Photocatalytic Nanofiber Membranes for the Degradation of Micropollutants and Their Antimicrobial Activity: Recent Advances and Future Prospects
Abstract
:1. Introduction
2. Photocatalytic Electrospun Nanofiber Membranes
2.1. Photocatalytic NanoMaterials and Electrospun Nanofiber Membranes
| Photocatalytic Materials | Crystal Structure | Eg (eV) | λe (nm) | ECB (V vs. NHE) | EVB (V vs. NHE) | Ref. |
|---|---|---|---|---|---|---|
| TiO2 | Tetragonal | 3.2 | <388 | −0.16 | +3.04 | [53,54,55] |
| ZnO | Hexagonal | 3.4 | <388 | +0.21 | +3.41 | [56,57] |
| CeO2 | Cubic | 3.2 | <388 | −0.07 | +3.13 | [58] |
| ZrO2 | Monoclinic | 5.0 | <248 | −0.69 | +4.31 | [59,60] |
| SnO2 | Tetragonal | 3.5 | <354 | +0.25 | +3.75 | [61,62,63] |
| WO3 | Monoclinic | 2.7 | <443 | +0.77 | +3.47 | [64,65] |
| α-Fe2O3 | Trigonal | 2.2 | <564 | +0.79 | +2.99 | [66,67] |
| BiVO4 | Monoclinic | 2.4 | <517 | +0.49 | +2.89 | [68,69] |
| SrTiO3 | Cubic | 3.4 | <365 | −0.75 | +2.65 | [70,71,72] |
| Ag3PO4 | Cubic | 2.4 | <517 | +0.50 | +2.90 | [73,74] |
| CdS | Hexagonal | 2.4 | <517 | −0.40 | +2.00 | [75] |
| g-C3N4 | 2D | 2.7 | <459 | −0.90 | +1.80 | [76,77,78,79] |
2.2. Coupled and Hybrid Photocatalytic Nanofibers
3. Antimicrobial Membranes
4. Other Applications of Hybrid Photocatalytic Membrane Processes
4.1. Filtration
4.2. Adsorption Technology
4.3. Electrocatalysis
5. Challenges and Future Direction
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipczynska-kochany, E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere 2018, 202, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Mart, C.A.; Rodrigo, M.A.; Sire, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef]
- Bi, O.; Charles, L.; Casado, C.; Marugán, J.; Septien, S.; Ndlovu, T.; Nsikayezwe, L. Photocatalytic degradation of atrazine in aqueous solution using hyperbranched polyethyleneimine templated morphologies of BiVO4 fused. J. Environ. Chem. Eng. 2020, 8, 104215. [Google Scholar] [CrossRef]
- Nieto-delgado, C.; Partida-gutierrez, D.; Rangel-mendez, J.R. Preparation of activated carbon cloths from renewable natural fabrics and their performance during the adsorption of model organic and inorganic pollutants in water. J. Clean. Prod. 2019, 213, 650–658. [Google Scholar] [CrossRef]
- Cai, W.; Yu, J.; Jaroniec, M. Template-free synthesis of hierarchical spindle-like g-Al2O3 materials and their adsorption affinity towards organic and inorganic pollutants in water. J. Mater. Chem. 2010, 20, 4587–4594. [Google Scholar] [CrossRef]
- Rivas, B.L.; Urbano, B.F.; Sánchez, J. Water-Soluble and Insoluble Polymers, Nanoparticles, