A Literature Review of Modelling and Experimental Studies of Water Treatment by Adsorption Processes on Nanomaterials
Abstract
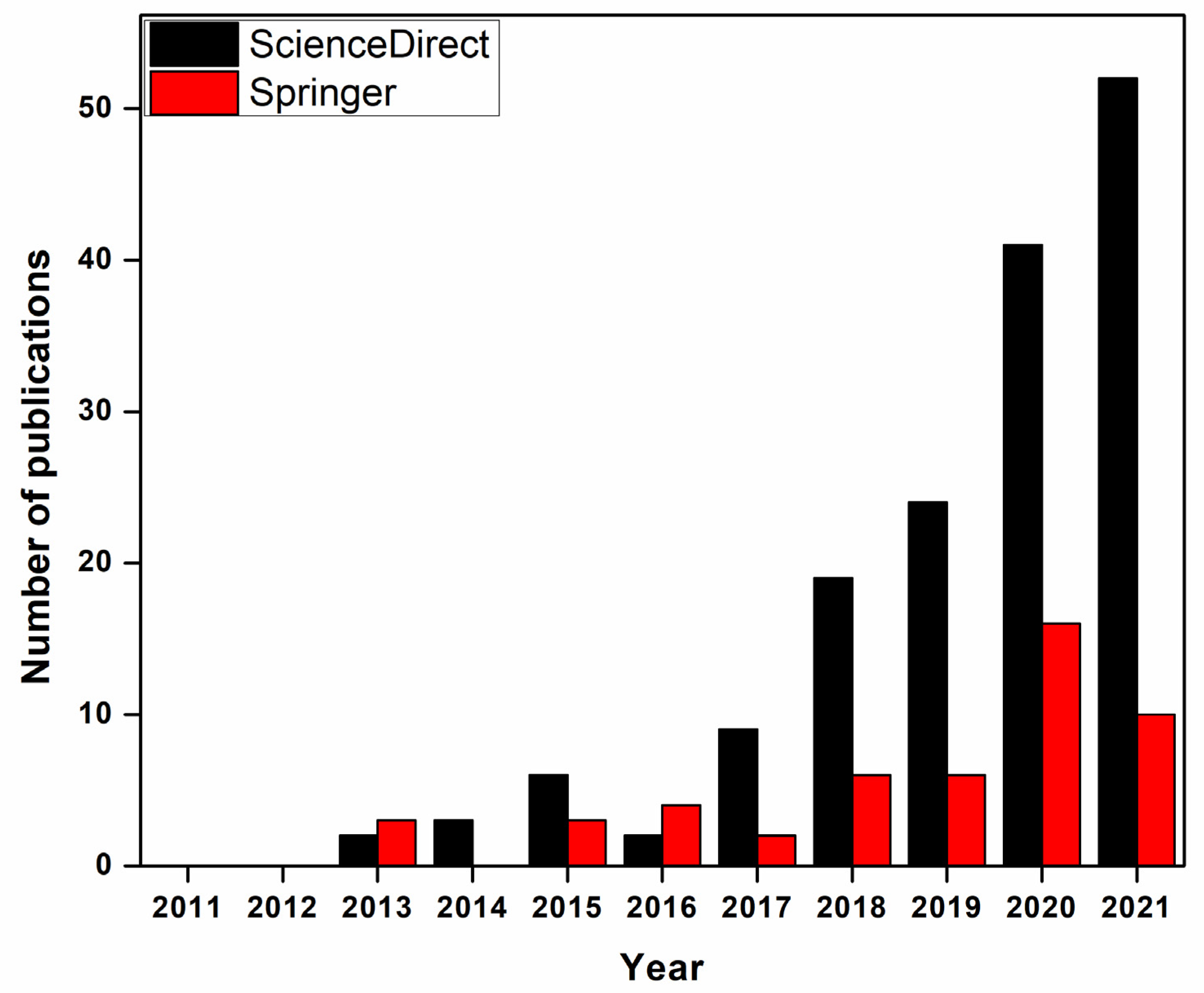
:1. Introduction
1.1. Water Filtration
1.2. Adsorption Techniques
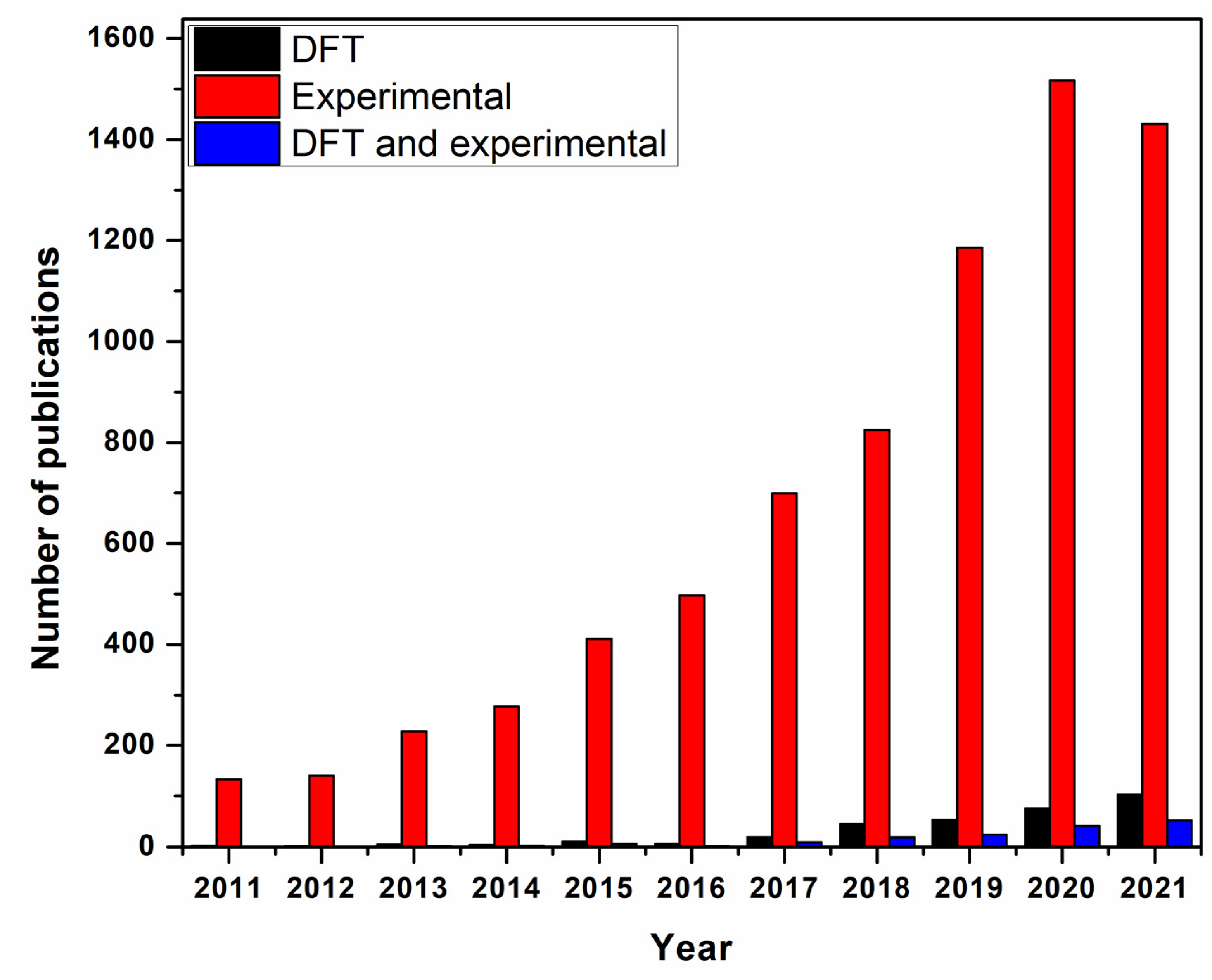
1.3. Computational Methods
Calibration and Validation
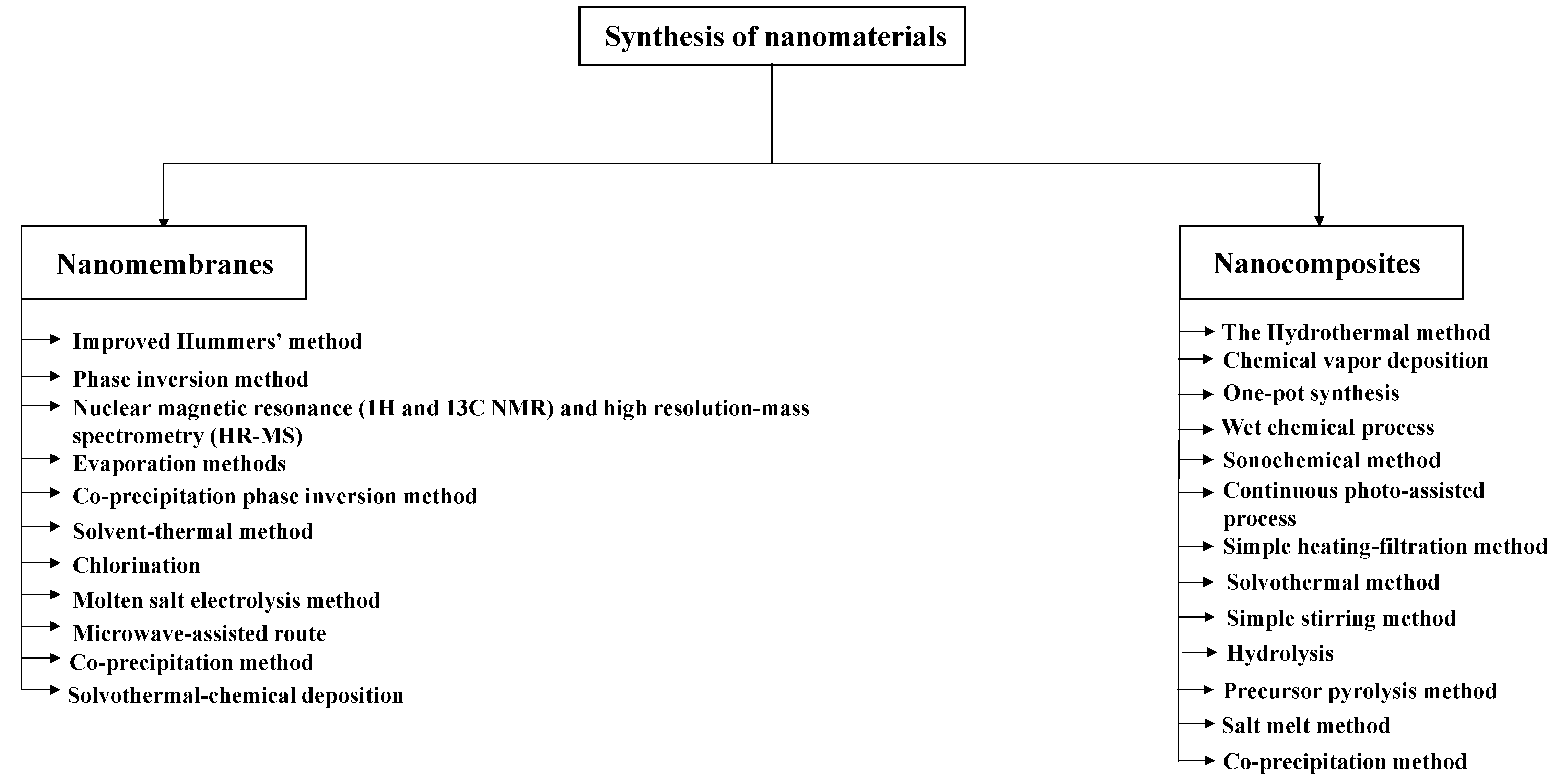
2. Synthesis and Simulation of Nanomaterials
2.1. Nanomembranes
2.1.1. Synthesis of Nanomembranes
2.1.2. Simulation of Nanomembranes
2.2. Nanocomposites
2.2.1. Synthesis of Nanocomposites
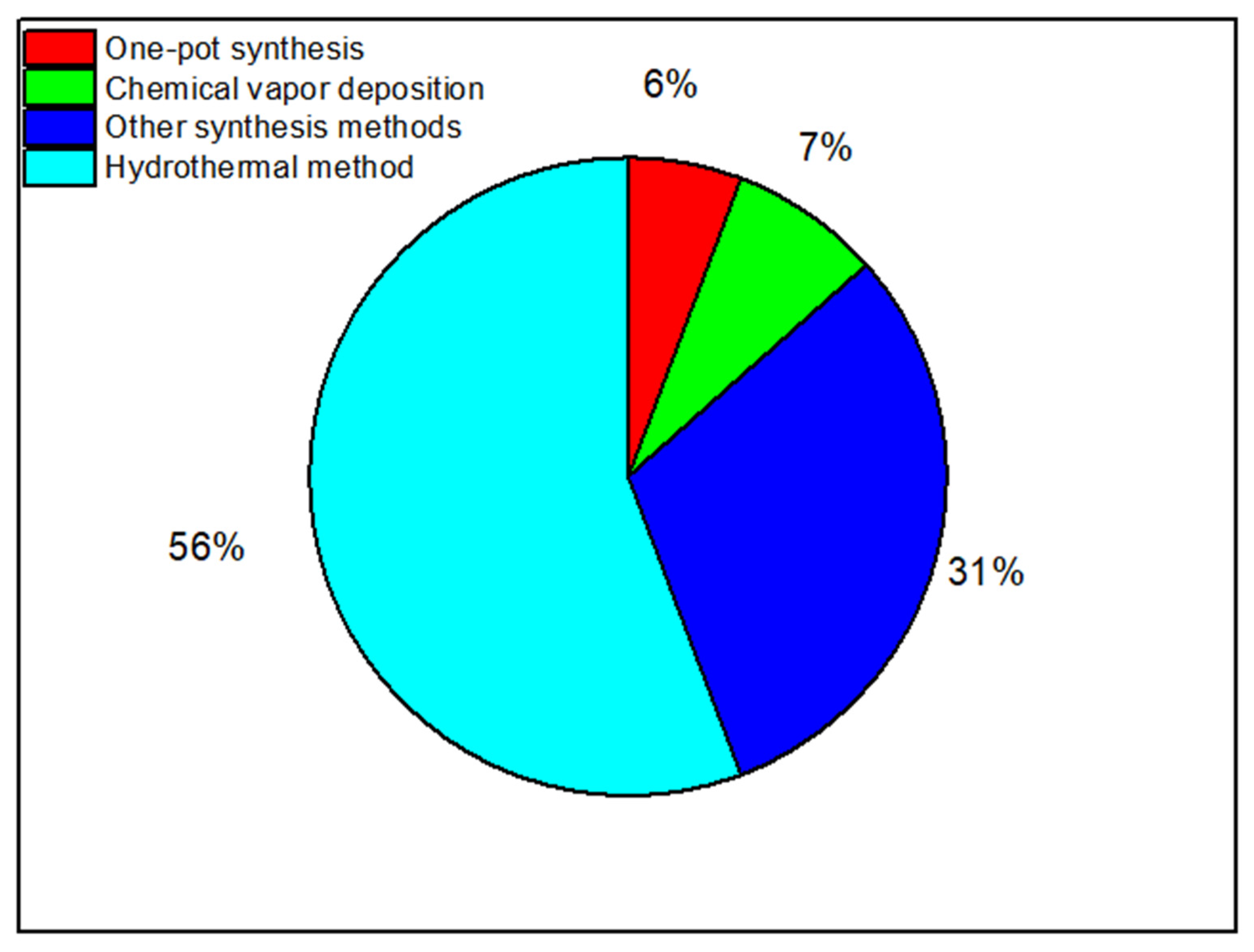
The Hydrothermal Method
Chemical Vapor Deposition
One-Pot Synthesis
Other Synthesis Methods
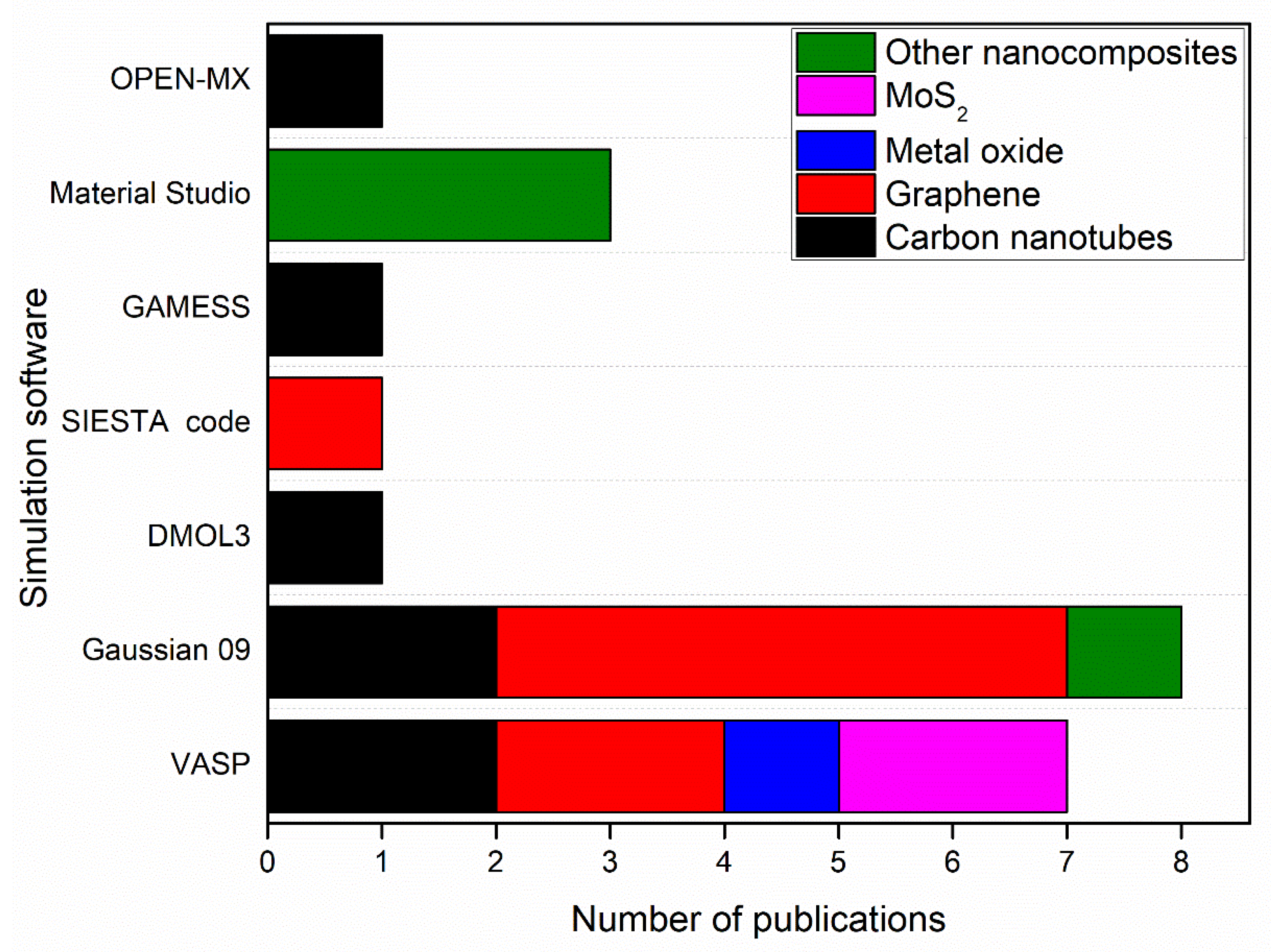
2.2.2. Simulation of Nanocomposites
3. Water Filtration by Membrane Technology
3.1. Carbon-Based Membranes
3.2. Metal Oxides
3.3. Other Nanocomposite Membranes
4. Photocatalytic Degradation of Organic Pollutants
4.1. Titanium Dioxide (TiO2)
4.2. Carbon Nanomaterials
4.3. Metal Oxides
4.4. Other Nanocomposites
5. Future Direction of Nanomembrane Adsorption Processes in Wastewater Treatment
- With the rapid development of simulation software in some important DFT codes such as PBE, B3LYP and PAW, it will be easier to understand the physical and chemical properties of the adsorption process to fill the scientific gaps in realizing the adsorption mechanism, isotherm, kinetics, thermodynamics and other aspects of the adsorption process.
