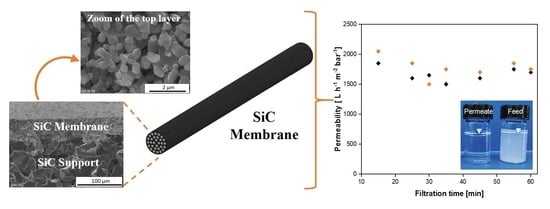
Ceramic Processing of Silicon Carbide Membranes with the Aid of Aluminum Nitrate Nonahydrate: Preparation, Characterization, and Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
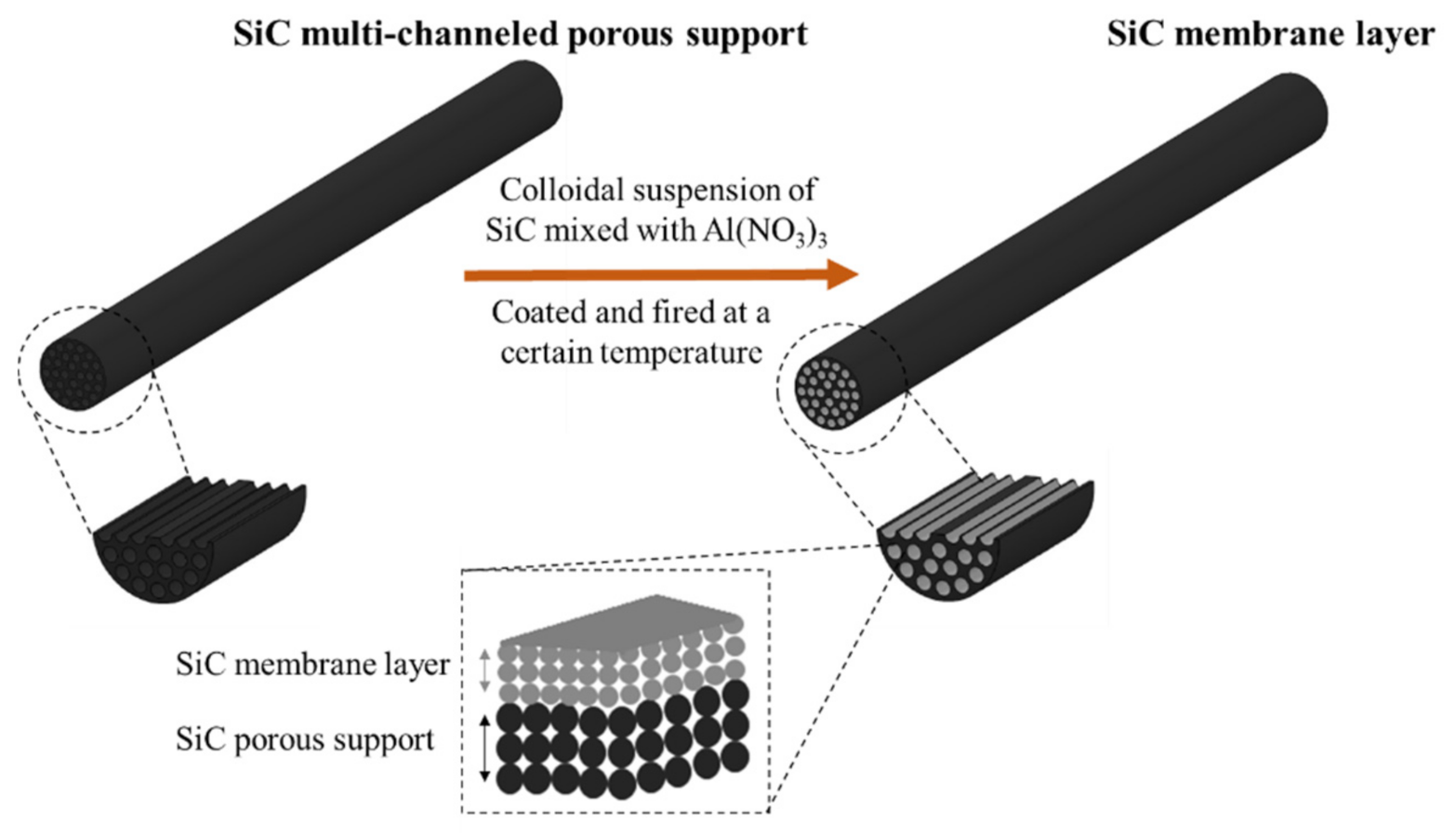
2.2. SiC Membrane Fabrication Process
2.3. Characterization Methods
2.4. Membrane Filtration Performance
3. Results and Discussion
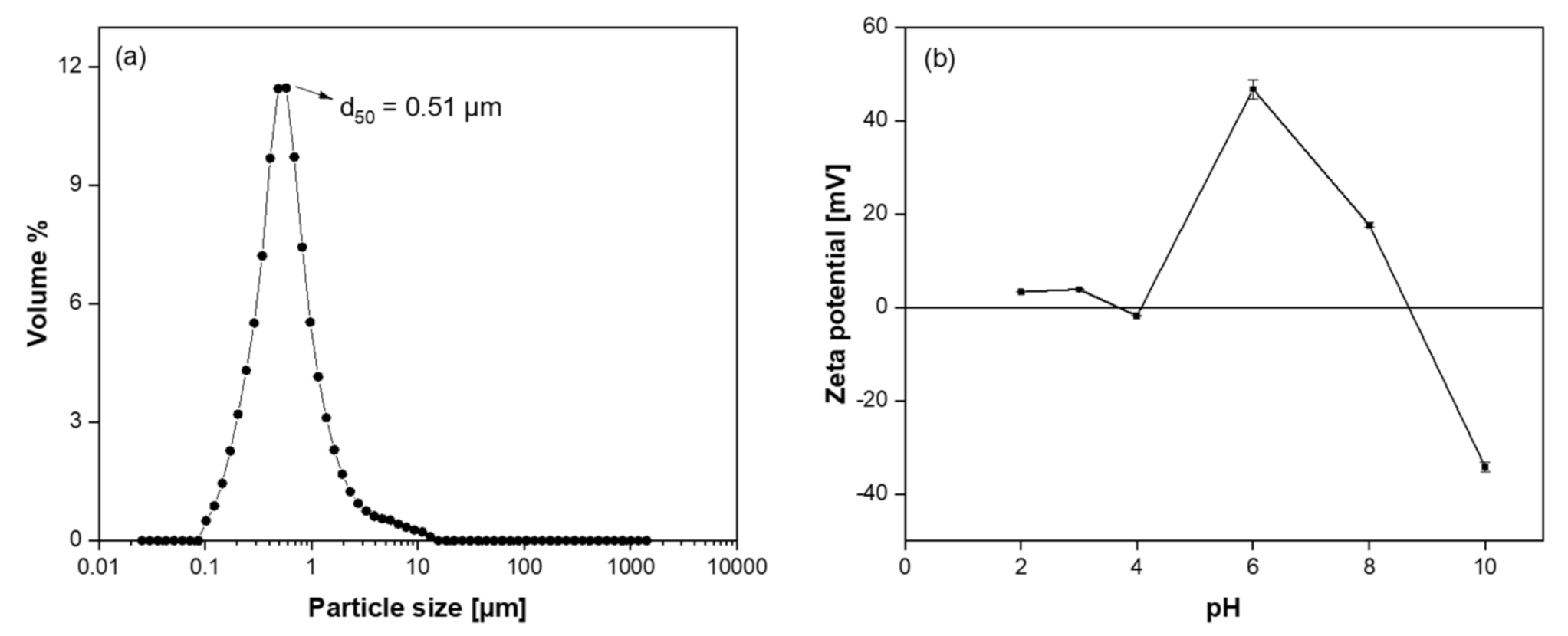
3.1. Characterization of Coating Suspension
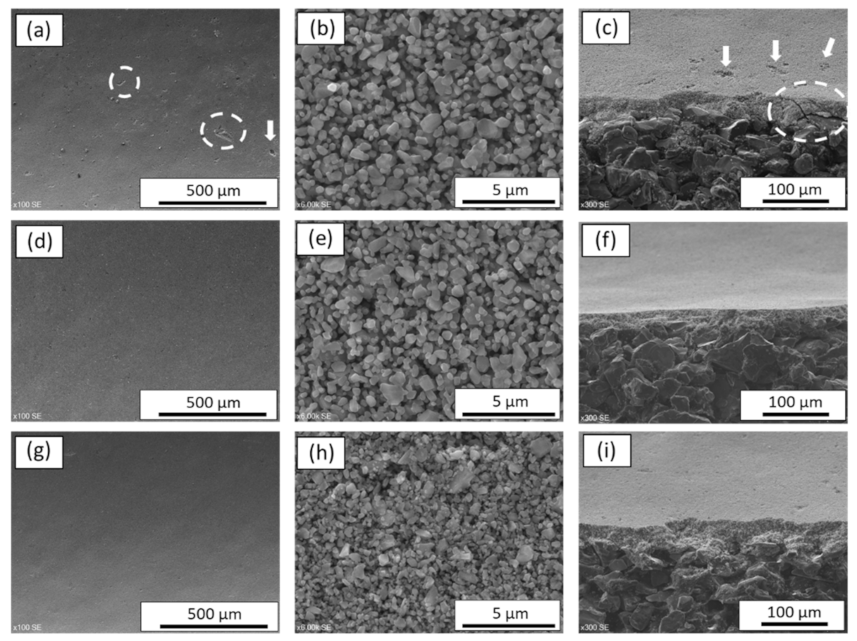
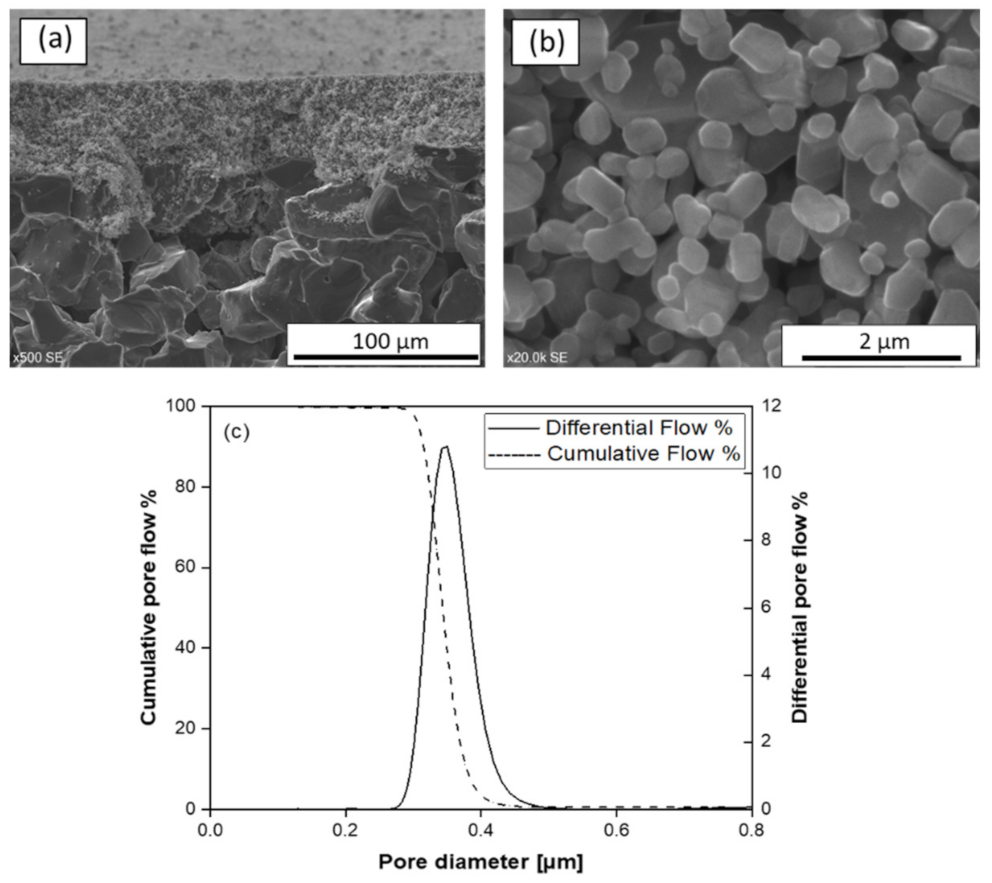
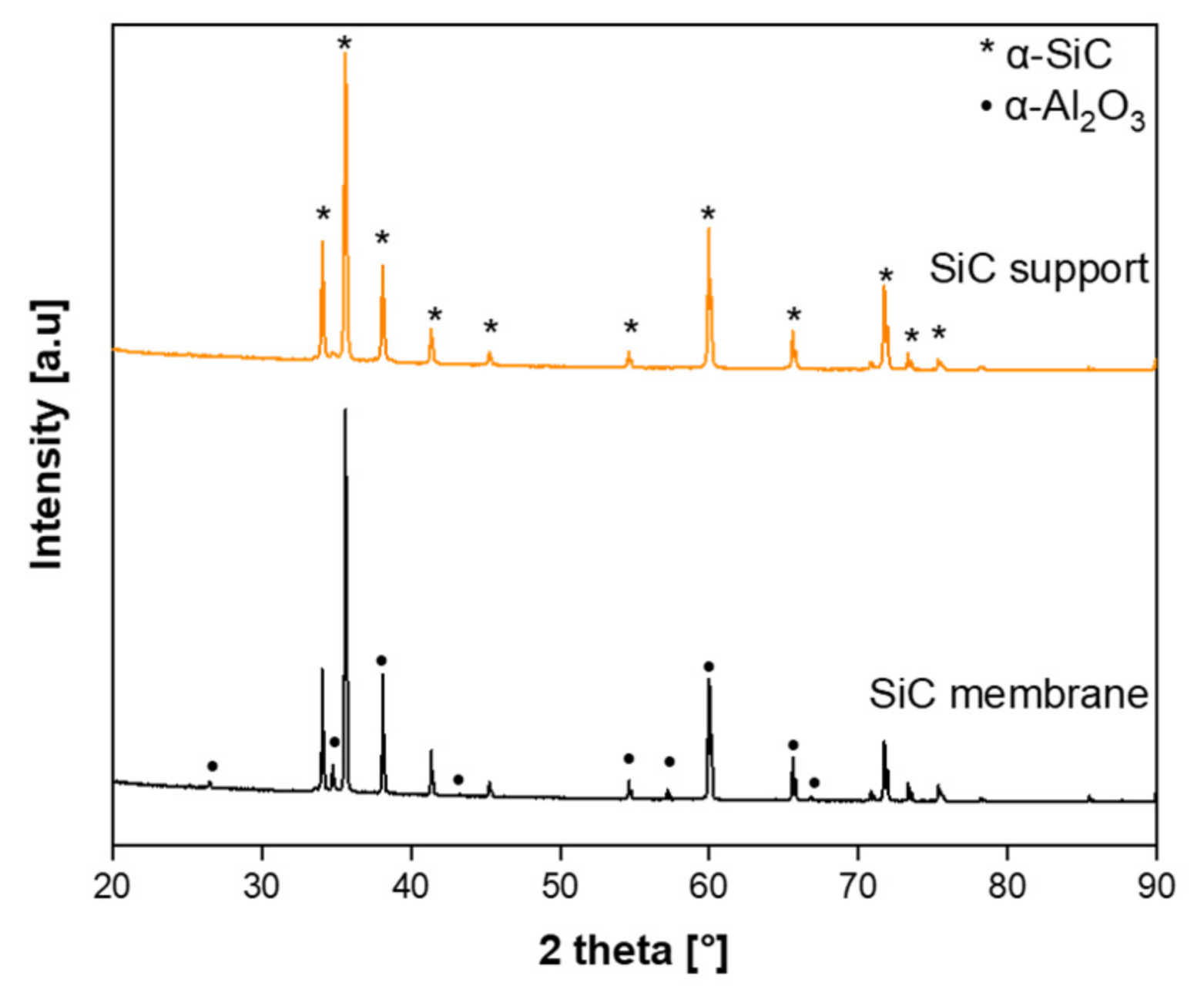
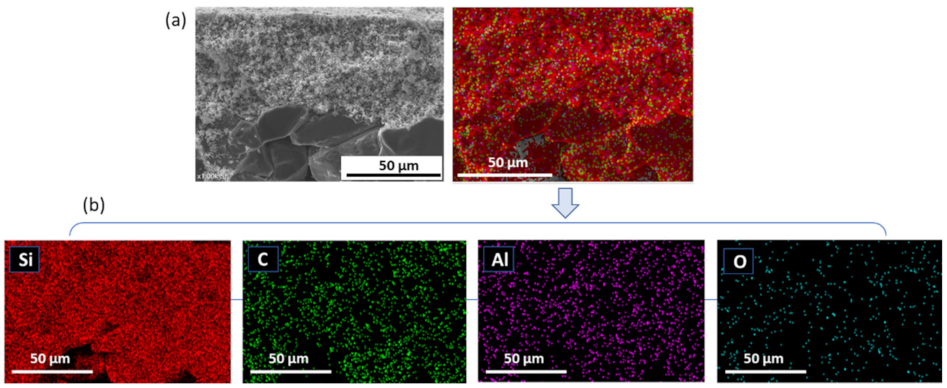
3.2. Structural Characterization of SiC Membrane
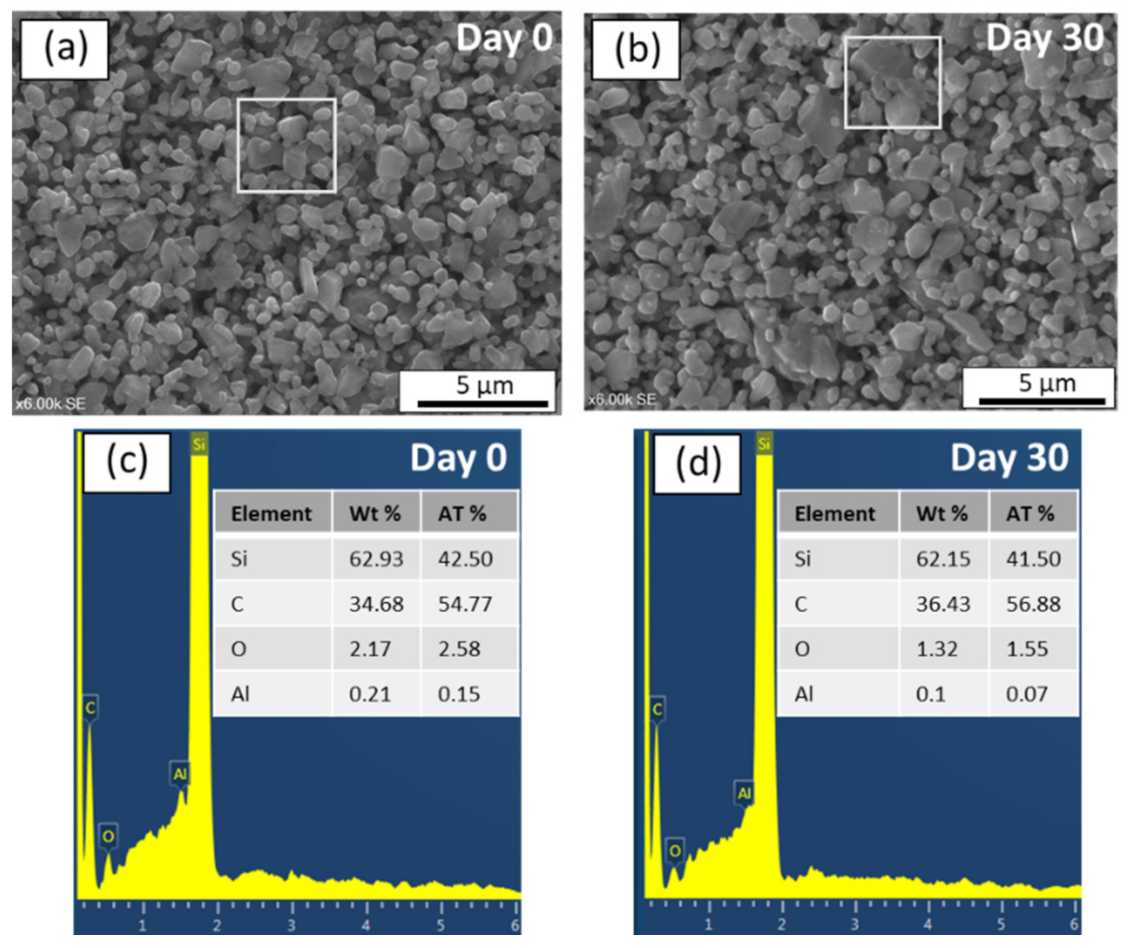
3.3. Chemical and Mechanical Stability
3.4. Filtration Performance of SiC Membrane
4. Conclusions
- The successful preparation of coating suspension containing two different α-SiC powders with the mixing ratio of 1:1 and 10−4 M aluminum nitrate nonahydrate as a sintering additive resulted in a homogeneous, defect-free, and smooth SiC membrane layer on macroporous multi-channeled SiC tubular support. With the use of aluminum nitrate nonahydrate, the sintering temperature was reduced approximately 200 °C with respect to the conventional SiC membrane fabrication. This decrease in the sintering temperature effectively reduces the cost of production of SiC membrane. The developed membrane has a narrow pore size distribution with an average pore size of 0.35 µm, which indicates the membranes are suitable for microfiltration.
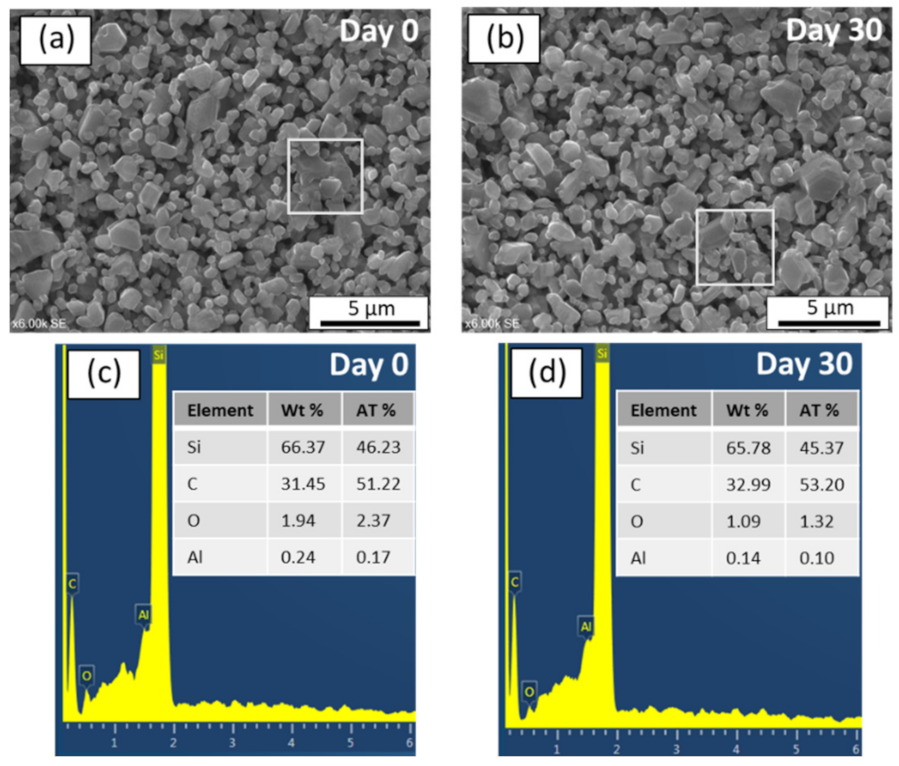
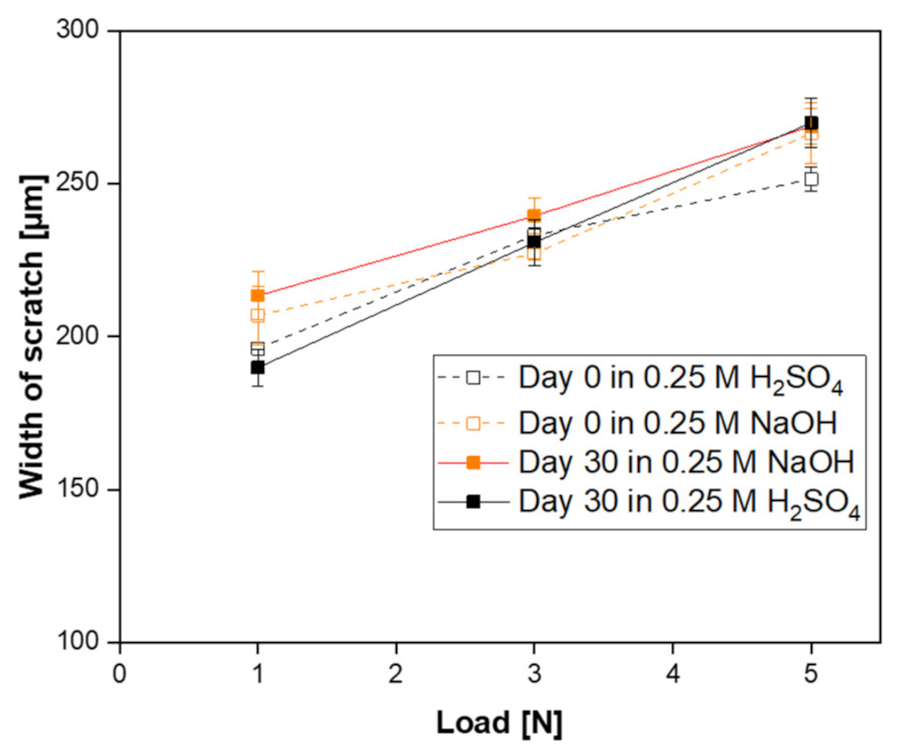
- The prepared SiC membrane demonstrated a good chemical resistance in both alkali and acid environments for 30 days. In both cases, pore size distributions, surface morphologies, and elemental compositions of the membranes were not changed significantly before and after exposure to a 30-day corrosion resistance test. Moreover, no change in the membrane strength was detected after corrosion tests, which shows that the developed membrane has good mechanical resistance.
