The Preparation of High-Performance and Stable MXene Nanofiltration Membranes with MXene Embedded in the Organic Phase
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of MXene
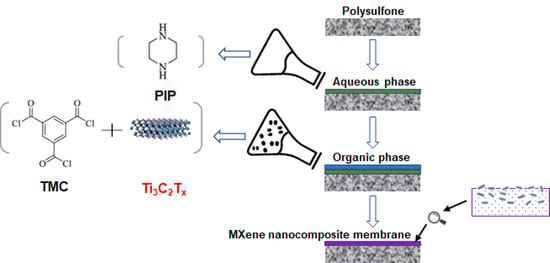
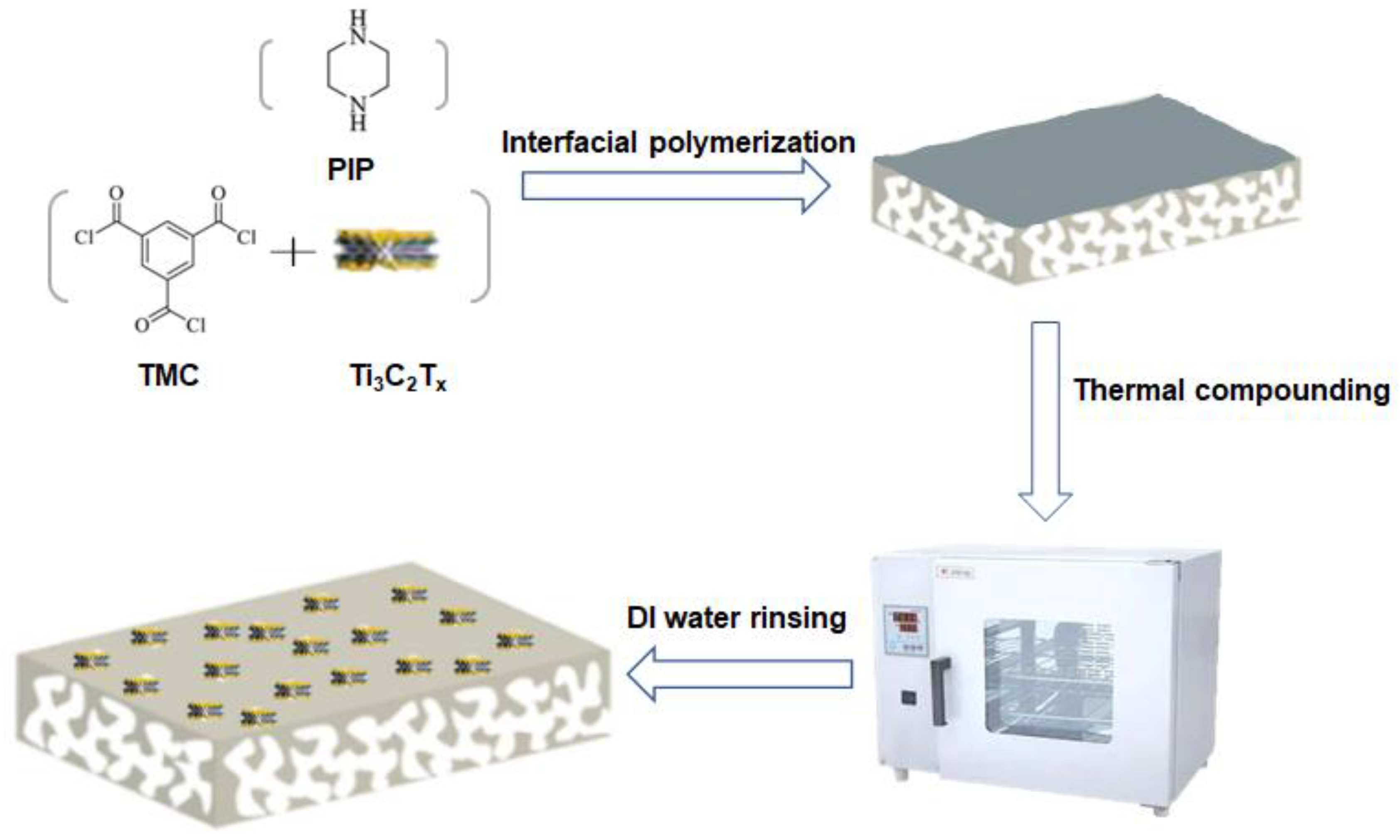
2.3. Preparation of MXene Membrane
2.4. Characterization of MXene
2.5. Characterization of MXene Membrane
2.6. Separation Performance of MXene Membrane
3. Results and Discussions
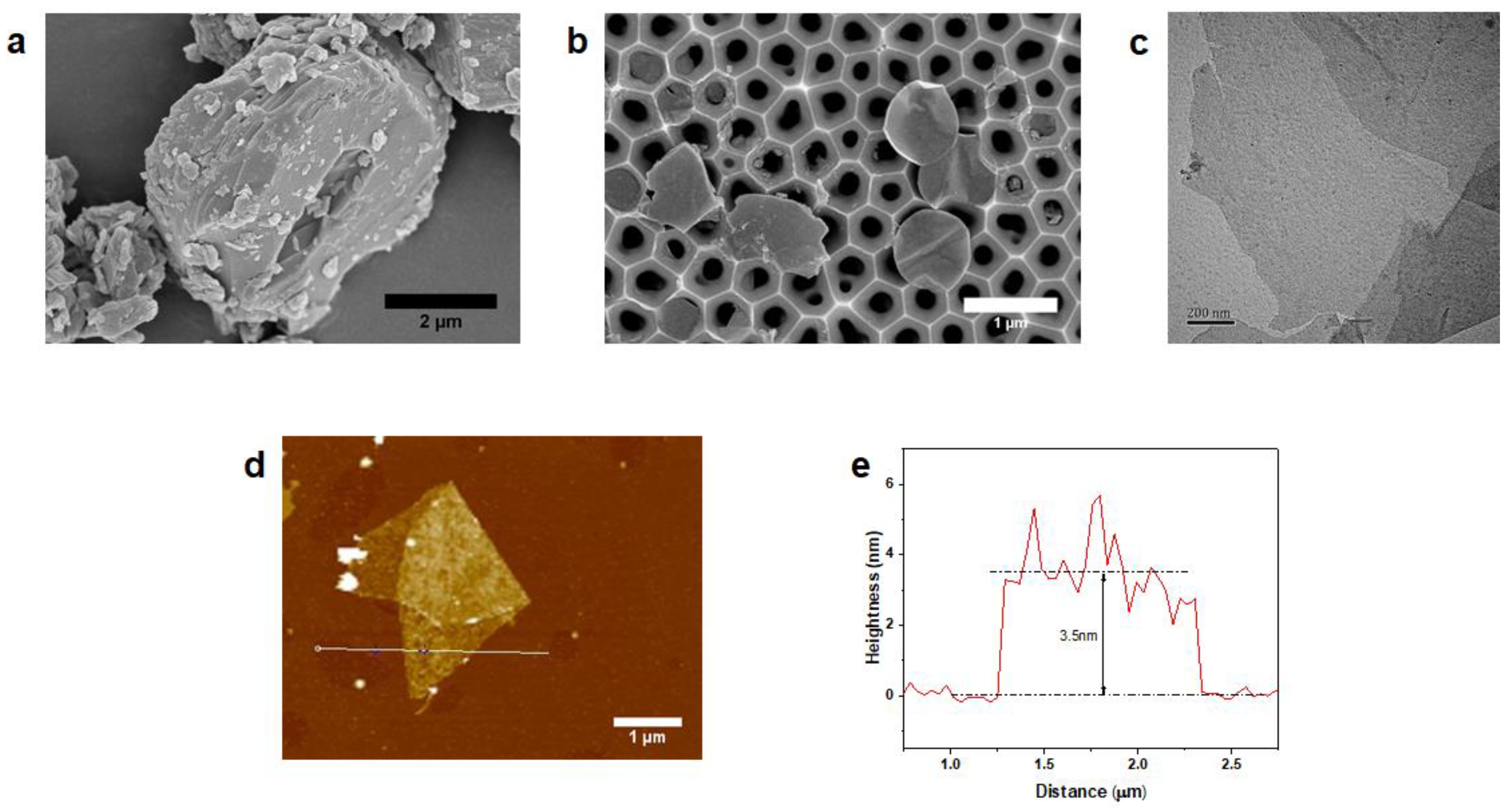
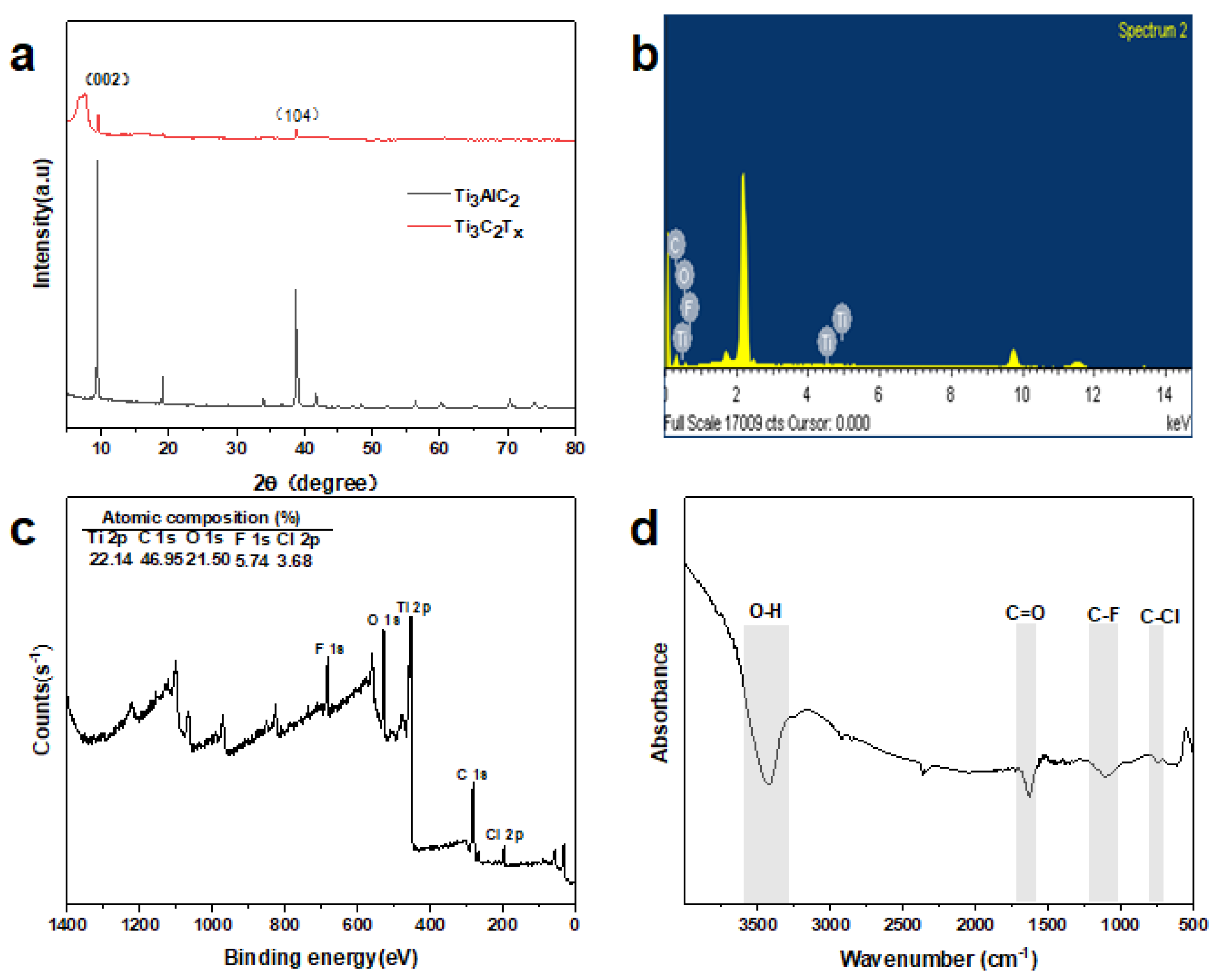
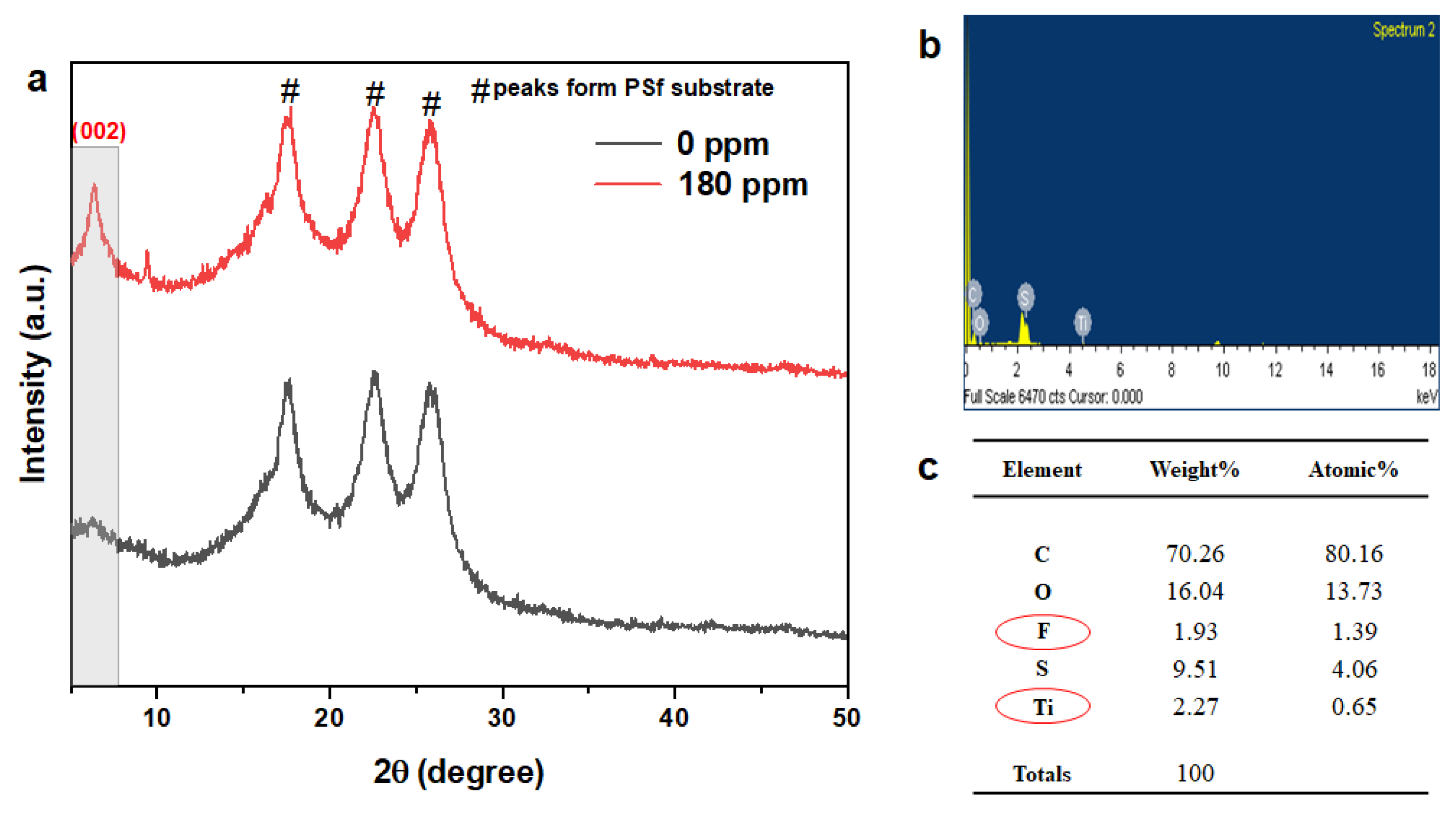
3.1. Physicochemical Characterization of MXene
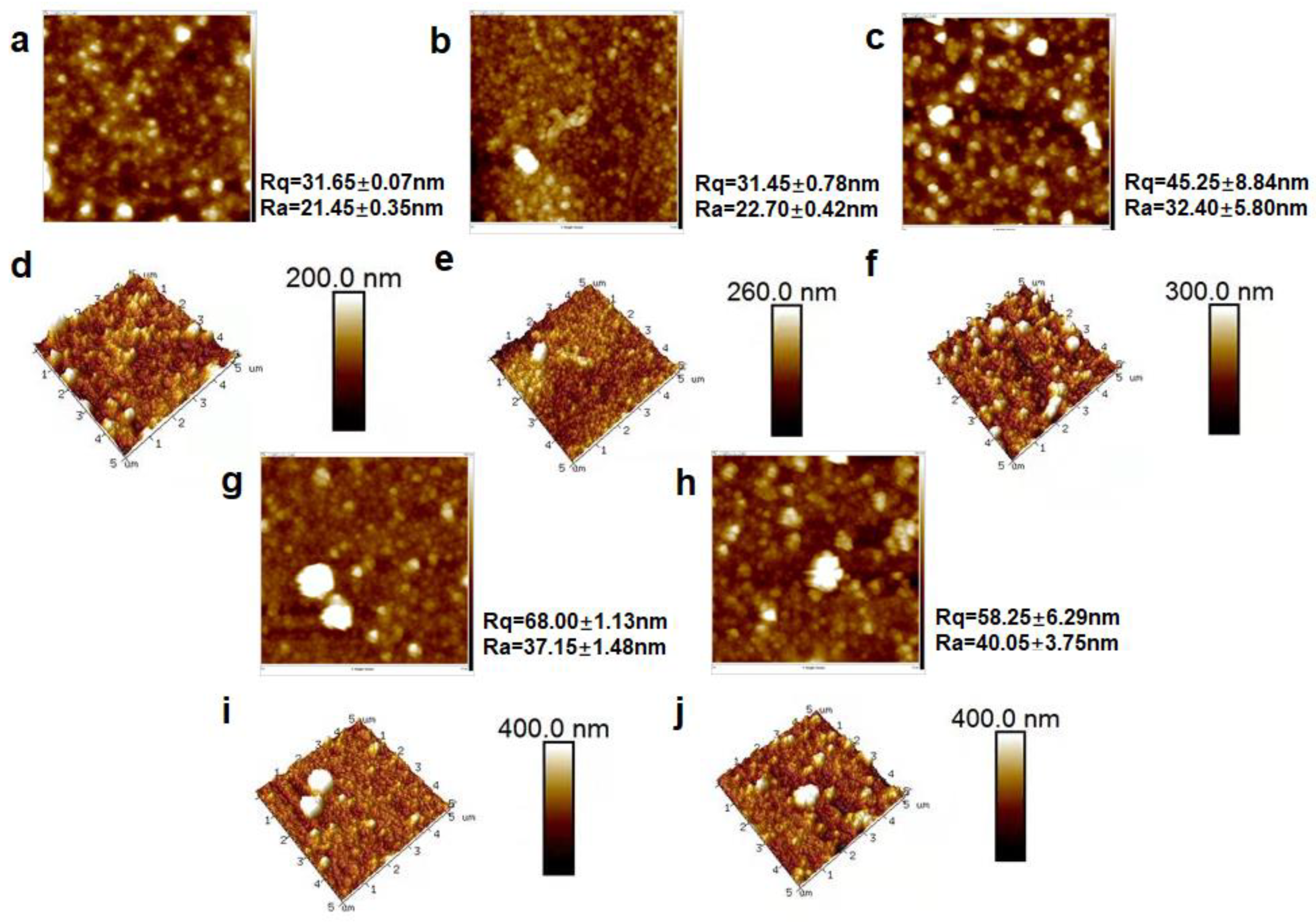
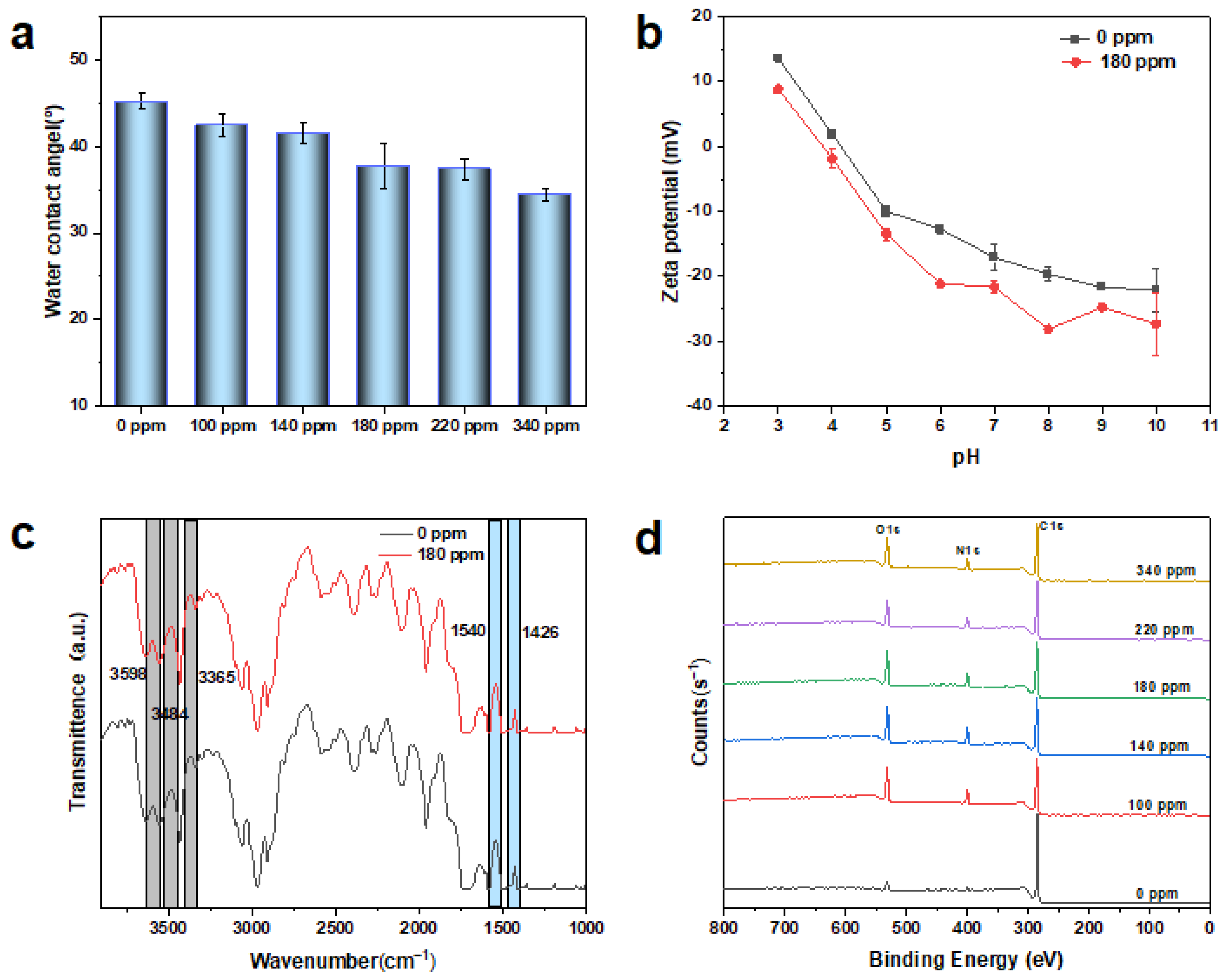
3.2. Characterization of MXene Membrane
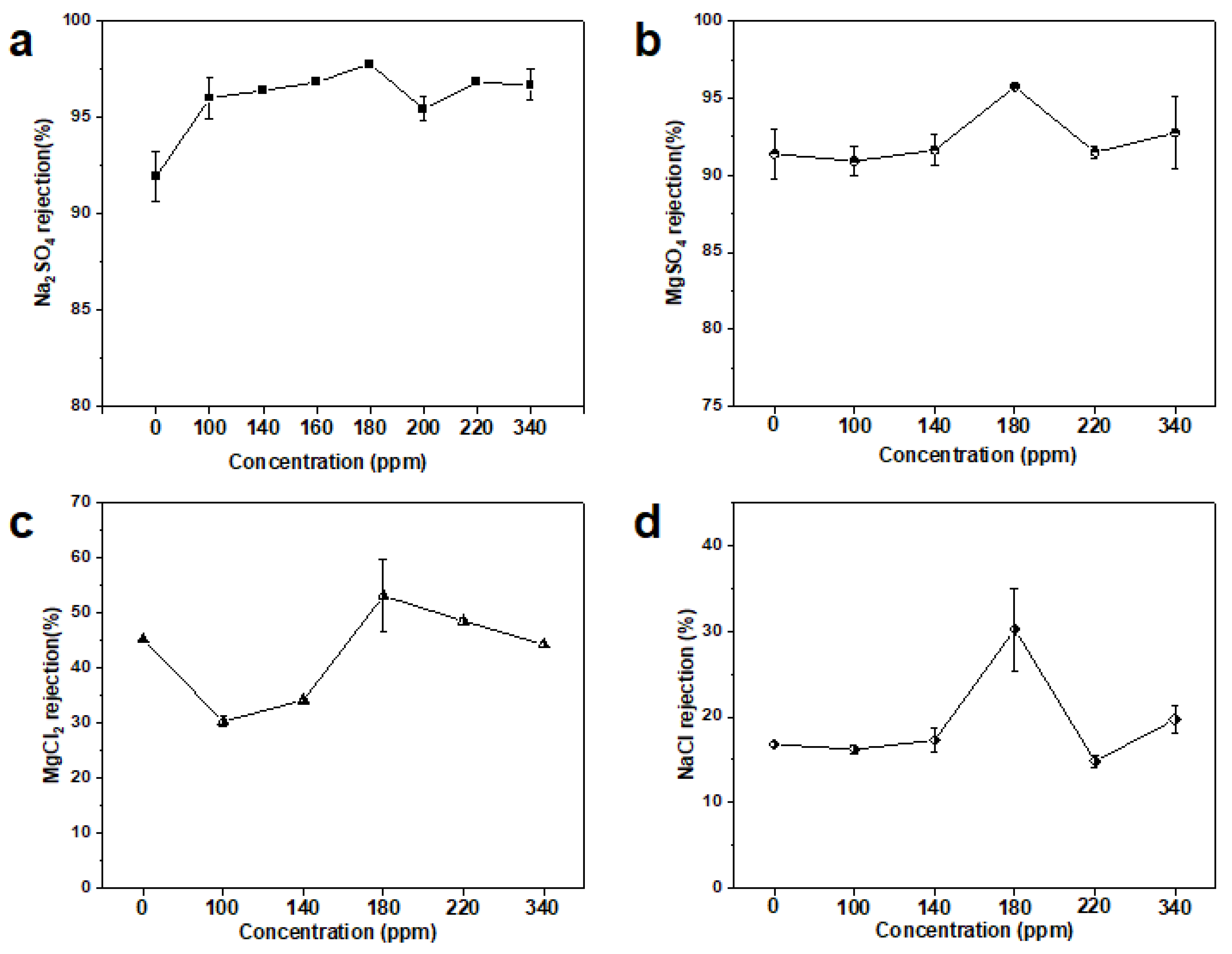
3.3. Separation Performance of MXene Membrane
3.4. Long-Time Water Immersion Test of MXene Membrane
3.5. Stability Test of MXene Membrane
3.6. Performance Comparison of MXene Membranes with Two Different Additions Way
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sari, M.A.; Chellam, S. Relative contributions of organic and inorganic fouling during nanofiltration of inland brackish surface water. J. Membr. Sci. 2017, 523, 68–76. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Mänttäri, M.; Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol. 2008, 63, 251–263. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Zaman, N.K.; Rohani, R.; Mohammad, A.W.; Isloor, A.M. Polyimide-graphene oxide nanofiltration membrane: Characterizations and application in enhanced high concentration salt removal. Chem. Eng. Sci. 2018, 177, 218–233. [Google Scholar] [CrossRef]
- Shin, M.G.; Seo, J.Y.; Park, H.; Park, Y.-I.; Lee, J.-H. Overcoming the permeability-selectivity trade-off of desalination membranes via controlled solvent activation. J. Membr. Sci. 2021, 620, 118870. [Google Scholar] [CrossRef]
- Mallya, D.S.; Dumée, L.F.; Muthukumaran, S.; Lei, W.; Baskaran, K. 2D nanosheet enabled thin film nanocomposite membranes for freshwater production—A review. Mater. Adv. 2021, 2, 3519–3537. [Google Scholar] [CrossRef]
- Yang, J.; Li, Z.; Wang, Z.; Yuan, S.; Li, Y.; Zhao, W.; Zhang, X. 2D Material Based Thin-Film Nanocomposite Membranes for Water Treatment. Adv. Mater. Technol. 2021, 6, 2000862. [Google Scholar] [CrossRef]
- Anand, A.; Unnikrishnan, B.; Mao, J.-Y.; Lin, H.-J.; Huang, C.-C. Graphene-based nanofiltration membranes for improving salt rejection, water flux and antifouling—A review. Desalination 2018, 429, 119–133. [Google Scholar] [CrossRef]
- Meng, B.; Liu, G.; Mao, Y.; Liang, F.; Liu, G.; Jin, W. Fabrication of surface-charged MXene membrane and its application for water desalination. J. Membr. Sci. 2021, 623, 119076. [Google Scholar] [CrossRef]
