Electrodialysis Can Lower the Environmental Impact of Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Real Dialysis RO Rejects Samples
2.2. Electrodialysis Equipment and Membranes
2.3. Experiments and Analysis Methods
2.3.1. Experimental Procedure
2.3.2. Water Analysis
2.3.3. Data Analysis
- Determination of Removal rate (R−%)
- Determination of the demineralization rate (DR %)
- Determination of the specific power consumption (SPC)
- Determination of ion transport flux (J)
- Determination of the productivity (W)
2.4. Parametric Study and Statistical Method
3. Results
3.1. Parametric Analysis and Modeling of the ED Process with Model Solution
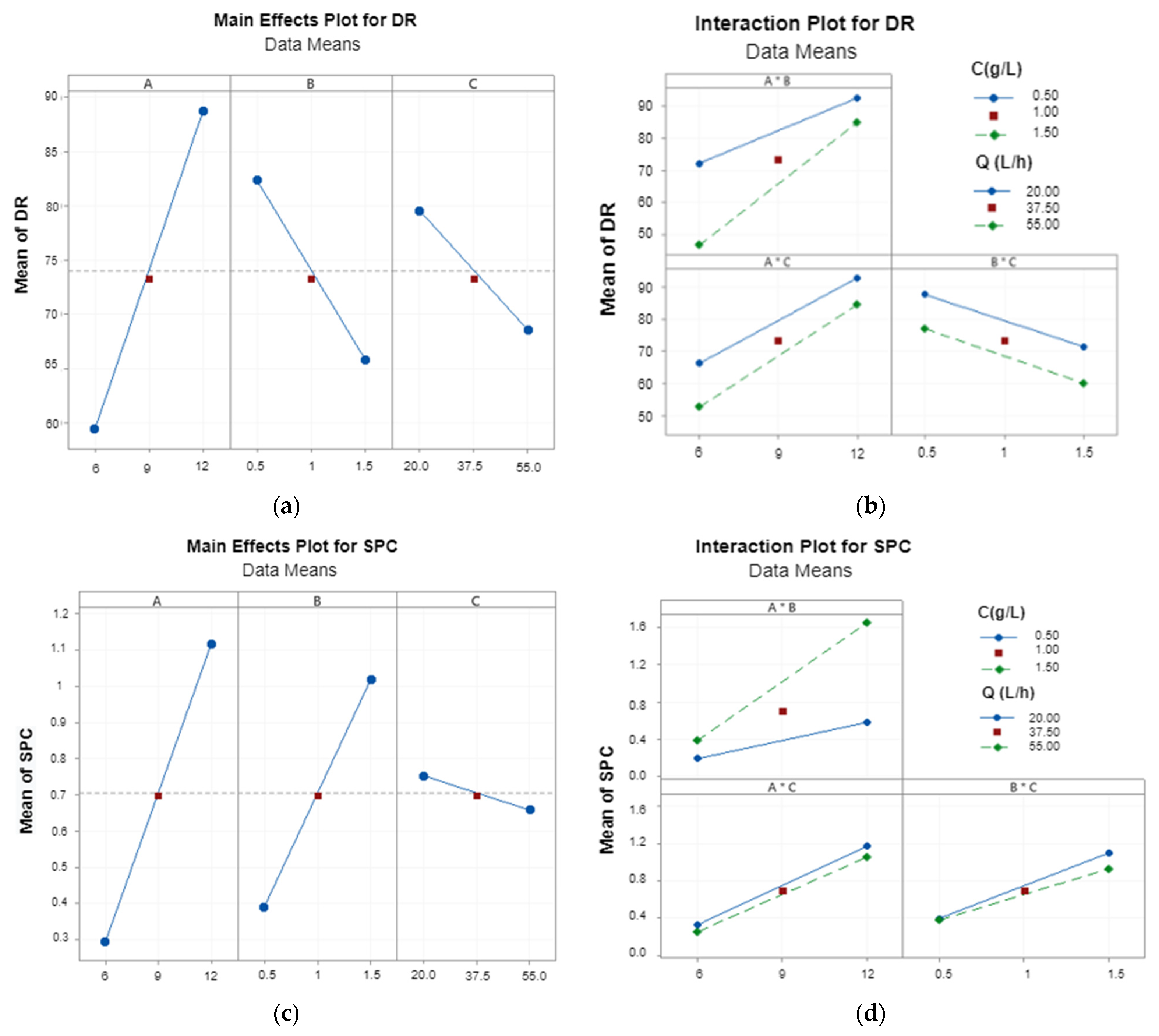
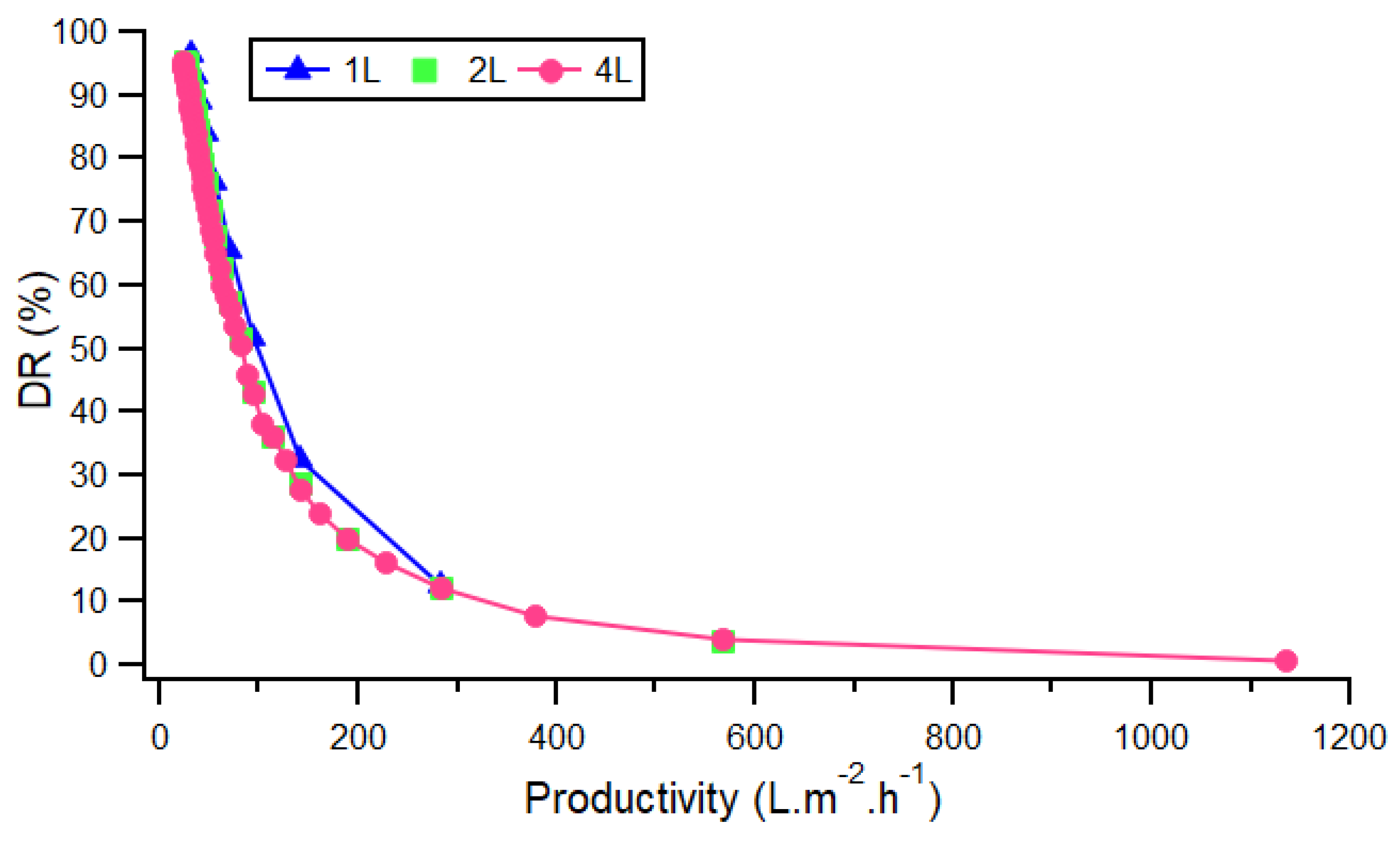
3.1.1. General Trends
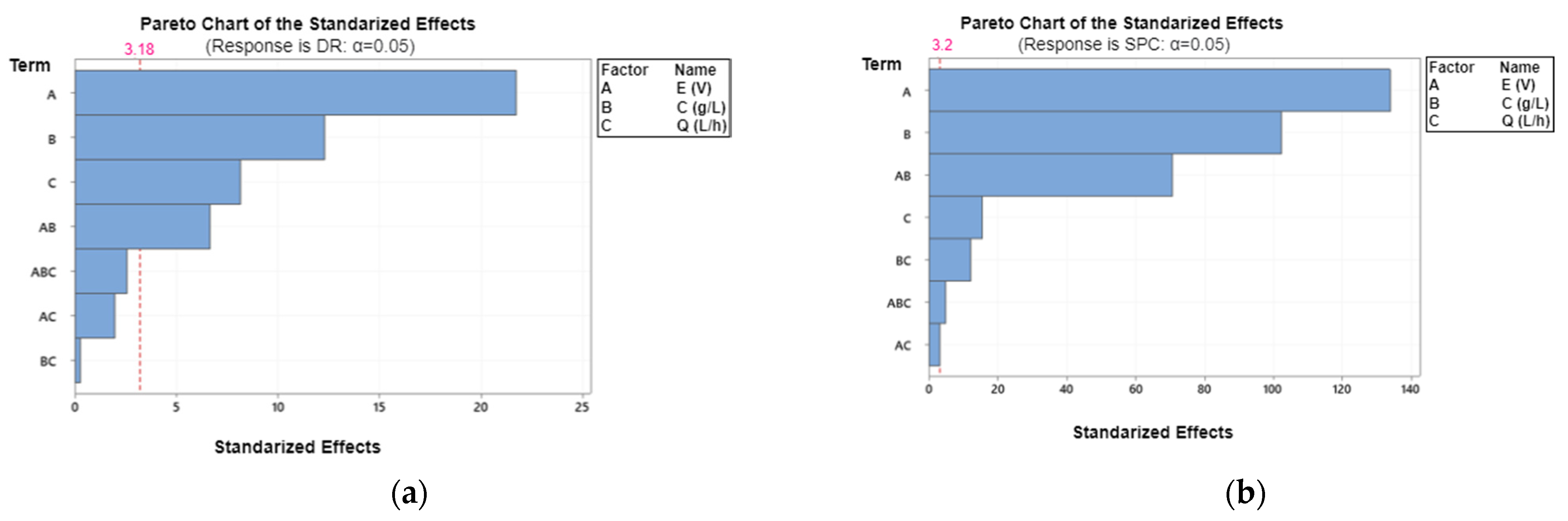
3.1.2. Main Effects and Interactions between Parameters
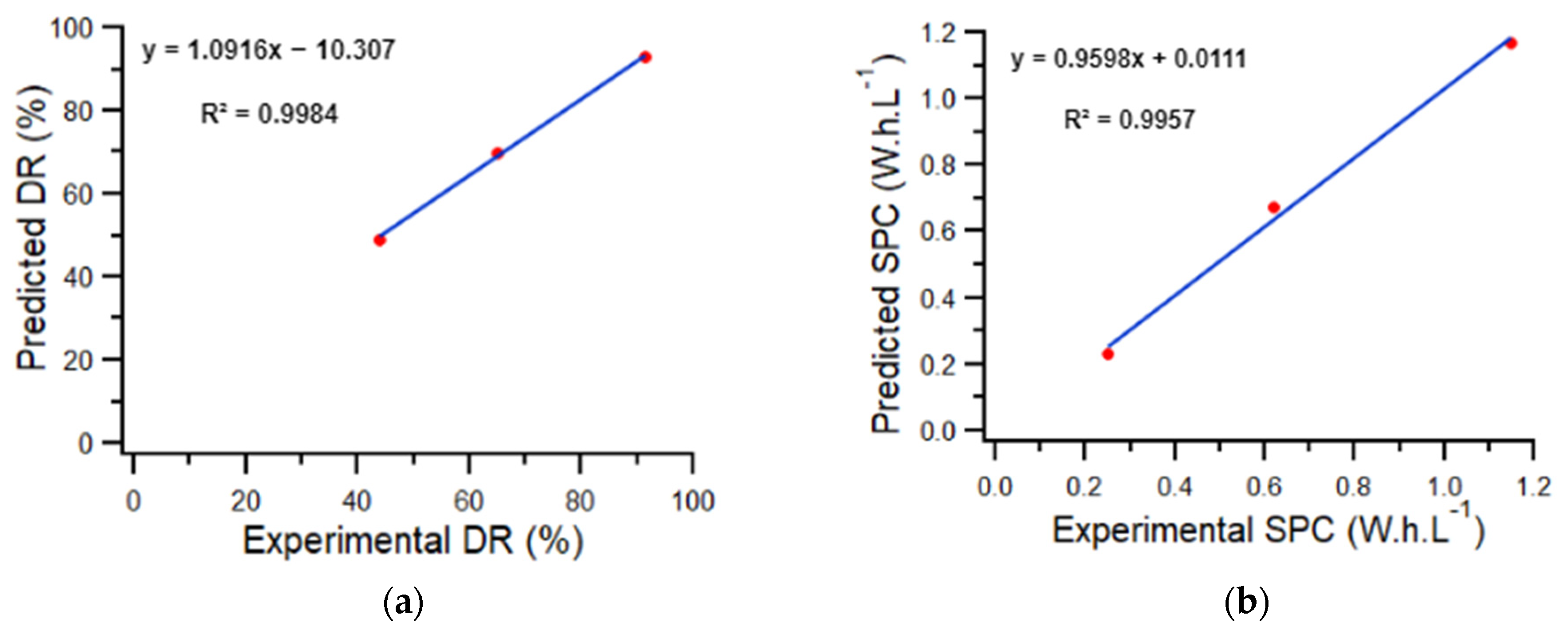
3.1.3. Model Validation
3.2. Application to RO Concentrate in Dialysis Unit
3.2.1. Comparison between Real and Model Solutions
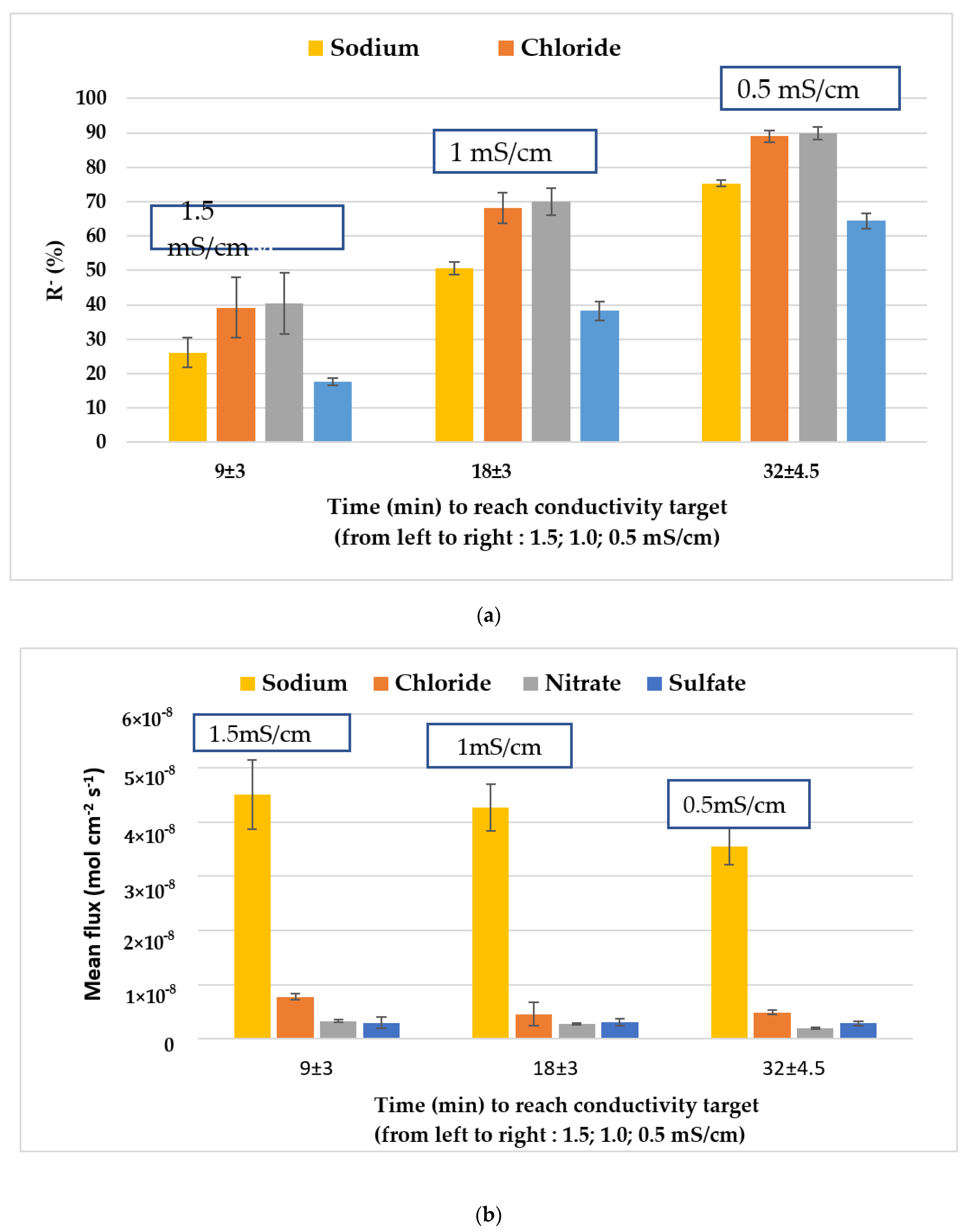
3.2.2. Physico-Chemical Characterization of the Diluate and Species’ Mass