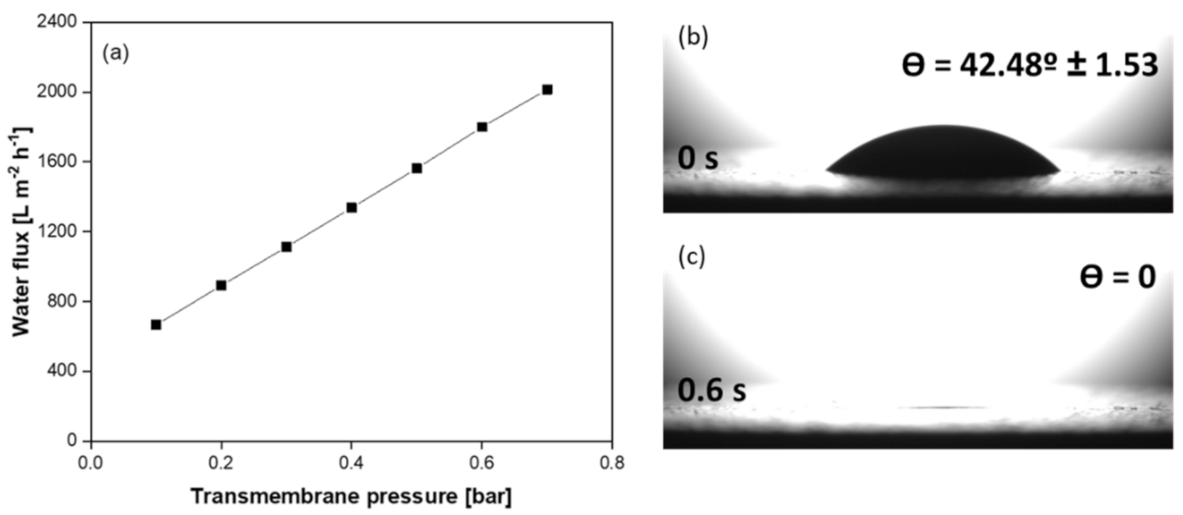
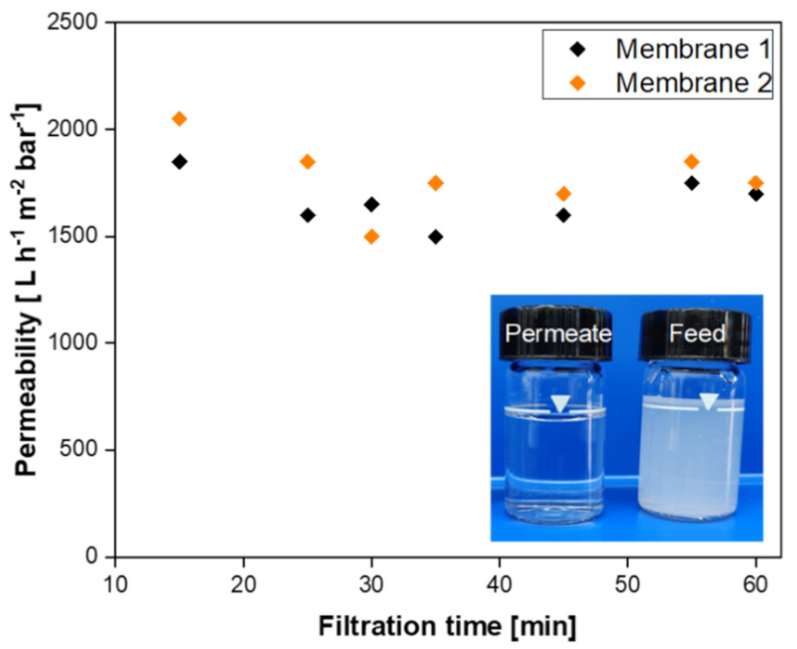
- The resultant SiC membrane showed outstanding water permeability (2252 L h−1 m−2 bar−1) and remarkable separation performance (99.7%) for oil/water emulsion with excellent permeability. Therefore, this membrane has the potential for application in the treatment of oily wastewater streams.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tang, C.Y.; Yang, Z.; Guo, H.; Wen, J.J.; Nghiem, L.D.; Cornelissen, E. Potable water reuse through advanced membrane technology. Environ. Sci. Technol. 2018, 52, 10215–10223. [Google Scholar] [CrossRef] [Green Version]
- Mohsen, S.; Gato-trinidad, S.; Altaee, A. The application of pressure-driven ceramic membrane technology for the treatment of industrial wastewaters—A review. Sep. Purif. Technol. 2018, 200, 198–220. [Google Scholar]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surf. A 2019, 578, 123513. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Coelho, L.L.; di Luccio, M.; Hotza, D.; de Fátima Peralta Muniz Moreira, R.; Moreira, A.C.; Fernandes, C.P.; Rezwan, K.; Wilhelm, M. Tailoring asymmetric Al2O3 membranes by combining tape casting and phase inversion. J. Memb. Sci. 2021, 623, 119056. [Google Scholar] [CrossRef]
- Zhang, L.; Ng, T.C.A.; Liu, X.; Gu, Q.; Pang, Y.; Zhang, Z.; Lyu, Z.; He, Z.; Ng, H.Y.; Wang, J. Hydrogenated TiO2 membrane with photocatalytically enhanced anti-fouling for ultrafiltration of surface water. Appl. Catal. B Environ. 2020, 264, 118528. [Google Scholar] [CrossRef]
- Li, S.; Wei, C.; Wang, P.; Gao, P.; Zhou, L.; Wen, G. Zirconia ultrafiltration membranes on silicon carbide substrate: Microstructure and water flux. J. Eur. Ceram. Soc. 2020, 40, 4290–4298. [Google Scholar] [CrossRef]
- Eray, E.; Candelario, V.M.; Boffa, V.; Safafar, H.; Østedgaard-Munck, D.N.; Zahrtmann, N.; Kadrispahic, H.; Jørgensen, M.K. A roadmap for the development and applications of silicon carbide membranes for liquid filtration: Recent advancements, challenges, and perspectives. Chem. Eng. J. 2021, 414, 128826. [Google Scholar] [CrossRef]
