Novel TiO2/GO-Al2O3 Hollow Fiber Nanofiltration Membrane for Desalination and Lignin Recovery
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of α-Al2O3 Hollow Fiber Support
2.3. Preparation of Graphene Oxide (GO)
2.4. Preparation of TiO2/GO-Al2O3 Hollow Fiber (HF) NANOFILTRATION (NF) Membrane
2.5. Characterization of Al2O3 HF Support and TiO2/GO-Al2O3 HF NF Membrane
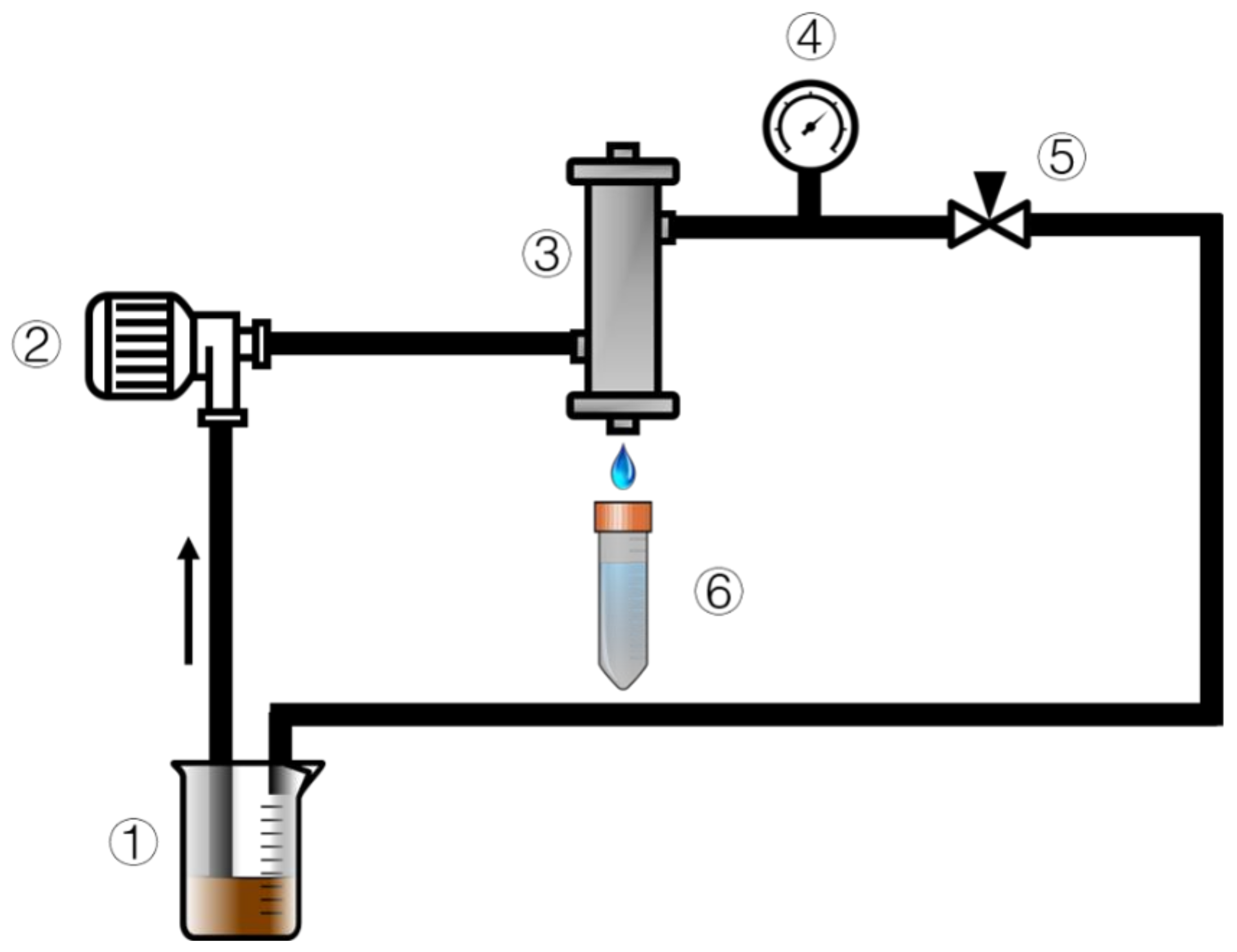
2.6. TiO2/GO-Al2O3 HF NF Membrane Permeation Test, Mean Pore Size and Flux Recovery Rate
2.7. Long-Term Test in Lignin Wastewater
3. Results and Discussion
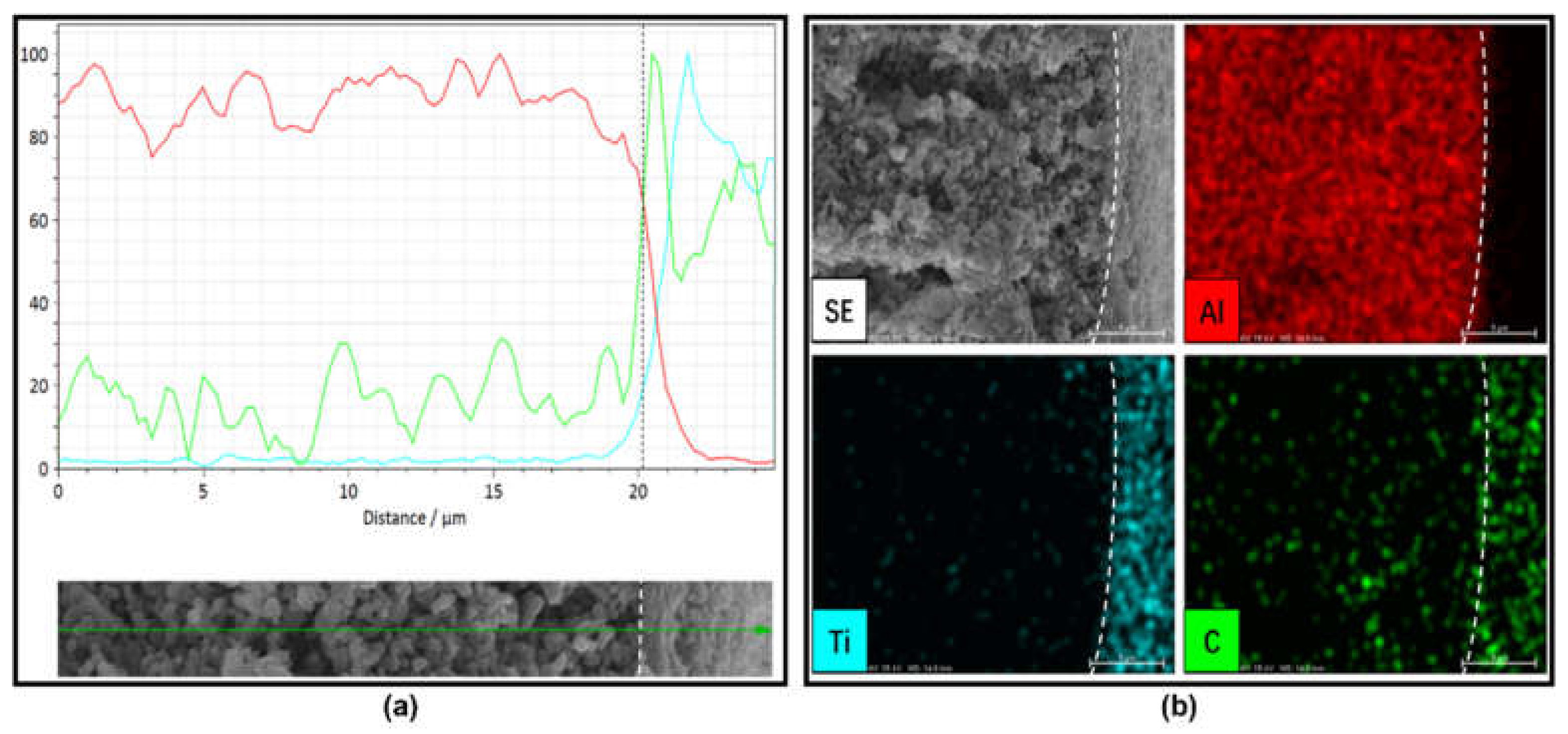
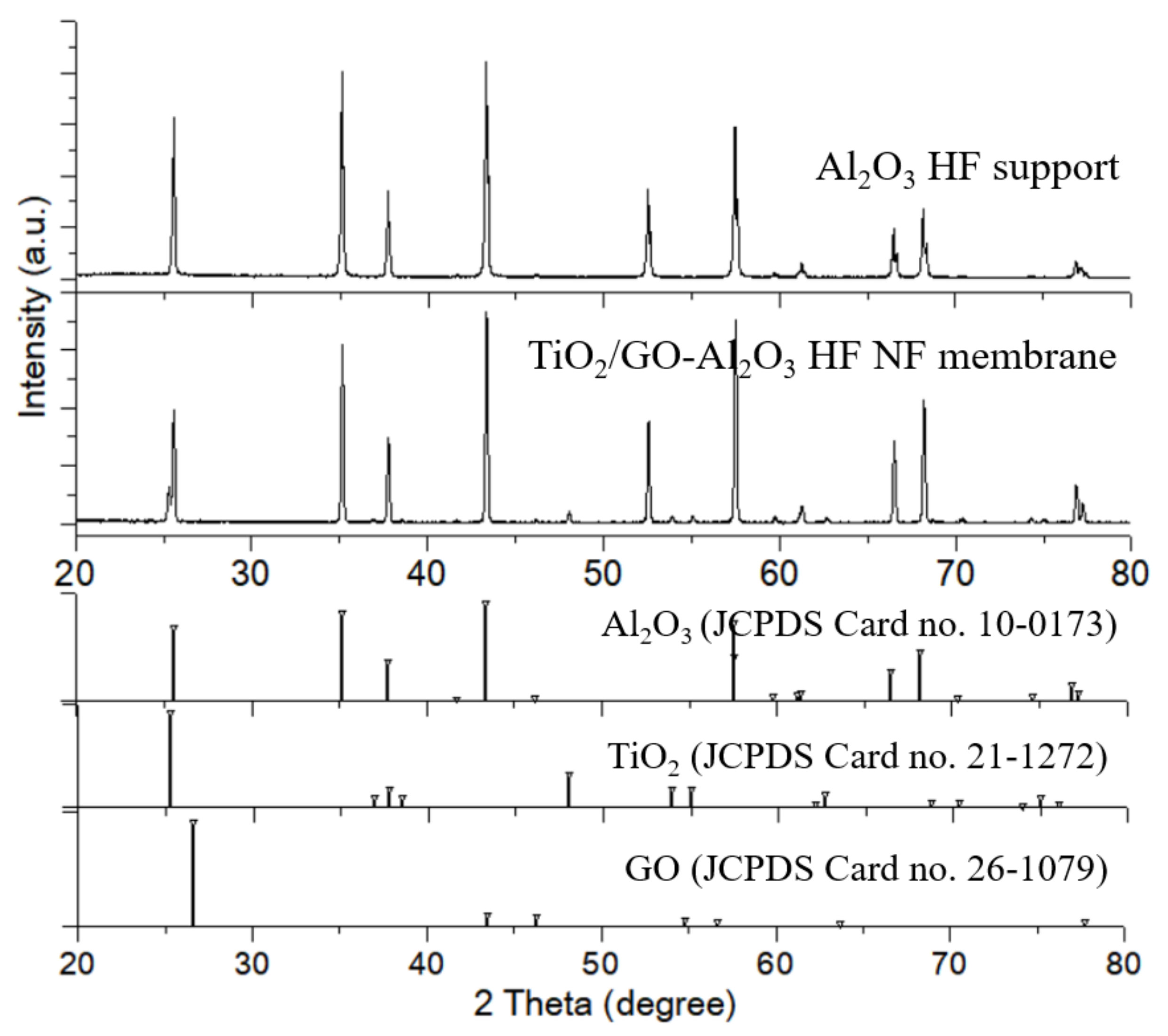
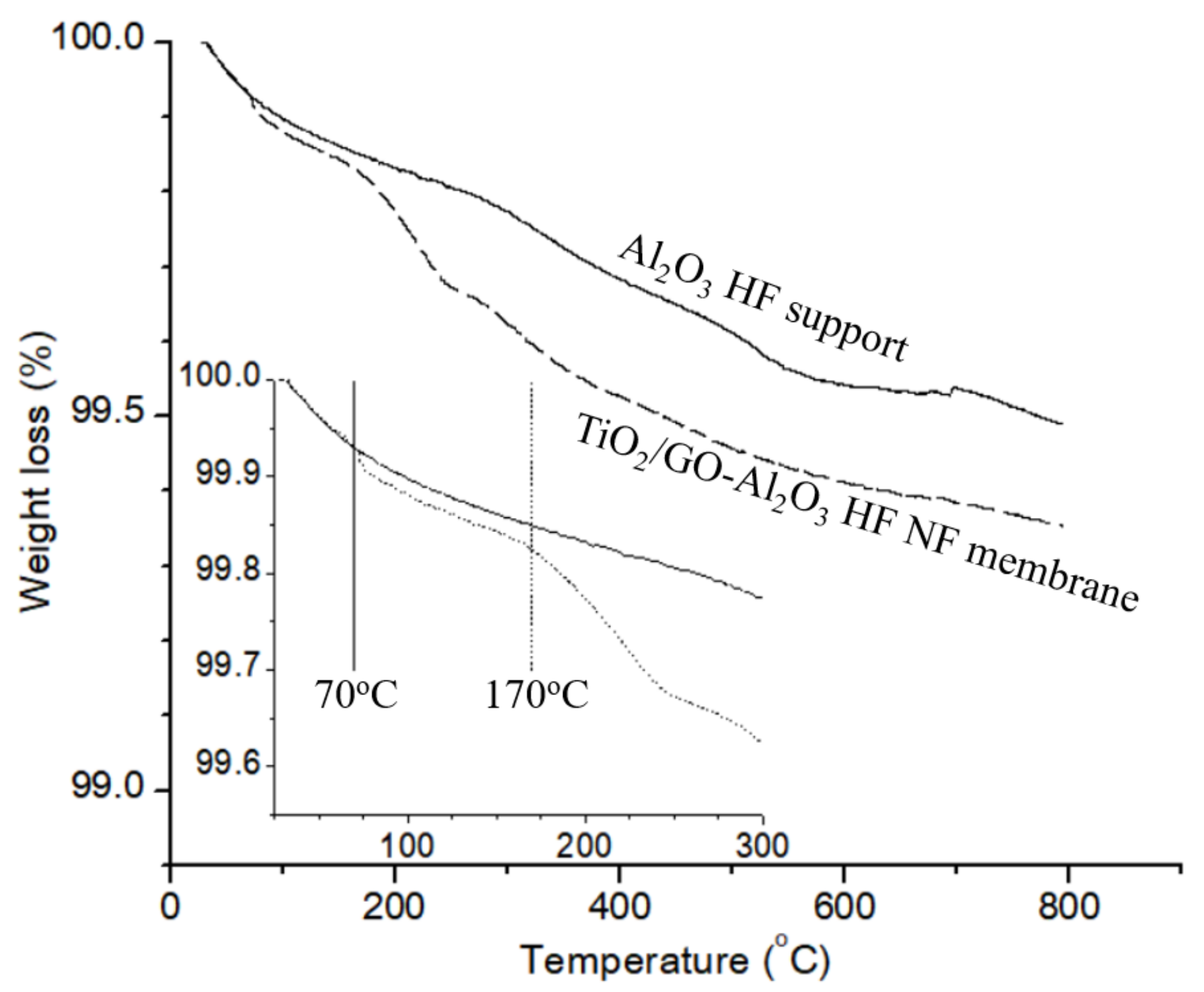
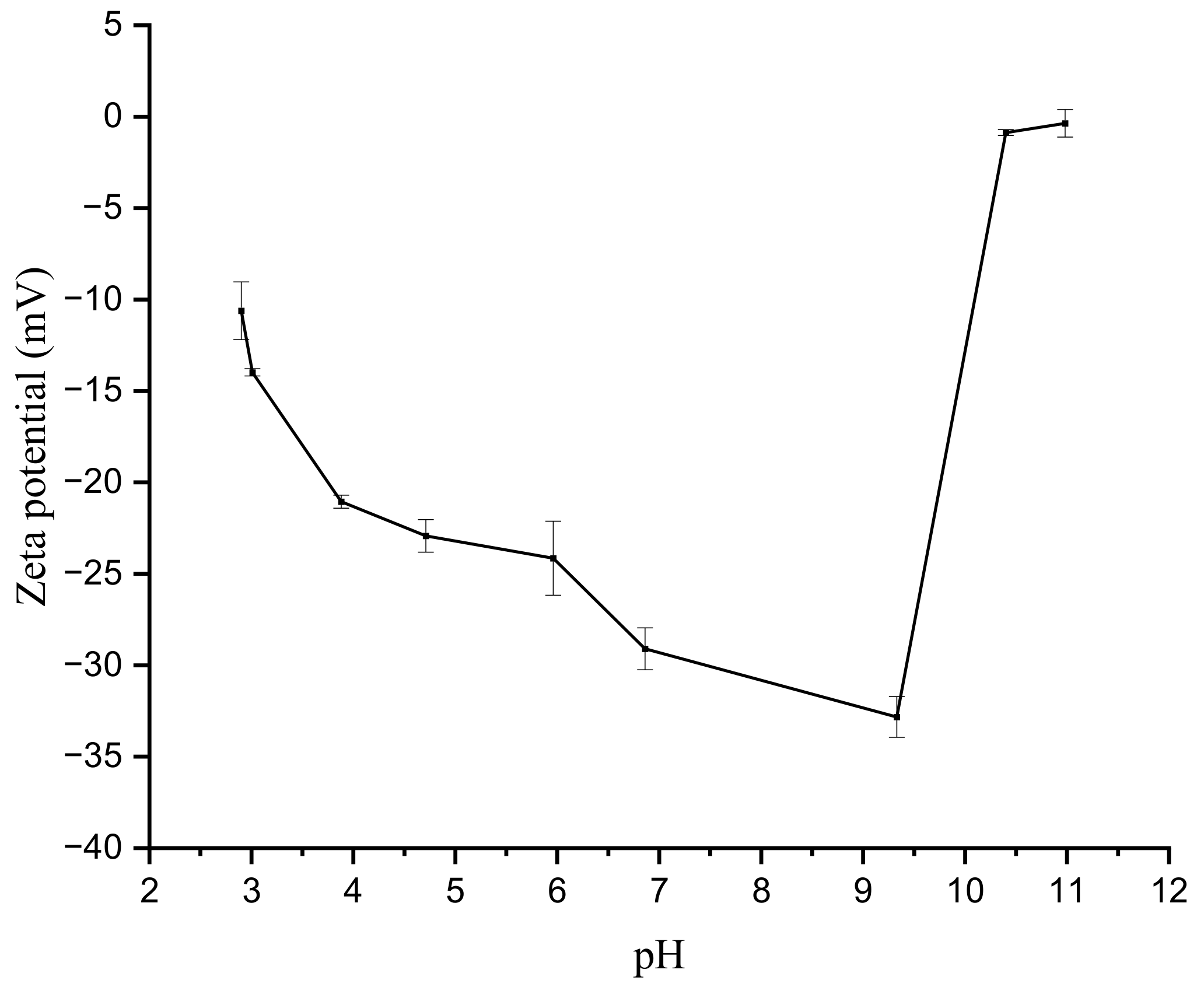
3.1. Characterization of Al2O3 HF Support and TiO2/GO-Al2O3 HF NF Membrane
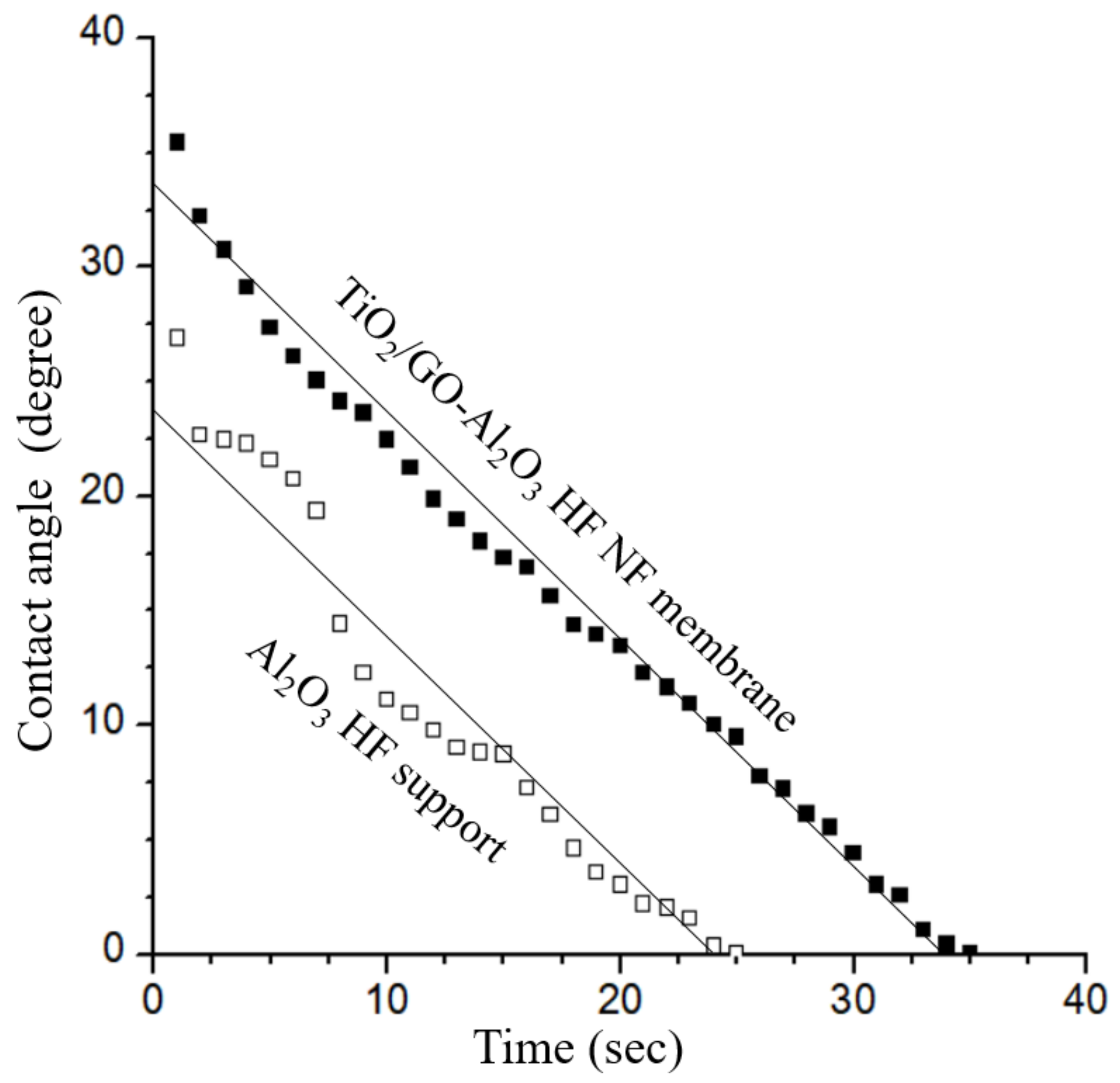
3.2. Surface Hydrophilicity and Porosity
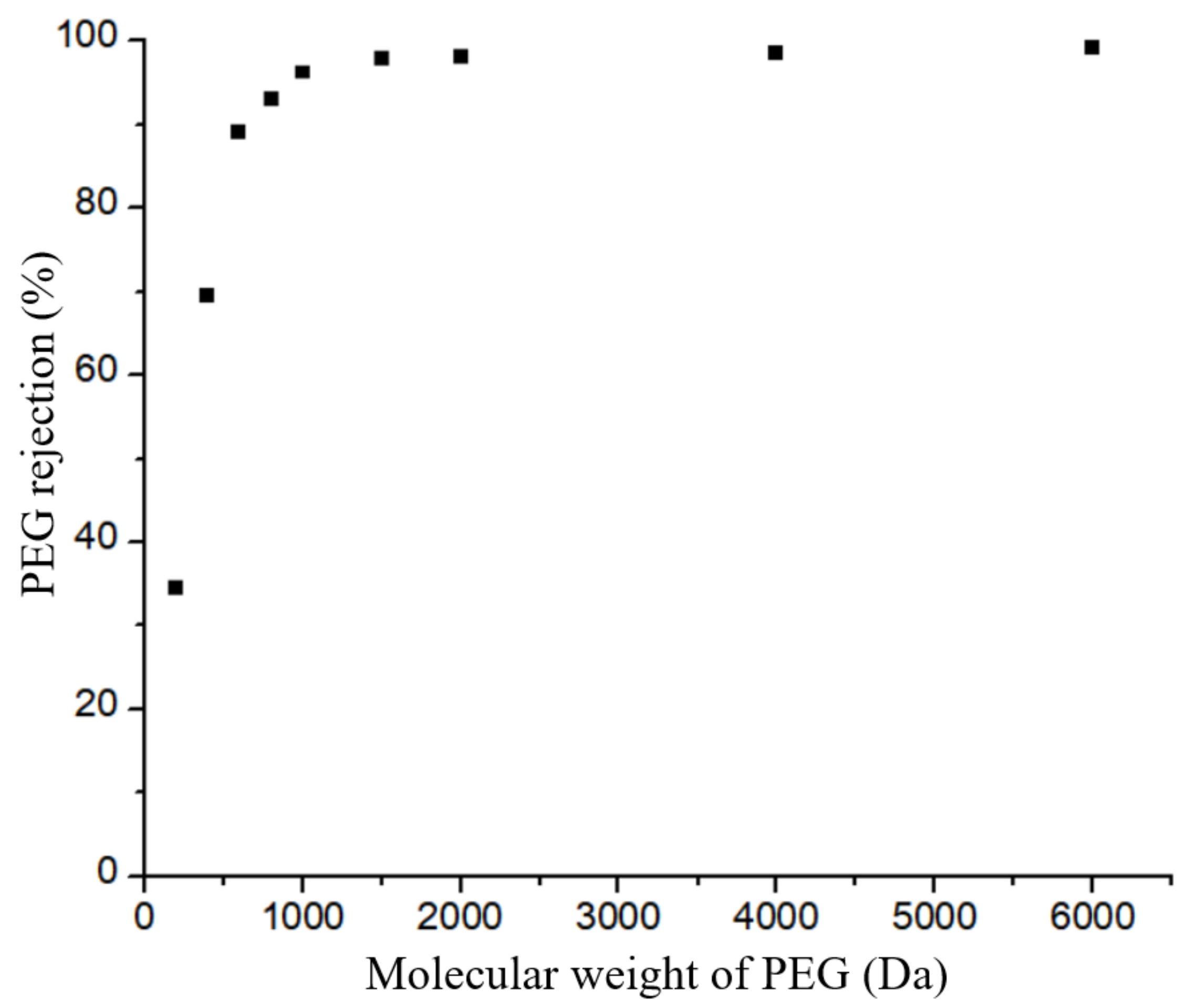
3.3. Permeation Test
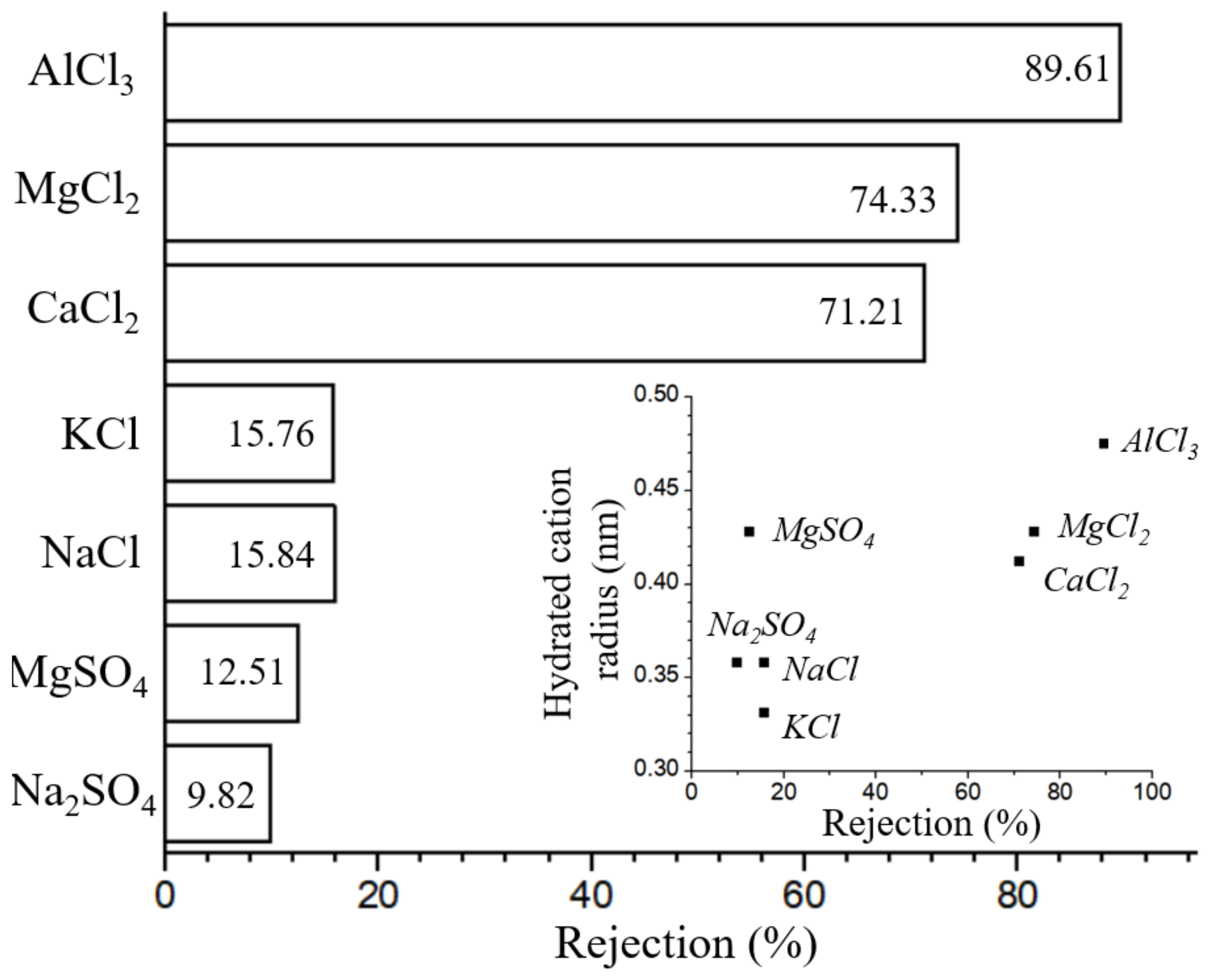
3.4. Long-Term Separation Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Małachowska, E.; Dubowik, M.; Boruszewski, P.; Łojewska, J.; Przybysz, P. Influence of lignin content in cellulose pulp on paper durability. Sci. Rep. 2020, 10, 19998. [Google Scholar] [CrossRef] [PubMed]
- Acciardo, E.; Tabasso, S.; Cravotto, G.; Bensaid, S. Process intensification strategies for lignin valorization. Chem. Eng. Process. 2022, 171, 108732. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Yang, Y.; Ge, B.; Meng, F. Research and application progress of lignin-based composite membrane. J. Polym. Eng. 2021, 41, 245–258. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Liu, Y.; Ruan, X.; Tsang, D.C.W.; Hunt, A.J.; Ok, Y.S.; Song, H.; Zhang, S. Lignin valorization for the production of renewable chemicals: State-of-the-art Review and Future Prospects. Bioresour. Technol. 2018, 269, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Norgren, M.; Edlund, H. Lignin: Recent advances and emerging applications. Curr. Opin. Colloid Interface Sci. 2014, 19, 409–416. [Google Scholar] [CrossRef]
- Jönsson, A.; Nordin, A.; Wallberg, O. Concentration and purification of lignin in hardwood kraft pulping liquor by ultrafiltration and nanofiltration. Chem. Eng. Res. Des. 2008, 86, 1271–1280. [Google Scholar] [CrossRef]
- Rashidi, F.; Kevlich, N.S.; Sinquefield, S.A.; Shofner, M.L.; Nair, S. Graphene oxide membranes in extreme operating environments: Concentration of Kraft black liquor by lignin retention. ACS Sustain. Chem. Eng. 2017, 5, 1002–1009. [Google Scholar] [CrossRef]
- Bai, L.; Ding, J.; Wang, H.; Ren, N.; Li, G.; Liang, H. High-performance nanofiltration membranes with a sandwiched layer and a surface layer for desalination and environmental pollutant removal. Sci. Total Environ. 2020, 743, 140766. [Google Scholar] [CrossRef]
