Properties of Anion Exchange Membranes with a Focus on Water Electrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
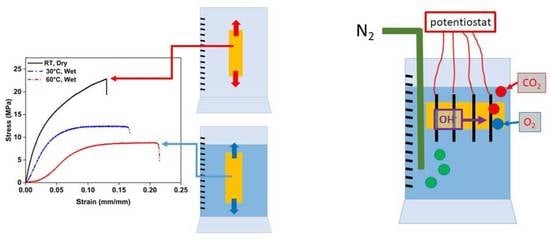
2.2. Mechanical Properties
2.3. Alkaline Stability
2.4. Swelling Behavior
2.5. Conductivity
2.6. Water Permeability
3. Results and Discussion
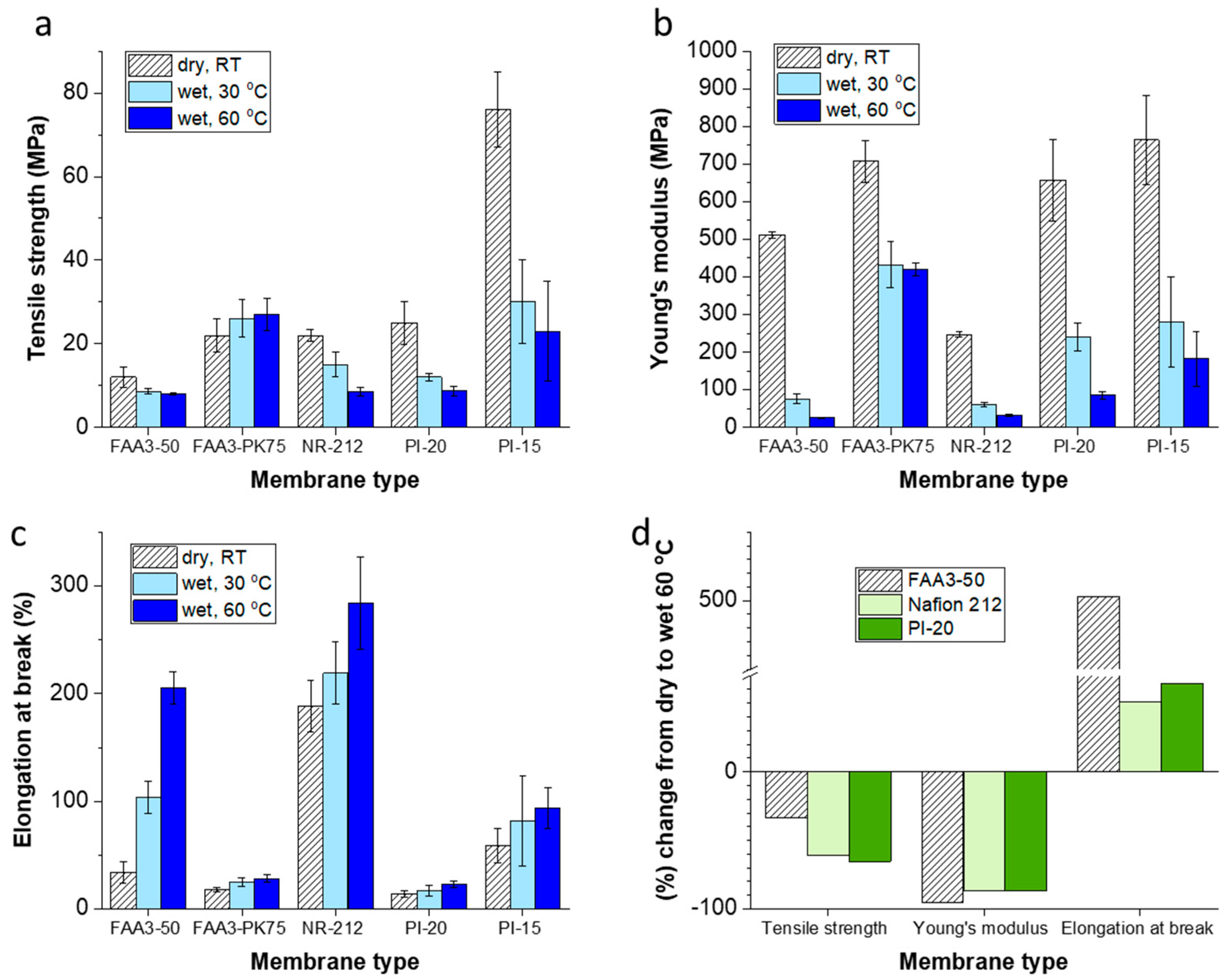
3.1. Mechanical Properties
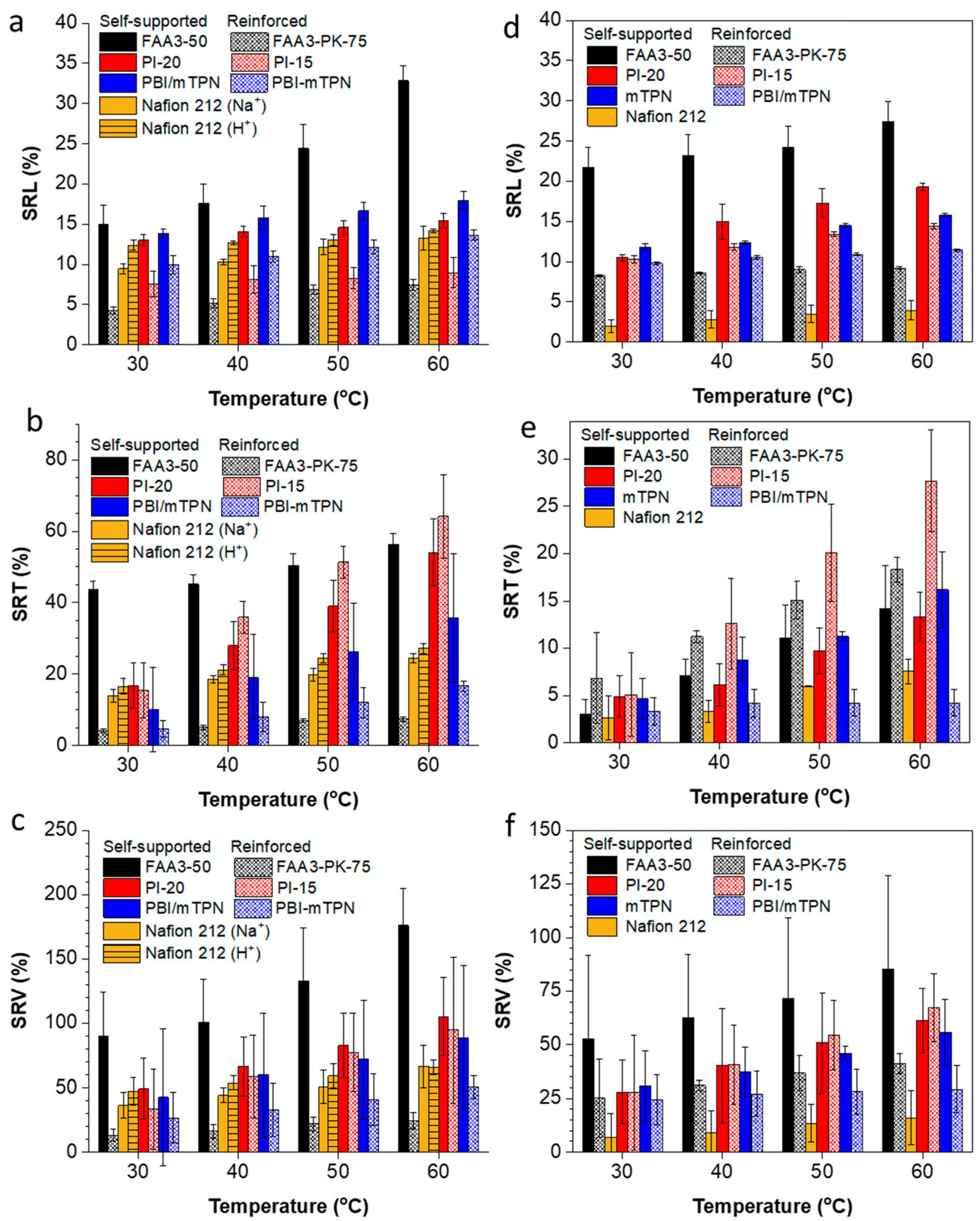
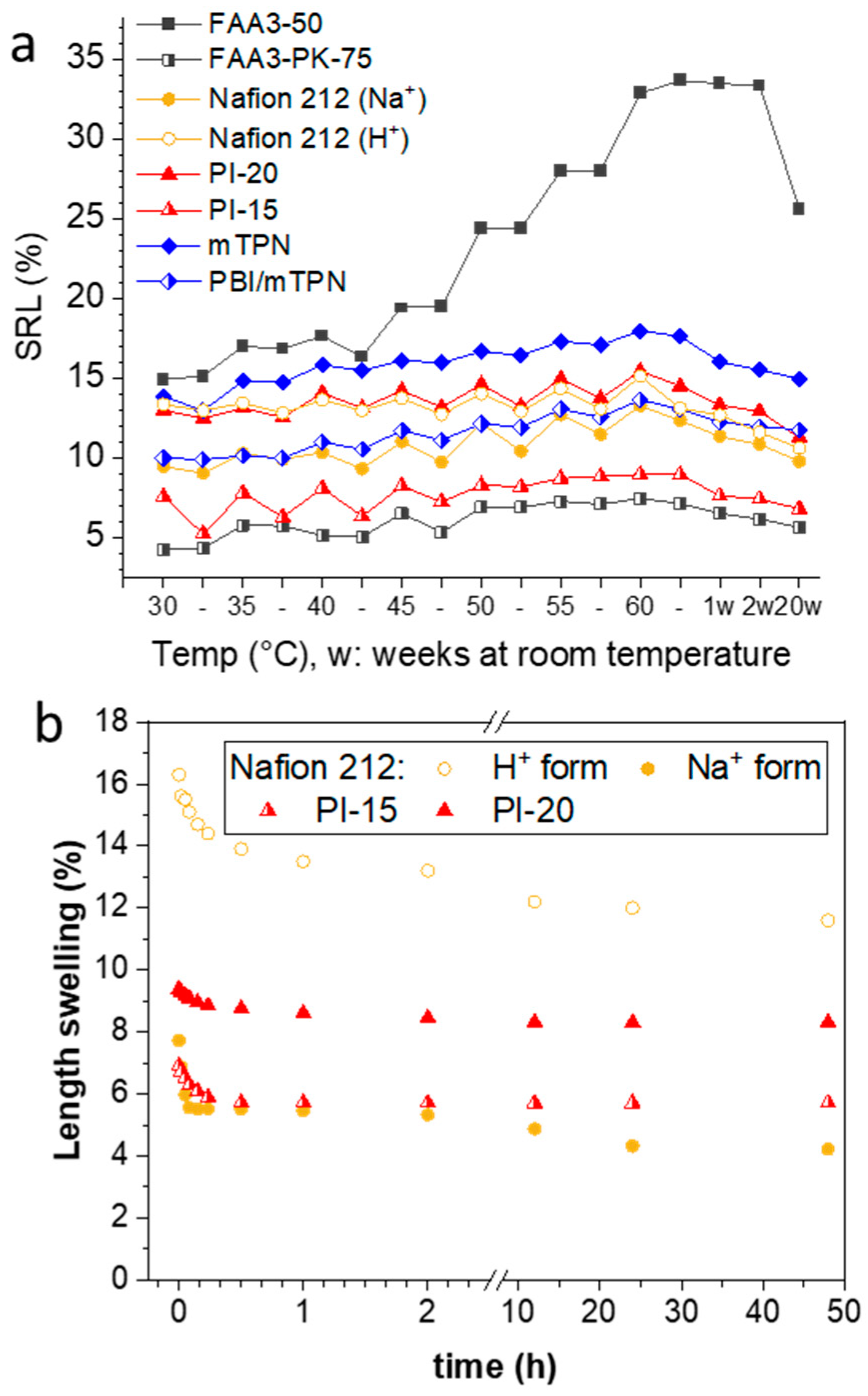
3.2. Swelling Behavior
3.3. Hydroxide Conductivity
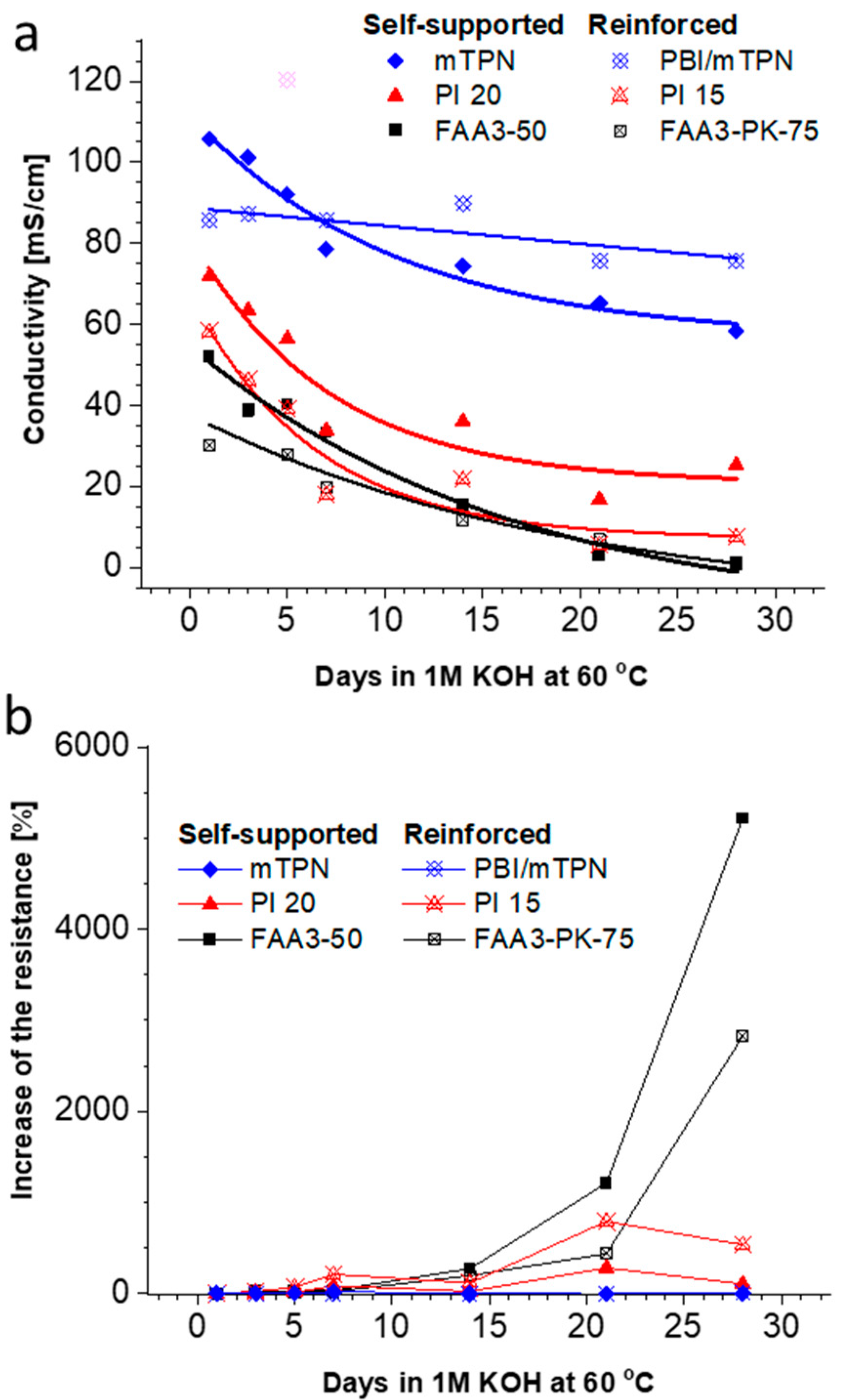
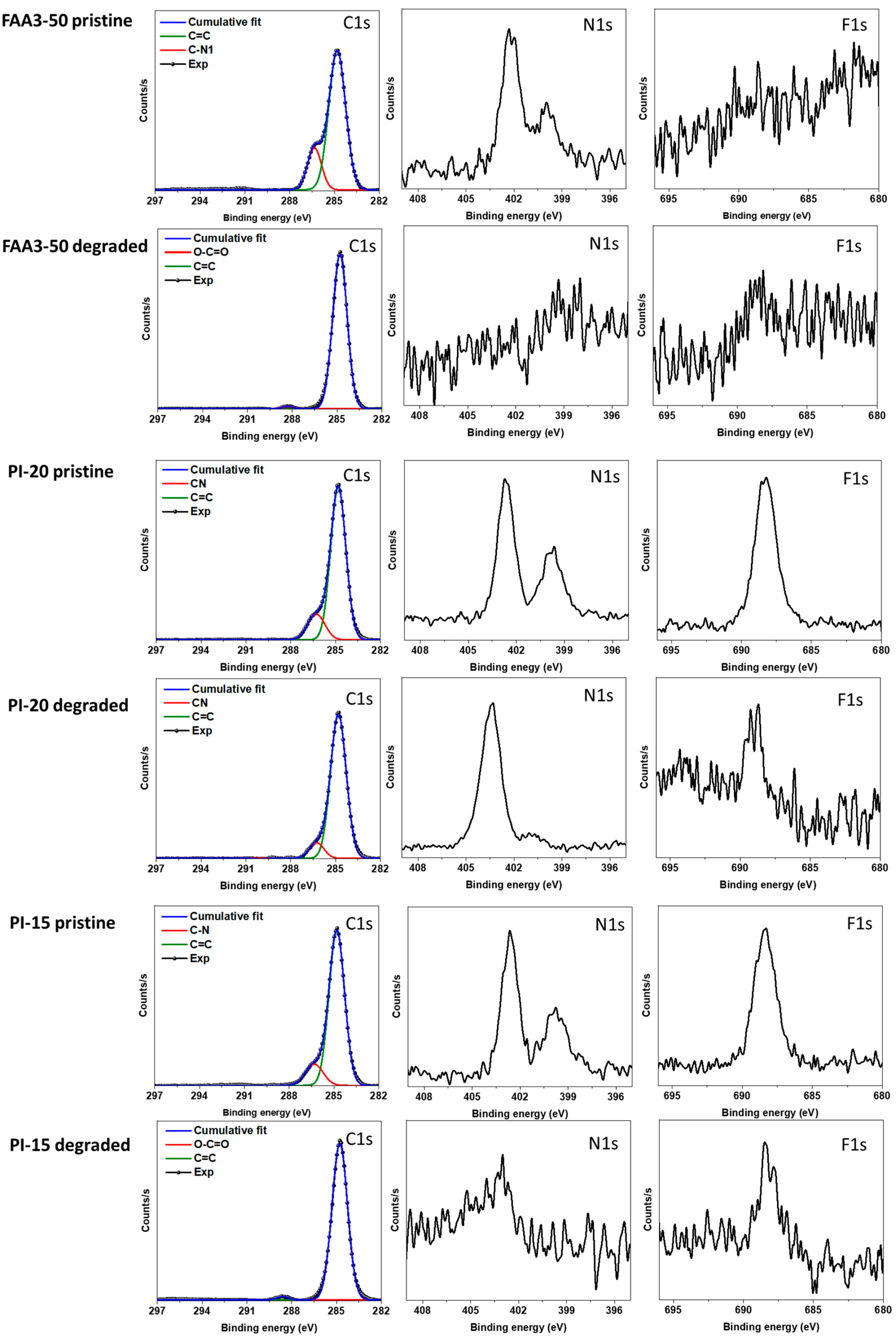
3.4. Alkaline Stability
3.5. Water Permeability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef]
- Kou, T.; Wang, S.; Li, Y. Perspective on High-Rate Alkaline Water Splitting. ACS Mater. Lett. 2021, 3, 224–234. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A Comprehensive Review on PEM Water Electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Feng, M.; Qu, R.; Wei, Z.; Wang, L.; Sun, P.; Wang, Z. Characterization of the Thermolysis Products of Nafion Membrane: A Potential Source of Perfluorinated Compounds in the Environment. Sci. Rep. 2015, 5, 9859. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, R.; Cousins, I.T.; Dewitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lindstrom, A.B.; Miller, M.F.; Ng, C.A.; Patton, S.; et al. Are Fluoropolymers Really of Low Concern for Human and Environmental Health and Separate from Other PFAS? Environ. Sci. Technol. 2020, 54, 12820–12828. [Google Scholar] [CrossRef] [PubMed]
