Increased Tropism of Extracellular Vesicles Derived from Palmitic Acid-Treated Hepatocytes to Activated Hepatic Stellate Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of PA
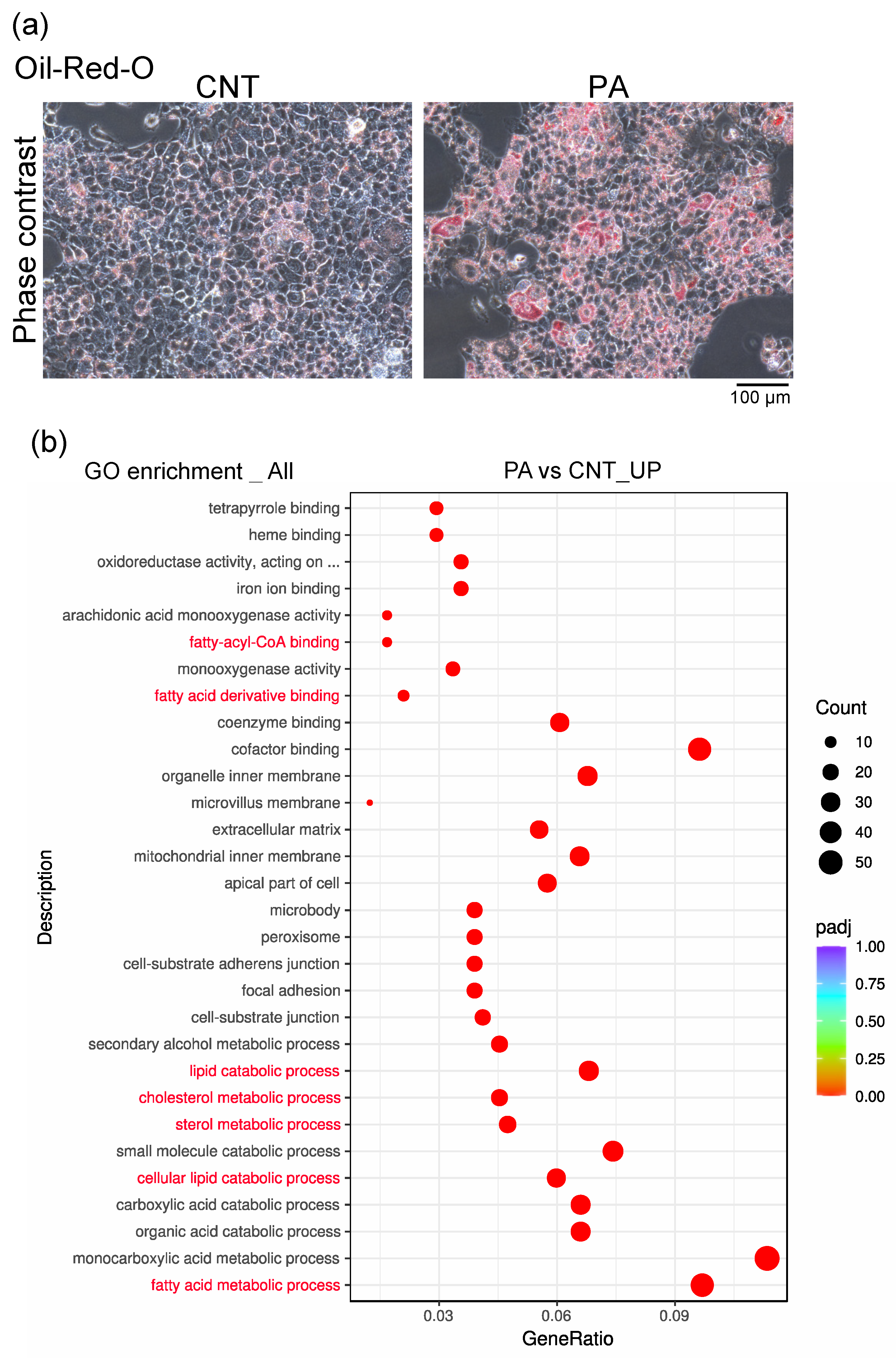
2.3. Oil-Red-O Stain
2.4. mRNA-Sequencing Analysis of AML12 Cells
2.5. Isolation of EVs from AML12 Cells
2.6. Characterization of EVs
2.7. Western Blot Analysis of EVs
2.8. Intracellular Uptake of EVs in Hepatocytes and HSCs
2.9. Statistics
3. Results
3.1. Isolation of EVs Derived from AML12 Cells
3.2. PA Induced the Accumulation of Fatty Acids in AML12 Cells
3.3. Characterization of EV-AML
3.4. Intracellular Uptake of EV-AML into AML12, and Quiescent or Activated LX-2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Koyama, Y.; Brenner, D.A. Liver Inflammation and Fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of Hepatic Stellate Cell Activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Cong, M.; Paik, Y.H.; Scholten, D.; Jiang, C.; Benner, C.; Iwaisako, K.; Moore-Morris, T.; Scott, B.; Tsukamoto, H.; et al. Myofibroblasts Revert to an Inactive Phenotype during Regression of Liver Fibrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 9448–9453. [Google Scholar] [CrossRef]
- Arroyo, N.; Villamayor, L.; Díaz, I.; Carmona, R.; Ramos-Rodríguez, M.; Muñoz-Chápuli, R.; Pasquali, L.; Toscano, M.G.; Martín, F.; Cano, D.A.; et al. GATA4 Induces Liver Fibrosis Regression by Deactivating Hepatic Stellate Cells. JCI Insight 2021, 6, e150059. [Google Scholar] [CrossRef] [PubMed]
- el Taghdouini, A.; Najimi, M.; Sancho-Bru, P.; Sokal, E.; van Grunsven, L.A. In Vitro Reversion of Activated Primary Human Hepatic Stellate Cells. Fibrogenesis Tissue Repair 2015, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Nederveen, J.P.; Warnier, G.; di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2021, 11, 1757. [Google Scholar] [CrossRef] [PubMed]
- Bazzan, E.; Tinè, M.; Casara, A.; Biondini, D.; Semenzato, U.; Cocconcelli, E.; Balestro, E.; Damin, M.; Radu, C.M.; Turato, G.; et al. Critical Review of the Evolution of Extracellular Vesicles’ Knowledge: From 1946 to Today. Int. J. Mol. Sci. 2021, 22, 6417. [Google Scholar] [CrossRef]
