Microtransplantation of Postmortem Native Synaptic mGluRs Receptors into Xenopus Oocytes for Their Functional Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Xenopus Oocytes
2.2. Brain Samples
2.3. Synaptosome Isolation
2.4. Injection of Synaptosome Preparations
2.5. Two Electrode Voltage Clamp
2.6. Drugs
2.7. Immunoblots
2.8. Statistics
3. Results
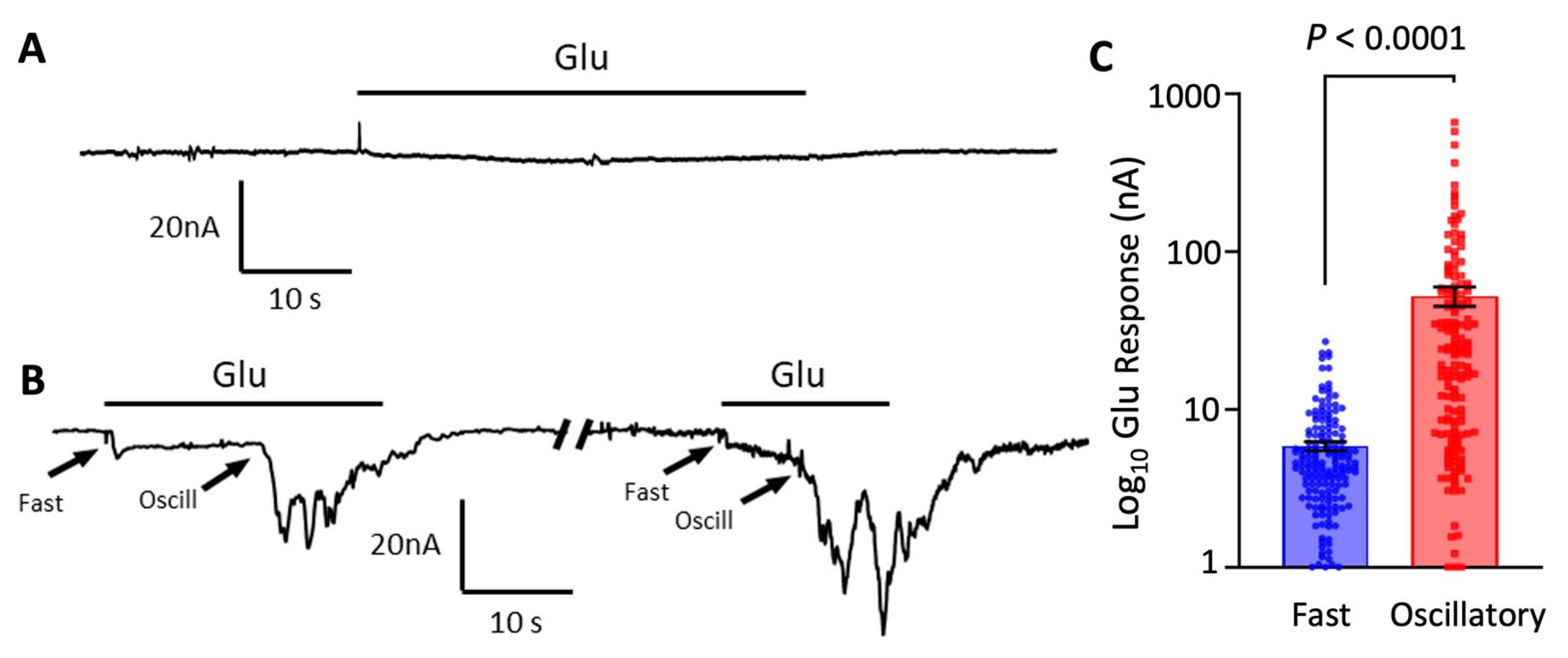
3.1. Oscillatory Responses to Glutamate in Oocytes Microtransplanted with Cortical Synaptic Membranes
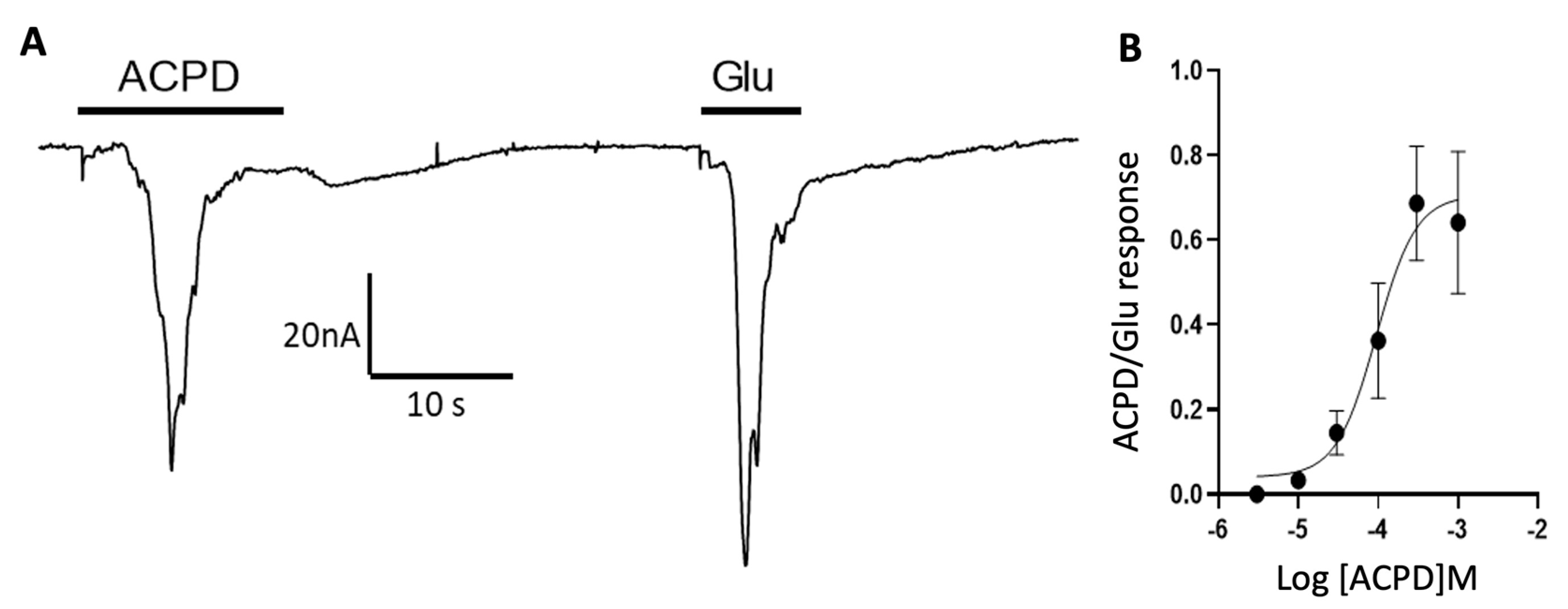
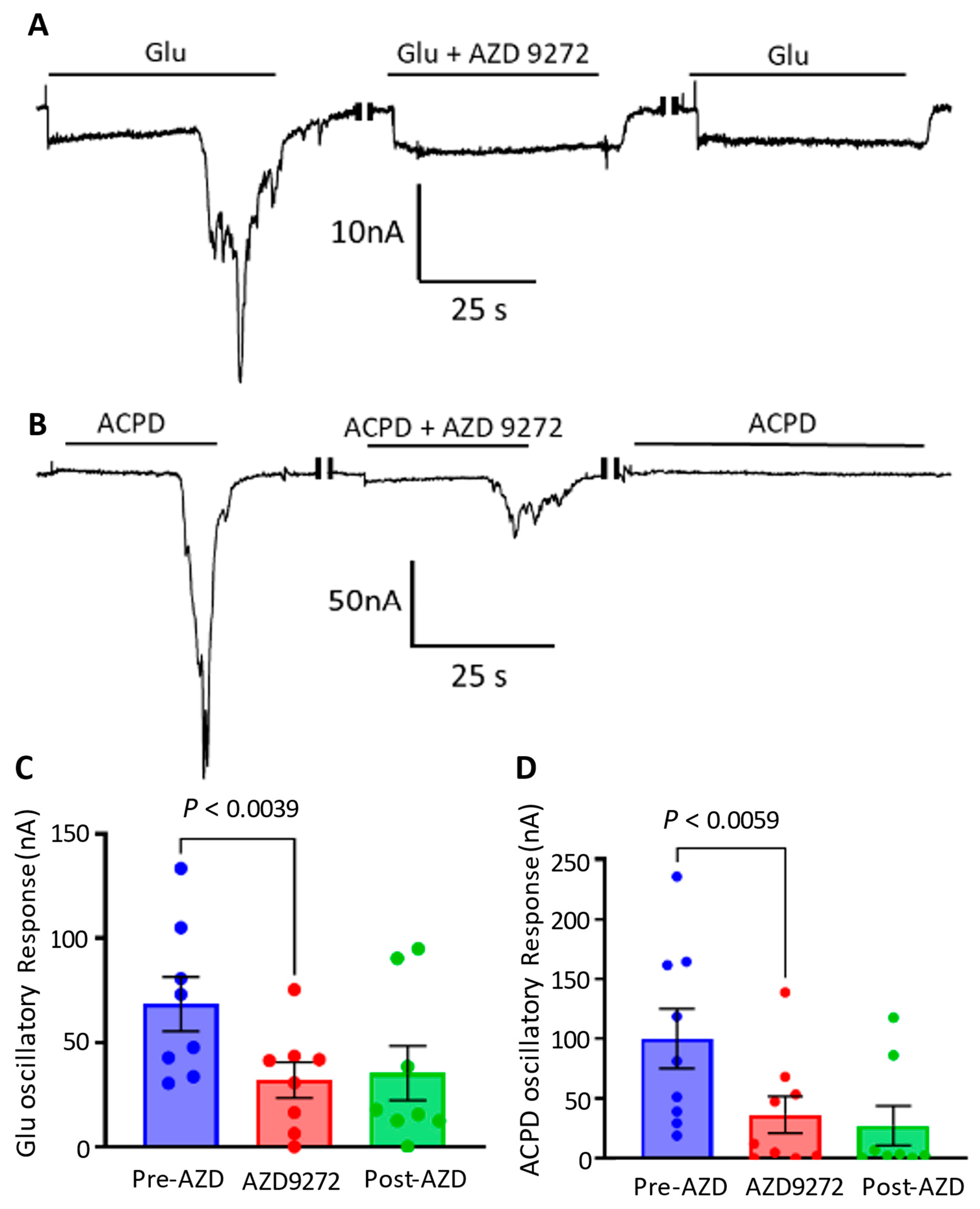
3.2. Participation of Cortical Group I Metabotropic Glutamate Receptors in Oscillatory Responses
3.3. Cortical mGluR 5 Type Receptors Are Mostly Responsible for Oscillatory Responses
3.4. Presence of mGluR1 and mGLuR5 in Cortical Synaptosomal Preparations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nedergaard, M.; Takano, T.; Hansen, A.J. Beyond the Role of Glutamate as a Neurotransmitter. Nat. Rev. Neurosci. 2002, 3, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Grewer, C.; Gameiro, A.; Zhang, Z.; Tao, Z.; Braams, S.; Rauen, T. Glutamate Forward and Reverse Transport: From Molecular Mechanism to Transporter-Mediated Release after Ischemia. IUBMB Life 2008, 60, 609–619. [Google Scholar] [CrossRef] [PubMed]
- DeLong, M.R.; Wichmann, T. Basal Ganglia Circuits as Targets for Neuromodulation in Parkinson Disease. JAMA Neurol. 2015, 72, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Bhandage, A.; Bazov, I.; Kononenko, O.; Bakalkin, G.; Korpi, E.; Birnir, B. Selective Increases of AMPA, NMDA, and Kainate Receptor Subunit MRNAs in the Hippocampus and Orbitofrontal Cortex but Not in Prefrontal Cortex of Human Alcoholics. Front. Cell. Neurosci. 2014, 8, 11. [Google Scholar] [CrossRef]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73, 298–487. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Mao, L.-M.; Bodepudi, A.; Chu, X.-P.; Wang, J.Q. Group I Metabotropic Glutamate Receptors and Interacting Partners: An Update. Int. J. Mol. Sci. 2022, 23, 840. [Google Scholar] [CrossRef]