- Minke, C.; Suermann, M.; Bensmann, B.; Hanke-Rauschenbach, R. Is iridium demand a potential bottleneck in the realization of large-scale PEM water electrolysis? Int. J. Hydrog. Energy 2021, 46, 23581–23590. [Google Scholar] [CrossRef]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green Hydrogen from Anion Exchange Membrane Water Electrolysis: A Review of Recent Developments in Critical Materials and Operating Conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Arges, C.G.; Ramani, V. Two-Dimensional NMR Spectroscopy Reveals Cation-Triggered Backbone Degradation in Polysulfone-Based Anion Exchange Membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 2490–2495. [Google Scholar] [CrossRef]
- Marino, M.G.; Kreuer, K.D. Alkaline Stability of Quaternary Ammonium Cations for Alkaline Fuel Cell Membranes and Ionic Liquids. ChemSusChem 2015, 8, 513–523. [Google Scholar] [CrossRef]
- Wright, A.G.; Fan, J.; Britton, B.; Weissbach, T.; Lee, H.F.; Kitching, E.A.; Peckham, T.J.; Holdcroft, S. Hexamethyl-p-terphenyl poly(benzimidazolium): A Universal Hydroxide-Conducting Polymer for Energy Conversion Devices. Energy Environ. Sci. 2016, 9, 2130–2142. [Google Scholar] [CrossRef]
- Thomas, O.D.; Soo, K.J.W.Y.; Peckham, T.J.; Kulkarni, M.P.; Holdcroft, S. A Stable Hydroxide-Conducting Polymer. J. Am. Chem. Soc. 2012, 134, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Henkensmeier, D.; Najibah, M.; Harms, C.; Žitka, J.; Hnát, J.; Bouzek, K. Overview: State-of-the Art Commercial Membranes for Anion Exchange Membrane Water Electrolysis. J. Electrochem. Energy Convers. Storage 2021, 18, 024001. [Google Scholar] [CrossRef]