- Manorma; Ferreira, I.; Alves, P.; Gil, M.H.; Gando-Ferreira, L.M. Lignin separation from black liquor by mixed matrix polysulfone nanofiltration membrane filled with multiwalled carbon nanotubes. Sci. Total Environ. 2021, 260, 118231. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Busse, N.; Kerker, S.; Schmitz, O.; Hilpert, M.; Czermak, P. Treatment of the bleaching effluent from sulfite pulp production by ceramic membrane filtration. Membranes 2016, 6, 7. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Yang, G.; Chen, J. Improvement membrane filterability in nanofiltration of prehydrolysis liquor of Kraft dissolving pulp by Laccase treatment. Bioresour. Technol. 2015, 181, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Arkell, A.; Olsson, J.; Wallberg, O. Process performance in lignin separation from softwood black liquor by membrane filtration. Chem. Eng. Res. Des. 2014, 92, 1792–1800. [Google Scholar] [CrossRef]
- Lee, K.H.; Khan, I.A.; Song, L.H.; Kim, J.Y.; Kim, J.-O. Evaluation of structural/performance variation between α-Al2O3 and polyvinylidene fluoride membranes under long-term clean-in-place treatment used for water treatment. Desalination 2022, 538, 115921. [Google Scholar] [CrossRef]
- Singha, I.; Kumar Mishrab, P. Nano-membrane filtration a novel application of nanotechnology for waste water treatment. Mater. Today Proc. 2020, 29, 327–332. [Google Scholar] [CrossRef]
- Benfer, S.; Árki, P.; Tomandl, G. Ceramic membranes for filtration applications—Preparation and characterization. Adv. Eng. Mater. 2004, 6, 495–500. [Google Scholar] [CrossRef]