- Ding, M.; Xu, H.; Chen, W.; Yang, G.; Kong, Q.; Ng, D.; Lin, T.; Xie, Z. 2D laminar maleic acid-crosslinked MXene membrane with tunable nanochannels for efficient and stable pervaporation desalination. J. Membr. Sci. 2020, 600, 117871. [Google Scholar] [CrossRef]
- Karahan, H.E.; Goh, K.; Zhang, C.; Yang, E.; Yıldırım, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, Ş.B.; Chen, Y.; et al. MXene Materials for Designing Advanced Separation Membranes. Adv. Mater. 2020, 32, 1906697. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Zhang, J.; Huang, H.; Wu, S.; Yang, Y. Novel thin-film reverse osmosis membrane with MXene Ti3C2Tx embedded in polyamide to enhance the water flux, anti-fouling and chlorine resistance for water desalination. J. Membr. Sci. 2020, 603, 118036. [Google Scholar] [CrossRef]
- Ihsanullah, I. MXenes (two-dimensional metal carbides) as emerging nanomaterials for water purification: Progress, challenges and prospects. Chem. Eng. J. 2020, 388, 124340. [Google Scholar] [CrossRef]
- Lee, H.S.; Im, S.J.; Kim, J.H.; Kim, H.J.; Kim, J.P.; Min, B.R. Polyamide thin-film nanofiltration membranes containing TiO2 nanoparticles. Desalination 2008, 219, 48–56. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, Y.; Wang, H.; Zhang, H.; Liu, J. Study on the modification of positively charged composite nanofiltration membrane by TiO2 nanoparticles. Desalination 2013, 313, 57–65. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y. Understanding water and solute transport in thin film nanocomposite membranes by resistance-in-series theory combined with Monte Carlo simulation. J. Membr. Sci. 2021, 626, 119106. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, K. MXene nanocomposite nanofiltration membrane for low carbon and long-lasting desalination. J. Membr. Sci. 2021, 640, 119808. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Sun, B.; Dong, X.; Li, H.; Shang, Y.; Zhang, Y.; Hu, F.; Gu, S.; Wu, Y.; Gao, T.; Zhou, G. Surface charge engineering for two-dimensional Ti2CTx MXene for highly efficient and selective removal of cationic dye from aqueous solution. Sep. Purif. Technol. 2021, 272, 118964. [Google Scholar] [CrossRef]
- Sheng, F.; Wu, B.; Li, X.; Xu, T.; Shehzad, M.A.; Wang, X.; Ge, L.; Wang, H.; Xu, T. Efficient Ion Sieving in Covalent Organic Framework Membranes with Sub-2-Nanometer Channels. Adv. Mater. 2021, 33, 2104404. [Google Scholar] [CrossRef]
- Arshadi, F.; Mohammad, M.; Hosseini, E.; Ahmadi, H.; Asadnia, M.; Orooji, Y.; Korayem, A.H.; Noorbakhsh, A.; Razmjou, A. The effect of D-spacing on the ion selectivity performance of MXene membrane. J. Membr. Sci. 2021, 639, 119752. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, S.; Peng, X.; Zhang, L.; Gao, C. Polyamide membranes with nanoscale Turing structures for water purification. Science 2018, 360, 518. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhang, K. Few-layers MoS2 nanosheets modified thin film composite nanofiltration membranes with improved separation performance. J. Membr. Sci. 2020, 595, 117526. [Google Scholar] [CrossRef]
- Mathelié-Guinlet, M.; Grauby-Heywang, C.; Martin, A.; Février, H.; Moroté, F.; Vilquin, A.; Béven, L.; Delville, M.-H.; Cohen-Bouhacina, T. Detrimental impact of silica nanoparticles on the nanomechanical properties of Escherichia coli, studied by AFM. J. Colloid Interface Sci. 2018, 529, 53–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Li, C.; Huang, W.; Mei, S.; Lin, H.; Ou, Q.; Zhang, Y.; Guo, J.; Zhang, F.; Xu, S.; et al. MXene/Polymer Membranes: Synthesis, Properties, and Emerging Applications. Chem. Mater. 2020, 32, 1703–1747. [Google Scholar] [CrossRef]
- Kwon, O.; Choi, Y.; Kang, J.; Kim, J.H.; Choi, E.; Woo, Y.C.; Kim, D.W. A comprehensive review of MXene-based water-treatment membranes and technologies: Recent progress and perspectives. Desalination 2022, 522, 115448. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Yusof, N.; Tan, Y.H. Graphene oxide incorporated thin film nanocomposite nanofiltration membrane for enhanced salt removal performance. Desalination 2016, 387, 14–24. [Google Scholar] [CrossRef]