- Iraci, N.; Leonardi, T.; Gessler, F.; Vega, B.; Pluchino, S. Focus on Extracellular Vesicles: Physiological Role and Signalling Properties of Extracellular Membrane Vesicles. Int. J. Mol. Sci. 2016, 17, 171. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, S.Y.; Ko, E.; Lee, J.H.; Yi, H.S.; Yoo, Y.J.; Je, J.; Suh, S.J.; Jung, Y.K.; Kim, J.H.; et al. Exosomes Derived from Palmitic Acid-Treated Hepatocytes Induce Fibrotic Activation of Hepatic Stellate Cells. Sci. Rep. 2017, 7, 3710. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Gao, H.; Jin, Z.; Bandyopadhyay, G.; Wang, G.; Zhang, D.; e Rocha, K.C.; Liu, X.; Zhao, H.; Kisseleva, T.; Brenner, D.A.; et al. Aberrant Iron Distribution via Hepatocyte-Stellate Cell Axis Drives Liver Lipogenesis and Fibrosis. Cell Metab. 2022, 34, 1201–1213.e5. [Google Scholar] [CrossRef] [PubMed]
- Marrone, G.; Shah, V.H.; Gracia-Sancho, J. Sinusoidal Communication in Liver Fibrosis and Regeneration. J. Hepatol. 2016, 65, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.S.; Cao, Z.; Lis, R.; Nolan, D.J.; Guo, P.; Simons, M.; Penfold, M.E.; Shido, K.; Rabbany, S.Y.; Rafii, S. Divergent Angiocrine Signals from Vascular Niche Balance Liver Regeneration and Fibrosis. Nature 2013, 505, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Giraudi, P.J.; Barbero Becerra, V.J.; Marin, V.; Chavez-Tapia, N.C.; Tiribelli, C.; Rosso, N. The Importance of the Interaction between Hepatocyte and Hepatic Stellate Cells in Fibrogenesis Induced by Fatty Accumulation. Exp. Mol. Pathol. 2015, 98, 85–92. [Google Scholar] [CrossRef] [PubMed]
- MABUCHI, A. Role of Hepatic Stellate Cell/Hepatocyte Interaction and Activation of Hepatic Stellate Cells in the Early Phase of Liver Regeneration in the Rat. J. Hepatol. 2004, 40, 910–916. [Google Scholar] [CrossRef]