- Hotza, D.; di Luccio, M.; Wilhelm, M.; Iwamoto, Y.; Bernard, S.; da Costa, J.C.D. Silicon carbide filters and porous membranes: A review of processing, properties, performance and application. J. Memb. Sci. 2020, 610, 118193. [Google Scholar] [CrossRef]
- Chen, M.; Shang, R.; Sberna, P.M.; Luiten-Olieman, M.W.J.; Rietveld, L.C.; Heijman, S.G.J. Highly permeable silicon carbide-alumina ultrafiltration membranes for oil-in-water filtration produced with low-pressure chemical vapor deposition. Sep. Purif. Technol. 2020, 253, 117496. [Google Scholar] [CrossRef]
- Nagano, T.; Sato, K.; Kawahara, K. Gas permeation property of silicon carbide membranes synthesized by counter-diffusion chemical vapor deposition. Membranes 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Yu, X.; Sahimi, M.; Tsotsis, T.T. Highly permeable porous silicon carbide support tubes for the preparation of nanoporous inorganic membranes. J. Memb. Sci. 2014, 451, 192–204. [Google Scholar] [CrossRef]
- Dabir, S.; Deng, W.; Sahimi, M.; Tsotsis, T. Fabrication of silicon carbide membranes on highly permeable supports. J. Memb. Sci. 2017, 537, 239–247. [Google Scholar] [CrossRef]
- Zhou, Y.; Fukushima, M.; Miyazaki, H.; Yoshizawa, Y.i.; Hirao, K.; Iwamoto, Y.; Sato, K. Preparation and characterization of tubular porous silicon carbide membrane supports. J. Memb. Sci. 2011, 369, 112–118. [Google Scholar] [CrossRef]
- Elyassi, B.; Sahimi, M.; Tsotsis, T.T. Silicon carbide membranes for gas separation applications. J. Memb. Sci. 2007, 288, 290–297. [Google Scholar] [CrossRef]
- Wang, Q.; Yokoji, M.; Nagasawa, H.; Yu, L.; Kanezashi, M.; Tsuru, T. Microstructure evolution and enhanced permeation of SiC membranes derived from allylhydridopolycarbosilane. J. Memb. Sci. 2020, 612, 118392. [Google Scholar] [CrossRef]
- Elyassi, B.; Deng, W.; Sahimi, M.; Tsotsis, T.T. On the use of porous and nonporous fillers in the fabrication of silicon carbide membranes. Ind. Eng. Chem. Res. 2013, 52, 10269–10275. [Google Scholar] [CrossRef]