- Trisno, M.L.A.; Dayan, A.; Lee, S.J.; Egert, F.; Gerle, M.; Kraglund, M.R.; Jensen, J.O.; Aili, D.; Roznowska, A.; Michalak, A.; et al. Reinforced gel-state polybenzimidazole hydrogen separators for alkaline water electrolysis. Energy Env. Sci. 2022. [Google Scholar] [CrossRef]
- Kang, Z.; Pak, M.; Bender, G. Introducing a novel technique for measuring hydrogen crossover in membrane-based electrochemical cells. Int. J. Hydrog. Energy 2021, 46, 15161–15167. [Google Scholar] [CrossRef]
- Ziv, N.; Dekel, D.R. A Practical Method for Measuring the True Hydroxide Conductivity of Anion Exchange Membranes. Electrochem. Commun. 2018, 88, 109–113. [Google Scholar] [CrossRef]
- Khaisa, A.Z.; Kubannek, F.; Krewer, U.; Dekel, D.R. Measuring the true hydroxide conductivity of anion exchange membranes. J. Membr. Sci. 2020, 612, 118461. [Google Scholar] [CrossRef]
- Najibah, M.; Tsoy, E.; Khalid, H.; Chen, Y.; Li, Q.; Bae, C.; Hnát, J.; Plevová, M.; Bouzek, K.; Jang, J.H.; et al. PBI Nanofiber Mat-Reinforced Anion Exchange Membranes with Covalently Linked Interfaces for Use in Water Electrolysers. J. Membr. Sci. 2021, 640, 119832. [Google Scholar] [CrossRef]
- Lee, W.H.; Park, E.J.; Han, J.; Shin, D.W.; Kim, Y.S.; Bae, C. Poly(Terphenylene) Anion Exchange Membranes: The Effect of Backbone Structure on Morphology and Membrane Property. ACS Macro Lett. 2017, 6, 566–570. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Setzler, B.P.; Rojas-Carbonell, S.; Ben Yehuda, C.; Amel, A.; Page, M.; Wang, L.; Hu, K.; Shi, L.; et al. Poly(Aryl Piperidinium) Membranes and Ionomers for Hydroxide Exchange Membrane Fuel Cells. Nat. Energy 2019, 4, 392–398. [Google Scholar] [CrossRef]