- Gohil, J.M.; Ray, P. A review on semi-aromatic polyamide TFC membranes prepared by interfacial polymerization: Potential for water treatment and desalination. Sep. Purif. Technol. 2017, 181, 159–182. [Google Scholar] [CrossRef]
- Zheng, J.; Li, M.; Yao, Y.; Zhang, X.; Wang, L. Zwitterionic carbon nanotube assisted thin-film nanocomposite membranes with excellent efficiency for separation of mono/divalent ions from brackish water. J. Mater. Chem. A 2017, 5, 13730–13739. [Google Scholar] [CrossRef]
- Ihsanullah, I. Potential of MXenes in Water Desalination: Current Status and Perspectives. Nano-Micro Lett. 2020, 12, 72. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, L.; Xu, Y.; Zhu, J.; Liu, F.; Shen, J.; Wang, Z.; Lin, J. MXene nanosheet stacks with tunable nanochannels for efficient molecular separation. Chem. Eng. J. 2022, 427, 132070. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, Q.; Zhang, K. Few-layers 2D O–MoS2 TFN nanofiltration membranes for future desalination. J. Membr. Sci. 2020, 604, 118052. [Google Scholar] [CrossRef]
- Ma, M.-Q.; Zhang, C.; Zhu, C.-Y.; Huang, S.; Yang, J.; Xu, Z.-K. Nanocomposite membranes embedded with functionalized MoS2 nanosheets for enhanced interfacial compatibility and nanofiltration performance. J. Membr. Sci. 2019, 591, 117316. [Google Scholar] [CrossRef]