- Grueter, B.A.; Winder, D.G. Group II and III Metabotropic Glutamate Receptors Suppress Excitatory Synaptic Transmission in the Dorsolateral Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology 2005, 30, 1302–1311. [Google Scholar] [CrossRef]
- Crupi, R.; Impellizzeri, D.; Cuzzocrea, S. Role of Metabotropic Glutamate Receptors in Neurological Disorders. Front. Mol. Neurosci. 2019, 12, 20. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, H. Role of Glutamate and NMDA in Alzheimer’s Desease. J. Alzheimer’s Desese 2017, 57, 1041–1048. [Google Scholar] [CrossRef]
- Platt, S.R. The Role of Glutamate in Central Nervous System Health and Disease–A Review. Vet. J. 2007, 173, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Madeira, C.; Vargas-Lopes, C.; Brandão, C.O.; Reis, T.; Laks, J.; Panizzutti, R.; Ferreira, S.T. Elevated Glutamate and Glutamine Levels in the Cerebrospinal Fluid of Patients With Probable Alzheimer’s Disease and Depression. Front. Psychiatry 2018, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, R.M.; Deng, A.; Wurtman, R.J.; Growdon, J.H. Metabotropic Glutamate Receptor Subtype MGlur1α Stimulates the Secretion of the Amyloid β-Protein Precursor Ectodomain. J. Neurochem. 1997, 69, 704–712. [Google Scholar] [CrossRef]
- Ishibashi, K.; Miura, Y.; Toyohara, J.; Ishiwata, K.; Ishii, K. Unchanged Type 1 Metabotropic Glutamate Receptor Availability in Patients with Alzheimer’s Disease: A Study Using (11)C-ITMM Positron Emission Tomography. NeuroImage Clin. 2019, 22, 101783. [Google Scholar] [CrossRef] [PubMed]
- Ostapchenko, V.G.; Beraldo, F.H.; Guimarães, A.L.S.; Mishra, S.; Guzman, M.; Fan, J.; Martins, V.R.; Prado, V.F.; Prado, M.A.M. Increased Prion Protein Processing and Expression of Metabotropic Glutamate Receptor 1 in a Mouse Model of Alzheimer’s Disease. J. Neurochem. 2013, 127, 415–425. [Google Scholar] [CrossRef]