- König, K.; Boffa, V.; Buchbjerg, B.; Farsi, A.; Christensen, M.L.; Magnacca, G.; Yue, Y. One-step deposition of ultrafiltation SiC membranes on macroporous SiC supports. J. Memb. Sci. 2014, 472, 232–240. [Google Scholar] [CrossRef]
- Eray, E.; Boffa, V.; Jørgensen, M.K.; Magnacca, G.; Candelario, V.M. Enhanced fabrication of silicon carbide membranes for wastewater treatment: From laboratory to industrial scale. J. Memb. Sci. 2020, 606, 118080. [Google Scholar] [CrossRef]
- Bukhari, S.Z.A.; Ha, J.H.; Lee, J.; Song, I.H. Oxidation-bonded SiC membrane for microfiltration. J. Eur. Ceram. Soc. 2018, 38, 1711–1719. [Google Scholar] [CrossRef]
- Bukhari, S.Z.A.; Ha, J.H.; Lee, J.; Song, I.H. Effect of different heat treatments on oxidation-bonded SiC membrane for water filtration. Ceram. Int. 2018, 44, 14251–14257. [Google Scholar] [CrossRef]
- Fukushima, M.; Zhou, Y.; Miyazaki, H.; Yoshizawa, Y.I.; Hirao, K.; Iwamoto, Y.; Yamazaki, S.; Nagano, T. Microstructural characterization of porous silicon carbide membrane support with and without alumina additive. J. Am. Ceram. Soc. 2006, 89, 1523–1529. [Google Scholar] [CrossRef]
- Eom, J.; Kim, Y.; Song, I. Effects of the initial α-SiC content on the microstructure, mechanical properties, and permeability of macroporous silicon carbide ceramics. J. Eur. Ceram. Soc. 2012, 32, 1283–1290. [Google Scholar] [CrossRef]
- Ding, S.; Zhu, S.; Zeng, Y.; Jiang, D. Effect of Y2O3 addition on the properties of reaction-bonded porous SiC ceramics. Ceram. Int. 2006, 32, 461–466. [Google Scholar] [CrossRef]
- Fukushima, M.; Zhou, Y.; Yoshizawa, Y. Fabrication and microstructural characterization of porous SiC membrane supports with Al2O3–Y2O3 additives. J. Membr. Sci. 2009, 339, 78–84. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, W.; Jiang, Q.; Xu, P.; Zhong, Z.; Zhang, F.; Xing, W. Preparation of non-oxide SiC membrane for gas purification by spray coating. J. Memb. Sci. 2017, 540, 381–390. [Google Scholar] [CrossRef]