- Xu, L.; Hui, A.Y.; Albanis, E.; Arthur, M.J.; O’Byrne, S.M.; Blaner, W.S.; Mukherjee, P.; Friedman, S.L.; Eng, F.J. Human Hepatic Stellate Cell Lines, LX-1 and LX-2: New Tools for Analysis of Hepatic Fibrosis. Gut 2005, 54, 142–151. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ohbayashi, S.; Ooka, A.; Yamashita, H.; Motohashi, N.; Kaneko, Y.K.; Kimura, T.; Saito, S.Y.; Ishikawa, T. Harmine Suppresses Collagen Production in Hepatic Stellate Cells by Inhibiting DYRK1B. Biochem. Biophys. Res. Commun. 2022, 600, 136–141. [Google Scholar] [CrossRef]
- Wu, J.C.; Merlino, G.; Fausto, N. Establishment and Characterization of Differentiated, Nontransformed Hepatocyte Cell Lines Derived from Mice Transgenic for Transforming Growth Factor Alpha. Proc. Natl. Acad. Sci. USA 1994, 91, 674–678. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Dohi, N.; Ooka, A.; Saito, S.Y.; Ishikawa, T. Caffeine-Induced Inversion of Prostaglandin E 2 Effects on Hepatic Stellate Cell Activation. Biomed. Pharmacother. 2021, 142, 111989. [Google Scholar] [CrossRef]
- Furukawa, S.; Yamaguchi, M.; Ooka, A.; Kikuchi, H.; Ishikawa, T.; Saito, S.Y. Differentiation-Inducing Factor-1 Prevents Hepatic Stellate Cell Activation through Inhibiting GSK3β Inactivation. Biochem. Biophys. Res. Commun. 2019, 520, 140–144. [Google Scholar] [CrossRef]
- Kuntsche, J.; Horst, J.C.; Bunjes, H. Cryogenic Transmission Electron Microscopy (Cryo-TEM) for Studying the Morphology of Colloidal Drug Delivery Systems. Int. J. Pharm. 2011, 417, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Ricchi, M.; Odoardi, M.R.; Carulli, L.; Anzivino, C.; Ballestri, S.; Pinetti, A.; Fantoni, L.I.; Marra, F.; Bertolotti, M.; Banni, S.; et al. Differential Effect of Oleic and Palmitic Acid on Lipid Accumulation and Apoptosis in Cultured Hepatocytes. J. Gastroenterol. Hepatol. 2009, 24, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Li, J.; Chen, Y.; Xu, Y.; Wei, Y.; Lu, J.; Dong, R. Hepatic Stellate Cell Reprogramming via Exosome-Mediated CRISPR/DCas9-VP64 Delivery. Drug Deliv. 2021, 28, 10. [Google Scholar] [CrossRef]