- Luo, X.; Rojas-Carbonell, S.; Yan, Y.; Kusoglu, A. Structure-Transport Relationships of Poly(Aryl Piperidinium) Anion-Exchange Membranes: Eeffect of Anions and Hydration. J. Membr. Sci. 2020, 598, 117680. [Google Scholar] [CrossRef]
- Meek, K.M.; Antunes, C.M.; Strasser, D.; Owczarczyk, Z.R.; Neyerlin, A.; Pivovar, B.S. High-Throughput Anion Exchange Membrane Characterization at NREL. ECS Trans. 2019, 92, 723–731. [Google Scholar] [CrossRef]
- Kamcev, J.; Paul, D.R.; Freeman, B.D. Ion Activity Coefficients in Ion Exchange Polymers: Applicability of Manning’s Counterion Condensation Theory. Macromolecules 2015, 48, 8011–8024. [Google Scholar] [CrossRef]
- Munchinger, A.; Kreuer, K.-D. Selective Ion Transport Through Hydrated Cation and Anion Exchange Membranes I. The Effect of Specific Interactions. J. Membr. Sci. 2019, 592, 117372. [Google Scholar] [CrossRef]
- Peron, J.; Mani, A.; Zhao, X.; Edwards, D.; Adachi, M.; Soboleva, T.; Shi, Z.; Xie, Z.; Navessin, T.; Holdcroft, S. Properties of Nafion® NR-211 Membranes for PEMFCs. J. Membr. Sci. 2010, 356, 44–51. [Google Scholar] [CrossRef]
- Krishnan, N.N.; Henkensmeier, D.; Jang, J.H.; Hink, S.; Kim, H.J.; Nam, S.W.; Lim, T.H. Locally Confined Membrane Modification of Sulfonated Membranes for Fuel Cell Application. J. Membr. Sci. 2014, 454, 174–183. [Google Scholar] [CrossRef]
- Choi, P.; Datta, R. Sorption in Proton-Exchange Membranes: An Explanation of Schroeder’s Paradox. J. Electrochem. Soc. 2003, 150, E601–E607. [Google Scholar] [CrossRef][Green Version]