- Albasanz, J.L.; Dalfó, E.; Ferrer, I.; Martín, M. Impaired Metabotropic Glutamate Receptor/Phospholipase C Signaling Pathway in the Cerebral Cortex in Alzheimer’s Disease and Dementia with Lewy Bodies Correlates with Stage of Alzheimer’s-Disease-Related Changes. Neurobiol. Dis. 2005, 20, 685–693. [Google Scholar] [CrossRef]
- Caraci, F.; Nicoletti, F.; Copani, A. Metabotropic Glutamate Receptors: The Potential for Therapeutic Applications in Alzheimer’s Disease. Curr. Opin. Pharmacol. 2018, 38, 1–7. [Google Scholar] [CrossRef]
- Um, J.W.; Kaufman, A.C.; Kostylev, M.; Heiss, J.K.; Stagi, M.; Takahashi, H.; Kerrisk, M.E.; Vortmeyer, A.; Wisniewski, T.; Koleske, A.J.; et al. Metabotropic Glutamate Receptor 5 Is a Coreceptor for Alzheimer Aβ Oligomer Bound to Cellular Prion Protein. Neuron 2013, 79, 887–902. [Google Scholar] [CrossRef]
- Hamilton, A.; Esseltine, J.L.; DeVries, R.A.; Cregan, S.P.; Ferguson, S.S.G. Metabotropic Glutamate Receptor 5 Knockout Reduces Cognitive Impairment and Pathogenesis in a Mouse Model of Alzheimer’s Disease. Mol. Brain 2014, 7, 40. [Google Scholar] [CrossRef]
- Hamilton, A.; Vasefi, M.; Vander Tuin, C.; McQuaid, R.J.; Anisman, H.; Ferguson, S.S.G. Chronic Pharmacological MGluR5 Inhibition Prevents Cognitive Impairment and Reduces Pathogenesis in an Alzheimer Disease Mouse Model. Cell Rep. 2016, 15, 1859–1865. [Google Scholar] [CrossRef]
- Paquet, M.; Asay, M.J.; Fam, S.R.; Inuzuka, H.; Castleberry, A.M.; Oller, H.; Smith, Y.; Yun, C.C.; Traynelis, S.F.; Hall, R.A. The PDZ Scaffold NHERF-2 Interacts with MGluR5 and Regulates Receptor Activity. J. Biol. Chem. 2006, 281, 29949–29961. [Google Scholar] [CrossRef]
- Tu, J.C.; Xiao, B.; Naisbitt, S.; Yuan, J.P.; Petralia, R.S.; Brakeman, P.; Doan, A.; Aakalu, V.K.; Lanahan, A.A.; Sheng, M.; et al. Coupling of MGluR/Homer and PSD-95 Complexes by the Shank Family of Postsynaptic Density Proteins. Neuron 1999, 23, 583–592. [Google Scholar] [CrossRef]