- Further economic feasibility studies should be conducted on adsorbents including the cost effectiveness of the choices of the materials, which is an important aspect of adsorption investigations.
- A huge improvement in the synthesis of nanomaterials using simulation will become possible by linking the density functional theory (DFT) codes using software such as Material Studio and Reactive forefield (ReaxFF) with the molecular dynamic (MD) simulation which will give more realism in acquiring accurate results before starting the experimental work. This step can reduce costs of conducting trials and save time.
- Evolution in the ability and durability of nanomembranes in selectivity of undesirable materials by adsorption which increase the adsorption capacity (qe). This is possible by improving the mechanical properties of the nanomembranes by creating special nanocomposites such as graphene/TiO2, and graphene/MoS2. These two nanocomposites have proven their ability to expel salt and permeate water with high efficiency, so we expect a high adsorption capacity (qe) from them.
- More comprehensive studies should be conducted on the effect of multi-layer membranes in the adsorption process, which is expected to increase the adsorption capacity (qe) due to the increase in attractions between organic pollutants and membranes. In addition, it is possible to use different layers in the same system which can adsorb different pollutants at the same time. We recommend simulating the system using a molecular dynamic simulation software using two different layers and then testing the possibility of adsorption on different organic pollutants.
- More studies should be conducted on the possibility of developing more effective forcefields which is highly required in some molecular dynamics simulation software. Creating and developing high effective forcefields will increase the possibility of simulating all kinds of atoms and molecules with high accuracy without errors.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Connor, R. The United Nations World Water Development Report 2015: Water for a Sustainable World; UNESCO Publishing: Paris, France, 2015; Volume 1. [Google Scholar]
- Cosgrove, W.J.; Loucks, D.P. Water management: Current and future challenges and research directions. Water Resour. Res. 2015, 51, 4823–4839. [Google Scholar] [CrossRef] [Green Version]
- Daud, M.; Nafees, M.; Ali, S.; Rizwan, M.; Bajwa, R.A.; Shakoor, M.B.; Arshad, M.U.; Chatha, S.A.S.; Deeba, F.; Murad, W. Drinking water quality status and contamination in Pakistan. BioMed Res. Int. 2017, 2017, 7908183. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme. Let It Flow: Improving Water Quantity and Quality in Tanzania’s Rufiji River Basin. Available online: https://www.unep.org/news-and-stories/story/let-it-flow-improving-water-quantity-and-quality-tanzanias-rufiji-river (accessed on 9 June 2021).
- Costa, D.; Burlando, P.; Priadi, C. The importance of integrated solutions to flooding and water quality problems in the tropical megacity of Jakarta. Sustain. Cities Soc. 2016, 20, 199–209. [Google Scholar] [CrossRef]
- Choubisa, S.L. Fluoride distribution in drinking groundwater in Rajasthan, India. Curr. Sci. 2018, 114, 1851–1857. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Odiyo, J.O.; Durowoju, O.S. Impact of wastewater on surface water quality in developing countries: A case study of South Africa. In Water Quality; IntechOpen: London, UK, 2017; pp. 401–416. [Google Scholar]
- Martínez-Santos, P. Does 91% of the world’s population really have “sustainable access to safe drinking water”? Int. J. Water Resour. Dev. 2017, 33, 514–533. [Google Scholar] [CrossRef]
- Hotza, D.; Di Luccio, M.; Wilhelm, M.; Iwamoto, Y.; Bernard, S.; da Costa, J.C.D. Silicon carbide filters and porous membranes: A review of processing, properties, performance, and application. J. Membr. Sci. 2020, 610, 118193. [Google Scholar] [CrossRef]
- Liu, H.; Cao, C.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. Progress on particulate matter filtration technology: Basic concepts, advanced materials, and performances. Nanoscale 2020, 12, 437–453. [Google Scholar] [CrossRef]
- Abejón, R.; Garea, A. A bibliometric analysis of research on arsenic in drinking water during the 1992–2012 period: An outlook to treatment alternatives for arsenic removal. J. Water Process Eng. 2015, 6, 105–119. [Google Scholar] [CrossRef]
- Boyd, C.E. Water quality protection. In Water Quality; Springer: Berlin/Heidelberg, Germany, 2020; pp. 379–409. [Google Scholar]
- Symons, G.E. Water treatment through the ages. J.-Am. Water Work. Assoc. 2006, 98, 87–98. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.; Tchobanoglous, G. MWH’s Water Treatment: Principles and Design; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Phu, N. Evaluating Pilot Scale Slow Sand Filtration Columns to Effectively Remove Emerging Contaminants in Recycled Water. Ph.D. Thesis, University of California, Los Angeles, CA, USA, 2016. [Google Scholar]
- Judd, S.J. Membrane technology costs and me. Water Res. 2017, 122, 1–9. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Umar, M.; Aziz, H.A. Photocatalytic degradation of organic pollutants in water. Org. Pollut.-Monit. Risk Treat. 2013, 8, 196–197. [Google Scholar]
- Abbasi-Garravand, E.; Catherine, N.M.; Laflamme, C.B.; Clairet, G. Investigation of the fouling effect on a commercial semi-permeable membrane in the pressure retarded osmosis (PRO) process. Sep. Purif. Technol. 2018, 193, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Puretec Industrial Waters. What is Reverse Osmosis? Available online: https://puretecwater.com/reverse-osmosis/what-is-reverse-osmosis (accessed on 9 June 2021).
- Alsarayreh, A.A.; Al-Obaidi, M.; Al-Hroub, A.; Patel, R.; Mujtaba, I. Evaluation and minimisation of energy consumption in a medium-scale reverse osmosis brackish water desalination plant. J. Clean. Prod. 2020, 248, 119220. [Google Scholar] [CrossRef]
- Gude, V.G. Energy consumption and recovery in reverse osmosis. Desalination Water Treat. 2011, 36, 239–260. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Homaeigohar, S.; Elbahri, M. Graphene membranes for water desalination. NPG Asia Mater. 2017, 9, e427. [Google Scholar] [CrossRef] [Green Version]
- Padaki, M.; Murali, R.S.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.; Hilal, N.; Ismail, A. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Belfort, G. Membrane filtration with liquids: A global approach with prior successes, new developments and unresolved challenges. Angew. Chem. Int. Ed. 2019, 58, 1892–1902. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [Green Version]
- Shon, H.; Phuntsho, S.; Chaudhary, D.; Vigneswaran, S.; Cho, J. Nanofiltration for water and wastewater treatment—A mini review. Drink. Water Eng. Sci. 2013, 6, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, Q.; Akbarzadeh, R. A photocatalytic TiO2/graphene bilayer membrane design for water desalination: A molecular dynamic simulation. J. Mol. Modeling 2020, 26, 165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Z.-R.; Fu, X.; Xu, Y.-J. Engineering the unique 2D mat of graphene to achieve graphene-TiO2 nanocomposite for photocatalytic selective transformation: What advantage does graphene have over its forebear carbon nanotube? ACS Nano 2011, 5, 7426–7435. [Google Scholar] [CrossRef] [PubMed]
- Bhanvase, B.; Shende, T.; Sonawane, S. A review on graphene–TiO2 and doped graphene–TiO2 nanocomposite photocatalyst for water and wastewater treatment. Environ. Technol. Rev. 2017, 6, 1–14. [Google Scholar] [CrossRef]
- Madhura, L.; Kanchi, S.; Sabela, M.I.; Singh, S.; Bisetty, K.; Inamuddin. Membrane technology for water purification. Environ. Chem. Lett. 2018, 16, 343–365. [Google Scholar] [CrossRef]
- Heiranian, M.; Farimani, A.B.; Aluru, N.R. Water desalination with a single-layer MoS2 nanopore. Nat. Commun. 2015, 6, 8616. [Google Scholar] [CrossRef] [Green Version]
- Cohen-Tanugi, D.; Grossman, J.C. Water desalination across nanoporous graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef]
- Kou, J.; Yao, J.; Wu, L.; Zhou, X.; Lu, H.; Wu, F.; Fan, J. Nanoporous two-dimensional MoS2 membranes for fast saline solution purification. Phys. Chem. Chem. Phys. 2016, 18, 22210–22216. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; Lin, L.-C.; Grossman, J.C. Multilayer nanoporous graphene membranes for water desalination. Nano Lett. 2016, 16, 1027–1033. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Sim, A.; Urban, J.J.; Mi, B. Removal and recovery of heavy metal ions by two-dimensional MoS2 nanosheets: Performance and mechanisms. Environ. Sci. Technol. 2018, 52, 9741–9748. [Google Scholar] [CrossRef]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the future of membranes: Perspectives for advanced and new membrane materials and manufacturing processes. J. Membr. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- Suresh, R.; Rajendran, S.; Kumar, P.S.; Vo, D.-V.N.; Cornejo-Ponce, L. Recent advancements of spinel ferrite based binary nanocomposite photocatalysts in wastewater treatment. Chemosphere 2021, 274, 129734. [Google Scholar] [CrossRef] [PubMed]
- Marcolongo, D.M.S.; Nocito, F.; Ditaranto, N.; Comparelli, R.; Aresta, M.; Dibenedetto, A. Opto-electronic characterization of photocatalysts based on p, n-junction ternary and quaternary mixed oxides semiconductors (Cu2O-In2O3 and Cu2O-In2O3-TiO2). Catalysts 2022, 12, 153. [Google Scholar] [CrossRef]
- Dineshbabu, N.; Jayaprakash, R.N.; Karuppasamy, P.; Arun, T.; Judith Vijaya, J.; Esther Nimshi, R.; Pandian, M.S.; Maria Packiam, S.; Ramasamy, P. Investigation on Tetracycline degradation and bactericidal properties of binary and ternary ZnO/NiO/g-C3N4 composites prepared by a facile co-precipitation method. J. Environ. Chem. Eng. 2022, 10, 107368. [Google Scholar] [CrossRef]
- Rassoulinejad-Mousavi, S.M.; Azamat, J.; Khataee, A.; Zhang, Y. Molecular dynamics simulation of water purification using zeolite MFI nanosheets. Sep. Purif. Technol. 2020, 234, 116080. [Google Scholar] [CrossRef]
- Guan, W.; Dai, Y.; Dong, C.; Yang, X.; Xi, Y. Zeolite imidazolate framework (ZIF)-based mixed matrix membranes for CO2 separation: A review. J. Appl. Polym. Sci. 2020, 137, 48968. [Google Scholar] [CrossRef]
- Aloulou, W.; Aloulou, H.; Khemakhem, M.; Duplay, J.; Daramola, M.; Ben Amar, R. Synthesis and characterization of clay-based ultrafiltration membranes supported on natural zeolite for removal of heavy metals from wastewater. Environ. Technol. Innov. 2020, 18, 100794. [Google Scholar] [CrossRef]
- Zalfani, M.; van der Schueren, B.; Mahdouani, M.; Bourguiga, R.; Yu, W.-B.; Wu, M.; Deparis, O.; Li, Y.; Su, B.-L. ZnO quantum dots decorated 3DOM TiO2 nanocomposites: Symbiose of quantum size effects and photonic structure for highly enhanced photocatalytic degradation of organic pollutants. Appl. Catal. B Environ. 2016, 199, 187–198. [Google Scholar] [CrossRef]
- Chacko, L.; Jayaraj, M.; Aneesh, P. Excitation-wavelength dependent upconverting surfactant free MoS2 nanoflakes grown by hydrothermal method. J. Lumin. 2017, 192, 6–10. [Google Scholar] [CrossRef]
- Abdelaziz Aboelazm, E.A.; Mohammed Ali, G.A.; Algarni, H.; Chong, K.F. Flakes size-dependent optical and electrochemical properties of MoS2. Curr. Nanosci. 2018, 14, 416–420. [Google Scholar] [CrossRef]
- Li, B.L.; Zou, H.L.; Lu, L.; Yang, Y.; Lei, J.L.; Luo, H.Q.; Li, N.B. Size-dependent optical absorption of layered MoS2 and dna oligonucleotides induced dispersion behavior for label-free detection of single-nucleotide polymorphism. Adv. Funct. Mater. 2015, 25, 3541–3550. [Google Scholar] [CrossRef]
- Yan, H.; Liu, L.; Wang, R.; Zhu, W.; Ren, X.; Luo, L.; Zhang, X.; Luo, S.; Ai, X.; Wang, J. Binary composite MoS2/TiO2 nanotube arrays as a recyclable and efficient photocatalyst for solar water disinfection. Chem. Eng. J. 2020, 401, 126052. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, S.; Li, X.; Han, Z.; Zhao, C.; Di, T.; Liu, S.; Cheng, Z. Controllable growth of MoS2 nanosheets on TiO2 burst nanotubes and their photocatalytic activity. RSC Adv. 2020, 10, 40904–40915. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B. Zinc oxide based photocatalytic degradation of persistent pesticides: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100290. [Google Scholar] [CrossRef]
- Cai, Z.; Dwivedi, A.D.; Lee, W.-N.; Zhao, X.; Liu, W.; Sillanpää, M.; Zhao, D.; Huang, C.-H.; Fu, J. Application of nanotechnologies for removing pharmaceutically active compounds from water: Development and future trends. Environ. Sci. Nano 2018, 5, 27–47. [Google Scholar] [CrossRef]