- Henkensmeier, D.; Gubler, L. Shape Memory Effect in Radiation Grafted Ion Exchange Membranes. J. Mater. Chem. A 2014, 2, 9482–9485. [Google Scholar] [CrossRef]
- Hink, S.; Henkensmeier, D.; Jang, J.H.; Kim, H.J.; Han, J.; Nam, S.W. Reduced In-Plane Swelling of Nafion by a Biaxial Modification Process. Macromol. Chem. Phys. 2015, 216, 1235–1243. [Google Scholar] [CrossRef]
- Babcock, E.; Szekely, N.; Konovalova, A.; Lin, Y.; Appavou, M.S.; Mangiapia, G.; Revay, Z.; Stieghorst, C.; Holderer, O.; Henkensmeier, D.; et al. Using Neutron Methods SANS and PGAA to Study Evolution of Structure and Composition of Alkali-Doped Polybenzimidazole Membranes. J. Membr. Sci. 2019, 577, 12–19. [Google Scholar] [CrossRef]
- Coury, L. Conductance Measurements Part 1: Theory. Current 1999, 3, 91–96. [Google Scholar]
- Kreuer, K.D. On the Development of Proton Conducting Polymer Membranes for Hydrogen and Methanol Fuel Cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ikhsan, M.M.; Jin, K.S.; Henkensmeier, D. Nanostructure-Property Relationship of Two Perfluorinated Sulfonic Acid (PFSA) Membranes. Int. J. Energy Res. 2022, 46, 11265–11277. [Google Scholar] [CrossRef]
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Facile Fabrication and Characterization of Improved Proton Conducting Sulfonated Poly(Arylene Biphenylether Sulfone) Blocks Containing Fluorinated Hydrophobic Units for Proton Exchange Membrane Fuel Cell Applications. Polymers 2018, 10, 1367. [Google Scholar] [CrossRef]