- Giuffrida, R.; Musumeci, S.; D’Antoni, S.; Bonaccorso, C.M.; Giuffrida-Stella, A.M.; Oostra, B.A.; Catania, M.V. A Reduced Number of Metabotropic Glutamate Subtype 5 Receptors Are Associated with Constitutive Homer Proteins in a Mouse Model of Fragile X Syndrome. J. Neurosci. 2005, 25, 8908–8916. [Google Scholar] [CrossRef]
- Ronesi, J.A.; Collins, K.A.; Hays, S.A.; Tsai, N.-P.; Guo, W.; Birnbaum, S.G.; Hu, J.-H.; Worley, P.F.; Gibson, J.R.; Huber, K.M. Disrupted Homer Scaffolds Mediate Abnormal MGluR5 Function in a Mouse Model of Fragile X Syndrome. Nat. Neurosci. 2012, 15, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Piers, T.; Kim, D.H.; Kim, B.; Regan, P.; Whitcomb, D.; Cho, K. Translational Concepts of MGluR5 in Synaptic Diseases of the Brain. Front. Pharmacol. 2012, 3, 199. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef]
- Mazzo, F.; Zwart, R.; Serratto, G.M.; Gardinier, K.M.; Porter, W.; Reel, J.; Maraula, G.; Sher, E. Reconstitution of Synaptic Ion Channels from Rodent and Human Brain in Xenopus Oocytes: A Biochemical and Electrophysiological Characterization. J. Neurochem. 2016, 138, 384–396. [Google Scholar] [CrossRef]
- Zhang, D.; Watson, J.F.; Matthews, P.M.; Cais, O.; Greger, I.H. Gating and Modulation of a Hetero-Octameric AMPA Glutamate Receptor. Nature 2021, 594, 454–458. [Google Scholar] [CrossRef]
- Menuz, K.; Stroud, R.M.; Nicoll, R.A.; Hays, F.A. TARP Auxiliary Subunits Switch AMPA Receptor Antagonists into Partial Agonists. Science 2007, 318, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Marsal, J.; Tigyi, G.; Miledi, R. Incorporation of Acetylcholine Receptors and Cl- Channels in Xenopus Oocytes Injected with Torpedo Electroplaque Membranes. Proc. Natl. Acad. Sci. USA 1995, 92, 5224–5228. [Google Scholar] [CrossRef]
