Development of an Integrated Salt Cartridge-Reverse Electrodialysis (Red) Device to Increase Electrolyte Concentrations to Biomedical Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hollow-Fiber Membrane Production and Salt Cartridge Preparation
2.3. Salt-Pick-Up Experiments
2.4. Reverse Electrodialysis (RED) Setup and Salt-Cartridge Coupling
3. Results and Discussion
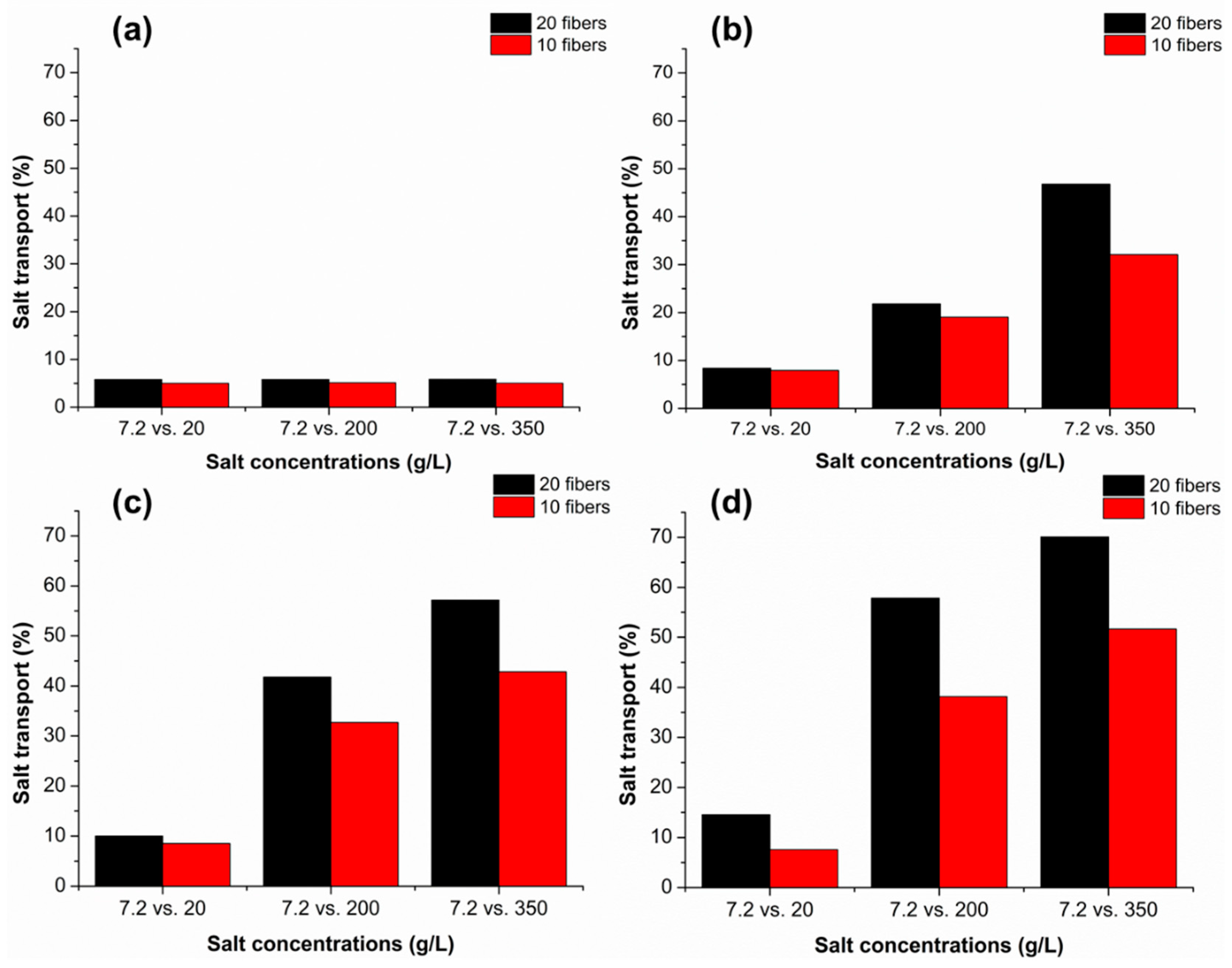
3.1. Salt Pick-Up Studies
3.2. Reverse Electrodialysis Operation with Salt Cartridge
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cadei, A.; Dionisi, A.; Sardini, E.; Serpelloni, M. Kinetic and thermal energy harvesters for implantable medical devices and biomedical autonomous sensors. Meas. Sci. Technol. 2013, 25, 012003. [Google Scholar] [CrossRef]
- Dean, J.; Sulke, N. Pacemaker battery scandal. BMJ 2016, 352, i228. [Google Scholar] [CrossRef]
- Uslan, D.Z.; Gleva, M.J.; Warren, D.K.; Mela, T.; Chung, M.K.; Gottipaty, V.; Borge, R.; Dan, D.; Shinn, T.; Mitchell, K.; et al. Cardiovascular Implantable Electronic Device Replacement Infections and Prevention: Results from the REPLACE Registry. Pacing Clin. Electrophysiol. 2011, 35, 81–87. [Google Scholar] [CrossRef] [PubMed]
- McKelvie, R. Heart failure, Search Date August 2010. BMJ Clinical Evidence. 2011. Available online: http://www.clinicalevidence.com (accessed on 20 September 2022).
- Haeberlin, A.; Zurbuchen, A.; Walpen, S.; Schaerer, J.; Niederhauser, T.; Huber, C.; Tanner, H.; Servatius, H.; Seiler, J.; Haeberlin, H.; et al. The first batteryless, solar-powered cardiac pacemaker. Heart Rhythm 2015, 12, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yi, Z.; Ma, Y.; Xie, F.; Huang, Y.; Tian, Y.; Dong, X.; Liu, Y.; Shao, X.; Jin, L.; et al. Direct Powering a Real Cardiac Pacemaker by Natural Energy of a Heartbeat. ACS Nano 2019, 13, 2822–2830. [Google Scholar] [CrossRef]
- Ashraf, M.; Masoumi, N. A Thermal Energy Harvesting Power Supply With an Internal Startup Circuit for Pacemakers. IEEE Trans. Very Large Scale Integr. (VLSI) Syst. 2015, 24, 26–37. [Google Scholar] [CrossRef]
- Nasar, A.; Perveen, R. Applications of enzymatic biofuel cells in bioelectronic devices—A review. Int. J. Hydrogen Energy 2019, 44, 15287–15312. [Google Scholar] [CrossRef]
- Rewatkar, P.; Hitaishi, V.P.; Lojou, E.; Goel, S. Enzymatic fuel cells in a microfluidic environment: Status and opportunities. A mini review. Electrochem. Commun. 2019, 107, 106533. [Google Scholar] [CrossRef]