- Schwämmlein, J.N.; Pham, N.L.T.; Mittermeier, T.; Egawa, M.; Bonorand, L.; Gasteiger, H.A. Through-Plane Conductivity of Anion Exchange Membranes at Sub-Freezing Temperatures-Hydroxide vs (Bi-)Carbonate Ions. J. Electrochem. Soc. 2020, 167, 084513. [Google Scholar] [CrossRef]
- Kim, Y.S.; Pivovar, B.S. Moving beyond Mass-Based Parameters for Conductivity Analysis of Sulfonated Polymers. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 123–148. [Google Scholar] [CrossRef]
- Ziv, N.; Mondal, A.N.; Weissbach, T.; Holdcroft, S.; Dekel, D.R. Effect of CO2 on the Properties of Anion Exchange Membranes for Fuel Cell Applications. J. Membr. Sci. 2019, 586, 140–150. [Google Scholar] [CrossRef]
- Lee, W.; Konovalova, A.; Tsoy, E.; Park, G.; Henkensmeier, D.; Kwon, Y. Alkaline Naphthoquinone-Based Redox Flow Batteries with a Crosslinked Sulfonated Polyphenylsulfone Membrane. Int. J. Energy Res. 2022, 46, 12988–13002. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-Exchange Membranes in Electrochemical Energy Systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion Exchange Membranes for Alkaline Fuel Cells: A Review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Dekel, D.; Schuster, M.; Ash, U.; Jaouen, F. Deliverable Report, Critical Raw Materials Elimination by a Top-down Approach to Hydrogen and Electricity Generation; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Lindquist, G.A.; Oener, S.Z.; Krivina, R.; Motz, A.R.; Keane, A.; Capuano, C.; Ayers, K.E.; Boettcher, S.W. Performance and Durability of Pure-Water-Fed Anion Exchange Membrane Electrolyzers Using Baseline Materials and Operation. ACS Appl. Mater. Interfaces 2021, 13, 51917–51924. [Google Scholar] [CrossRef]