- Eusebi, F.; Palma, E.; Amici, M.; Miledi, R. Microtransplantation of Ligand-Gated Receptor-Channels from Fresh or Frozen Nervous Tissue into Xenopus Oocytes: A Potent Tool for Expanding Functional Information. Prog. Neurobiol. 2009, 88, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zwart, R.; Mazzo, F.; Sher, E. Microtransplantation of Human Brain Receptors into Oocytes to Tackle Key Questions in Drug Discovery. Drug Discov. Today 2019, 24, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Gulyássy, P.; Puska, G.; Györffy, B.A.; Todorov-Völgyi, K.; Juhász, G.; Drahos, L.; Kékesi, K.A. Proteomic Comparison of Different Synaptosome Preparation Procedures. Amino Acids 2020, 52, 1529–1543. [Google Scholar] [CrossRef] [PubMed]
- Zeppillo, T.; Schulmann, A.; Macciardi, F.; Hjelm, B.E.; Föcking, M.; Sequeira, P.A.; Guella, I.; Cotter, D.; Bunney, W.E.; Limon, A.; et al. Functional Impairment of Cortical AMPA Receptors in Schizophrenia. Schizophr. Res. 2020; in press. [Google Scholar] [CrossRef] [PubMed]
- Lauterborn, J.C.; Scaduto, P.; Cox, C.D.; Schulmann, A.; Lynch, G.; Gall, C.M.; Keene, C.D.; Limon, A. Increased Excitatory to Inhibitory Synaptic Ratio in Parietal Cortex Samples from Individuals with Alzheimer’s Disease. Nat. Commun. 2021, 12, 2603. [Google Scholar] [CrossRef]
- Miller, B.; Powell, A.; Gutierrez, B.A.; Limon, A. Microtransplantation of Synaptic Membranes to Reactivate Human Synaptic Receptors for Functional Studies. JoVE 2022, 185, e64024. [Google Scholar] [CrossRef]
- Bernareggi, A.; Dueñas, Z.; Reyes-Ruiz, J.M.; Ruzzier, F.; Miledi, R. Properties of Glutamate Receptors of Alzheimer’s Disease Brain Transplanted to Frog Oocytes. Proc. Natl. Acad. Sci. USA 2007, 104, 2956–2960. [Google Scholar] [CrossRef]
- Agenor, L.; Mauricio, R.-R.J.; Ricardo, M. Loss of Functional GABAA Receptors in the Alzheimer Diseased Brain. Proc. Natl. Acad. Sci. USA 2012, 109, 10071–10076. [Google Scholar] [CrossRef]
- Putney, J.W.J. Type 3 Inositol 1,4,5-Trisphosphate Receptor and Capacitative Calcium Entry. Cell Calcium 1997, 21, 257–261. [Google Scholar] [CrossRef]
- Palma, E.; Trettel, F.; Fucile, S.; Renzi, M.; Miledi, R.; Eusebi, F. Microtransplantation of Membranes from Cultured Cells to Xenopus Oocytes: A Method to Study Neurotransmitter Receptors Embedded in Native Lipids. Proc. Natl. Acad. Sci. USA 2003, 100, 2896–2900. [Google Scholar] [CrossRef]
- Parker, I.; Ivorra, I. Localized All-or-None Calcium Liberation by Inositol Trisphosphate. Science 1990, 250, 977–979. [Google Scholar] [CrossRef] [PubMed]
- DeLisle, S.; Krause, K.H.; Denning, G.; Potter, B.V.; Welsh, M.J. Effect of Inositol Trisphosphate and Calcium on Oscillating Elevations of Intracellular Calcium in Xenopus Oocytes. J. Biol. Chem. 1990, 265, 11726–11730. [Google Scholar] [CrossRef]
- Flint, A.; Dammerman, R.; Kriegstein, R. Endogenous Activation of Metabotropic Glutamate Receptors in Neocortical Development Causes Neuronal Calcium Oscillations. Proc. Natl. Acad. Sci. USA 1999, 96, 12144–12149. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, J.A.; Segerson, T.P.; Westbrook, G.L. Metabotropic Glutamate Receptors Activate G-Protein-Coupled Inwardly Rectifying Potassium Channels in Xenopus Oocytes. J. Neurosci. 1996, 16, 5979–5985. [Google Scholar] [CrossRef] [PubMed]
- Ryo, Y.; Miyawaki, A.; Furuichi, T.; Mikoshiba, K. Expression of the Metabotropic Glutamate Receptor MGluR1? And the Ionotropic Glutamate Receptor GluR1 in the Brain during the Postnatal Development of Normal Mouse and in the Cerebellum from Mutant Mice. J. Neurosci. Res. 1993, 36, 19–32. [Google Scholar] [CrossRef]
- Enz, R. Metabotropic Glutamate Receptors and Interacting Proteins: Evolving Drug Targets. Curr. Drug Targets 2012, 13, 145–156. [Google Scholar] [CrossRef]
- Jin, D.-Z.; Guo, M.-L.; Xue, B.; Fibuch, E.E.; Choe, E.S.; Mao, L.-M.; Wang, J.Q. Phosphorylation and Feedback Regulation of Metabotropic Glutamate Receptor 1 by Calcium/Calmodulin-Dependent Protein Kinase II. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 3402–3412. [Google Scholar] [CrossRef]