- Zheng, L.; Teng, F.; Ye, X.; Zheng, H.; Fang, X. Photo/electrochemical applications of metal sulfide/TiO2 heterostructures. Adv. Energy Mater. 2020, 10, 1902355. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. A review on plasmonic metal-TiO2 composite for generation, trapping, storing and dynamic vectorial transfer of photogenerated electrons across the Schottky junction in a photocatalytic system. Appl. Surf. Sci. 2016, 360, 601–622. [Google Scholar] [CrossRef]
- Duan, Y.; Liang, L.; Lv, K.; Li, Q.; Li, M. TiO2 faceted nanocrystals on the nanofibers: Homojunction TiO2 based Z-scheme photocatalyst for air purification. Appl. Surf. Sci. 2018, 456, 817–826. [Google Scholar] [CrossRef]
- Liu, C.; Faria, A.F.; Jackson, J.; He, Q.; Ma, J. Enhancing the anti-fouling and fouling removal properties of thin-film composite membranes through an intercalated functionalization method. Environ. Sci. Water Res. Technol. 2021, 7, 1336–1347. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- Zou, W.; Gao, B.; Ok, Y.S.; Dong, L. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review. Chemosphere 2019, 218, 845–859. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef] [Green Version]
- Patnaik, S.; Kumar Das, K.; Mohanty, A.; Parida, K. Enhanced photo catalytic reduction of Cr(VI) over polymer-sensitized g-C3N4/ZnFe2O4 and its synergism with phenol oxidation under visible light irradiation. Catal. Today 2018, 315, 52–66. [Google Scholar] [CrossRef]
- Cai, X.; Li, J.; Liu, Y.; Yan, Z.; Tan, X.; Liu, S.; Zeng, G.; Gu, Y.; Hu, X.; Jiang, L. Titanium dioxide-coated biochar composites as adsorptive and photocatalytic degradation materials for the removal of aqueous organic pollutants. J. Chem. Technol. Biotechnol. 2018, 93, 783–791. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Bechelany, M.; Viter, R.; Khranovskyy, V.; Smyntyna, V.; Starodub, N.; Yakimova, R. Optical biosensors based on ZnO nanostructures: Advantages and perspectives. A review. Sens. Actuators B Chem. 2016, 229, 664–677. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.N.; You, S.J.; Chao, H.P. Fast and efficient adsorption of methylene green 5 on activated carbon prepared from new chemical activation method. J. Environ. Manag. 2017, 188, 322–336. [Google Scholar] [CrossRef]
- Awual, M.R.; Khraisheh, M.; Alharthi, N.H.; Luqman, M.; Islam, A.; Karim, M.R.; Rahman, M.M.; Khaleque, M.A. Efficient detection and adsorption of cadmium (II) ions using innovative nano-composite materials. Chem. Eng. J. 2018, 343, 118–127. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Rashed, M.N. Adsorption technique for the removal of organic pollutants from water and wastewater. Org. Pollut.-Monit. Risk Treat. 2013, 7, 167–194. [Google Scholar]
- Clark, A. The Theory of Adsorption and Catalysis; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Wang, C.; Lin, G.; Zhao, J.; Wang, S.; Zhang, L. Enhancing Au (III) adsorption capacity and selectivity via engineering MOF with mercapto-1,3,4-thiadiazole. Chem. Eng. J. 2020, 388, 124221. [Google Scholar] [CrossRef]
- Deb, A.; Debnath, A.; Saha, B. Sono-assisted enhanced adsorption of eriochrome Black-T dye onto a novel polymeric nanocomposite: Kinetic, isotherm, and response surface methodology optimization. J. Dispers. Sci. Technol. 2021, 42, 1579–1592. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Y.; Li, P.; Zhao, Y.; Zou, R. Selective H2S/CO2 separation by metal–organic frameworks based on chemical-physical adsorption. J. Phys. Chem. C 2017, 121, 13249–13255. [Google Scholar] [CrossRef]
- Ruiz, V.G.; Liu, W.; Tkatchenko, A. Density-functional theory with screened van der Waals interactions applied to atomic and molecular adsorbates on close-packed and non-close-packed surfaces. Phys. Rev. B 2016, 93, 035118. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Evans, J. Metering and dispensing of powder; the quest for new solid freeforming techniques. Powder Technol. 2007, 178, 56–72. [Google Scholar] [CrossRef]
- Li, Q.; Rudolph, V.; Peukert, W. London-van der Waals adhesiveness of rough particles. Powder Technol. 2006, 161, 248–255. [Google Scholar] [CrossRef]
- Welker, R.W. Basics and sampling of particles for size analysis and identification. In Developments in Surface Contamination and Cleaning; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–80. [Google Scholar]
- Sotomayor, F.J.; Cychosz, K.A.; Thommes, M. Characterization of micro/mesoporous materials by physisorption: Concepts and case studies. Acc. Mater. Surf. Res. 2018, 3, 34–50. [Google Scholar]
- Pan, J.H.; Zhang, X.; Du, A.J.; Bai, H.; Ng, J.; Sun, D. A hierarchically assembled mesoporous ZnO hemisphere array and hollow microspheres for photocatalytic membrane water filtration. Phys. Chem. Chem. Phys. 2012, 14, 7481–7489. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Ren, P.-F.; Yang, H.-C.; Xu, Z.-K. Fabrication of antifouling membrane surface by poly(sulfobetaine methacrylate)/polydopamine co-deposition. J. Membr. Sci. 2014, 466, 18–25. [Google Scholar] [CrossRef]
- Peng, B.; Li, Y.; Zhao, Z.; Chen, Y.; Han, C.C. Facile surface modification of PVDF microfiltration membrane by strong physical adsorption of amphiphilic copolymers. J. Appl. Polym. Sci. 2013, 130, 3112–3121. [Google Scholar] [CrossRef]
- Bikerman, J.J. Surface Chemistry: Theory and Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Matouq, M.; Jildeh, N.; Qtaishat, M.; Hindiyeh, M.; Al Syouf, M.Q. The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. J. Environ. Chem. Eng. 2015, 3, 775–784. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; Tan, J.; Wang, B.; Huang, F. Advance of the treatment of heavy metal wastewater by adsorption. Chem. Ind. Eng. Prog. 2013, 32, 2749–2756. [Google Scholar]
- Renu, M.A.; Singh, K.; Upadhyaya, S.; Dohare, R. Removal of heavy metals from wastewater using modified agricultural adsorbents. Mater. Today Proc. 2017, 4, 10534–10538. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, C.; Wu, Y.; Geng, L.; Zhang, X.; Zhang, D.; Hu, H.; Zhang, Y.; Li, X.; Liu, W. Synthesis of uniform-sized and microporous MIL-125 (Ti) to boost arsenic removal by chemical adsorption. Polyhedron 2021, 196, 114980. [Google Scholar] [CrossRef]
- Martínez, C.M.; Canle L., M.; Fernández, M.I.; Santaballa, J.A.; Faria, J. Kinetics and mechanism of aqueous degradation of carbamazepine by heterogeneous photocatalysis using nanocrystalline TiO2, ZnO and multi-walled carbon nanotubes–anatase composites. Appl. Catal. B Environ. 2011, 102, 563–571. [Google Scholar] [CrossRef]
- Son, H.-S.; Lee, S.-J.; Cho, I.-H.; Zoh, K.-D. Kinetics and mechanism of TNT degradation in TiO2 photocatalysis. Chemosphere 2004, 57, 309–317. [Google Scholar] [CrossRef]
- Ji, P.; Zhang, J.; Chen, F.; Anpo, M. Study of adsorption and degradation of acid orange 7 on the surface of CeO2 under visible light irradiation. Appl. Catal. B Environ. 2009, 85, 148–154. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, X.; Gao, B.; Zou, W.; Zheng, Y.; Yang, Y.; Zhang, Y.; Tong, Q.; Dong, L. Synergistic adsorption-photocatalysis processes of graphitic carbon nitrate (g-C3N4) for contaminant removal: Kinetics, models, and mechanisms. Chem. Eng. J. 2019, 375, 122019. [Google Scholar] [CrossRef]
- Lan, L.; Huang, Y.; Dan, Y.; Jiang, L. Conjugated porous polymers for gaseous toluene adsorption in humid atmosphere. React. Funct. Polym. 2021, 159, 104804. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, D.; Zhang, G.; Cai, C.; Zhang, C.; Qiu, G.; Zheng, K.; Wu, Z. Adsorption of methylene blue from aqueous solution onto multiporous palygorskite modified by ion beam bombardment: Effect of contact time, temperature, pH and ionic strength. Appl. Clay Sci. 2013, 83, 137–143. [Google Scholar] [CrossRef]
- Han, Y.; Cao, X.; Ouyang, X.; Sohi, S.P.; Chen, J. Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr (VI) from aqueous solution: Effects of production conditions and particle size. Chemosphere 2016, 145, 336–341. [Google Scholar] [CrossRef]
- Thakur, K.; Kandasubramanian, B. Graphene and graphene oxide-based composites for removal of organic pollutants: A review. J. Chem. Eng. Data 2019, 64, 833–867. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Wang, H.; Ni, Y.; Zhu, Z. Absorption behaviors study on doped Li4SiO4 under a humidified atmosphere with low CO2 concentration. Int. J. Hydrogen Energy 2014, 39, 17913–17920. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations–a review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Zhu, J.; Shuai, Y.; Jiang, B.; Huang, Y. An experimental investigation on sunlight absorption characteristics of silver nanofluids. Sol. Energy 2015, 115, 85–94. [Google Scholar] [CrossRef]
- Reimhult, E.; Höök, F.; Kasemo, B. Intact vesicle adsorption and supported biomembrane formation from vesicles in solution: Influence of surface chemistry, vesicle size, temperature, and osmotic pressure. Langmuir 2003, 19, 1681–1691. [Google Scholar] [CrossRef]
- Woellner, M.; Hausdorf, S.; Klein, N.; Mueller, P.; Smith, M.W.; Kaskel, S. Adsorption and detection of hazardous trace gases by metal–organic frameworks. Adv. Mater. 2018, 30, 1704679. [Google Scholar] [CrossRef]
- Laabd, M.; El Jaouhari, A.; Bazzaoui, M.; Albourine, A.; El Jazouli, H. Adsorption of benzene-polycarboxylic acids on the electrosynthesized polyaniline films: Experimental and DFT calculation. J. Polym. Environ. 2017, 25, 359–369. [Google Scholar] [CrossRef]
- Bergaoui, M.; Nakhli, A.; Benguerba, Y.; Khalfaoui, M.; Erto, A.; Soetaredjo, F.E.; Ismadji, S.; Ernst, B. Novel insights into the adsorption mechanism of methylene blue onto organo-bentonite: Adsorption isotherms modeling and molecular simulation. J. Mol. Liq. 2018, 272, 697–707. [Google Scholar] [CrossRef]
- Zhang, J.; Tong, H.; Pei, W.; Liu, W.; Shi, F.; Li, Y.; Huo, Y. Integrated photocatalysis-adsorption-membrane separation in rotating reactor for synergistic removal of RhB. Chemosphere 2021, 270, 129424. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, W.; Xu, C.; Liu, X.; Yang, K.; Li, D.; Hou, Y.; Dionysiou, D.D. Novel hierarchical carbon quantum dots-decorated BiOCl nanosheet/carbonized eggshell membrane composites for improved removal of organic contaminants from water via synergistic adsorption and photocatalysis. Chem. Eng. J. 2021, 420, 129582. [Google Scholar] [CrossRef]
- Huang, J.; Huang, D.; Zeng, F.; Ma, L.; Wang, Z. Photocatalytic MOF fibrous membranes for cyclic adsorption and degradation of dyes. J. Mater. Sci. 2021, 56, 3127–3139. [Google Scholar] [CrossRef]
- Schmidt, J.; Shi, J.; Borlido, P.; Chen, L.; Botti, S.; Marques, M.A. Predicting the thermodynamic stability of solids combining density functional theory and machine learning. Chem. Mater. 2017, 29, 5090–5103. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Tu, C.-H.; Hsu, H.-H.; Chen, J.-H.; Chen, C.-H.; Hung, S.-H. Performance and power profiling for emulated android systems. ACM Trans. Des. Autom. Electron. Syst. 2014, 19, 1–25. [Google Scholar] [CrossRef]
- Mourtzis, D.; Doukas, M.; Bernidaki, D. Simulation in manufacturing: Review and challenges. Procedia Cirp 2014, 25, 213–229. [Google Scholar] [CrossRef] [Green Version]
- Brandimarte, P. Handbook in Monte Carlo Simulation: Applications in Financial Engineering, Risk Management, and Economics; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Hensen, J.L.; Lamberts, R. Building Performance Simulation for Design and Operation; Routledge: London, UK, 2012. [Google Scholar]
- Vo, H.T. On Efficient Algorithms for Stochastic Simulation of Biochemical Reaction Systems. Ph.D. Thesis, University of Trento, Trento, Italy, 2013. [Google Scholar]
- Fatemi, S.M.; Abbasi, Z.; Rajabzadeh, H.; Hashemizadeh, S.A.; Deldar, A.N. A review of recent advances in molecular simulation of graphene-derived membranes for gas separation. Eur. Phys. J. D 2017, 71, 194. [Google Scholar] [CrossRef]
- Yang, P.-Y.; Ju, S.-P.; Hsieh, H.-S.; Lin, J.-S.; Hsieh, J.-Y. Electrolytic molecule in-pore structure and capacitance of supercapacitors with nanoporous carbon electrodes: A coarse-grained molecular dynamics study. Comput. Mater. Sci. 2019, 166, 293–302. [Google Scholar] [CrossRef]
- Hu, Q.; Xue, M.; Shen, C.; Zhang, Z.; Guo, W. Graphynes for water desalination and gas separation. Adv. Mater. 2019, 42, 1803772. [Google Scholar]
- Rebelo, P.; Pacheco, J.G.; Voroshylova, I.V.; Melo, A.; Cordeiro, M.N.D.; Delerue-Matos, C. Rational development of molecular imprinted carbon paste electrode for Furazolidone detection: Theoretical and experimental approach. Sens. Actuators B Chem. 2021, 329, 129112. [Google Scholar] [CrossRef]