- Naumkin, V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/query_chem_name_detail.aspx?ID_NO=2222&CName=acetamide (accessed on 10 August 2022).
- Chen, S.; Peng, H.; Hu, M.; Wang, G.; Xiao, L.; Lu, J.; Zhuang, L. Ultrathin Self-Cross-Linked Alkaline Polymer Electrolyte Membrane for APEFC Applications. ACS Appl. Energy Mater. 2021, 4, 4297–4301. [Google Scholar] [CrossRef]
- Bass, M.; Berman, A.; Singh, A.; Konovalov, O.; Freger, V. Surface Structure of Nafion in Vapor and Liquid. J. Phys. Chem. B 2010, 114, 3784–3790. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Klaus, S.; Benziger, J. Wetting and Absorption of Water Drops on Nafion Films. Langmuir 2008, 24, 8627–8633. [Google Scholar] [CrossRef]
- Cho, M.K.; Park, H.Y.; Lee, H.J.; Kim, H.J.; Lim, A.; Henkensmeier, D.; Yoo, S.J.; Kim, J.Y.; Lee, S.Y.; Park, H.S.; et al. Alkaline Anion Exchange Membrane Water Electrolysis: Effects of Electrolyte Feed Method and Electrode Binder Content. J. Power Sources 2018, 382, 22–29. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Dang, Q.K.; Nambi Krishnan, N.; Jang, J.H.; Kim, H.J.; Nam, S.W.; Lim, T.H. Ortho-Dichlorobenzene as a Pore Modifier for PEMFC Catalyst Electrodes and Dense Nafion Membranes with One Porous Surface. J. Mater. Chem. 2012, 22, 14602–14607. [Google Scholar] [CrossRef]
- Luo, X.; Wright, A.; Weissbach, T.; Holdcroft, S. Water Permeation through Anion Exchange Membranes. J. Power Sources 2018, 375, 442–451. [Google Scholar] [CrossRef]
- Adachi, M.; Navessin, T.; Xie, Z.; Frisken, B.; Holdcroft, S. Correlation of In Situ and Ex Situ Measurements of Water Permeation Through Nafion NRE211 Proton Exchange Membranes. J. Electrochem. Soc. 2009, 156, B782–B790. [Google Scholar] [CrossRef]