- Ivorra, I.; Henriquez, M.; Lax, P.; Riquelme, G.; Morales, A. Functional Transplantation of Chloride Channels from the Human Syncytiotrophoblast Microvillous Membrane to Xenopus Oocytes. Pflugers Arch. 2002, 444, 685–691. [Google Scholar] [CrossRef]
- Murenzi, E.; Toltin, A.C.; Symington, S.B.; Morgan, M.M.; Clark, J.M. Evaluation of Microtransplantation of Rat Brain Neurolemma into Xenopus Laevis Oocytes as a Technique to Study the Effect of Neurotoxicants on Endogenous Voltage-Sensitive Ion Channels. Neurotoxicology 2017, 60, 260–273. [Google Scholar] [CrossRef]
- Rousset, M.; Humez, S.; Laurent, C.; Buée, L.; Blum, D.; Cens, T.; Vignes, M.; Charnet, P. Mammalian Brain Ca(2+) Channel Activity Transplanted into Xenopus Laevis Oocytes. Membranes 2022, 12, 496. [Google Scholar] [CrossRef]
- Clark, J.; Symington, S. Neurolemma-Injected Xenopus Oocytes: An Innovative Ex Vivo Approach to Study the Effects of Pyrethroids on Ion Channels in Their Native State. Engineering 2020, 6, 515–521. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, B.; Moreno, N.; Gutierrez, B.A.; Limon, A. Microtransplantation of Postmortem Native Synaptic mGluRs Receptors into Xenopus Oocytes for Their Functional Analysis. Membranes 2022, 12, 931. https://doi.org/10.3390/membranes12100931
Miller B, Moreno N, Gutierrez BA, Limon A. Microtransplantation of Postmortem Native Synaptic mGluRs Receptors into Xenopus Oocytes for Their Functional Analysis. Membranes. 2022; 12(10):931. https://doi.org/10.3390/membranes12100931
Chicago/Turabian StyleMiller, Brice, Naomi Moreno, Berenice A. Gutierrez, and Agenor Limon. 2022. "Microtransplantation of Postmortem Native Synaptic mGluRs Receptors into Xenopus Oocytes for Their Functional Analysis" Membranes 12, no. 10: 931. https://doi.org/10.3390/membranes12100931
APA StyleMiller, B., Moreno, N., Gutierrez, B. A., & Limon, A. (2022). Microtransplantation of Postmortem Native Synaptic mGluRs Receptors into Xenopus Oocytes for Their Functional Analysis. Membranes, 12(10), 931. https://doi.org/10.3390/membranes12100931