- Shi, W.; Yang, C.; Qiu, M.; Chen, X.; Fan, Y. A new method for preparing α-Alumina ultrafiltration membrane at low sintering temperature. J. Membr. Sci. 2022, 642, 119992. [Google Scholar] [CrossRef]
- Zhuang, X.; Shin, M.C.; Jeong, B.J.; Hwang, J.Y.; Choi, Y.C.; Park, J.H. Desalination and lignin concentration in a lignin aqueous solution by nano-filtration process: Advanced γ-Al2O3 film-coated porous α-Al2O3 hollow fiber membrane. Korean J. Chem. Eng. 2022, 39, 1588–1596. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, Y.-M.; Xu, Z.-L.; Cao, Y.; Dong, Z.-Q.; Shi, X.-L. Preparation, characterization and solvent resistance of γ-Al2O3/α-Al2O3 inorganic hollow fiber nanofiltration membrane. J. Membr. Sci. 2016, 503, 69–80. [Google Scholar] [CrossRef]
- Zahir, M.H.; Nagano, T.; Rahman, M.M.; Alhooshani, K.; Chowdhury, S.; Aziz, M.A. Microstructural investigations of tubular α-Al2O3-supported γ-Al2O3 membranes and their hydrothermal improvement. J. Eur. Ceram. Soc. 2017, 37, 2637–2647. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Balta, S.; Darvishmanash, S.; Kim, J.; Van der Bruggen, B. Nanofiltration membranes enhanced with TiO2 nanoparticles: A comprehensive study. Desalin. Wate Treat. 2011, 34, 179–183. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, B.X.; Li, J.M.; Chen, J.H.; Tao, J. Preparation and characterisation of a TiO2/α-Al2O3 nanofiltration membrane by a sol–gel method. Mater. Res. Innov. 2015, 19, S2-2–S2-9. [Google Scholar] [CrossRef]
- Khalili, M.; Sabbaghi, S.; Zerafat, M.M. Preparation of ceramic γ-Al2O3-TiO2 nanofiltration membranes for desalination. Chem. Pap. 2015, 69, 309–315. [Google Scholar] [CrossRef]