- de Oliveira, P.V.; Zanella, I.; Bulhoes, L.O.S.; Fagan, S.B. Adsorption of 17 β-estradiol in graphene oxide through the competing methanol co-solvent: Experimental and computational analysis. J. Mol. Liq. 2021, 321, 114738. [Google Scholar] [CrossRef]
- Arnold, J.G.; Moriasi, D.N.; Gassman, P.W.; Abbaspour, K.C.; White, M.J.; Srinivasan, R.; Santhi, C.; Harmel, R.D.; van Griensven, A.; van Liew, M.W.; et al. SWAT: Model use, calibration, and validation. Trans. ASABE 2012, 55, 1491–1508. [Google Scholar] [CrossRef]
- Malleson, N. Calibration of simulation models. Encycl. Criminol. Crim. Justice 2014, 40, 115–118. [Google Scholar]
- Zhu, R.; Zhu, Y.; Xian, H.; Yan, L.; Fu, H.; Zhu, G.; Xi, Y.; Zhu, J.; He, H. CNTs/ferrihydrite as a highly efficient heterogeneous Fenton catalyst for the degradation of bisphenol A: The important role of CNTs in accelerating Fe(III)/Fe(II) cycling. Appl. Catal. B Environ. 2020, 270, 118891. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Chen, G.; Zheng, X.; Dai, M.; Peng, C. Metal-organic framework membranes: Recent development in the synthesis strategies and their application in oil-water separation. Chem. Eng. J. 2021, 405, 127004. [Google Scholar] [CrossRef]
- Jakšić, Z.; Jakšić, O. Biomimetic nanomembranes: An overview. Biomimetics 2020, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Nosheen, S. Nanomembrane applications in environmental engineering. In Nanotechnology Applications in Environmental Engineering; IGI Global: Hershey, PA, USA, 2019; pp. 103–120. [Google Scholar]
- Jani, A.M.; Anglin, E.J.; McInnes, S.J.P.; Losic, D.; Shapter, J.G.; Voelcker, N.H. Nanoporous anodic aluminium oxide membranes with layered surface chemistry. Chem. Commun. 2009, 21, 3062–3064. [Google Scholar] [CrossRef]
- Mei, Y.; Thurmer, D.J.; Deneke, C.; Kiravittaya, S.; Chen, Y.-F.; Dadgar, A.; Bertram, F.; Bastek, B.; Krost, A.; Christen, J.; et al. Fabrication, self-assembly, and properties of ultrathin AlN/GaN porous crystalline nanomembranes: Tubes, spirals, and curved sheets. Acs Nano 2009, 3, 1663–1668. [Google Scholar] [CrossRef]
- De Wolf, I.; Senez, V.; Balboni, R.; Armigliato, A.; Frabboni, S.; Cedola, A.; Lagomarsino, S. Techniques for mechanical strain analysis in sub-micrometer structures: TEM/CBED, micro-Raman spectroscopy, X-ray micro-diffraction and modeling. Microelectron. Eng. 2003, 4, 425–435. [Google Scholar] [CrossRef]
- Dhanabal, R.; Naveena, D.; Velmathi, S.; Bose, A.C. Reduced graphene oxide supported molybdenum oxide hybrid nanocomposites: High performance electrode material for supercapacitor and photocatalytic applications. J. Nanosci. Nanotechnol. 2020, 20, 4035–4046. [Google Scholar] [CrossRef]
- Agboola, O.; Sadiku, E.R.; Mokrani, T. Nanomembrane materials based on polymer blends. In Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 101–123. [Google Scholar]
- Won, Y.-J.; Lee, J.; Choi, D.-C.; Chae, H.R.; Kim, I.; Lee, C.-H.; Kim, I.-C. Preparation and application of patterned membranes for wastewater treatment. Environ. Sci. Technol. 2012, 46, 11021–11027. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Armelin, E.; Puiggalí, J.; Alemán, C. Insulating and semiconducting polymeric free-standing nanomembranes with biomedical applications. J. Mater. Chem. B 2015, 3, 5904–5932. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Chen, W.; Zhu, M.; Murray, S. Embedding lauric acid into polystyrene nanofibers to make high-capacity membranes for efficient thermal energy storage. ACS Sustain. Chem. Eng. 2017, 5, 7249–7259. [Google Scholar] [CrossRef]
- Saleh, T.A. Protocols for synthesis of nanomaterials, polymers, and green materials as adsorbents for water treatment technologies. Environ. Technol. Innov. 2021, 24, 101821. [Google Scholar] [CrossRef]
- Gracheva, I.E.; Moshnikov, V.; Maraeva, E.; Karpova, S.S.; Alexsandrova, O.A.; Alekseyev, N.I.; Kuznetsov, V.V.; Olchowik, G.; Semenov, K.; Startseva, A.V.; et al. Nanostructured materials obtained under conditions of hierarchical self-assembly and modified by derivative forms of fullerenes. J. Non-Cryst. Solids 2012, 358, 433–439. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Yahaya, N.Z.S.; Paiman, S.H.; Abdullah, N.; Mahpoz, N.M.; Raffi, A.A.; Rahman, M.A.; Abas, K.H.; Aziz, A.A.; Othman, M.H.D.; Jaafar, J. Synthesis and characterizations of MIL-140B-Al2O3/YSZ ceramic membrane using solvothermal method for seawater desalination. J. Aust. Ceram. Soc. 2020, 56, 291–300. [Google Scholar] [CrossRef]
- Maina, J.W.; Gonzalo, C.P.; Merenda, A.; Kong, L.; Schütz, J.A.; Dumée, L.F. The growth of high density network of MOF nano-crystals across macroporous metal substrates–Solvothermal synthesis versus rapid thermal deposition. Appl. Surf. Sci. 2018, 427, 401–408. [Google Scholar] [CrossRef]
- Ahn, E.; Gaiji, H.; Kim, T.; Abderrabba, M.; Lee, H.-W.; Kim, B.-S. Graphene oxide nanosheet as a two-dimensional polyelectrolyte: pH-responsive behavior of a multilayered nanomembrane. J. Membr. Sci. 2019, 585, 191–198. [Google Scholar] [CrossRef]
- Kohn, J. Small-scale membrane filter electrophoresis and immuno-electrophoresis. Clin. Chim. Acta 1958, 3, 450–454. [Google Scholar] [CrossRef]
- Lagashetty, A.; Havanoor, V.; Basavaraja, S.; Balaji, S.D.; Venkataraman, A. Microwave-assisted route for synthesis of nanosized metal oxides. Sci. Technol. Adv. Mater. 2007, 8, 484. [Google Scholar] [CrossRef]
- Dahiya, M.S.; Tomer, V.K.; Duhan, S. Metal–ferrite nanocomposites for targeted drug delivery. In Applications of Nanocomposite Materials in Drug Delivery; Woodhead Publishing: Sawston, UK, 2018; pp. 737–760. [Google Scholar]
- Al-Hamadani, Y.A.; Jung, C.; Im, J.-K.; Boateng, L.K.; Flora, J.R.; Jang, M.; Heo, J.; Park, C.M.; Yoon, Y. Sonocatalytic degradation coupled with single-walled carbon nanotubes for removal of ibuprofen and sulfamethoxazole. Chem. Eng. Sci. 2017, 162, 300–308. [Google Scholar] [CrossRef]
- Abdulkhair, B.; Salih, M.; Modwi, A.; Adam, F.; Elamin, N.; Seydou, M.; Rahali, S. Adsorption behavior of barium ions onto ZnO surfaces: Experiments associated with DFT calculations. J. Mol. Struct. 2021, 1223, 128991. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, C.; Yin, L.; Wen, T.; Yang, Y.; Ai, Y.; Wang, X. Combining batch technique with theoretical calculation studies to analyze the highly efficient enrichment of U(VI) and Eu(III) on magnetic MnFe2O4 nanocubes. Chem. Eng. J. 2018, 349, 347–357. [Google Scholar] [CrossRef]
- Tian, Y.L.; Hua, H.L.; Yue, W.W.; Chen, M.N.; Hu, G.C.; Ren, J.F.; Yuan, X.B. Adsorption properties of chloroform molecule on graphene: Experimental and first-principles calculations. Mod. Phys. Lett. B 2017, 31, 1750335. [Google Scholar] [CrossRef]
- Kang, W.; Cui, Y.; Yang, Y.; Zhao, Z.; Wang, X.; Liu, X. An acid induction strategy to construct an ultralight and durable amino-functionalized graphene oxide aerogel for enhanced quinoline pollutants extraction from coking wastewater. Chem. Eng. J. 2021, 412, 128686. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, Q.; Liu, Y.; Chen, P.; Zheng, X.; Zhuang, X.; Fu, D.; Liu, H.; Liu, G.; Lv, W. A novel nitrogen-containing covalent organic framework adsorbent for the efficient removal of bisphenol A from aqueous solution. J. Taiwan Inst. Chem. Eng. 2020, 113, 204–213. [Google Scholar] [CrossRef]
- Khajouei, M.; Najafi, M.; Jafari, S.A. Development of ultrafiltration membrane via in-situ grafting of nano-GO/PSF with anti-biofouling properties. Chem. Eng. Res. Des. 2019, 142, 34–43. [Google Scholar] [CrossRef]
- Han, J.; Lee, S.; Choi, K.; Kim, J.; Ha, D.; Lee, C.-G.; An, B.; Lee, S.-H.; Mizuseki, H.; Choi, J.-W.; et al. Effect of nitrogen doping on titanium carbonitride-derived adsorbents used for arsenic removal. J. Hazard. Mater. 2016, 302, 375–385. [Google Scholar] [CrossRef]
- Reynosa-Martínez, A.C.G.; Tovar, N.; Gallegos, W.R.; Rodríguez-Meléndez, H.; Torres-Cadena, R.; Mondragón-Solórzano, G.; Barroso-Flores, J.; Alvarez-Lemus, M.A.; Montalvo, V.G.; López-Honorato, E. Effect of the degree of oxidation of graphene oxide on As(III) adsorption. J. Hazard. Mater. 2020, 384, 121440. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hu, X.; Zhou, Q. Sunlight-assisted tailoring of surface nanostructures on single-layer graphene nanosheets for highly efficient cation capture and high-flux desalination. Carbon 2020, 161, 674–684. [Google Scholar] [CrossRef]
- Lawal, I.A.; Lawal, M.M.; Akpotu, S.O.; Azeez, M.A.; Ndungu, P.; Moodley, B. Theoretical and experimental adsorption studies of sulfamethoxazole and ketoprofen on synthesized ionic liquids modified CNTs. Ecotoxicol. Environ. Saf. 2018, 161, 542–552. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Yang, S.; Hobiny, A.; Alsaedi, A.; Wang, X. Understanding the adsorption mechanism of Ni(II) on graphene oxides by batch experiments and density functional theory studies. Sci. China Chem. 2016, 59, 412–419. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Shi, C.; Yan, B.; Gong, L.; Chen, J.; Xiang, L.; Xu, H.; Liu, Q.; Zeng, H. Unraveling the molecular interaction mechanism between graphene oxide and aromatic organic compounds with implications on wastewater treatment. Chem. Eng. J. 2019, 358, 842–849. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, C.; Zhong, W.; Wei, Q.; Wang, Y.; Dai, Y.; Wang, Y.; Zhang, Z.; Liu, Y. Effective adsorption of uranyl ions with different MoS2-exposed surfaces in aqueous solution. Surf. Interfaces 2020, 18, 100409. [Google Scholar] [CrossRef]
- Zhang, L.; He, X.; Zhou, Q.; Hu, X. Fabrication of 1T-MoS2 nanosheets and the high-efficiency removal of toxic metals in aquatic systems: Performance and mechanisms. Chem. Eng. J. 2020, 386, 123996. [Google Scholar] [CrossRef]
- Younes, H.A.; Khaled, R.; Mahmoud, H.M.; Nassar, H.F.; Abdelrahman, M.M.; El-Ela, F.I.A.; Taha, M. Computational and experimental studies on the efficient removal of diclofenac from water using ZnFe-layered double hydroxide as an environmentally benign absorbent. J. Taiwan Inst. Chem. Eng. 2019, 102, 297–311. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Z.; Lei, D.; Liu, F.; He, Y.; Wang, H.; Luo, J. Experimental and modeling studies for adsorbing different species of fluoride using lanthanum-aluminum perovskite. Chemosphere 2021, 263, 128089. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Liu, T.; Cao, W.; Zhang, L.; Li, M.; Chen, Z. Synthesis of BiOBr/Ag3PO4 heterojunctions on carbon-fiber cloth as filter-membrane-shaped photocatalyst for treating the flowing antibiotic wastewater. J. Colloid Interface Sci. 2020, 575, 183–193. [Google Scholar] [CrossRef]
- Neugebauer, J.; Hickel, T. Density functional theory in materials science. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 438–448. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Kumar, P.; Chandra, R. Applications of BIOVIA materials studio, LAMMPS, and GROMACS in various fields of science and engineering. In Molecular Dynamics Simulation of Nanocomposites Using BIOVIA Materials Studio, Lammps and Gromacs; Elsevier: Amsterdam, The Netherlands, 2019; pp. 329–341. [Google Scholar]
- Robinson, S. Simulation: The Practice of Model Development and Use; Wiley: Chichester, UK, 2004; Volume 50. [Google Scholar]
- Krogel, J.T. Nexus: A modular workflow management system for quantum simulation codes. Comput. Phys. Commun. 2016, 198, 154–168. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Zhang, W.-B.; Tang, B.-Y. Electronic structures and elastic properties of monolayer and bilayer transition metal dichalcogenides MX2 (M = Mo, W; X = O, S, Se, Te): A comparative first-principles study. Chin. Phys. B 2015, 24, 097103. [Google Scholar]
- Lehner, A.J.; Fabini, D.H.; Evans, H.A.; Hébert, C.-A.; Smock, S.R.; Hu, J.; Wang, H.; Zwanziger, J.W.; Chabinyc, M.L.; Seshadri, R. Crystal and electronic structures of complex bismuth iodides A3Bi2I9 (A = K, Rb, Cs) related to perovskite: Aiding the rational design of photovoltaics. Chem. Mater. 2015, 27, 7137–7148. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, L.; Li, W.; Carrete, J.; Mingo, N.; Broido, D.A.; Reinecke, T.L. Phonon thermal transport in strained and unstrained graphene from first principles. Phys. Rev. B 2014, 89, 155426. [Google Scholar] [CrossRef]