- Cobbs, A.; Chen, X.; Zhang, Y.; George, J.; Huang, M.B.; Bond, V.; Thompson, W.; Zhao, X. Saturated Fatty Acid Stimulates Production of Extracellular Vesicles by Renal Tubular Epithelial Cells. Mol. Cell. Biochem. 2019, 458, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Hirsova, P.; Ibrahim, S.H.; Krishnan, A.; Verma, V.K.; Bronk, S.F.; Werneburg, N.W.; Charlton, M.R.; Shah, V.H.; Malhi, H.; Gores, G.J. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles from Hepatocytes. Gastroenterology 2016, 150, 956. [Google Scholar] [CrossRef] [PubMed]
- Moravcová, A.; Červinková, Z.; Kučera, O.; Mezera, V.; Rychtrmoc, D.; Lotková, H. The Effect of Oleic and Palmitic Acid on Induction of Steatosis and Cytotoxicity on Rat Hepatocytes in Primary Culture. Physiol. Res. 2015, 64, S627–S636. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- French, K.C.; Antonyak, M.A.; Cerione, R.A. Extracellular Vesicle Docking at the Cellular Port: Extracellular Vesicle Binding and Uptake. Semin. Cell Dev. Biol. 2017, 67, 48–55. [Google Scholar] [CrossRef]
- Li, Y.; Pu, S.; Liu, Q.; Li, R.; Zhang, J.; Wu, T.; Chen, L.; Li, H.; Yang, X.; Zou, M.; et al. An Integrin-Based Nanoparticle That Targets Activated Hepatic Stellate Cells and Alleviates Liver Fibrosis. J. Control. Release 2019, 303, 77–90. [Google Scholar] [CrossRef]
- Nishimichi, N.; Tsujino, K.; Kanno, K.; Sentani, K.; Kobayashi, T.; Chayama, K.; Sheppard, D.; Yokosaki, Y. Induced Hepatic Stellate Cell Integrin, A8β1, Enhances Cellular Contractility and TGFβ Activity in Liver Fibrosis. J. Pathol. 2021, 253, 366–373. [Google Scholar] [CrossRef]
- Kastanis, G.J.; Hernandez-Nazara, Z.; Nieto, N.; Rincón-Sanchez, A.R.; Popratiloff, A.; Dominguez-Rosales, J.A.; Lechuga, C.G.; Rojkind, M. The Role of Dystroglycan in PDGF-BB-Dependent Migration of Activated Hepatic Stellate Cells/Myofibroblasts. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G464–G474. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duggan, M.E.; Hutchinson, J.H. Ligands to the Integrin Receptor Avβ3. Expert Opin. Ther. Pat. 2005, 10, 1367–1383. [Google Scholar] [CrossRef]