- Rasmussen, M.; Abdellaoui, S.; Minteer, S.D. Enzymatic biofuel cells: 30 years of critical advancements. Biosens. Bioelectron. 2016, 76, 91–102. [Google Scholar] [CrossRef]
- Xiao, X.; Xia, H.-Q.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A. Tackling the Challenges of Enzymatic (Bio)Fuel Cells. Chem. Rev. 2019, 119, 9509–9558. [Google Scholar] [CrossRef]
- Ramon, G.Z.; Feinberg, B.J.; Hoek, E.M.V. Membrane-based production of salinity-gradient power. Energy Environ. Sci. 2011, 4, 4423–4434. [Google Scholar] [CrossRef]
- Yip, N.Y.; Vermaas, D.A.; Nijmeijer, K.; Elimelech, M. Thermodynamic, Energy Efficiency, and Power Density Analysis of Reverse Electrodialysis Power Generation with Natural Salinity Gradients. Environ. Sci. Technol. 2014, 48, 4925–4936. [Google Scholar] [CrossRef] [PubMed]
- Długołeçki, P.; Antoine, G.; Nijmeijer, K.; Wessling, M. Practicalpotential of reverse electrodialysis as process for sustainable energy generation. Environ. Sci. Technol. 2009, 43, 6888–6894. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Güler, E.; Saakes, M.; Nijmeijer, K. Theoretical power density from salinity gradients using reverse electrodialysis. Energy Procedia 2012, 20, 170–184. [Google Scholar] [CrossRef]
- Baek, S.; Kwon, S.-R.; Yeon, S.Y.; Yoon, S.-H.; Kang, C.M.; Han, S.H.; Lee, D.; Chung, T.D. Miniaturized Reverse Electrodialysis-Powered Biosensor Using Electrochemiluminescence on Bipolar Electrode. Anal. Chem. 2018, 90, 4749–4755. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-R.; Nam, S.H.; Park, C.Y.; Baek, S.; Jang, J.; Che, X.; Kwak, S.H.; Choi, Y.-R.; Park, N.-R.; Choi, J.-Y.; et al. Electrodeless Reverse Electrodialysis Patches as an Ionic Power Source for Active Transdermal Drug Delivery. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Tsai, T.-C.; Liu, C.-W.; Yang, R.-J. Power Generation by Reverse Electrodialysis in a Microfluidic Device with a Nafion Ion-Selective Membrane. Micromachines 2016, 7, 205. [Google Scholar] [CrossRef]
- Pakkaner, E.; Smith, C.; Trexler, C.; Hestekin, J.; Hestekin, C. Blood driven biopower cells: Acquiring energy from reverse electrodialysis using sodium concentrations from the flow of human blood. J. Power Sources 2021, 488, 229440. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Roberts, J.; Harrison, G.; Alshammari, N.; Hestekin, J.; Servoss, S.L. Low Fouling, Peptoid-Coated Polysulfone Hollow Fiber Membranes—the Effect of Grafting Density and Number of Side Chains. Appl. Biochem. Biotechnol. 2019, 191, 824–837. [Google Scholar] [CrossRef]