- Repetsky, S.P.; Vyshyvana, I.G.; Kruchinin, S.P.; Melnyk, R.M.; Polishchuk, A.P. The energy spectrum and the electrical conductivity of graphene with substitution impurity. arXiv 2020, arXiv:2003.02084v1. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.; Li, X.; Liu, Y.; Li, N.; Wang, Y.; Li, X. A key role of inner-cation-π interaction in adsorption of Pb(II) on carbon nanotubes: Experimental and DFT studies. J. Hazard. Mater. 2021, 412, 125187. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Tian, D.; Cheng, C.; Liu, Y.; Zhao, Z.; Liu, Y.; Bao, Y.; Xue, C. Carbon nanotube arrays hybrid membrane with excellent separation performance and conductivity. J. Membr. Sci. 2021, 620, 118874. [Google Scholar] [CrossRef]
- Ren, X.; Feng, J.; Si, P.; Zhang, L.; Lou, J.; Ci, L. Enhanced heterogeneous activation of peroxydisulfate by S, N co-doped graphene via controlling S, N functionalization for the catalytic decolorization of dyes in water. Chemosphere 2018, 210, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, W.; Qi, Z.; Zhang, L.; Peng, Y. Phosphate removal by ZIF-8@ MWCNT hybrids in presence of effluent organic matter: Adsorbent structure, wastewater quality, and DFT analysis. Sci. Total Environ. 2020, 745, 141054. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, Y.; Ai, Y.; Wang, X.; Zhang, R.; Chen, Z.; Chen, Z.; Zhao, G.; Wang, X. Rational design of carbonaceous nanofiber/Ni-Al layered double hydroxide nanocomposites for high-efficiency removal of heavy metals from aqueous solutions. Environ. Pollut. 2018, 242, 1–11. [Google Scholar] [CrossRef]
- Joseph, L.; Boateng, L.K.; Flora, J.R.; Park, Y.-G.; Son, A.; Badawy, M.; Yoon, Y. Removal of bisphenol A and 17α-ethinyl estradiol by combined coagulation and adsorption using carbon nanomaterials and powdered activated carbon. Sep. Purif. Technol. 2013, 107, 37–47. [Google Scholar] [CrossRef]
- do Céu Teixeira, M.; Santini, A.; Souto, E.B. Delivery of antimicrobials by chitosan-composed therapeutic nanostructures. In Nanostructures for Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 203–222. [Google Scholar]
- Zhang, S.; Sun, G.; He, Y.; Fu, R.; Gu, Y.; Chen, S. Preparation, characterization, and electrochromic properties of nanocellulose-based polyaniline nanocomposite films. ACS Appl. Mater. Interfaces 2017, 9, 16426–16434. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Ali, A. Nanomaterials in biosensors: Fundamentals and applications. In Nanomaterials for Biosensors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–74. [Google Scholar]
- Chen, Y.; Lai, Z.; Zhang, X.; Fan, Z.; He, Q.; Tan, C.; Zhang, H. Phase engineering of nanomaterials. Nat. Rev. Chem. 2020, 4, 243–256. [Google Scholar] [CrossRef]
- Chkirida, S.; Zari, N.; El Kacem Qaiss, A.; Bouhfid, R. Nanocomposite materials based on TiO2/clay for wastewater treatment. In Advanced Research in Nanosciences for Water Technology; Springer: Cham, Switzerland, 2019; pp. 363–380. [Google Scholar]
- Wang, J.; Li, Y.; Zheng, D.; Mikulčić, H.; Vujanović, M.; Sundén, B. Preparation and thermophysical property analysis of nanocomposite phase change materials for energy storage. Renew. Sustain. Energy Rev. 2021, 151, 111541. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Nayak, A.K. Nanocomposites for improved orthopedic and bone tissue engineering applications. In Applications of Nanocomposite Materials in Orthopedics; Woodhead Publishing: Sawston, UK, 2019; pp. 145–177. [Google Scholar]
- Singh, S.; Kumar, V.; Romero, R.; Sharma, K.; Singh, J. Applications of nanoparticles in wastewater treatment. In Nanobiotechnology in Bioformulations; Springer: Cham, Switzerland, 2019; pp. 395–418. [Google Scholar]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in situ remediation: A review of the benefits and potential risks. Environ. Health Perspect. 2009, 117, 1813–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Qian, L.; Zhao, Q.; Wang, Z.; Lin, D.; Liu, W.; Chen, Y.; Zhang, J. Carbon nanotube: Controlled synthesis determines its future. Sci. China Mater. 2020, 63, 16–34. [Google Scholar] [CrossRef] [Green Version]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for synthesis of nanoparticles and fabrication of nanocomposites. In Synthesis of Inorganic Nanomaterials; Woodhead Publishing: Sawston, UK, 2018; pp. 121–139. [Google Scholar]
- Manawi, Y.M.; Samara, A.; Al-Ansari, T.; Atieh, M.A. A review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Q.; Cao, M.; Hu, H.; Yang, D.; Chen, M.; Li, P.; Wu, L.; Zhang, Q. One-pot synthesis of highly stable CsPbBr3@ SiO2 core–shell nanoparticles. Acs Nano 2018, 12, 8579–8587. [Google Scholar] [CrossRef]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M. Hydrothermal synthesis of nanomaterials. J. Nanomater. 2020, 2020, 8917013. [Google Scholar] [CrossRef]
- Kaflé, B.P. Chemical Analysis and Material Characterization by Spectrophotometry; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Adeniji, Q.A.; Odunaike, R.K.; Bamijoko, B.A.; Adeleke, A.T.; Dahunsi, K.T. Synthesis and characterization of Zinc Tin sulphide (ZTS) thin films via chemical bath deposition route. J. Appl. Sci. Inf. Comput. 2020, 1, 22–31. [Google Scholar]
- Yang, G.; Park, S.-J. Conventional and microwave hydrothermal synthesis and application of functional materials: A review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Magdassi, S.; Gao, Y.; Long, Y. Hydrothermal synthesis of VO2 polymorphs: Advantages, challenges and prospects for the application of energy efficient smart windows. Small 2017, 13, 1701147. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Chen, M.; Yan, X.H.; Ren, J.; Dai, Y.; Wang, J.J.; Pan, J.M.; Wang, Y.P.; Cheng, X.N. Hydrothermal synthesis of Fe3O4 nanorods/graphitic C3N4 composite with enhanced supercapacitive performance. Mater. Lett. 2017, 198, 114–117. [Google Scholar] [CrossRef]
- Modan, E.M.; Plăiașu, A.G. Advantages and disadvantages of chemical methods in the elaboration of nanomaterials. Ann. “Dunarea de Jos” Univ. Galati. Fascicle IX Met. Mater. Sci. 2020, 43, 53–60. [Google Scholar] [CrossRef]
- Das, S.; Dhara, S. Chemical solution synthesis for materials design and thin film device applications. In Chemical Solution Synthesis for Materials Design and Thin Film Device Applications; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Yang, G.; Xie, J.; Deng, Y.; Bian, Y.; Hong, F. Hydrothermal synthesis of bacterial cellulose/AgNPs composite: A “green” route for antibacterial application. Carbohydr. Polym. 2012, 87, 2482–2487. [Google Scholar] [CrossRef]
- Byrappa, K.; Yoshimura, M. Handbook of Hydrothermal Technology; William Andrew: Norwich, NY, USA, 2012. [Google Scholar]
- Wang, X.L.; Li, J.; Liu, W.M. Synthesizing pyridinic-N dominate-doped graphene/BiVO4 nanocomposite as a superior photocatalyst for degradation under visible-irradiation. Opt. Mater. 2021, 114, 110922. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Ji, Y.; Ma, T.; Zhang, F.; Wang, Y.; Ci, M.; Chen, D.; Jiang, A.; Wang, W. Photocatalytic degradation of methylene blue with ZnO@ C nanocomposites: Kinetics, mechanism, and the inhibition effect on monoamine oxidase A and B. NanoImpact 2019, 15, 100174. [Google Scholar] [CrossRef]
- Li, P.; Guo, M.; Wang, Q.; Li, Z.; Wang, C.; Chen, N.; Wang, C.-C.; Wan, C.; Chen, S. Controllable synthesis of cerium zirconium oxide nanocomposites and their application for photocatalytic degradation of sulfonamides. Appl. Catal. B Environ. 2019, 259, 118107. [Google Scholar] [CrossRef]
- Nie, Q.; Xie, Y.; Ma, J.; Wang, J.; Zhang, G. High piezo–catalytic activity of ZnO/Al2O3 nanosheets utilizing ultrasonic energy for wastewater treatment. J. Clean. Prod. 2020, 242, 118532. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.L.; Guo, D.; Ma, L.L.; Zhu, B.L.; Wang, P.; Wang, G.C.; Zhang, S.M.; Huang, W.P. Fabrication and photocatalytic performance of C,N,F-tridoped TiO2 nanotubes. Catal. Today 2019, 327, 182–189. [Google Scholar] [CrossRef]
- Ke, T.; Shen, S.; Rajavel, K.; Yang, K.; Lin, D. In situ growth of TiO2 nanoparticles on nitrogen-doped Ti3C2 with isopropyl amine toward enhanced photocatalytic activity. J. Hazard. Mater. 2021, 402, 124066. [Google Scholar] [CrossRef]
- Zhao, Y.; Chi, Y.; Tian, C.; Liu, Y.; Li, H.; Wang, A. Recycling of titanium-coagulated algae-rich sludge for enhanced photocatalytic oxidation of phenolic contaminants through oxygen vacancy. Water Res. 2020, 177, 115789. [Google Scholar] [CrossRef]
- Cheng, K.; Cai, Z.; Fu, J.; Sun, X.; Sun, W.; Chen, L.; Zhang, D.; Liu, W. Synergistic adsorption of Cu(II) and photocatalytic degradation of phenanthrene by a jaboticaba-like TiO2/titanate nanotube composite: An experimental and theoretical study. Chem. Eng. J. 2019, 358, 1155–1165. [Google Scholar] [CrossRef]
- Wang, W.; Niu, Q.; Zeng, G.; Zhang, C.; Huang, D.; Shao, B.; Zhou, C.; Yang, Y.; Liu, Y.; Guo, H.; et al. 1D porous tubular g-C3N4 capture black phosphorus quantum dots as 1D/0D metal-free photocatalysts for oxytetracycline hydrochloride degradation and hexavalent chromium reduction. Appl. Catal. B Environ. 2020, 273, 119051. [Google Scholar] [CrossRef]
- Saleh, R.; Zaki, A.H.; El-Ela, F.I.A.; Farghali, A.A.; Taha, M.; Mahmoud, R. Consecutive removal of heavy metals and dyes by a fascinating method using titanate nanotubes. J. Environ. Chem. Eng. 2021, 9, 104726. [Google Scholar] [CrossRef]
- Maji, T.K.; Hasan, N.; Ghosh, S.; Wulferding, D.; Bhattacharya, C.; Lemmens, P.; Karmakar, D.; Pal, S.K. Development of a magnetic nanohybrid for multifunctional application: From immobile photocatalysis to efficient photoelectrochemical water splitting: A combined experimental and computational study. J. Photochem. Photobiol. A Chem. 2020, 397, 112575. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Ramteke, M.S.; Rondiya, S.R.; Mulani, S.R.; Patil, M.S.; Cross, R.W.; Dzade, N.Y.; Devan, R.S. DFT and experimental investigations on the photocatalytic activities of NiO nanobelts for removal of organic pollutants. J. Alloys Compd. 2021, 855, 157337. [Google Scholar] [CrossRef]
- Duan, Y.; Deng, L.; Shi, Z.; Liu, X.; Zeng, H.; Zhang, H.; Crittenden, J. Efficient sulfadiazine degradation via in-situ epitaxial grow of Graphitic Carbon Nitride (g-C3N4) on carbon dots heterostructures under visible light irradiation: Synthesis, mechanisms and toxicity evaluation. J. Colloid Interface Sci. 2020, 561, 696–707. [Google Scholar] [CrossRef]
- Cai, Z.; Song, Y.; Jin, X.; Wang, C.-C.; Ji, H.; Liu, W.; Sun, X. Highly efficient AgBr/h-MoO3 with charge separation tuning for photocatalytic degradation of trimethoprim: Mechanism insight and toxicity assessment. Sci. Total Environ. 2021, 781, 146754. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, R.; Zhang, N.; Su, Y.; Liu, Z.; Gao, R.; Du, C. Insight to unprecedented catalytic activity of double-nitrogen defective metal-free catalyst: Key role of coal gangue. Appl. Catal. B Environ. 2020, 263, 118316. [Google Scholar] [CrossRef]
- Regmi, C.; Kshetri, Y.K.; Kim, T.-H.; Dhakal, D.; Lee, S.W. Mechanistic understanding of enhanced photocatalytic activity of N-doped BiVO4 towards degradation of ibuprofen: An experimental and theoretical approach. Mol. Catal. 2019, 470, 8–18. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, J.; Zhu, K.; Wei, W.; Ma, X.; Zhu, Y. Self-assembled polymer phenylethnylcopper nanowires for photoelectrochemical and photocatalytic performance under visible light. Appl. Catal. B Environ. 2018, 226, 616–623. [Google Scholar] [CrossRef]
- Huang, L.-Z.; Wei, X.; Gao, E.; Zhang, C.; Hu, X.-M.; Chen, Y.; Liu, Z.; Finck, N.; Lützenkirchen, J.; Dionysiou, D. Single Fe atoms confined in two-dimensional MoS2 for sulfite activation: A biomimetic approach towards efficient radical generation. Appl. Catal. B Environ. 2020, 268, 118459. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Q.; Lin, Q.; Chen, Y.; Liao, X.; Yu, H.; Yu, C. Three-dimensional P-doped porous g-C3N4 nanosheets as an efficient metal-free photocatalyst for visible-light photocatalytic degradation of Rhodamine B model pollutant. J. Taiwan Inst. Chem. Eng. 2020, 114, 249–262. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, C.; Tang, L.; Zhou, Y.; Chen, Z.; Feng, H.; Wang, J.; Yu, J.; Liu, Y. Ultrathin low dimensional heterostructure composites with superior photocatalytic activity: Insight into the multichannel charge transfer mechanism. Chem. Eng. J. 2020, 393, 124718. [Google Scholar] [CrossRef]
- Wang, P.; Tian, Y.; Wang, H.; Zhang, J.; Kong, L.; Zuo, W.; Li, D.; Yin, L. Strong adsorption of tetracycline on octahedral Cu2O nanocrystals exposed with {111} facets: Adsorption behavior and mechanism insight. Appl. Surf. Sci. 2021, 542, 148545. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Li, X.; Chen, H.; Wen, T.; Jiang, Z.; Ai, Y.; Sun, Y.; Hayat, T.; Wang, X. Highly uranium elimination by crab shells-derived porous graphitic carbon nitride: Batch, EXAFS and theoretical calculations. Chem. Eng. J. 2018, 346, 406–415. [Google Scholar] [CrossRef]