- Wang, J.; Niu, S.; Fei, Y.; Qi, H. Fabrication and stability of GO/Al2O3 composite nanofiltration membrane. CIESC J. 2020, 71, 2795–2803. [Google Scholar]
- Hu, X.; Yu, Y.; Lin, N.; Ren, S.; Zhang, X.; Wang, Y.; Zhou, J. Graphene oxide/Al2O3 membrane with efficient salt rejection for water purification. Water Supply 2018, 18, 2162–2169. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, G.; Xu, N.; Shi, J. Preparation and application in oil–water separation of ZrO2/α-Al2O3 MF membrane. J. Membr. Sci. 1998, 142, 235–243. [Google Scholar] [CrossRef]
- Van Gestel, T.; Vandecasteele, C.; Buekenhoudt, A.; Dotremont, C.; Luyten, J.; Van der Bruggen, B.; Maes, G. Corrosion Properties of Alumina and Titania NF membranes. J. Membr. Sci. 2003, 214, 21–29. [Google Scholar] [CrossRef]
- Xu, C.; Cui, A.; Xu, Y.; Fu, X. Graphene oxide-TiO2 composite filtration membranes and their potential application for water purification. Carbon 2013, 62, 465–471. [Google Scholar] [CrossRef]
- Zhuang, X.; Shin, M.C.; Jeong, B.J.; Lee, S.H.; Park, J.H. Fabrication of K-PHI Zeolite coated Alumina hollow fiber membrane and study on removal characteristics of metal ions in lignin wastewater. Korean Chem. Eng. Res. 2021, 59, 174–179. [Google Scholar]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Zhu, Y.; Kong, G.; Pan, Y.; Liu, L.; Yang, B.; Zhang, S.; Lai, D.; Che, C. An improved Hummers method to synthesize graphene oxide using much less concentrated sulfuric acid. Chin. Chem. Lett. 2022, 33, 4541–4544. [Google Scholar] [CrossRef]