- Han, F.; Zhong, Z.; Zhang, F.; Xing, W.; Fan, Y. Preparation and characterization of SiC whisker-reinforced SiC porous ceramics for hot gas filtration. Ind. Eng. Chem. Res. 2015, 54, 226–232. [Google Scholar] [CrossRef]
- Han, F.; Xu, C.; Wei, W.; Zhang, F.; Xu, P.; Zhong, Z. Corrosion behaviors of porous reaction-bonded silicon carbide ceramics incorporated with CaO. Ceram. Int. 2018, 44, 12225–12232. [Google Scholar] [CrossRef]
- Qiao, H.; Feng, S.; Low, Z.; Chen, J.; Zhang, F.; Zhong, Z. Al-DTPA microfiber assisted formwork construction technology for high-performance SiC membrane preparation. J. Memb. Sci. 2020, 594, 117464. [Google Scholar] [CrossRef]
- Yang, Y.; Han, F.; Xu, W.; Wang, Y.; Zhong, Z.; Xing, W. Low-temperature sintering of porous silicon carbide ceramic support with SDBS as sintering aid. Ceram. Int. 2017, 43, 3377–3383. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhou, J.; Miao, Y.; Yang, S.; Zhou, M.; Zhong, Z.; Xing, W. Lower-temperature preparation of SiC ceramic membrane using zeolite residue as sintering aid for oil-in-water separation. J. Memb. Sci. 2020, 610, 118238. [Google Scholar] [CrossRef]
- Li, S.; Wei, C.; Zhou, L.; Wang, P.; Xie, Z. Evaporation-condensation derived silicon carbide membrane from silicon carbide particles with different sizes. J. Eur. Ceram. Soc. 2019, 39, 1781–1787. [Google Scholar] [CrossRef]
- Riedel, H.S.R.; Passsing, G. Synthesis of dense silicon-based ceramics at low temperatures. Nature 1992, 355, 714–717. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Wei, C.; Wang, P.; Gao, P.; Zhou, L.; Wen, G. One step co-sintering of silicon carbide ceramic membrane with the aid of boron carbide. J. Eur. Ceram. Soc. 2021, 41, 1181–1188. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1996; pp. 286–300. [Google Scholar]
- Facciotti, M.; Boffa, V.; Magnacca, G.; Bjerg, L.; Kjær, P.; Farsi, A.; König, K.; Lykkegaard, M.; Yue, Y. Deposition of thin ultrafiltration membranes on commercial SiC microfiltration tubes. Ceram. Int. 2014, 40, 3277–3285. [Google Scholar] [CrossRef]