- Gu, P.; Zhao, C.; Wen, T.; Ai, Y.; Zhang, S.; Chen, W.; Wang, J.; Hu, B.; Wang, X. Highly U(VI) immobilization on polyvinyl pyrrolidine intercalated molybdenum disulfide: Experimental and computational studies. Chem. Eng. J. 2019, 359, 1563–1572. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, R.; Lu, Z.; Ai, Y. Experimental and theoretical studies of spherical β-cyclodextrin modified titanium dioxide composites for uranium removal. Ecol. Eng. 2020, 149, 105835. [Google Scholar] [CrossRef]
- Tokalıoğlu, Ş.; Yavuz, E.; Demir, S.; Patat, Ş. Zirconium-based highly porous metal-organic framework (MOF-545) as an efficient adsorbent for vortex assisted-solid phase extraction of lead from cereal, beverage and water samples. Food Chem. 2017, 237, 707–715. [Google Scholar] [CrossRef]
- Senapati, S.; Maiti, P. Emerging bio-applications of two-dimensional nanoheterostructure materials. In 2D Nanoscale Heterostructured Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–255. [Google Scholar]
- Carlsson, J.-O.; Martin, P.M. Chemical vapor deposition. In Handbook of Deposition Technologies for Films and Coatings; William Andrew Publishing: Norwich, NY, USA, 2010; pp. 314–363. [Google Scholar]
- Badv, M.; Jaffer, I.H.; Weitz, J.I.; Didar, T.F. An omniphobic lubricant-infused coating produced by chemical vapor deposition of hydrophobic organosilanes attenuates clotting on catheter surfaces. Sci. Rep. 2017, 7, 11639. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of chemical vapor deposition of graphene and related applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef]
- Pourmand, S.; Abdouss, M.; Rashidi, A. Fabrication of nanoporous graphene by chemical vapor deposition (CVD) and its application in oil spill removal as a recyclable nanosorbent. J. Ind. Eng. Chem. 2015, 22, 8–18. [Google Scholar] [CrossRef]
- Creighton, J.R.; Ho, P. Introduction to chemical vapor deposition (CVD). Chem. Vap. Depos. 2001, 2, 1–22. [Google Scholar]
- Haubner, R. The history of hard CVD coatings for tool applications at the University of Technology Vienna. Int. J. Refract. Met. Hard Mater. 2013, 41, 22–34. [Google Scholar] [CrossRef]
- Tietjen, J.J. Chemical vapor deposition of electronic materials. Annu. Rev. Mater. Sci. 1973, 3, 317–326. [Google Scholar] [CrossRef]
- Gao, L.; Ren, W.; Zhao, J.; Ma, L.-P.; Chen, Z.; Cheng, H.-M. Efficient growth of high-quality graphene films on Cu foils by ambient pressure chemical vapor deposition. Appl. Phys. Lett. 2010, 97, 183109. [Google Scholar]
- Park, H.J.; Meyer, J.; Roth, S.; Skákalová, V. Growth and properties of few-layer graphene prepared by chemical vapor deposition. Carbon 2010, 48, 1088–1094. [Google Scholar] [CrossRef] [Green Version]
- Vasudev, M.C.; Anderson, K.D.; Bunning, T.J.; Tsukruk, V.V.; Naik, R.R. Exploration of plasma-enhanced chemical vapor deposition as a method for thin-film fabrication with biological applications. ACS Appl. Mater. Interfaces 2013, 5, 3983–3994. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.-M. Chemical vapor deposition growth and applications of two-dimensional materials and their heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef]
- Cong, C.; Shang, J.; Wu, X.; Cao, B.; Peimyoo, N.; Qiu, C.; Sun, L.; Yu, T. Synthesis and optical properties of large-area single-crystalline 2D semiconductor WS2 monolayer from chemical vapor deposition. Adv. Opt. Mater. 2014, 2, 131–136. [Google Scholar] [CrossRef]
- Liu, B.; Song, W.; Wu, H.; Liu, Z.; Teng, Y.; Sun, Y.; Xu, Y.; Zheng, H. Degradation of norfloxacin with peroxymonosulfate activated by nanoconfinement Co3O4@CNT nanocomposite. Chem. Eng. J. 2020, 398, 125498. [Google Scholar] [CrossRef]
- Dastgerdi, Z.H.; Meshkat, S.S.; Esrafili, M.D. Enhanced adsorptive removal of Indigo carmine dye performance by functionalized carbon nanotubes based adsorbents from aqueous solution: Equilibrium, kinetic, and DFT study. J. Nanostruct. Chem. 2019, 9, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Shah, K.A.; Tali, B.A. Tali. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Mater. Sci. Semicond. Process. 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Wang, F.; Li, R.; Yu, X.; Kang, L.; Zhao, J.; Li, A. One-pot biosynthesis of 1, 6-hexanediol from cyclohexane by de novo designed cascade biocatalysis. Green Chem. 2020, 22, 7476–7483. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.; Zhu, Z.; Cui, F.; Zhu, Y.-A.; Duan, X.; Wang, S. Magnetic nitrogen-doped nanocarbons for enhanced metal-free catalytic oxidation: Integrated experimental and theoretical investigations for mechanism and application. Chem. Eng. J. 2018, 354, 507–516. [Google Scholar] [CrossRef]
- Du, X.; Fu, W.; Su, P.; Cai, J.; Zhou, M. Internal-micro-electrolysis-enhanced heterogeneous electro-Fenton process catalyzed by Fe/Fe3C@ PC core–shell hybrid for sulfamethazine degradation. Chem. Eng. J. 2020, 398, 125681. [Google Scholar] [CrossRef]
- Mingos, D.; Crabtree, M.P.; Robert, H. Comprehensive Organometallic Chemistry, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Andrews, D.; Nann, T.; Lipson, R.H. Comprehensive Nanoscience and Nanotechnology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Sun, H.; Cao, L.; Lu, L. Magnetite/reduced graphene oxide nanocomposites: One step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Res. 2011, 4, 550–562. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, H.; Sharma, A.K.; Hong, W.G.; Shin, K.; Song, H.; Kim, H.Y.; Hong, Y.J. Recyclable aqueous metal adsorbent: Synthesis and Cu(II) sorption characteristics of ternary nanocomposites of Fe3O4 nanoparticles@ graphene–poly-N-phenylglycine nanofibers. J. Hazard. Mater. 2021, 401, 123283. [Google Scholar] [CrossRef]
- de Santiago Colín, D.M.; Martínez-Chávez, L.A.; Cuán, Á.; Elizalde-Peña, E.A.; Rivera, J.A.; Guzmán, C.; Escobar-Alarcón, L.; Esquivel, K. Sonochemical coupled synthesis of Cr-TiO2 supported on Fe3O4 structures and chemical simulation of the degradation mechanism of Malachite Green dye. J. Photochem. Photobiol. A Chem. 2018, 364, 250–261. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Xia, Y.; Li, P.; Wu, Y.; Yang, K.; Song, Y.; Jiang, S.; Zhang, T.; Li, B. Boosted electron-transfer by coupling Ag and Z-scheme heterostructures in CdSe-Ag-WO3-Ag for excellent photocatalytic H2 evolution with simultaneous degradation. Chem. Eng. J. 2021, 417, 129298. [Google Scholar] [CrossRef]
- Gong, J.; Lee, C.-S.; Chang, Y.-Y. Novel self-assembled bimetallic structure of Bi/Fe0: The oxidative and reductive degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX). J. Hazard. Mater. 2015, 286, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Deng, S.; Zhao, T.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of regenerable granular carbon nanotubes by a simple heating-filtration method for efficient removal of typical pharmaceuticals. Chem. Eng. J. 2016, 294, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, Z.; Yue, R.; Gao, F.; Ren, R.; Wei, J.; Wang, X.; Kong, Z. Functional group-rich hyperbranched magnetic material for simultaneous efficient removal of heavy metal ions from aqueous solution. J. Hazard. Mater. 2020, 384, 121288. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huo, Y.; Wang, X.; Yu, S.; Ai, Y.; Chen, Z.; Zhang, P.; Chen, L.; Song, G.; Alharbi, N.S.; et al. Impact of metal ions and organic ligands on uranium removal properties by zeolitic imidazolate framework materials. J. Clean. Prod. 2021, 278, 123216. [Google Scholar] [CrossRef]
- Yan, L.; Tu, H.; Chan, T.; Jing, C. Mechanistic study of simultaneous arsenic and fluoride removal using granular TiO2-La adsorbent. Chem. Eng. J. 2017, 313, 983–992. [Google Scholar] [CrossRef]
- Song, Q.; Liang, J.; Fang, Y.; Guo, Z.; Du, Z.; Zhang, L.; Liu, Z.; Huang, Y.; Lin, J.; Tang, C. Nickel(II) modified porous boron nitride: An effective adsorbent for tetracycline removal from aqueous solution. Chem. Eng. J. 2020, 394, 124985. [Google Scholar] [CrossRef]
- Liu, H.; Chen, P.; Yuan, X.; Zhang, Y.; Huang, H.; Wang, L.; Dong, F. Pivotal roles of artificial oxygen vacancies in enhancing photocatalytic activity and selectivity on Bi2O2CO3 nanosheets. Chin. J. Catal. 2019, 40, 620–630. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Wang, J.; Tang, Y.; Zhang, Z. Selective adsorption of Pb2+ and Cu2+ on amino-modified attapulgite: Kinetic, thermal dynamic and DFT studies. J. Hazard. Mater. 2021, 404, 124140. [Google Scholar] [CrossRef]
- Shen, C.; Chen, C.; Wen, T.; Zhao, Z.; Wang, X.; Xu, A. Superior adsorption capacity of g-C3N4 for heavy metal ions from aqueous solutions. J. Colloid Interface Sci. 2015, 456, 7–14. [Google Scholar] [CrossRef]
- Liang, H.; Liu, R.; Hu, C.; An, X.; Zhang, X.; Liu, H.; Qu, J. Synergistic effect of dual sites on bimetal-organic frameworks for highly efficient peroxide activation. J. Hazard. Mater. 2021, 406, 124692. [Google Scholar] [CrossRef]
- Valadi, F.M.; Gholami, M.R. Synthesis of CuCo2O4/BiVO4 composites as promise and efficient catalysts for 4-nitrophenol reduction in water: Experimental and theoretical study. J. Environ. Chem. Eng. 2021, 9, 105408. [Google Scholar] [CrossRef]
- Zaher, A.; Taha, M.; Farghali, A.A.; Mahmoud, R.K. Zn/Fe LDH as a clay-like adsorbent for the removal of oxytetracycline from water: Combining experimental results and molecular simulations to understand the removal mechanism. Environ. Sci. Pollut. Res. 2020, 27, 12256–12269. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, N.; Zhu, L.; Xu, X. Application of ZnTiO3 in quantum-dot-sensitized solar cells and numerical simulations using first-principles theory. J. Alloys Compd. 2016, 681, 88–95. [Google Scholar] [CrossRef]
- Zahid, M.; Abd-Elsalam, K.A. Applications of nanomaterials in water remediation: A note from the Editors. In Aquananotechnology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 15–114. [Google Scholar]
- Zhang, Y.; Li, L.; Su, H.; Huang, W.; Dong, X. Binary metal oxide: Advanced energy storage materials in supercapacitors. J. Mater. Chem. A 2015, 3, 43–59. [Google Scholar] [CrossRef]
- Abdelwahab, A.; Carrasco-Marín, F.; Pérez-Cadenas, A. Binary and ternary 3D nanobundles metal oxides functionalized carbon xerogels as electrocatalysts toward oxygen reduction reaction. Materials 2020, 13, 3531. [Google Scholar] [CrossRef]
- Parkinson, G.S.; Diebold, U. Adsorption on metal oxide surfaces. Surf. Interface Sci. Solid-Gas Interfaces II 2016, 6, 793–817. [Google Scholar]
- Ani, I.J.; Akpan, U.G.; Olutoye, M.A.; Hameed, B.H. Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO2-and ZnO-based photocatalysts: Recent development. J. Clean. Prod. 2018, 205, 930–954. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic principles, diverse forms of implementations and emerging scientific opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef] [Green Version]
- Coronado, J.M. A historical introduction to photocatalysis. In Design of Advanced Photocatalytic Materials for Energy and Environmental Applications; Springer: London, UK, 2013; pp. 1–4. [Google Scholar]
- Landau, M. Le phénomène de la photocatalyse. Compt. Rend. 1913, 156, 1894–1896. [Google Scholar]
- Kim, S.-M.; Vogelpohl, A. Degradation of organic pollutants by the photo-Fenton-process. Chem. Eng. Technol. Ind. Chem.-Plant Equip.-Process Eng.-Biotechnol. 1998, 21, 187–191. [Google Scholar] [CrossRef]
- Bonelli, B.; Freyria, F.S.; Rossetti, I.; Sethi, R. (Eds.) Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]


| Membrane | Material Type | Synthesis Method | Reference |
|---|---|---|---|
| SWCNTs | Carbon nanotube (CNT) | Obtained from Cheap Tubes, Inc. | [137] |
| Graphene oxide | Oxidized graphene oxide | Obtained commercially from Sigma Aldrich | [113] |
| ZnO surface | Zinc oxide (ZnO) | Evaporation methods | [138] |
| MnFe2O4 nanocubes | Manganese ferrite nanoparticles (MnFe2O4) | Co-precipitation phase inversion method | [139] |
| Graphene | 3D foam graphene | Obtained commercially | [140] |
| MGOA | Graphene oxide (GO), ammonium (NH4+) | Modified Hummers’ method | [141] |
| PyTTA-Dva-COF | Nitrogen (N), covalent organic framework | Solvent-thermal method | [142] |
| Ultrafiltration PSF/GO membrane | Graphene oxide (GO), polysulfone (PSF) | Phase inversion method | [143] |
| Nitrogen doped carbon (CNs) | Carbon (C), nitrogen (N), titanium (Ti) | Chlorination | [144] |