- Bano, S.; Mahmood, A.; Kim, S.-J.; Lee, K.-H. Graphene oxide modified polyamide nanofiltration membrane with improved flux and antifouling properties. J. Mater. Chem. A 2015, 3, 2065–2071. [Google Scholar] [CrossRef]
- Xue, S.-M.; Ji, C.-H.; Xu, Z.-L.; Tang, Y.-J.; Li, R.-H. Chlorine resistant TFN nanofiltration membrane incorporated with octadecylamine-grafted GO and fluorine-containing monomer. J. Membr. Sci. 2018, 545, 185–195. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Chen, J.; Li, Z.; Yin, Y.; Cao, L.; Zhong, Y.; Wu, H. Covalent organic framework modified polyamide nanofiltration membrane with enhanced performance for desalination. J. Membr. Sci. 2017, 523, 273–281. [Google Scholar] [CrossRef]

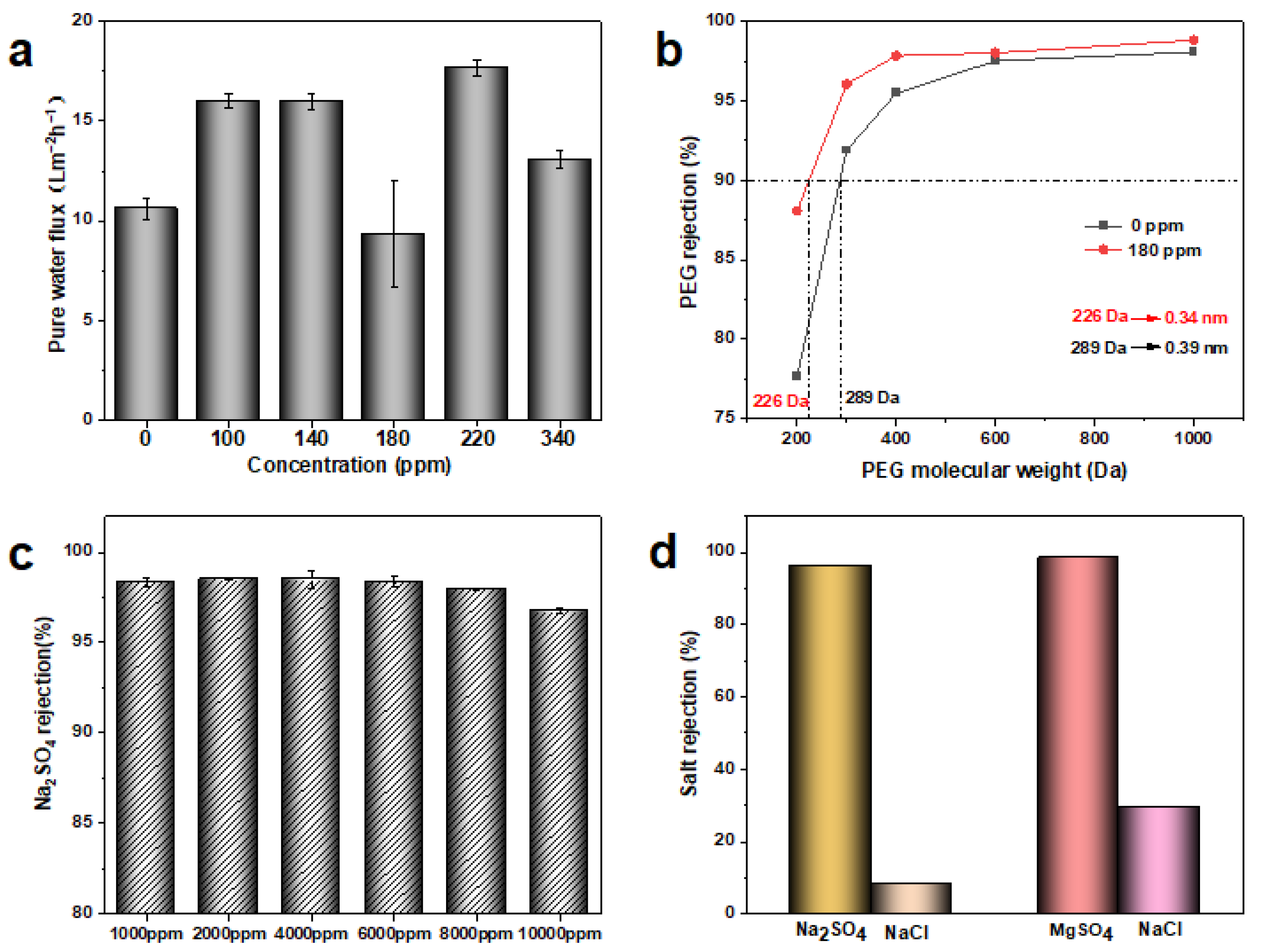

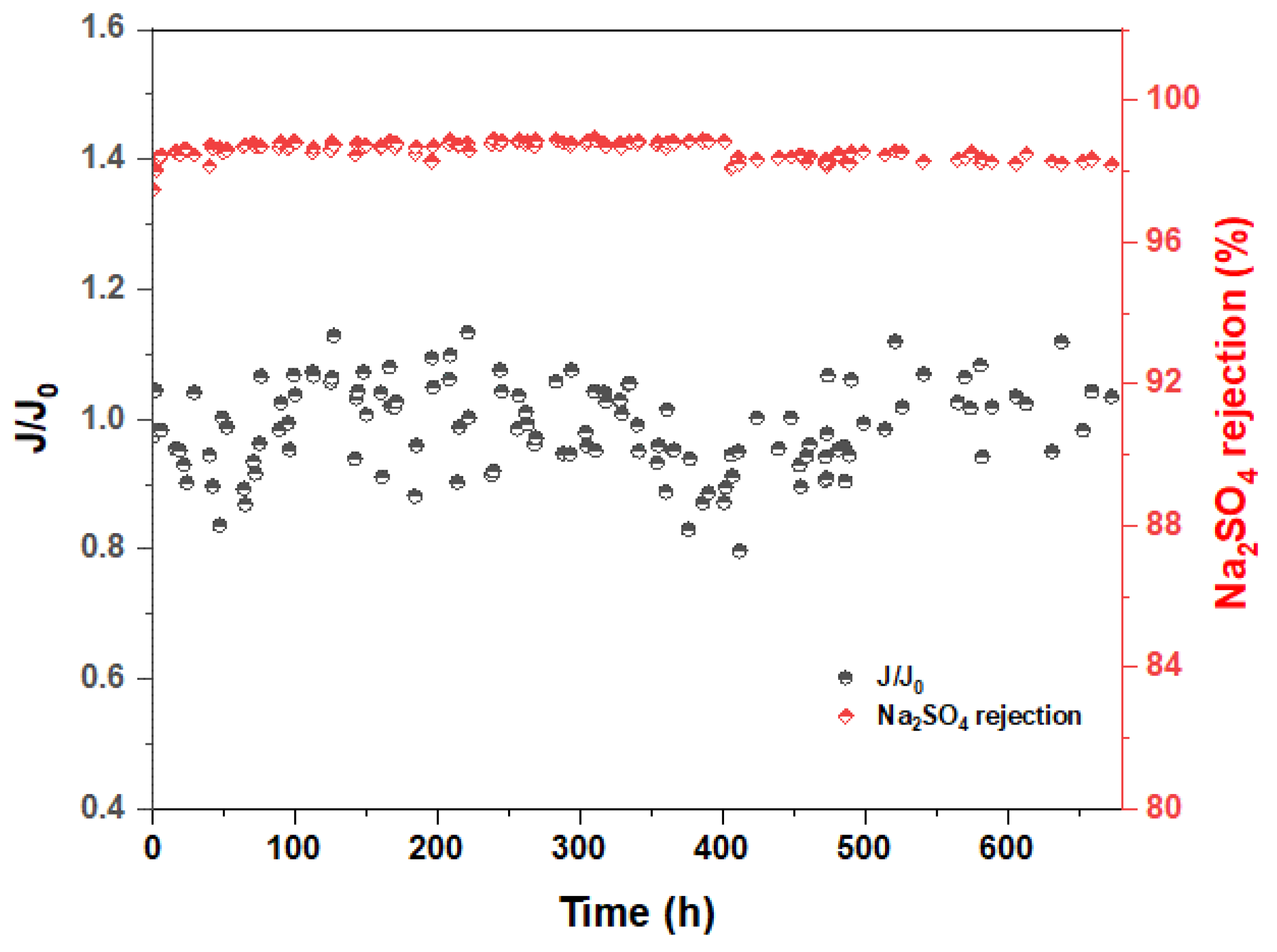
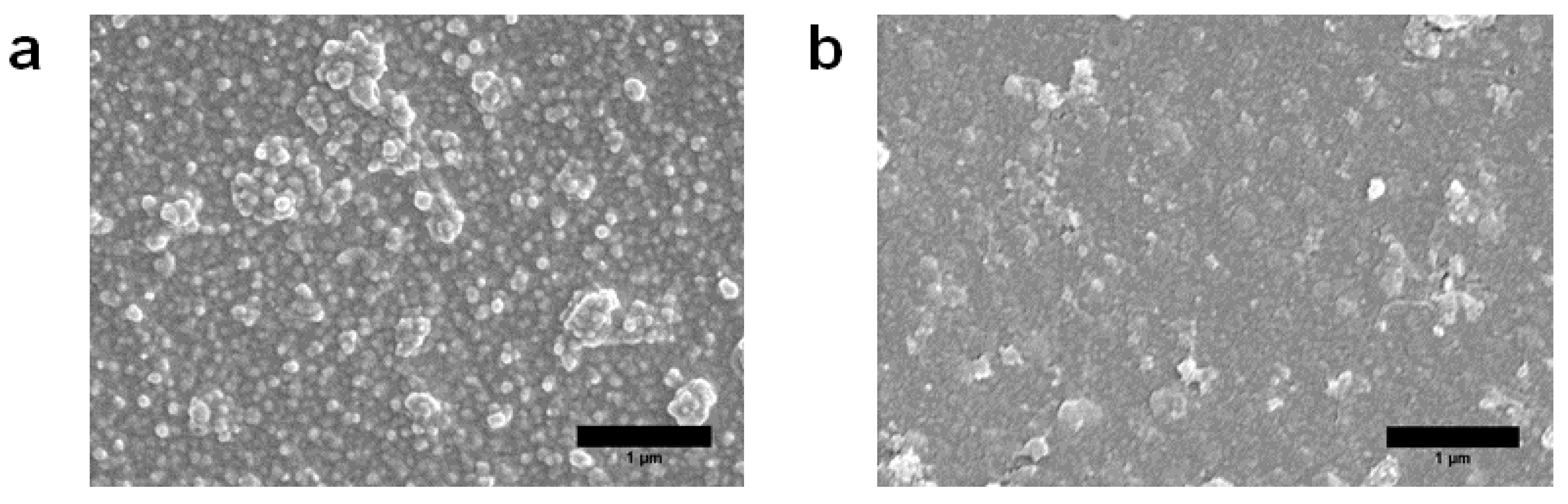
| Membranes | Na2SO4 Concentration | Operating Pressure (bar) | Long-Duration Time | Rejection (%) | Ref. |
|---|---|---|---|---|---|
| MXene-OP | 2000 ppm | 4 | 28 days | 98.6 | this work |
| MXene-AP | 2000 ppm | 3 | 58 days | 97.6 | [17] |
| MXene-PEI | 1000 ppm | 4 | —— | 65.7 | [31] |
| MoS2 | 2000 ppm | 3.5 | 16 h | 94.4 | [23] |
| O-MoS2 | 2000 ppm | 3.5 | 15 h | 97.9 | [32] |
