Cai, Z.; Shang, Y.; Li, J.; Chen, Y.; Wu, Z.; Jiang, S. In-Situ Synchrotron SAXS and WAXS Investigations on Deformation and α–β Transformation of Uniaxial Stretched Poly(Vinylidene Fluoride). CrystEngComm 2013, 15, 1597–1606. [Google Scholar] [CrossRef]
- Rowe, M.C.; Brewer, B.J. AMORPH: A Statistical Program for Characterizing Amorphous Materials by X-Ray Diffraction. Comput. Geosci. 2018, 120, 21–31. [Google Scholar] [CrossRef]
- Esterly, D.M.; Love, B.J. Phase Transformation to β-Poly(Vinylidene Fluoride) by Milling. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 91–97. [Google Scholar] [CrossRef]
- Cataldi, P.; Bayer, I.S.; Cingolani, R.; Marras, S.; Chellali, R.; Athanassiou, A. A Thermochromic Superhydrophobic Surface. Sci. Rep. 2016, 6, 27984. [Google Scholar] [CrossRef]
- Liu, X.; Liang, B.; Long, J. Preparation of Novel Thick Sheet Graphene and Its Effect on the Properties of Polyolefins with Different Crystallinities. Polym. Bull. 2021. [Google Scholar] [CrossRef]
- Tripathi, M.; Bobade, S.M.; Kumar, A. Preparation of Polyvinylidene Fluoride-Co-Hexafluoropropylene-Based Polymer Gel Electrolyte and Its Performance Evaluation for Application in EDLCs. Bull. Mater. Sci. 2019, 42, 27. [Google Scholar] [CrossRef]
- Parangusan, H.; Ponnamma, D.; Al-Maadeed, M.A.A. Stretchable Electrospun PVDF-HFP/Co-ZnO Nanofibers as Piezoelectric Nanogenerators. Sci. Rep. 2018, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Park, Y.J.; Hwang, J.Y.; Jeong, H.J.; Lee, J.S.; Kim, K.J.; Kim, H.-C.; Huh, J.; Park, C. Localized Pressure-Induced Ferroelectric Pattern Arrays of Semicrystalline Poly(Vinylidene Fluoride) by Microimprinting. Adv. Mater. 2007, 19, 581–586. [Google Scholar] [CrossRef]
- Xu, P.; Cui, Z.-P.; Ruan, G.; Ding, Y.-S. Enhanced Crystallization Kinetics of PLLA by Ethoxycarbonyl Ionic Liquid Modified Graphene. Chin. J. Polym. Sci. 2019, 37, 243–252. [Google Scholar] [CrossRef]
- Maiz, J.; Martin, J.; Mijangos, C. Confinement Effects on the Crystallization of Poly(Ethylene Oxide) Nanotubes. Langmuir 2012, 28, 12296–12303. [Google Scholar] [CrossRef]
- Gonçalves, R.; Martins, P.M.; Caparrós, C.; Martins, P.; Benelmekki, M.; Botelho, G.; Lanceros-Mendez, S.; Lasheras, A.; Gutiérrez, J.; Barandiarán, J.M. Nucleation of the Electroactive β-Phase, Dielectric and Magnetic Response of Poly(Vinylidene Fluoride) Composites with Fe2O3 Nanoparticles. J. Non-Cryst. Solids 2013, 361, 93–99. [Google Scholar] [CrossRef]
- Tong, Y.; Que, M.; Su, S.; Chen, L. Design of Amphiphilic Poly(Vinylidene Fluoride-Co-Hexafluoropropylene)-Based Gel Electrolytes for High-Performance Lithium-Ion Batteries. Ionics 2016, 22, 1311–1318. [Google Scholar] [CrossRef]
- Wu, L.; Huang, G.; Hu, N.; Fu, S.; Qiu, J.; Wang, Z.; Ying, J.; Chen, Z.; Li, W.; Tang, S. Improvement of the Piezoelectric Properties of PVDF-HFP Using AgNWs. RSC Adv. 2014, 4, 35896–35903. [Google Scholar] [CrossRef]
- Chacko, S.K.; Rahul, M.T.; Raneesh, B.; Kalarikkal, N. Enhanced Magnetoelectric Coupling and Dielectric Constant in Flexible Ternary Composite Electrospun Fibers of PVDF-HFP Loaded with Nanoclay and NiFe2O4 Nanoparticles. New J. Chem. 2020, 44, 11356–11364. [Google Scholar] [CrossRef]
- Kim, K.M.; Park, N.-G.; Ryu, K.S.; Chang, S.H. Characteristics of PVdF-HFP/TiO2 Composite Membrane Electrolytes Prepared by Phase Inversion and Conventional Casting Methods. Electrochim. Acta 2006, 51, 5636–5644. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.; Bavarian, M.; Nejati, S. Correlating the Macrostructural Variations of an Ion Gel with Its Carbon Dioxide Sorption Capacity. Membranes 2022, 12, 1087. https://doi.org/10.3390/membranes12111087
Nguyen T, Bavarian M, Nejati S. Correlating the Macrostructural Variations of an Ion Gel with Its Carbon Dioxide Sorption Capacity. Membranes. 2022; 12(11):1087. https://doi.org/10.3390/membranes12111087
Chicago/Turabian StyleNguyen, Tung, Mona Bavarian, and Siamak Nejati. 2022. "Correlating the Macrostructural Variations of an Ion Gel with Its Carbon Dioxide Sorption Capacity" Membranes 12, no. 11: 1087. https://doi.org/10.3390/membranes12111087
APA StyleNguyen, T., Bavarian, M., & Nejati, S. (2022). Correlating the Macrostructural Variations of an Ion Gel with Its Carbon Dioxide Sorption Capacity. Membranes, 12(11), 1087. https://doi.org/10.3390/membranes12111087