Transfer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dheda, S.; van Eps, C.; Hawley, C.; Johnson, D.W. Water Treatment for Centre and Home-Based Haemodialysis. In Updates in Hemodialysis; Suzuki, H., Ed.; InTech: Brisbane, Australia, 2015. [Google Scholar] [CrossRef]
- Salomon, J.-N. Le dessalement de l’eau de mer est-il une voie d’avenir? GOT 2012, 1, 237–262. [Google Scholar] [CrossRef][Green Version]
- Liang, J.; Deng, A.; Xie, R.; Gomez, M.; Hu, J.; Zhang, J.; Ong, C.N.; Adin, A. Impact of seawater reverse osmosis (SWRO) product remineralization on the corrosion rate of water distribution pipeline materials. Desalination 2013, 311, 54–61. [Google Scholar] [CrossRef]
- Barraclough, K.A.; Agar, J.W.M. Green nephrology. Nat. Rev. Nephrol. 2020, 16, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Abarkan, A.; Metayer, H.; Housseini, T.S.; Legallais, C. La Dialyse Verte: Diminuer L’Impact Environnemental des Eaux de Dialyse: C’est Possible! Techniques Hospitalières. Available online: https://www.techniques-hospitalieres.fr/article/2058-la-dialyse-verte-diminuer-limpact-environnemental-des-eaux-de-dialyse-cest-possible-.html (accessed on 5 November 2021).