| TA-MoS2 | 1000 ppm | 6 | 120 h | 98.0 | [33] |
| GO | 2000 ppm | 15 | 5.5 h | 97.0 | [34] |
| GO-ODA | 2000 ppm | 6 | 10 days | 98.4 | [35] |
| COFs | 1000 ppm | 2 | 72 h | 80 | [36] |
| Membranes | MWCO (Da) | Flux (Lm−2h−1bar−1) | Na2SO4 Rejection (%) | Long-Duration Time (day) | Ref. |
|---|---|---|---|---|---|
| MXene in organic phase | 226 | 2.33 | 98.6 | 28 | this work |
| MXene in aqueous phase | 288 | 4.70 | 97.6 | 58 | [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Q.; Zhang, K. The Preparation of High-Performance and Stable MXene Nanofiltration Membranes with MXene Embedded in the Organic Phase. Membranes 2022, 12, 2. https://doi.org/10.3390/membranes12010002
Xue Q, Zhang K. The Preparation of High-Performance and Stable MXene Nanofiltration Membranes with MXene Embedded in the Organic Phase. Membranes. 2022; 12(1):2. https://doi.org/10.3390/membranes12010002
Chicago/Turabian StyleXue, Qiang, and Kaisong Zhang. 2022. "The Preparation of High-Performance and Stable MXene Nanofiltration Membranes with MXene Embedded in the Organic Phase" Membranes 12, no. 1: 2. https://doi.org/10.3390/membranes12010002
APA StyleXue, Q., & Zhang, K. (2022). The Preparation of High-Performance and Stable MXene Nanofiltration Membranes with MXene Embedded in the Organic Phase. Membranes, 12(1), 2. https://doi.org/10.3390/membranes12010002