- Lee, H.J.; Magnone, E.; Park, J.H. Preparation, characterization and laboratory-scale application of modified hydrophobic aluminum oxide hollow fiber membrane for CO2 capture using H2O as low-cost absorbent. J. Membr. Sci. 2015, 494, 143–153. [Google Scholar] [CrossRef]
- Magnone, E.; Lee, H.J.; Che, J.W.; Park, J.H. High-performance of modified Al2O3 hollow fiber membranes for CO2 absorption at room temperature. J. Ind. Eng. Chem. 2016, 42, 19–22. [Google Scholar] [CrossRef]
- Kim, M.K.; Pak, S.-H.; Shin, M.C.; Park, C.; Magnone, E.; Park, J.H. Development of an advanced hybrid process coupling TiO2 photocatalysis and zeolite-based adsorption for water and wastewater treatment. Korean J. Chem. Eng. 2019, 36, 1201–1207. [Google Scholar] [CrossRef]
- Magnone, E.; Hwang, J.Y.; Shin, M.C.; Zhuang, X.; Lee, J.I.; Park, J.H. Al2O3-based hollow fiber membranes functionalized by nitrogen-doped titanium dioxide for photocatalytic degradation of ammonia gas. Membranes 2022, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Zhu, S.; Luo, X.; Zou, M.; Huang, S. Ultraviolet-visible spectroscopy of graphene oxides. AIP Adv. 2012, 2, 032146. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Çiplak, Z.; Yildiz, N.; Çalimli, A. Investigation of graphene/Ag nanocomposites synthesis parameters for two different synthesis methods. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 361–370. [Google Scholar] [CrossRef]