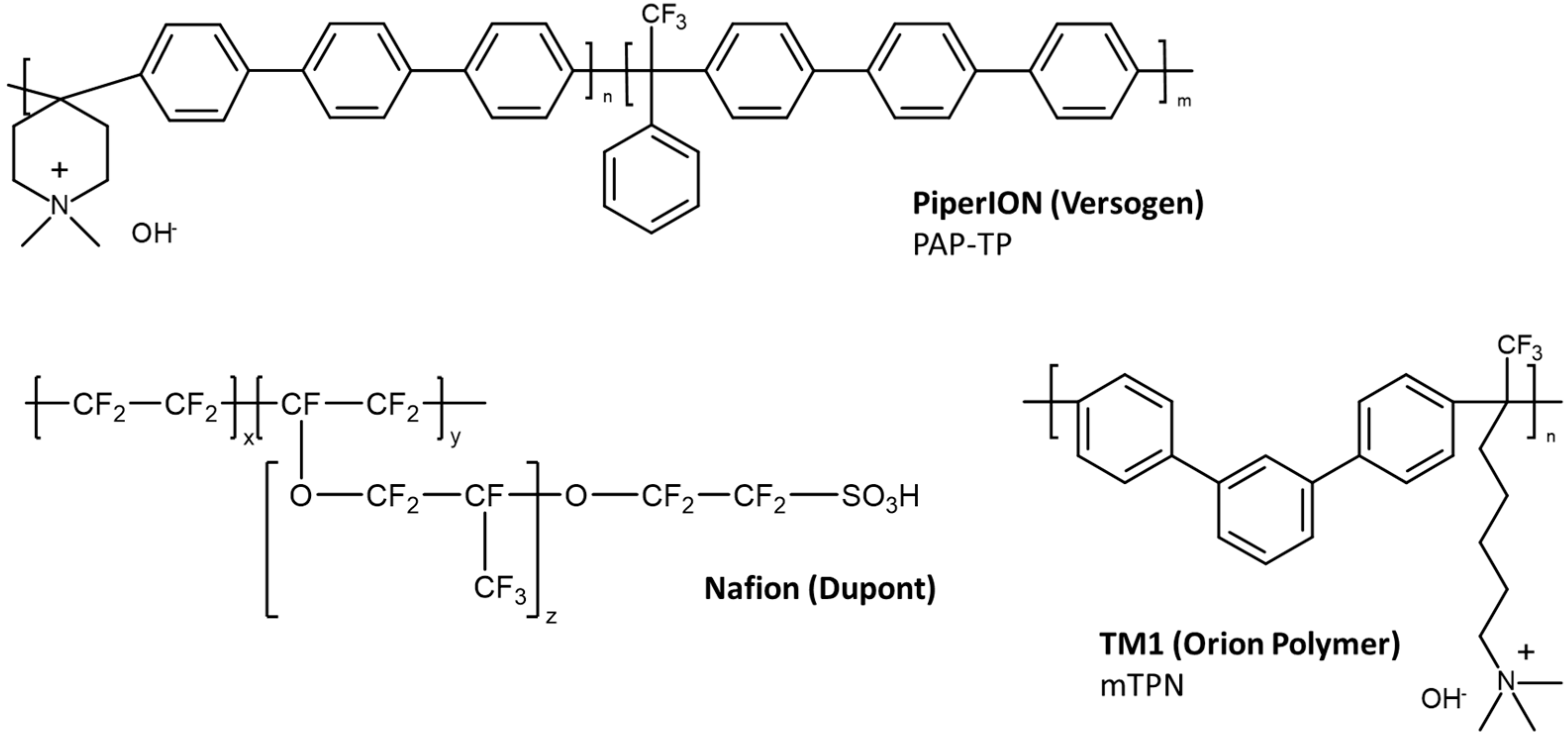


| Dupont Nafion 212 | PiperION PI-15 | PiperION PI-20 | Orion Polymer TM1 (mTPN) | PBI/mTPN | Fumatech FAA3-50 | Fumatech FAA3-PK-75 | |
|---|---|---|---|---|---|---|---|
| Type | CEM | AEM, reinforced with ePTFE | AEM | AEM | AEM, reinforced with a PBI nanofiber mat | AEM | AEM, reinforced with polyetherketone |
| Nominal thickness [µm] | 50 | 15 | 20 | Variable; made by casting from mTPBr [18] | Variable; made according to [17] | 50 | 75 |
| IEC [mmol/g] | 0.92 | na | 2.35 a | 2.20 b | 1.62 c [17] | 1.65–2.18 c | 1.23–1.44 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, H.; Najibah, M.; Park, H.S.; Bae, C.; Henkensmeier, D. Properties of Anion Exchange Membranes with a Focus on Water Electrolysis. Membranes 2022, 12, 989. https://doi.org/10.3390/membranes12100989
Khalid H, Najibah M, Park HS, Bae C, Henkensmeier D. Properties of Anion Exchange Membranes with a Focus on Water Electrolysis. Membranes. 2022; 12(10):989. https://doi.org/10.3390/membranes12100989
Chicago/Turabian StyleKhalid, Hamza, Malikah Najibah, Hyun S. Park, Chulsung Bae, and Dirk Henkensmeier. 2022. "Properties of Anion Exchange Membranes with a Focus on Water Electrolysis" Membranes 12, no. 10: 989. https://doi.org/10.3390/membranes12100989
APA StyleKhalid, H., Najibah, M., Park, H. S., Bae, C., & Henkensmeier, D. (2022). Properties of Anion Exchange Membranes with a Focus on Water Electrolysis. Membranes, 12(10), 989. https://doi.org/10.3390/membranes12100989