- Zhang, Y.; Binner, J. In-situ surface modification of silicon carbide particles using Al3+ complexes and polyelectrolytes in aqueous suspensions. J. Am. Ceram. Soc. 2002, 85, 529–534. [Google Scholar] [CrossRef]
- Hofmann, T.; von der Kammer, F. Particles in Water: Properties and Processes; CRC Press: New York, NY, USA, 2007; p. 3611. [Google Scholar]
- Candelario, V.M.; Moreno, R.; Shen, Z.; Guiberteau, F.; Ortiz, A.L. Liquid-phase assisted spark-plasma sintering of SiC nanoceramics and their nanocomposites with carbon nanotubes. J. Eur. Ceram. Soc. 2017, 37, 1929–1936. [Google Scholar] [CrossRef]
- Raju, K.; Yoon, D.H. Sintering additives for SiC based on the reactivity: A review. Ceram. Int. 2016, 42, 17947–17962. [Google Scholar] [CrossRef]
- Gu, Q.; Ng, T.C.A.; Bao, Y.; Ng, H.Y.; Tan, S.C.; Wang, J. Developing better ceramic membranes for water and wastewater Treatment: Where microstructure integrates with chemistry and functionalities. Chem. Eng. J. 2021, 428, 130456. [Google Scholar] [CrossRef]
- Ortiz, A.L.; Sánchez-Bajo, F.; Cumbrera, F.L.; Guiberteau, F. X-ray powder diffraction analysis of a silicon carbide-based ceramic. Mater. Lett. 2001, 49, 137–145. [Google Scholar] [CrossRef]
- Roque-Ruiz, J.H.; Medellín-Castillo, N.A.; Reyes-López, S.Y. Fabrication of α-alumina fibers by sol-gel and electrospinning of aluminum nitrate precursor solutions. Results Phys. 2019, 12, 193–204. [Google Scholar] [CrossRef]
- Myronyuk, I.F.; Mandzyuk, V.I.; Sachko, V.M.; Gun’ko, V.M. Structural and morphological features of disperse alumina synthesized using aluminum nitrate nonahydrate. Nanoscale Res. Lett. 2016, 11, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, D.; Baitalik, S.; Haldar, B.; Saha, R.; Kayal, N. Preparation and characterization of macroporous SiC ceramic membrane for treatment of wastewater. J. Porous Mater. 2018, 25, 1183–1193. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, Y.W.; Song, I.H. Processing and properties of glass-bonded silicon carbide membrane supports. J. Eur. Ceram. Soc. 2017, 37, 1225–1232. [Google Scholar] [CrossRef]