- Arrêté du 11 Janvier 2007 Relatif aux Limites et Références de Qualité des eaux Brutes et des eaux Destinées à la Consommation Humaine Mentionnées aux Articles R. 1321-2, R. 1321-3, R. 1321-7 et R. 1321-38 du Code de la Santé PubliqueLegifrance. Available online: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000000465574 (accessed on 6 November 2021).
- Lazarova, V.; Hills, S.; Birks, R. Using recycled water for non-potable, urban uses: A review with particular reference to toilet flushing. Water Supply 2003, 3, 69–77. [Google Scholar] [CrossRef]
- Gmar, S.; Helali, N.; Boubakri, A.; Sayadi, I.B.S.; Tlili, M.; Amor, M.B. Electrodialytic desalination of brackish water: Determination of optimal experimental parameters using full factorial design. Appl. Water Sci. 2017, 7, 4563–4572. [Google Scholar] [CrossRef]
- Mourad, B.S.A.; Mnif, A.; Hamrouni, B.; Dhahbi, M. Desalination of brackish water using electrodialysis: Effect of operational conditions. Zaštita Mater. 2009, 50, 141–146. [Google Scholar]
- Gude, V.G. Desalination and sustainability—An appraisal and current perspective. Water Res. 2016, 89, 87–106. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Mohammadi, T. Sea water desalination using electrodialysis. Desalination 2008, 221, 440–447. [Google Scholar] [CrossRef]
- McGovern, R.K.; Zubair, S.M.; Lienhard V, J.H. Hybrid electrodialysis reverse osmosis system design and its optimization for treatment of highly saline brines. IDA J. Desalination Water Reuse 2014, 6, 15–23. [Google Scholar] [CrossRef]
- Praneeth, K.; Manjunath, D.; Bhargava, S.K.; Tardio, J.; Sridhar, S. Economical treatment of reverse osmosis reject of textile industry effluent by electrodialysis–evaporation integrated process. Desalination 2014, 333, 82–91. [Google Scholar] [CrossRef]
- Dach, H. Comparison of Nanofiltration and Reverse Osmosis Processes for a Selective Desalination of Brackish Water Feeds. Ph.D. Thesis, Université d’Angers, Angers, France, 2008. Available online: https://tel.archives-ouvertes.fr/tel-00433513 (accessed on 6 November 2021).
- Kobayashi, S.; Müllen, K. Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Sata, T.; Sata, T.; Yang, W. Studies on cation-exchange membranes having permselectivity between cations in electrodialysis. J. Membr. Sci. 2002, 206, 31–60. [Google Scholar] [CrossRef]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Tongwen, X. Electrodialysis processes with bipolar membranes (EDBM) in environmental protection—A review. Resour. Conserv. Recycl. 2002, 37, 1–22. [Google Scholar] [CrossRef]
- McGovern, R.K.; Weiner, A.M.; Sun, L.; Chambers, C.G.; Zubair, S.M.; Lienhard V, J.H. On the cost of electrodialysis for the desalination of high salinity feeds. Appl. Energy 2014, 136, 649–661. [Google Scholar] [CrossRef]
- McGovern, R.K.; Zubair, S.M.; Lienhard V, J.H. The cost effectiveness of electrodialysis for diverse salinity applications. Desalination 2014, 348, 57–65. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Khalil, A.; Hilal, N. Emerging desalination technologies: Current status, challenges and future trends. Desalination 2021, 517, 115183. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Xu, P.; Cath, T.Y.; Robertson, A.P.; Reinhard, M.; Leckie, J.O.; Drewes, J.E. Critical Review of Desalination Concentrate Management, Treatment and Beneficial Use. Environ. Eng. Sci. 2013, 30, 502–514. [Google Scholar] [CrossRef]
- Zhang, Y.; Desmidt, E.; van Looveren, A.; Pinoy, L.; Meesschaert, B.; van der Bruggen, B. Phosphate Separation and Recovery from Wastewater by Novel Electrodialysis. Environ. Sci. Technol. 2013, 47, 5888–5895. [Google Scholar] [CrossRef]
- Ali-Taleshi, M.S.; Nejadkoorki, F. Characterization of Hemodialysis Reverse Osmosis Wastewater From Yazd Educational Hospitals. Avicenna J. Environ. Health Eng. 2016, 3, e5067. [Google Scholar] [CrossRef]
- Gmar, S.; Ben Salah Sayadi, I.; Helali, N.; Tlili, M.; Ben Amor, M. Desalination and Defluoridation of Tap Water by Electrodialysis. Environ. Process. 2015, 2, 209–222. [Google Scholar] [CrossRef]