- Takada, Y.; Ye, X.; Simon, S. The Integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef] [PubMed]
- Deregibus, M.C.; Figliolini, F.; D’Antico, S.; Manzini, P.M.; Pasquino, C.; de Lena, M.; Tetta, C.; Brizzi, M.F.; Camussi, G. Charge-Based Precipitation of Extracellular Vesicles. Int. J. Mol. Med. 2016, 38, 1359. [Google Scholar] [CrossRef] [PubMed]

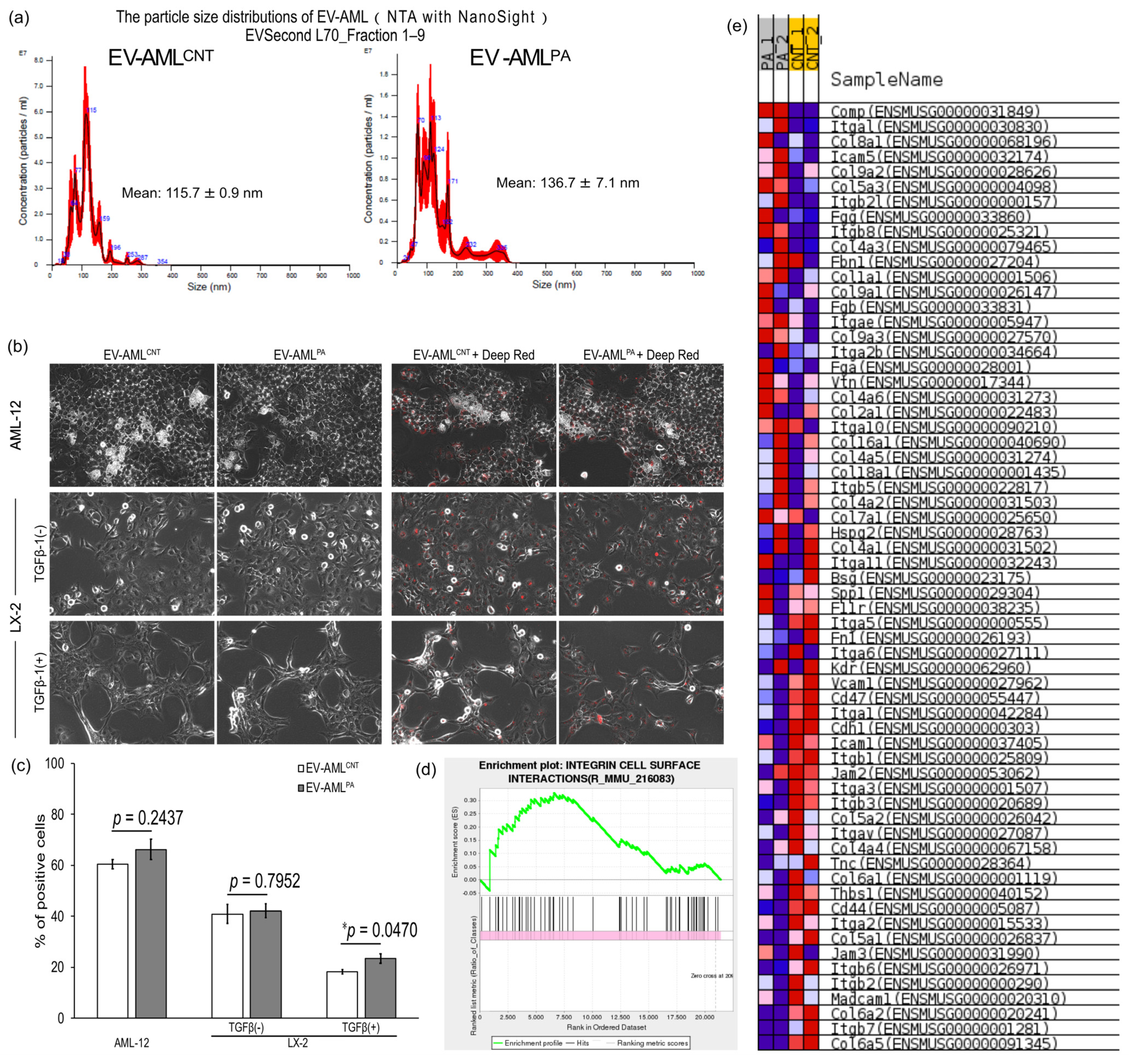
| Physical Properties | EV-AMLCNT | EV-AMLPA |
|---|---|---|
| Mean particle diameter (nm), NTA | 115.7 ± 0.9 | 136.7 ± 7.1 |
| Particle concentration (109 particles/mL), NTA | 3.26 ± 0.291 | 1.09 ± 0.124 |
| Protein concentration (μg/mL), BCA | 23.444 | 17.000 |
| Amount of protein/particle (10−9 μg protein/particle) | 7.191 | 15.596 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, M.; Kanazawa, T.; Morino, S.; Iioka, S.; Watanabe, Y.; Dohi, N.; Higashi, K.; Kondo, H.; Ishikawa, T. Increased Tropism of Extracellular Vesicles Derived from Palmitic Acid-Treated Hepatocytes to Activated Hepatic Stellate Cells. Membranes 2022, 12, 1023. https://doi.org/10.3390/membranes12101023
Yamaguchi M, Kanazawa T, Morino S, Iioka S, Watanabe Y, Dohi N, Higashi K, Kondo H, Ishikawa T. Increased Tropism of Extracellular Vesicles Derived from Palmitic Acid-Treated Hepatocytes to Activated Hepatic Stellate Cells. Membranes. 2022; 12(10):1023. https://doi.org/10.3390/membranes12101023
Chicago/Turabian StyleYamaguchi, Momoka, Takanori Kanazawa, Sumire Morino, Shingo Iioka, Yuta Watanabe, Naoki Dohi, Kenjirou Higashi, Hiromu Kondo, and Tomohisa Ishikawa. 2022. "Increased Tropism of Extracellular Vesicles Derived from Palmitic Acid-Treated Hepatocytes to Activated Hepatic Stellate Cells" Membranes 12, no. 10: 1023. https://doi.org/10.3390/membranes12101023
APA StyleYamaguchi, M., Kanazawa, T., Morino, S., Iioka, S., Watanabe, Y., Dohi, N., Higashi, K., Kondo, H., & Ishikawa, T. (2022). Increased Tropism of Extracellular Vesicles Derived from Palmitic Acid-Treated Hepatocytes to Activated Hepatic Stellate Cells. Membranes, 12(10), 1023. https://doi.org/10.3390/membranes12101023