- Cifuentes-Cabezas, M.; Carbonell-Alcaina, C.; Vincent-Vela, M.C.; Mendoza-Roca, J.A.; Álvarez-Blanco, S. Comparison of different ultrafiltration membranes as first step for the recovery of phenolic compounds from olive-oil washing wastewater. Process Saf. Environ. Prot. 2021, 149, 724–734. [Google Scholar] [CrossRef]
- Rasouli, S.; Rezaei, N.; Hamedi, H.; Zendehboudi, S.; Duan, X. Superhydrophobic and superoleophilic membranes for oil-water separation application: A comprehensive review. Mater. Des. 2021, 204, 109599. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, T.; Sun, H.; Wang, W. Preparation and application of titanium dioxide dynamic membranes in microfiltration of oil-in-water emulsions. Sep. Purif. Technol. 2012, 89, 78–83. [Google Scholar] [CrossRef]
- Fraga, M.C.; Sanches, S.; Pereira, V.J.; Crespo, J.G.; Yuan, L.; Marcher, J.; de Yuso, M.M.; Rodríguez-Castellón, E.; Benavente, J. Morphological, chemical surface and filtration characterization of a new silicon carbide membrane. J. Eur. Ceram. Soc. 2017, 37, 899–905. [Google Scholar] [CrossRef]
- Fraga, M.C.; Sanches, S.; Crespo, G.; Pereira, V.J. Assessment of a new silicon carbide tubular honeycomb membrane for treatment of olive mill wastewaters. Membranes 2017, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Xu, C.; Rakesh, K.P.; Cui, Y.; Yin, J.; Chen, C.; Wang, S.; Chen, B.; Zhu, L. Hydrophilic SiC hollow fiber membranes for low fouling separation of oil-in-water emulsions with high flux. RSC Adv. 2020, 10, 4832–4839. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, S.; Abadi, H.; Reza, M.; Hemati, M.; Rekabdar, F.; Mohammadi, T. Ceramic membrane performance in microfiltration of oily wastewater. Desalination 2011, 265, 222–228. [Google Scholar]
- Zhou, J.; Chang, Q.; Wang, Y.; Wang, J.; Meng, G. Separation of stable oil–water emulsion by the hydrophilic nano-sized ZrO2 modified Al2O3 microfiltration membrane. Sep. Purif. Technol. 2010, 75, 243–248. [Google Scholar] [CrossRef]

| Membrane Material | Membrane Geometry | Pore Size [µm] | Operating Parameters | Oil Rejection (%) | Ref. |