- Casademont, C.; Farias, M.; Pourcelly, G.; Bazinet, L. Impact of electrodialytic parameters on cation migration kinetics and fouling nature of ion-exchange membranes during treatment of solutions with different magnesium/calcium ratios. J. Membr. Sci. 2008, 325, 570–579. [Google Scholar] [CrossRef]
- Wang, Q.; Ying, T.; Jiang, T.; Yang, D.; Jahangir, M.M. Demineralization of soybean oligosaccharides extract from sweet slurry by conventional electrodialysis. J. Food Eng. 2009, 95, 410–415. [Google Scholar] [CrossRef]
- Fadel, A.; Lafi, R.; Aouni, A.; Hafiane, A.; Nacef, S. Separation of zinc ions from synthetically prepared brackish water using electrodialysis: Effect of operating parameters. Desalination Water Treat. 2016, 57, 17852–17860. [Google Scholar] [CrossRef]
- Ergun, E.; Tor, A.; Cengeloglu, Y.; Kocak, I. Electrodialytic removal of fluoride from water: Effects of process parameters and accompanying anions. Sep. Purif. Technol. 2008, 64, 147–153. [Google Scholar] [CrossRef]
- Lopez, A.M.; Williams, M.; Paiva, M.; Demydov, D.; Do, T.D.; Fairey, J.L.; Lin, Y.J.; Hestekin, J.A. Potential of electrodialytic techniques in brackish desalination and recovery of industrial process water for reuse. Desalination 2017, 409, 108–114. [Google Scholar] [CrossRef]
- Turan, N.G.; Elevli, S.; Mesci, B. Adsorption of copper and zinc ions on illite: Determination of the optimal conditions by the statistical design of experiments. Appl. Clay Sci. 2011, 52, 392–399. [Google Scholar] [CrossRef]
- Minitab 18 Support. Available online: https://support.minitab.com/en-us/minitab/18/ (accessed on 6 November 2021).
- Aponte, V.M.; Colón, G. Sodium chloride removal from urine via a six-compartment ED cell for use in Advanced Life Support Systems (Part 1: Salt removal as a function of applied voltage and fluid velocity). Desalination 2001, 140, 121–132. [Google Scholar] [CrossRef]
- Camacho, L.M.; Fox, J.A.; Ajedegba, J.O. Optimization of electrodialysis metathesis (EDM) desalination using factorial design methodology. Desalination 2017, 403, 136–143. [Google Scholar] [CrossRef]
- Kabay, N.; Demircioglu, M.; Ersöz, E.; Kurucaovali, I. Removal of calcium and magnesium hardness by electrodialysis. Desalination 2002, 149, 343–349. [Google Scholar] [CrossRef]
- Chindapan, N.; Devahastin, S.; Chiewchan, N. Electrodialysis Desalination of Fish Sauce: Electrodialysis Performance and Product Quality. J. Food Sci. 2009, 74, E363–E371. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.-Y.; Sun, S.-Y.; Song, X.; Yu, J.-G. Further investigation into lithium recovery from salt lake brines with different feed characteristics by electrodialysis. J. Membr. Sci. 2017, 530, 185–191. [Google Scholar] [CrossRef]
- Ghoniem, A.A.; El-Naggar, N.E.-A.; Saber, W.I.A.; El-Hersh, M.S.; El-Khateeb, A.Y. Statistical modeling-approach for optimization of Cu2+ biosorption by Azotobacter nigricans NEWG-1; characterization and application of immobilized cells for metal removal. Sci. Rep. 2020, 10, 9491. [Google Scholar] [CrossRef] [PubMed]
- Cherif, M.; Mkacher, I.; Dammak, L.; Ben Salah, A.; Walha, K.; Nikonenko, V.; Korchane, S.; Grande, D. Fractional factorial design of water desalination by neutralization dialysis process: Concentration, flow rate, and volume effects. Desalination Water Treat. 2015, 57, 14403–14413. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Kabay, N.; İpek, Ö.; Kahveci, H.; Yüksel, M. Effect of salt combination on separation of monovalent and divalent salts by electrodialysis. Desalination 2006, 198, 84–91. [Google Scholar] [CrossRef]
- Le Pen, N. Le contrôle sanitaire de l’eau des piscines-Comment interpréter les résultats et agir pour le bien-être des baigneurs. Cah. de L’association Sci. Eur. pour l’Eau et la St. (ASEES) 2015, 20, 6. [Google Scholar] [CrossRef]
- Guide SF2S—Société Française des Sciences de la Stérilisation. Available online: https://www.sf2s-sterilisation.fr/mediatheque-en-ligne/documents/architecture-locaux-et-infrastructure/guide-sf2s-4/ (accessed on 6 November 2021).