- Rokmana, A.W.; Asriani, A.; Suhendar, H.; Triyana, K.; Kusumaatmaja, A.; Santoso, I. The optical properties of thin film reduced graphene oxide/poly (3,4 ethylenedioxtriophene):poly (styrene sulfonate)(PEDOT:PSS) fabricated by spin coating. J. Phys. Conf. Ser. 2018, 1011, 012007. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Wang, B.; Gao, T.; Wang, D. A reduced graphene oxide modified metallic Cobalt composite with superior electrochemical performance for supercapacitors. RSC Adv. 2015, 5, 63553–63560. [Google Scholar] [CrossRef]
- Shawon, A.K.M.A.; Ur, S.-C. Mechanical and thermoelectric properties of bulk AlSb synthesized by controlled melting, pulverizing and subsequent vacuum hot pressing. Appl. Sci. 2019, 9, 1609. [Google Scholar] [CrossRef]
- Newman, J.A.; Schmitt, P.D.; Toth, S.J.; Deng, F.; Zhang, S.; Simpson, G.J. Parts per million powder X-ray diffraction. Anal. Chem. 2015, 87, 10950–10955. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.Y.S.; Huang, N.M.; An’amt, M.N.; Marlinda, A.R.; Norazriena, Y.; Muhamad, M.R.; Harrison, I.; Lim, H.N.; Chia, C.H. Facile hydrothermal preparation of titanium dioxide decorated reduced graphene oxide nanocomposite. Int. J. Nanomed. 2012, 7, 3379–3387. [Google Scholar]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]


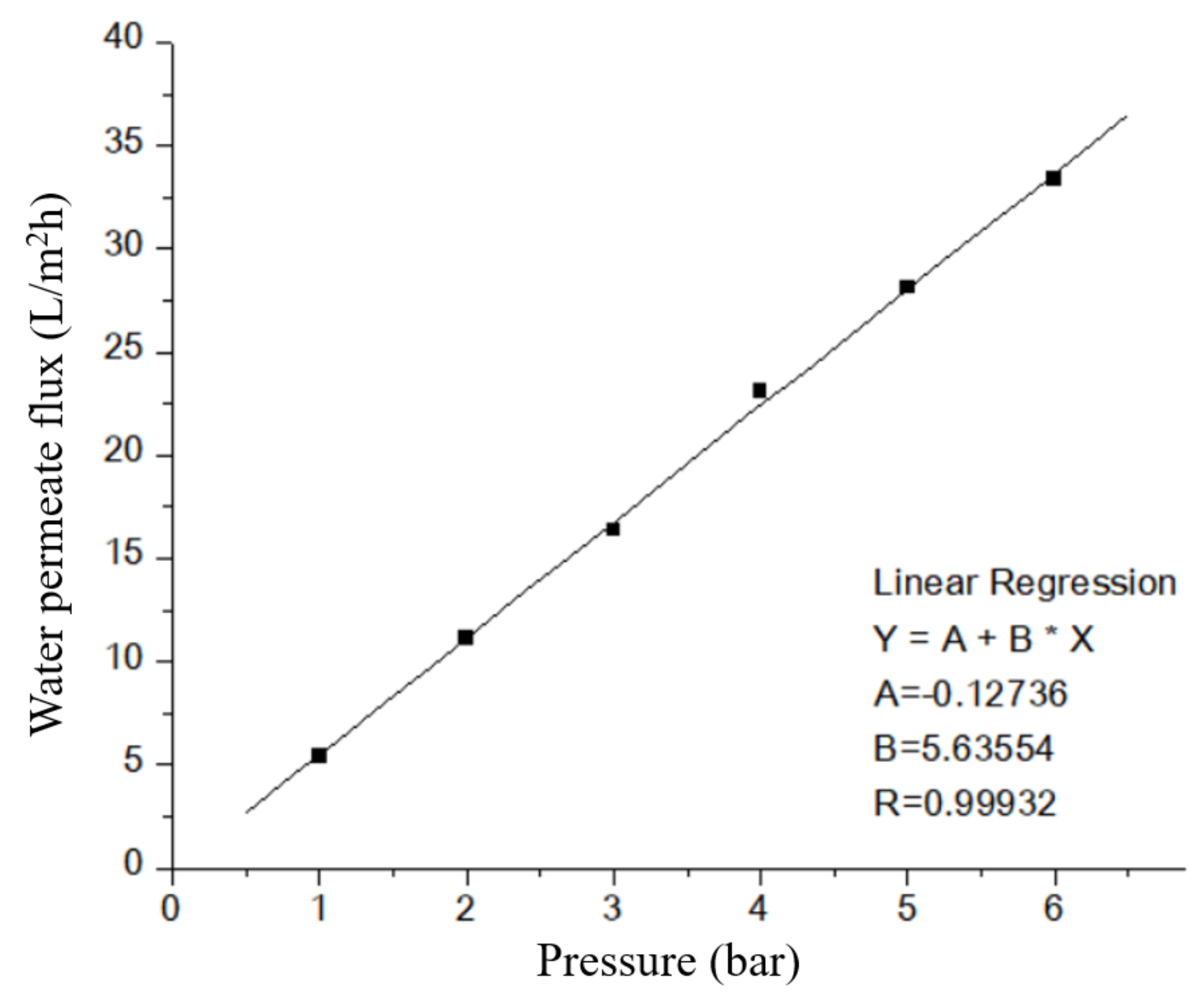

| Pressure (bar) | Water Permeate Flux (L/m2h) | ||
|---|---|---|---|
| 1st | 2nd | 3rd | |
| 1 | 5.452677 | 5.619573 | 5.328018 |
| 2 | 11.16083 | 10.98572 | 11.1781 |