- Cohen-Adad, R.; John, W.L. (Eds.) Alkali Metal and Ammonium Chlorides in Water and Heavy Water (Binary Systems); Elsevier: Amsterdam, The Netherlands, 2013; Volume 47. [Google Scholar]
- Tedesco, M.; Hamelers, H.V.M.; Biesheuvel, P.M. Nernst-Planck transport theory for (reverse) electrodialysis: I. Effect of co-ion transport through the membranes. J. Membr. Sci. 2016, 510, 370–381. [Google Scholar] [CrossRef]
- Klarhöfer, M.; Csapo, B.; Balassy, C.; Szeles, J.C.; Moser, E. High-resolution blood flow velocity measurements in the human finger. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2001, 45, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Jaffrin, M.Y. Convective Mass Transfer in Hemodialysis. Artif. Organs 1995, 19, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Coulliette, A.D.; Arduino, M. Hemodialysis and Water Quality. Semin. Dial. 2013, 26, 427–438. [Google Scholar] [CrossRef]
- Maduell, F.; Ojeda, R.; Arias-Guillen, M.; Bazan, G.; Vera, M.; Fontseré, N.; Masso, E.; Gómez, M.; Rodas, L.; Jiménez-Hernández, M.; et al. Assessment of dialyzer surface in online hemodiafiltration; objective choice of dialyzer surface area. Nefrología 2015, 35, 280–286. [Google Scholar] [CrossRef][Green Version]
- Seader, J.; Ernest, D.; Henley, J.; Keith Roper, D. Separation Process Principles; Wiley: New York, NY, USA, 1998; Volume 25. [Google Scholar]
- Kunikata, S.; Fukuda, M.; Yamamoto, K.-I.; Yagi, Y.; Matsuda, M.; Sakai, K. Technical Characterization of Dialysis Fluid Flow of Newly Developed Dialyzers Using Mass Transfer Correlation Equations. ASAIO J. 2009, 55, 231–235. [Google Scholar] [CrossRef]
- Sigler, M.H.; Teehan, B.P.; Van Valkenburgh, W.T.T.A.O.D. Solute transport in continuous hemodialysis: A new treatment for acute renal failure. Kidney Int. 1987, 32, 562–571. [Google Scholar] [CrossRef]
- Micari, M.; Bevacqua, M.; Cipollina, A.; Tamburini, A.; Van Baak, W.; Putts, T.; Micale, G. Effect of different aqueous solutions of pure salts and salt mixtures in reverse electrodialysis systems for closed-loop applications. J. Membr. Sci. 2018, 551, 315–325. [Google Scholar] [CrossRef]