- Jabrane, M.; Fadili, W.; Kennou, B.; Labaali, A.; Zahlane, K.; Laouad, I. Évaluation de l’impact d’un centre d’hémodialyse sur l’environnement et l’écologie locale. Néphrol. Thér. 2013, 9, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Biesheuvel, P.M.; Elimelech, M. Energy Consumption of Brackish Water Desalination: Identifying the Sweet Spots for Electrodialysis and Reverse Osmosis. ACS EST Eng. 2021, 1, 851–864. [Google Scholar] [CrossRef]
- Anderson, M.A.; Cudero, A.L.; Palma, J. Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: Will it compete? Electrochim. Acta 2010, 55, 3845–3856. [Google Scholar] [CrossRef]
- Fatima, E.; Elazhar, M.; Hafsi, M.; Elmidaoui, A. Performances of electrodialysis process in desalination of brackish waters at various salinities and voltage. Int. J. Adv. Chem. 2014, 2, 49–52. [Google Scholar] [CrossRef]
- Singh, R. Analysis of energy usage at membrane water treatment plants. Desalination Water Treat. 2011, 29, 63–72. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Molina, S.; Ortiz, J.M.; Navarro, R.; García-Calvo, E. Circular economy in membrane technology: Using end-of-life reverse osmosis modules for preparation of recycled anion exchange membranes and validation in electrodialysis. J. Membr. Sci. 2020, 593, 117423. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, C.; Dominguez-Ramos, A.; Ibañez, R.; Irabien, A. Sustainability assessment of electrodialysis powered by photovoltaic solar energy for freshwater production. Renew. Sustain. Energy Rev. 2015, 47, 604–615. [Google Scholar] [CrossRef]



| Parameter | Dialysis RO Loop Water Reject |
|---|---|
| Mean (±SD) | |
| Conductivity (μS·cm−1) | 1960 ± 0.086 |
| pH | 8.12 ± 0.22 |
| Calcium, mmol·L−1 | 0.025 ± 3.06 × 10−3 |
| Chloride, mmol·L−1 | 2.183 ± 0.115 |
| Total Hardness, °F | 0.32 ± 0.04 |
| Magnesium, mmol·L−1 | 0.005 ± 9.12 × 10−4 |
| Sodium, mmol·L−1 | 19.164 ± 0.7844 |
| Sulfates, mmol·L−1 | 1.77 ± 0.104 |
| Turbidity, NFU | 0.10 ± 0,00 |
| Ammonium, mmol·L−1 | <0.0027 ± 0.00 |
| Nitrates, mmol·L−1 | 0.0715 ± 0.0042 |
| Free Chlorine, mmol·L−1 | <0.00006 ± 0.00 |
| Total Chlorine, mmol·L−1 | 0.00007 ± 2 × 10−5 |
| Iron, μmol·L−1 | 0.07 ± 0.01 |
| Arsenic, μmol·L−1 | 0.00253 ± 2.02 × 10−4 |
| Cadmium, μmol·L−1 | <0.000088 ± 0.00 |
| Mercury, μmol·L−1 | <0.00005 ± 0.00 |
| Membrane Type | Membrane Characteristic | Resistance/ Ω·cm2 | Water Content (wt%) | Thickness (μm) | Ion Exchange Capacity Strong Basic (mequiv·g−1) | Chemical Stability (pH) | Permselectivity |
|---|---|---|---|---|---|---|---|
| PC-SK | Strongly acidic (Sulfonic acid) | ~2.5 | ~9 | 100–120 | c.a. 1.2 | 0–11 | >0.95 |
| PC-SA | Strongly alkaline (Ammonium) | ~1.8 | ~14 | 100–110 | 3 | 0–9 | >0.95 |
| PC -MTE | Strongly acidic (Sulfonic acid) | ~4.5 | - | 220 | 1.8 | 1–13 | >0.94 |