| 3 | 16.36402 | 16.43361 | 16.37582 |
| 4 | 23.1098 | 22.73496 | 23.19718 |
| 5 | 28.11514 | 27.69619 | 28.07752 |
| 6 | 33.37971 | 33.37116 | 33.29028 |
| Fitting results | |||
| A | −0.12736 | −0.04553 | −0.15861 |
| B | 5.63554 | 5.57688 | 5.63803 |
| R | 0.99932 | 0.9997 | 0.99914 |
| Bending Strength (MPa) | Stand. Dev. | FRR (%) | Stand. Dev. |
|---|---|---|---|
| 28.97 | 7.81 | 86.03 | 2.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, X.; Magnone, E.; Shin, M.C.; Lee, J.I.; Hwang, J.Y.; Choi, Y.C.; Park, J.H. Novel TiO2/GO-Al2O3 Hollow Fiber Nanofiltration Membrane for Desalination and Lignin Recovery. Membranes 2022, 12, 950. https://doi.org/10.3390/membranes12100950
Zhuang X, Magnone E, Shin MC, Lee JI, Hwang JY, Choi YC, Park JH. Novel TiO2/GO-Al2O3 Hollow Fiber Nanofiltration Membrane for Desalination and Lignin Recovery. Membranes. 2022; 12(10):950. https://doi.org/10.3390/membranes12100950
Chicago/Turabian StyleZhuang, Xuelong, Edoardo Magnone, Min Chang Shin, Jeong In Lee, Jae Yeon Hwang, Young Chan Choi, and Jung Hoon Park. 2022. "Novel TiO2/GO-Al2O3 Hollow Fiber Nanofiltration Membrane for Desalination and Lignin Recovery" Membranes 12, no. 10: 950. https://doi.org/10.3390/membranes12100950
APA StyleZhuang, X., Magnone, E., Shin, M. C., Lee, J. I., Hwang, J. Y., Choi, Y. C., & Park, J. H. (2022). Novel TiO2/GO-Al2O3 Hollow Fiber Nanofiltration Membrane for Desalination and Lignin Recovery. Membranes, 12(10), 950. https://doi.org/10.3390/membranes12100950