|---|---|---|---|---|---|
| SiC | Disc | 0.4 | Constant permeate flux: 162 LMH | 98.52 | [32] |
| Cross-flow velocity: 1.3 m/s | |||||
| TMP: 0.5 bar | |||||
| SiC | Multi-channeled tubular | 0.269–0.282 | Cross-flow velocity: 0.5 m/s TMP: 1.5 bar | 93.8 | [51] |
| SiC | Multi-channeled tubular | 0.269–0.282 | Constant permeate flux: 67 LMH Cross-flow velocity: 2 m/s | 84 | [52] |
| SiC | Hollow fiber | 0.71 | Constant permeate flux: 103.9 LMH | 93.5 | [53] |
| Cross-flow velocity: 0.15 m/s TMP: 0.25 bar | |||||
| Al2O3 | Multi-channeled tubular | 0.2 | Cross-flow velocity: 2.25 m/s TMP: 1.25 bar | 85 | [54] |
| ZrO2-Al2O3 | Tubular | 0.2 | Cross-flow velocity: 5 m/s TMP: 0.16 bar | 97.8 | [55] |
| TiO2 | Tubular | 1.4–2.8 | Constant permeate flux: 599.7 LMH Cross-flow velocity: 1.13 m/s | 99.1 | [46] |
| This work | Multi-channeled tubular | 0.35 | Constant permeate flux: 150 LMH Cross-flow: 1527 L/h TMP: 0.08 bar | 99.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eray, E.; Candelario, V.M.; Boffa, V. Ceramic Processing of Silicon Carbide Membranes with the Aid of Aluminum Nitrate Nonahydrate: Preparation, Characterization, and Performance. Membranes 2021, 11, 714. https://doi.org/10.3390/membranes11090714
Eray E, Candelario VM, Boffa V. Ceramic Processing of Silicon Carbide Membranes with the Aid of Aluminum Nitrate Nonahydrate: Preparation, Characterization, and Performance. Membranes. 2021; 11(9):714. https://doi.org/10.3390/membranes11090714
Chicago/Turabian StyleEray, Esra, Victor Manuel Candelario, and Vittorio Boffa. 2021. "Ceramic Processing of Silicon Carbide Membranes with the Aid of Aluminum Nitrate Nonahydrate: Preparation, Characterization, and Performance" Membranes 11, no. 9: 714. https://doi.org/10.3390/membranes11090714
APA StyleEray, E., Candelario, V. M., & Boffa, V. (2021). Ceramic Processing of Silicon Carbide Membranes with the Aid of Aluminum Nitrate Nonahydrate: Preparation, Characterization, and Performance. Membranes, 11(9), 714. https://doi.org/10.3390/membranes11090714