| Parameter | Unit | Dialysis Loop Water RO before ED | Target1.5 mS·cm−1 | ED Target1 mS·cm−1 | ED Target0.5 mS·cm−1 |
|---|---|---|---|---|---|
| Mean (±SD) | Mean (±SD) | Mean (±SD) | Mean (±SD) | ||
| Conductivity | (mS·cm−1) | 1.96 ± 0.092 | 1.50 ± 0.030 | 1.04 ± 0.060 | 0.54 ± 0.0081 |
| pH | 8.12 ± 0.22 | 7.79 ± 0.34 | 7.72 ± 0.37 | 7.36 ± 0.39 | |
| Calcium | mg·L−1 | 1.06 ± 0.13 | 0.77 ± 0.12 | 0.49 ± 0.06 | 0.26 ± 0.02 |
| Chloride | mg·L−1 | 77.67 ± 4.04 | 50.33 ± 9.71 | 24.67 ± 2.08 | 8.50 ± 0.87 |
| Total Hardness | ° f | 0.32 ± 0.04 | 0.23 ± 0.04 | 0.15 ± 0.02 | 0.07 ± 0.00 |
| Magnesium | mg·L−1 | 0.12 ± 0.2 | 0.09 ± 0.03 | 0.05 ± 0.01 | 0.02 ± 0.01 |
| Sodium | mg·L−1 | 440.67 ± 17.93 | 325.00 ± 10.58 | 217.00 ± 1.73 | 108.67 ± 1.15 |
| Sulfates | mg·L−1 | 170.00 ± 10.00 | 140.00 ± 10.00 | 105.00 ± 8.66 | 60.67 ± 6.81 |
| Turbidity | NFU | 0.10 ± 000 | 0.23 ± 0.06 | 0.20 ± 0.10 | 0.13 ± 0.06 |
| Ammonium | mg·L−1 | <0.05 ± 0.00 | <0.05 ± 0.00 | <0.05 ± 0.00 | <0.05 ± 0.00 |
| Nitrates | mg·L−1 | 55.67 ± 3.21 | 33.00 ± 3.00 | 16.67 ± 1.15 | 5.60 ± 0.70 |
| Free Chlorine | mg·L−1 | <0.02 ± 0.00 | <0.02 ± 0.00 | <0.02 ± 0.00 | <0.02 ± 0.00 |
| Total Chlorine | mg·L−1 | 0.03 ± 0.00 | 0.03 ± 0.00 | <0.02 ± 0.00 | 0.03 ± 0.00 |
| Iron | μg·L−1 | 3.67 ± 0.58 | 3.50 ± 2.12 | 1.33 ± 0.58 | 1.67 ± 0.58 |
| Arsenic | μg·L−1 | 0.19 ± 0.02 | 0.21 ± 0.11 | 0.16 ± 0.06 | 0.09 ± 0.02 |
| Cadmium | μg·L−1 | <0.01 ± 0.00 | <0.01 ± 0.00 | <0.01 ± 0.00 | <0.01 ± 0.00 |
| Mercury | μg·L−1 | <0.01 ± 0.00 | <0.01 ± 0.00 | <0.01 ± 0.00 | <0.01 ± 0.00 |
| Potential Use of Treated RO Effluents | Re-Use Conductivity (mS·cm−1) | DR (%) | SPC (kW·h·m−3) | W (L·m−2·h−1) |
|---|---|---|---|---|
| Rehabilitation pool [45] | 1.05 | 45 | 0.69 | 107 |
| Sterilization: ManualWashing [46] | 1 | 48 | 0.72 | 102 |
| Sterilization: Machine wash and vacuum pump [46] | 0.65 | 66 | 0.98 | 70 |
| Irrigation of green areas [47] | 0.5 | 74 | 1.09 | 59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abarkan, A.; Grimi, N.; Métayer, H.; Sqalli Houssaïni, T.; Legallais, C. Electrodialysis Can Lower the Environmental Impact of Hemodialysis. Membranes 2022, 12, 45. https://doi.org/10.3390/membranes12010045
Abarkan A, Grimi N, Métayer H, Sqalli Houssaïni T, Legallais C. Electrodialysis Can Lower the Environmental Impact of Hemodialysis. Membranes. 2022; 12(1):45. https://doi.org/10.3390/membranes12010045
Chicago/Turabian StyleAbarkan, Ahmed, Nabil Grimi, Hubert Métayer, Tarik Sqalli Houssaïni, and Cécile Legallais. 2022. "Electrodialysis Can Lower the Environmental Impact of Hemodialysis" Membranes 12, no. 1: 45. https://doi.org/10.3390/membranes12010045
APA StyleAbarkan, A., Grimi, N., Métayer, H., Sqalli Houssaïni, T., & Legallais, C. (2022). Electrodialysis Can Lower the Environmental Impact of Hemodialysis. Membranes, 12(1), 45. https://doi.org/10.3390/membranes12010045