| Graphene oxide | Graphene oxide | Improved Hummers’ method | [145] |
| Single-layer graphene nanosheets | Graphite | Solution-phase exfoliation integrating bath sonication and microwave irradiation in organic solvents | [146] |
| Carbon nanotubes (CNTs) | Carbon nanotube (CNT) | Nuclear magnetic resonance (1H and 13C NMR) and high resolution-mass spectrometry (HR-MS) | [147] |
| Graphene oxide | Graphene oxide | Modified Hummers’ method | [148] |
| Graphene oxide | Graphene oxide | Modified Hummers’ method | [149] |
| MoS2 nanosheets | Molybdenum disulphide | Molten salt electrolysis method | [150] |
| MoS2 nanosheets | Molybdenum disulphide | Microwave-assisted route | [151] |
| Zn–Fe LDH | Zinc (Zn), iron (Fe) | Co-precipitation method | [152] |
| Lanthanum-aluminium perovskite (La2Al4O9) | Lanthanum (La), aluminium (Al) | Obtained commercially from Aladdin company | [153] |
| CF/BiOBr/Ag3PO4 cloth | Carbon fibre (CF), bismuth oxybromide (BiOBr), silver phosphate (Ag3PO4) | Solvothermal-chemical deposition | [154] |
| Membrane | Software | Simulation Method | Mathematical Model | Reference |
|---|---|---|---|---|
| (O-CNTs), (G-CNTs) | Gaussian 09W | DFT (B3LYP functional group) | Integral Equation Formalism Polarized Continuum Model (IEFPCM) | [163] |
| Graphene | VASP | DFT (PAW) | Kohn-Sham equations | [140] |
| Graphene oxide | SIESTA code | DFT (LDA) | Kohn-Sham equations | [113] |
| MGOA | Gaussian 09 | DFT (B3LYP functional group) | Thomas, Yoon–Nelson, and Adams–Bohart models | [141] |
| PyTTA-Dva-COF | Gaussian 09 | DFT (B3LYP functional group) | ONIOM model | [142] |
| Vertically aligned (VA) CNT (open-end) hybrid membrane | DMOL3 package | DFT (PW91) | Exchange-Correlation functional | [164] |
| Ultrafiltration PSF/GO membrane | OPEN-MX software | DFT (LDA) | Hoffmann’s model | [143] |
| Graphene oxide | Gaussian 09 | DFT (Gaussian-Lorentzian function) | Exchange-Correlation functional | [145] |
| S, N co-doped graphene aerogel (SN-rGO-A) | Gaussian 09 | DFT (B3LYP functional group) | Thomas, Yoon–Nelson, and Adams–Bohart models | [165] |
| ZIF8@carbon nanotube | VASP | DFT (PBE) | Exchange-Correlation functional | [166] |
| Carbonaceous nanofiber/Ni-Al layered double hydroxide (CNF/LDH) | VASP | DFT (PAW) | Kohn-Sham equations | [167] |
| SWCNTs, MWCNTs, and PAC | GAMESS | DFT (B3LYP5 functional) | Exchange-Correlation functional | [168] |
| Single-layer graphene nanosheets | VASP | DFT (PAW) | Kohn-Sham equations | [146] |
| Graphene oxide | Gaussian 09 | DFT (PBE1PBE functional model) | Exchange-Correlation functional | [148] |
| Graphene oxide | Gaussian 09 | DFT (B3LYP/6-31G* level) | Exchange-Correlation functional | [149] |
| ZnO surface | VASP | DFT (PBE) | Exchange-Correlation functional | [138] |
| MoS2 nanosheets | VASP | DFT (PAW) | Kohn-Sham equations | [150] |
| Zn–Fe LDH | Materials Studio (BIOVIA, 2017) | DFT (DMol3) code | Exchange-Correlation functional | [152] |
| Lanthanum-aluminium perovskite (La2Al4O9) | Materials Studio | DFT (PBE) | Exchange-Correlation functional | [153] |
| MoS2 nanosheets | VASP | DFT (PAW) | Kohn-Sham equations | [151] |
| SWCNTs | Gaussview | DFT (B3LYP5) functional | Exchange-Correlation functional | [137] |
| CF/BiOBr/Ag3PO4 cloth | Materials Studio | DFT (GGA-PBE) | Exchange-Correlation functional | [154] |
| Nanocomposite Material | Material Type | Reference |
|---|---|---|
| Heterogeneous Fenton catalysts (CNTs/Fh) | Oxidized carbon nanotubes (CNTs), ferrihydrite (Fh) | [116] |
| (N-rGO/BiVO4) | Bismuth vanadate (BiVO4), reduced graphene oxide (rGO), nitrogen (N) | [193] |
| ZnO@C | Zinc Oxide (ZnO), carbon (C) | [194] |
| Cerium zirconium oxide (CexZryO2) | Cerium (Ce), zirconium oxide (ZrO2) | [195] |
| ZnO/Al2O3 | Zinc oxide (ZnO), aluminium oxide (Al2O3). | [196] |
| C, N, F/TiO2NTs | Carbon (C), nitrogen (N), fluoride (F), titanium dioxide nanotubes (TiO2NTs) | [197] |
| iN-Ti3C2/TiO2 hybrid | Titanium carbide (Ti3C2), titanium dioxide (TiO2), isopropyl amine, nitrogen (N) | [198] |
| TiO2 nanoflowers (TNFs) | Titanium dioxide (TiO2) | [199] |
| Titanate nanotubes supported TiO2 (TiO2/TiNTs) | Titanium dioxide (TiO2), titanate nanotubes | [200] |
| Black phosphorus quantum dots/Tubular g-C3N4 (BPQDs/TCN) | Black phosphorus (BP), tubular g-C3N4 | [201] |
| Sodium titanate nanotubes (Na-TNT) | Sodium (Na), titanate nanotubes (TNT) | [202] |
| Fe2O3-PC nanohybrids | Iron oxide (Fe2O3) | [203] |
| NiO nanobelt | Nickel oxide (NiO) | [204] |
| Carbon dots/g-C3N4 (C-CN) heterostructures | Graphitic Carbon Nitride (g-C3N4) | [205] |
| AgBr/h-MoO3 | Silver bromide (AgBr), hexagonal molybdenum oxide (h-MoO3) | [206] |
| Hybrid catalysts (CN-CGs) | Coal gangue (CG), graphitic carbon nitride g-C3N4 (CN) | [207] |
| N-doped BiVO4 | Nitrogen (N), bismuth vanadate (BiVO4) | [208] |
| PPECu thin film electrode | Copper (Cu), phenylacetylene (PPE) | [209] |
| FexMo1-xS2 catalysts | Iron (Fe), Molybdenum disulfide (MoS2) | [210] |
| P-doped porous g-C3N4 | Graphitic carbon nitride (g-C3N4), phosphorus (P) | [211] |
| 1D/2D W18O49/g-C3N4 nanocomposites | Graphitic carbon nitride (g-C3N4), oxygen-deficient tungsten oxide (W18O49) | [212] |
| Oct-Cu2O NCs | Cuprous oxide (Cu2O) | [213] |
| g-C3N4 | Graphitic carbon nitride (g-C3N4) | [214] |
| ZIF8@carbon nanotube | Carbon nanotube (CNT), zeolitic imidazole framework-8 (ZIF8) | [166] |
| CNF/LDH | Carbonaceous nanofiber (CNF), nickel (Ni), aluminium (Al) | [167] |
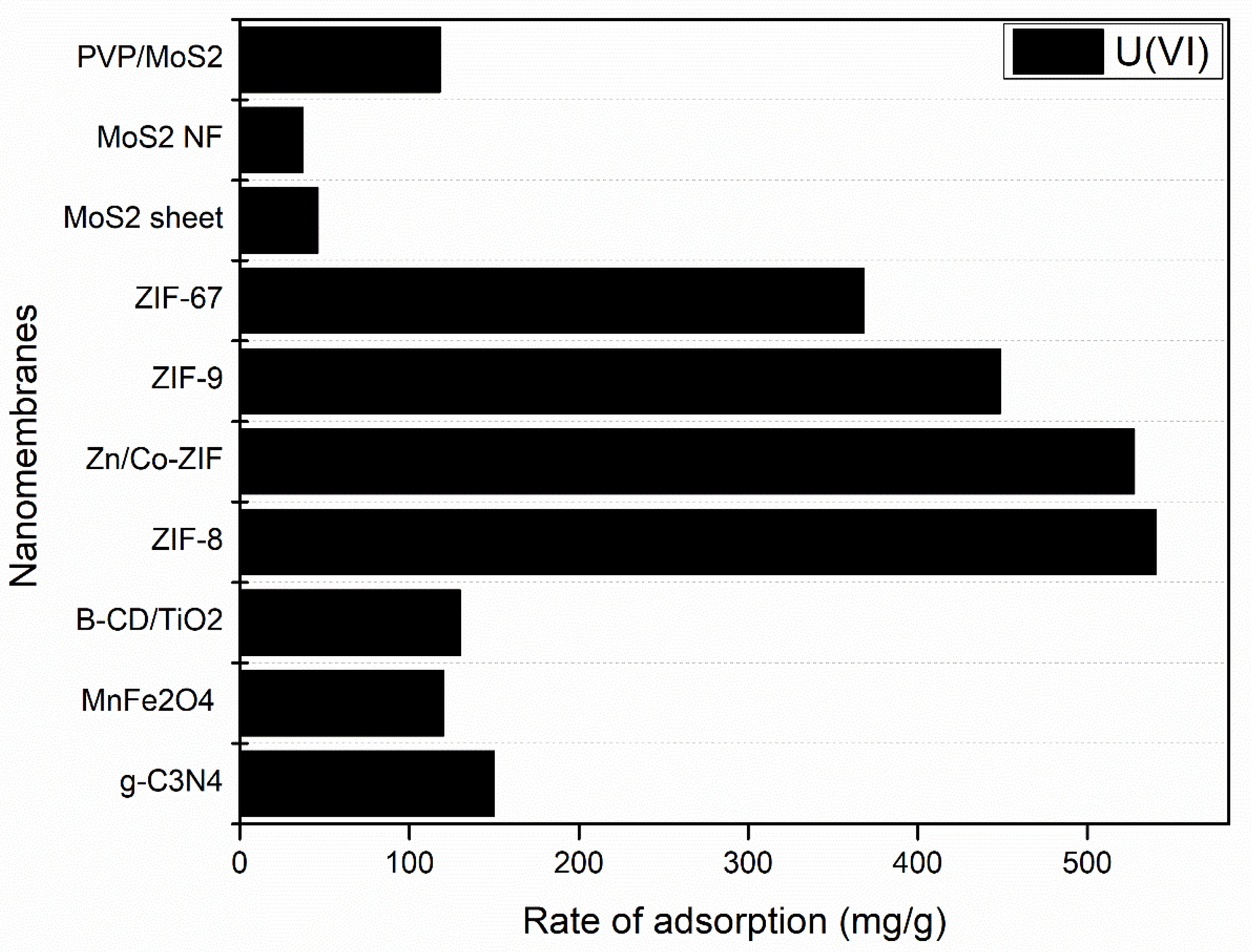
| PVP/MoS2 | Molybdenum disulphide, polyvinylpyrrolidone | [215] |
| β-CD/TiO2 | Titanium dioxide (TiO2), β-cyclodextrin C42H70O35 | [216] |
| MOF-545 | Zirconyl chloride octahydrate, Sigma-Aldrich; porphyrin, H4-Tcpp-H2, TCl | [217] |
| Nanocomposite Material | Material Type | Reference |
|---|---|---|
| Co3O4/CNTs | Carbon nanotubes (CNTs), cobalt tetra-oxide (Co3O4) | [231] |
| O-CNTs, G-CNTs | Oxidized carbon nanotubes (O-CNTs), graphitized carbon nanotubes (G-CNTs). | [163] |
| Vertically aligned (VA) CNT (open-end) hybrid membrane | Carbon nanotube (CNT), polydimethylsiloxane (PDMS) membrane | [164] |
| COOH/CNTs | Carbon nanotubes (CNTs), carboxylic functionalized groups (COOH) | [232] |
| Nanocomposite Material | Material Type | Reference |
|---|---|---|
| S, N co-doped graphene aerogel (SN-rGO-A) | Graphene oxide (GO), sulfur (S), nitrogen (N). | [165] |
| ZIF-67 Carbocatalysts, Nitrogen-doped magnetic carbon (Co@N-C) | Cobalt (Co), nitrogen (N), carbon (C) | [235] |
| Fe/Fe3C@PC | Graphitized porous carbon (PC), Fe-based nanoparticle core (Fe/Fe3C) | [236] |
| Nanocomposite Material | Material Type | Synthesis Method | Reference |
|---|---|---|---|
| Ternary nanocomposites of Fe3O4 nanoparticles@ graphene–poly-N-phenylglycine nanofibers | Graphene oxide (GO), nitrogen (N), iron oxide (Fe3O4), phenylglycine (C6H5CHCO2H). | Wet chemical process | [241] |
| Cr-TiO2 supported on Fe3O4 | Titanium dioxide (TiO2), chromium (Cr), iron oxide black (Fe3O4). | Sonochemical method | [242] |
| CdSe-Ag-WO3-Ag photocatalyst | Cadmium selenide (CdSe), silver (Ag), tungsten trioxide (WO3). | Continuous photo-assisted process | [243] |
| Bi/Fe0 | Bismuth (Bi), iron (Fe) | Simple chemical reactions | [244] |
| Granular carbon nanotubes (CNTs) | Carbon nanotubes (CNTs) | Simple heating-filtration method | [245] |
| SWCNTs, MWCNTs, and PAC | Carbon nanotubes (CNTs) | SWCNTs: Obtained commercially from Cheap Tubes, Inc. MWCNTs: Obtained commercially from Sigma Aldrich. | [168] |
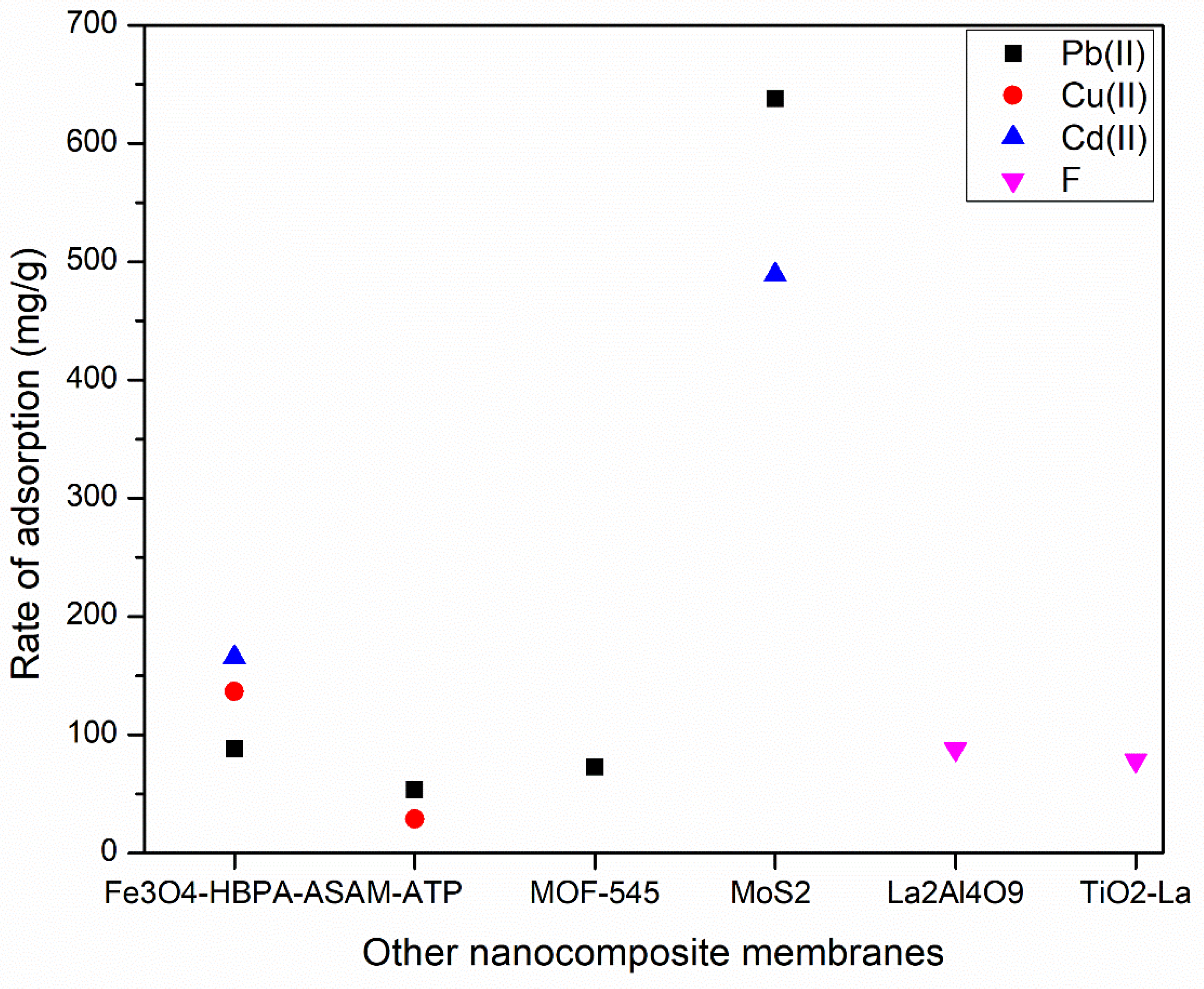
| Fe3O4-HBPA-ASA | Magnetite (Fe3O4) | Solvothermal method | [246] |
| Highly porous zeolitic imidazolate frameworks (ZIFs) | Highly porous zeolitic imidazolate frameworks | Simple stirring method | [247] |
| Granular TiO2-La | Titanium dioxide (TiO2), lanthanum (La) | Hydrolysis | [248] |
| Ni (II) modified porous BN | Nickel (Ni), boron nitride (BN) | Precursor pyrolysis method | [249] |
| Bi2O2CO3 nanosheets | Bismuth carbonate | Simple stirring method | [250] |
| Amino-modified attapulgite (M-ATP) | Attapulgite clay, the 3-aminopropyltriethoxysilane, Pb (NO3)2 and Cu(NO3)2 | Simple stirring method | [251] |
| g-C3N4 | Graphitic carbon nitride (g-C3N4) | Salt melt method | [252] |
| MIL-101(Fe) and MIL-101(Fe,Co) | MIL-101(Fe) | Solvothermal method | [253] |
| CuCo2O4/BiVO4 | Bismuth vanadate (BiVO4) | Solvothermal method | [254] |
| Zn/Fe LDH | Zinc (Zn), iron (Fe) | Co-precipitation method | [255] |
| Nanocomposite Material | Software | Simulation Method | Mathematical Model | Reference |
|---|---|---|---|---|
| Nitrogen doped carbon (CNs) | VASP | DFT (PAW) | Kohn-Sham equations | [144] |
| COOH/CNTs | DMol3 program | DFT (PBE) | Exchange-Correlation functional | [232] |
| Porous graphitic carbon nitride (g-C3N4) | VASP | DFT (PAW) | Kohn-Sham equations | [214] |
| Granular carbon nanotubes (CNTs) | Not supplied | DFT | The Langmuir model | [245] |
| Carbon nanotubes (CNTs) | Gaussian 09 | DFT (Minnesota dispersion functional, M06-2×/6–31G(d) level) | Exchange-Correlation functional | [147] |
| MnFe2O4 nanocubes | VASP | DFT (PAW) | Kohn-Sham equations | [139] |
| Oct-Cu2O NCs | VASP | DFT (PW91) | Exchange-Correlation functional | [213] |
| Amino-modified attapulgite (M-ATP) | VASP | DFT (PBE) | Exchange-Correlation functional | [251] |
| β-CD/TiO2 | VASP | DFT (PAW) | Kohn-Sham equations | [216] |
| Fe3O4-HBPA-ASA | Gaussian 16 package | DFT (B3LYP) | Exchange-Correlation functional | [246] |
| PVP/MoS2 | VASP | DFT (PAW) | Kohn-Sham equations | [215] |
| Highly porous zeolitic imidazolate frameworks (ZIFs) | Gaussian 09 | DFT (B3LYP) | Exchange-Correlation functional | [247] |
| Ni (II) modified porous BN | VASP | DFT (PAW) | Kohn-Sham equations | [249] |
| CuCo2O4/BiVO4 | Materials Studio 6.0 (2011) | DFT (PBE) | Exchange-Correlation functional | [254] |
| Granular TiO2-La | Materials Studio 7.0 | DFT (PBE) | Exchange-Correlation functional | [248] |
| g-C3N4 | Not supplied | DFT | Langmuir model, and Freundlich model | [252] |
| MOF-545 | Not supplied | DFT | Exchange-Correlation functional | [217] |
| MIL-101(Fe) and MIL-101(Fe, Co) | DMol3 code | DFT (PBE) | Exchange-Correlation functional | [253] |
| Bi2O2CO3 nanosheets | VASP 5.4 | DFT (HSE06) | Exchange-Correlation functional | [250] |
| Zn/Fe LDH | Materials Studio (BIOVIA, 2017) | DFT (GGA-RPBE) | Exchange-Correlation functional | [255] |
| Heterogeneous Fenton catalysts (CNTs/Fh) | VASP | DFT (PAW) | Kohn-Sham equations | [116] |
| Co3O4/CNTs | Material studio 2017 | DFT (PBE) | Exchange-Correlation functional | [231] |
| (ZIF-67 Carbocatalysts), Nitrogen-doped magnetic carbon (Co@N-C) | VASP | DFT (PBE) | Exchange-Correlation functional | [235] |
| ternary nanocomposites of Fe3O4 nanoparticles@ graphene–poly-N-phenylglycine nanofibers | VASP | DFT (RPBE) | Exchange-Correlation functional | [241] |
| (N-rGO/BiVO4) | Not supplied | DFT | Exchange-Correlation functional | [193] |
| Cerium zirconium oxide (CexZryO2) | VASP | DFT (PBE) | Exchange-Correlation functional | [195] |
| NiO nanobelt | VASP | DFT (PAW) | Kohn-Sham equations | [204] |
| ZnO/Al2O3 | VASP, COMSOL | DFT (PBE) | Exchange-Correlation functional | [196] |
| ZnO@C | Molecular Operating Environment software (MOE, 2008.10) | DFT | Exchange-Correlation functional | [194] |
| C, N, F/TiO2NTs | VASP | DFT | Exchange-Correlation functional | [197] |
| iN-Ti3C2/TiO2 hybrid | VASP | DFT (PBE) | Exchange-Correlation functional | [198] |
| TiO2 nanoflowers (TNFs) | VASP | DFT (PBE) | Exchange-Correlation functional | [199] |
| Cr-TiO2 supported on Fe3O4 | Not supplied | DFT (M06 L) | a Langmuir-Hinshelwood model | [242] |
| Titanate nanotubes supported TiO2 (TiO2/TiNTs) | Gaussian 03 | DFT (B3LYP) | Exchange-Correlation functional | [200] |
| Black phosphorus quantum dots/Tubular g-C3N4 (BPQDs/TCN) | Materials Studio | DFT (PBE) | Exchange-Correlation functional | [201] |
| CdSe-Ag-WO3-Ag photocatalyst | VASP | DFT (PBE) | Exchange-Correlation functional | [243] |
| Sodium titanate nanotubes (Na-TNT) | Materials Studio | DFT (RPBE) | Exchange-Correlation functional | [202] |
| Fe2O3-PC nanohybrids | VASP | DFT (PBE) | Exchange-Correlation functional | [203] |
| Carbon dots/g-C3N4 (C-CN) heterostructures | VASP | DFT (PBE) | Exchange-Correlation functional | [205] |
| AgBr/h-MoO3 | Toxicity Estimation Software Tool (T.E.S.T.) | DFT (QSAR) | Exchange-Correlation functional | [206] |
| Hybrid catalysts (CN-CGs) | VASP | DFT (GGA-PBE) | Exchange-Correlation functional | [207] |
| Fe/Fe3C@PC | VASP, Version 5.4.1 | DFT (PAW) | Kohn-Sham equations | [236] |