- Zhang, W.; Han, B.; Tufa, R.A.; Tang, C.; Liu, X.; Zhang, G.; Chang, J.; Zhang, R.; Mu, R.; Liu, C.; et al. Tracing the impact of stack configuration on interface resistances in reverse electrodialysis by in situ electrochemical impedance spectroscopy. Front. Environ. Sci. Eng. 2022, 16, 1–12. [Google Scholar] [CrossRef]
- Haeberlin, A.; Zurbuchen, A.; Schaerer, J.; Wagner, J.; Walpen, S.; Huber, C.; Haeberlin, H.; Fuhrer, J.; Vogel, R. Successful pacing using a batteryless sunlight-powered pacemaker. Europace 2014, 16, 1534–1539. [Google Scholar] [CrossRef]
- NIST Reference on Constants, Units and Uncertainty. Available online: https://physics.nist.gov/cgi-bin/cuu/Value?jev (accessed on 1 June 2021).
- Hoenich, N.A.; Ronco, C. Haemodialysis Fluid: Composition and Clinical Importance. Blood Purif. 2007, 25, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; Leclercq, C. Sodium. Adv. Nutr. 2014, 5, 188–190. [Google Scholar] [CrossRef] [PubMed]


| Feed (Acceptor) NaCl Concentration (g/L) | Cartridge (Donor) NaCl Concentration (g/L) | % Average Salt Transport | Acceptor Final NaCl Concentration (g/L) |
|---|---|---|---|
| 7.2 | 20 | 14.08 ± 7.3 | 9.37 ± 0.05 |
| 7.2 | 100 | 51.07 ± 24.3 | 29.59 ± 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakkaner, E.; Orton, J.L.; Campbell, C.G.; Hestekin, J.A.; Hestekin, C.N. Development of an Integrated Salt Cartridge-Reverse Electrodialysis (Red) Device to Increase Electrolyte Concentrations to Biomedical Devices. Membranes 2022, 12, 990. https://doi.org/10.3390/membranes12100990
Pakkaner E, Orton JL, Campbell CG, Hestekin JA, Hestekin CN. Development of an Integrated Salt Cartridge-Reverse Electrodialysis (Red) Device to Increase Electrolyte Concentrations to Biomedical Devices. Membranes. 2022; 12(10):990. https://doi.org/10.3390/membranes12100990
Chicago/Turabian StylePakkaner, Efecan, Jessica L. Orton, Caroline G. Campbell, Jamie A. Hestekin, and Christa N. Hestekin. 2022. "Development of an Integrated Salt Cartridge-Reverse Electrodialysis (Red) Device to Increase Electrolyte Concentrations to Biomedical Devices" Membranes 12, no. 10: 990. https://doi.org/10.3390/membranes12100990
APA StylePakkaner, E., Orton, J. L., Campbell, C. G., Hestekin, J. A., & Hestekin, C. N. (2022). Development of an Integrated Salt Cartridge-Reverse Electrodialysis (Red) Device to Increase Electrolyte Concentrations to Biomedical Devices. Membranes, 12(10), 990. https://doi.org/10.3390/membranes12100990