| N-doped BiVO4 | VASP | DFT (PBE) | Exchange-Correlation functional | [208] |
| Bi/Fe0 | Materials Studio | DFT (PBE) | Exchange-Correlation functional | [244] |
| PPECu thin film electrode | VASP | DFT (PAW) | Kohn-Sham equations | [209] |
| FexMo1-xS2 catalysts | VASP | DFT (PBE) | Exchange-Correlation functional | [210] |
| P-doped porous g-C3N4 | VASP | DFT (PBE) | Exchange-Correlation functional | [211] |
| 1D/2D W18O49/g-C3N4 nanocomposites | VASP | DFT (PAW) | Kohn-Sham equations | [212] |
| Membrane | Role of Carbon-Based Membrane | Rejected/Adsorbed Material | Rate of Rejection (%)/Adsorption Capacity (qe) (mg/g) | Reference |
|---|---|---|---|---|
| O-CNTs, G-CNTs | Adsorption of Pb2+ on O-CNTs and G-CNTs | Pb2+ | <9.03% | [163] |
| Vertically aligned (VA) CNT (open-end) hybrid membrane | Gas separation |
| Not supplied | [164] |
| COOH/CNTs | Adsorptive removal of Indigo carmine (IC) dye onto nanotube carbon (CNTs) | Indigo carmine (IC) dye | CNT: (88.5 mg/g) COOH-CNT: (136 mg/g) | [232] |
| Granular carbon nanotubes (CNTs) | Efficient removal of typical pharmaceuticals | Typical pharmaceuticals | CBZ: 0.3695 mg/g TC: 0.2842 mg/g DS: 0.2031 mg/g | [245] |
| ZIF8@carbon nanotube | Adsorption of Phosphate on ZIF-8@MWCNT | Phosphate | (92.8–100%) | [166] |
| SWCNTs, MWCNTs, and PAC | Adsorption of bisphenol A and 17a-ethinyl estradiol (EE2) using carbon nanomaterials and powdered activated carbon | bisphenol A, 17a-ethinyl estradiol (EE2) | 90% removal of both BPA and EE2 | [168] |
| Carbon nanotubes (CNTs) | Adsorption of Sulfamethoxazole (SMZ) and ketoprofen (KET) on modified carbon nanotubes (CNTs) | Sulfamethoxazole (SMZ) and ketoprofen (KET) | Adsorption percentage: SMZ: >70% KET >80% Removal percentage: SMZ: 30% KET: >50% | [147] |
| Graphene | Adsorption of CHCl3 on graphene | Chloroform molecule (CHCl3) | Not supplied | [140] |
| Single-layer graphene nanosheets | Desalination and ion capture by sunlight single layer graphene nanosheet | Na+, Pb2+ and Fe3+ | Na+: 86.1% Pb2+: 77.3% Fe3+: 46.1% | [146] |
| Graphene oxide | Adsorption of 17 β- estradiol on graphene oxide | 17 β- estradiol | 169.49 mg/g | [113] |
| Graphene oxide | Adsorption of As(III) on graphene oxide | As(III) | 288 mg/g | [145] |
| Graphene oxide | Removal of Ni(II) from wastewater by adsorption on graphene oxide surface | Ni(II) | 197.8 mg/g | [148] |
| Graphene oxide | Adsorption of Methylene blue (MB) on graphene oxide surface | Methylene blue (MB) | Not supplied | [149] |
| MGOA | Adsorption of quinoline in wastewater | Quinoline pollutants | 103 mg/g | [141] |
| Ultrafiltration PSF/GO membrane | Nitrate rejection, antifouling property | Nitrate | 22.5% at 0.5 weight percent of GO | [143] |
| SN-rGO-A | Adsorb oils and organic solvents by SN-rGO-A | Oils and organic solvents | qe: 65–192 times its weight | [165] |
| Nitrogen doped carbon (CNs) | Adsorbent for the removal of anionic heavy metals from wastewater and sewage | Arsenic | 31.08 mg/g | [144] |
| g-C3N4 | Adsorptive removal of uranyl by porous graphitic carbon nitride (g-C3N4) | Uranium | 149.70 mg/g | [214] |
| g-C3N4 | Removal of heavy metal ions from aqueous solutions | Pb(II), Cu(II), Cd(II) and Ni(II)) | Pb(II): 1.36 mmol/g Cu(II): 2.09 mmol/g Cd(II): 1.00 mmol/g Ni(II): 0.64 mmol/g | [252] |
| Carbonaceous nanofiber/Ni-Al layered double hydroxide (CNF/LDH) | Removal of heavy metals from aqueous solutions | Cu(II), Cr(VI) | Cu(II): 219.6 mg/g Cr(VI): 341.2 mg/g | [167] |
| Membrane | Role of Metal Oxide | Rejected Material | Adsorption Capacity (qe) (mg/g) | Reference |
|---|---|---|---|---|
| ZnO surface | Removal of barium (Ba2+) ions on ZnO spherical nanoparticles | Barium ions | 64.6 mg/g | [138] |
| MnFe2O4 nanocubes | High adsorption capacity of U(VI) and Eu(III) on magnetic MnFe2O4 nanocubes | Uranium U(VI) Eu(III) | U(VI): 119.90 mg/g Eu(III): 473.93 mg/g | [139] |
| Oct-Cu2O NCs | Adsorption of tetracycline on octahedral Cu2O nanocrystals | Tetracycline | 1112.6 mg/g | [213] |
| Membrane | Role of Nanocomposite Membrane | Rejected Material | Rate of Rejection (%)/Adsorption Capacity (qe) (mg/g) | Reference |
|---|---|---|---|---|
| PyTTA-Dva-COF | Removal of bisphenol A from aqueous solution | bisphenol A | 285 mg/g | [142] |
| Zn–Fe LDH | Removal of diclofenac from water using Zn–Fe LDH | Diclofenac | 74.50 mg/g | [152] |
| Lanthanum-aluminium perovskite (La2Al4O9) | Adsorption mechanisms for removing fluoride using lanthanum-aluminum perovskite | Fluoride (F) | 87.75 mg/g | [153] |
| β-CD/TiO2 | Adsorption mechanisms for uranium removal by β-CD/TiO2 | U(VI) | 129.8 mg/g | [216] |
| Fe3O4-HBPA-ASA | Removal of heavy metal ions from aqueous solution by Fe3O4-HBPA-ASA | Heavy metal ions | Cu(II): 136.66 mg/g Pb(II): 88.36 mg/g Cd(II): 165.46 mg/g | [246] |
| ZIFs | Highly efficient removal of U(IV) | U(VI) | ZIF-8: 540.4 mg/g Zn/Co-ZIF: 527.5 mg/g ZIF-9: 448.6 mg/g ZIF-67: 368.2 mg/g | [247] |
| Granular TiO2-La | Adsorption of arsenic and fluoride using granular TiO2-La | Arsenic (As III), fluoride (F) | As(III): 114 mg/g F: 78.4 mg/g | [248] |
| Ni (II) modified porous BN | Removal of tetracycline from aqueous solution | Tetracycline (Tc) | 429.582 mg/g Removal percentage: 99.769% | [249] |
| Bi2O2CO3 (BOC) nanosheets with oxygen vacancies | Removal of (NO) by BOC nanosheets | Nitric oxide (NO) | Removal percentage: 50.2% | [250] |
| Amino-modified attapulgite (M-ATP) | Removal of Pb2+, and Cu2+ by adsorption on Amino-modified attapulgite (M-ATP) | Pb2+, Cu2+ | Pb2+: 53.58 mg/g Cu2+: 28.86 mg/g | [251] |
| MIL-101(Fe) and MIL-101(Fe,Co) | Removal of Ciprofloxacin (CIP) by MIL-101(Fe) and MIL-101(Fe,Co) | Ciprofloxacin (CIP) | Removal percentage: 97.8% | [253] |
| CuCo2O4/BiVO4 | Removal of 4-Nitrophenol | 4-Nitrophenol | Not supplied | [254] |
| MOF-545 | Removal of lead by adsorption on (MOF-545) | Pb(II) | Pb(II): 73 mg/g | [217] |
| Zn/Fe LDH | Removal of oxytetracycline hydrochloride (OTC) by adsorption on Zn/Fe LDH | Oxytetracycline hydrochloride (OTC) | Removal percentage: 77.23% | [255] |
| MoS2 | Removal of uranyl ions U(VI) by adsorption on MoS2 | U(VI) | MoS2 nanosheets: 45.7 mg/g MoS2 nanoflowers: 37.1 mg/g | [150] |
| MoS2 nanosheets | Removal of Pb2+ in aquatic systems by MoS2 nanosheets | Toxic metals (Pb2+), (Cd2+), | Pb2+: 638 mg/g under 1 sun illuminations, 902 mg/g under 4 sun illuminations Cd2+: 489 mg/g under 1 sun illuminations, 719 mg/g under 4 sun illuminations | [151] |
| PVP/MoS2 | Removal of uranyl ions by adsorption on PVP/MoS2 | U(VI) | U(VI): 117.9 mg/g | [215] |
| Nanocomposite Material | Role of TiO2 | Degraded Material | Decomposition Rate (min−1)/Degradation Efficiency (%) | Reference |
|---|---|---|---|---|
| C, N, F/TiO2NTs | High photocatalytic activity under UV-light | Methyl orange | Under UV-light:
| [197] |
| iN-Ti3C2/TiO2 hybrid | Achieved a high photocatalytic performance in degrading MB. | Methylene blue (MB) | Under UV-light:0.02642 min−1 | [198] |
| TiO2 nanoflowers(TNFs) | high photocatalytic performance for the degradation of diverse phenolic organic contaminants | Bisphenol A (BPA), diphenyl phenol, P-tert-butyl phenol, and resorcinol | Under UV-light:>95% | [199] |
| (TiO2/TiNTs) | TiO2/TiNTs showed about 10 times higher degradation for phenanthrene compared to the unmodified TiNTs | Cu(II), phenanthrene |
| [200] |
| Cr-TiO2 supported on Fe3O4 | High photocatalytic activity under solar radiation | Malachite green dye (MG), total organic carbon (TOC) | Under solar radiation:
| [242] |
| Nanocomposite Material | Role of Carbon Nanomaterials | Degraded Material | Decomposition Rate (min−1)/Degradation Efficiency (%) | Reference |
|---|---|---|---|---|
| Heterogeneous Fenton catalysts (CNTs/Fh) | Degradation of bisphenol A | bisphenol A | 3% CNTs/Fh: 79.1% | [116] |
| Co3O4/CNTs | Degradation of norfloxacin (NX) | NX | 97.5% | [231] |
| ZIF-67 Carbocatalysts, Nitrogen-doped magnetic carbon (Co@N-C) | Degradation of BPA | BPA | 60% | [235] |
| Ternary nanocomposites of Fe3O4 nanoparticles@ graphene–poly-N-phenylglycine nanofibers | Adsorption of Cu2+ | Cu2+ | 95% | [241] |
| SWCNTs | Degradation of pharmaceutical: PhACs, ibuprofen (IBP) and sulfamethoxazole (SMX) | ibuprofen and sulfamethoxazole | At pH = 3.5: 99% for IBP and SMX | [137] |
| CF/BiOBr/Ag3PO4 cloth | Degradation of tetracycline hydrochloride (TCH) | TCH | 90% | [154] |
| (N-rGO/BiVO4) | Degradation of methylene blue (MB) | MB | 99.3% | [193] |
| Nanocomposite Material | Role of Metal Oxide | Degraded Material | Decomposition Rate (min−1)/Degradation Efficiency (%) | Reference |
|---|---|---|---|---|
| ZnO@C | Photocatalytic degradation of methylene blue | Methylene blue (MB) | 99.8% | [194] |
| Cerium zirconium oxide (CexZryO2) | Photocatalytic degradation of sulfonamides | Sulfonamides | 91.33% | [195] |
| ZnO/Al2O3 | Wastewater treatment | Methyl orange dye (MO), TOC | TOC: 80.4% | [196] |
| NiO nanobelt | Removal of organic pollutants such as RhB, MO, MB, and CV | Removal of organic pollutants | RhB: 89% CV: 76.7% MB: 82.7% MO: 79.1% | [204] |
| Nanocomposite Material | Role of the Nanocomposite | Degraded Material | Decomposition Rate (min−1)/Degradation Efficiency (%) | Reference |
|---|---|---|---|---|
| Black phosphorus quantum dots/Tubular g-C3N4 (BPQDs/TCN) | Facilitates the charge spatial separation in the photocatalytic process which improves the process efficiency | Oxytetracycline hydrochloride, hexavalent chromium reduction | Oxytetracycline hydrochloride: 0.0276 min−1, Hexavalent chromium: 0.0404 min−1 | [201] |
| CdSe-Ag-WO3-Ag photocatalyst | Strong redox capacity, enhanced optical absorption and accelerated transfer and separation of carriers | Cefazolin (CFZ) | CFZ: 96.32% in 30 min | [243] |
| Sodium titanate nanotubes (Na-TNT) | Photocatalytic degradation of nickel (Ni(II)), methylene blue (MB) | Nickel (Ni(II)), methylene blue (MB) | 90% of Ni(II) ions within the first 15 min. Removed 99.5% of MB | [202] |
| Fe2O3-PC nanohybrids | Photocatalytic degradation of methylene blue (MB) | Methylene blue (MB) | Fe2O3: Removed 56% of MB Fe2O3-PC: Removed 75% of MB | [203] |
| Carbon dots/g-C3N4 (C-CN) heterostructures | Photocatalytic degradation of sulfamethoxazole (SDZ) | Sulfamethoxazole (SDZ) | 0.5C-CN: 62.7% 1C-CN: 75.2% 3C-CN: 92.8% 5C-CN: 85.7% | [205] |
| AgBr/h-MoO3 composite | Photocatalytic degradation of trimethoprim (TMP) | Trimethoprim (TMP) | TMP: 97% | [206] |
| CN-CGs | Photocatalytic degradation of Total organic carbon (TOC), bisphenol A (BPA) | Total organic carbon (TOC), bisphenol A (BPA) | BPA: 90% TOC: 80% | [207] |
| Fe/Fe3C@PC | Photocatalytic degradation of sulfamethazine (SMT) | Sulfamethazine (SMT) | SMT: 99.2% | [236] |
| N-doped BiVO4 | Photocatalytic degradation of ibuprofen (IBP) | Ibuprofen (IBF) | IBP: 90% | [208] |
| Bi/Fe0 | Photocatalytic degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) | hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) | RDX: The best RDX degradation was achieved using 4%-Bi/Fe0 (atomic ratio) NPs | [244] |
| PPECu thin film electrode | Photocatalytic degradation of phenol and 2,4-DCP | Phenol, 2,4-DCP | Photocatalytic degradation of phenol and 2,4-DCP activity was 2.52 and 3.85 times higher than g-C3N4 | [209] |
| FexMo1-xS2 catalysts | Photocatalytic degradation of propranolol | Propranolol | 90% at pH = 4.0 | [210] |
| P-doped porous g-C3N4 | Photocatalytic degradation of rhodamine B (RhB) | Rhodamine B (RhB) | RhB: 99.5% | [211] |
| 1D/2D W18O49/g-C3N4 nanocomposites | Photocatalytic degradation of ibuprofen (IBF) | Ibuprofen (IBF) | IBF: 96.3% | [212] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, Q.; Creedon, L.; Gharbia, S. A Literature Review of Modelling and Experimental Studies of Water Treatment by Adsorption Processes on Nanomaterials. Membranes 2022, 12, 360. https://doi.org/10.3390/membranes12040360
Ibrahim Q, Creedon L, Gharbia S. A Literature Review of Modelling and Experimental Studies of Water Treatment by Adsorption Processes on Nanomaterials. Membranes. 2022; 12(4):360. https://doi.org/10.3390/membranes12040360
Chicago/Turabian StyleIbrahim, Qusai, Leo Creedon, and Salem Gharbia. 2022. "A Literature Review of Modelling and Experimental Studies of Water Treatment by Adsorption Processes on Nanomaterials" Membranes 12, no. 4: 360. https://doi.org/10.3390/membranes12040360
APA StyleIbrahim, Q., Creedon, L., & Gharbia, S. (2022). A Literature Review of Modelling and Experimental Studies of Water Treatment by Adsorption Processes on Nanomaterials. Membranes, 12(4), 360. https://doi.org/10.3390/membranes12040360









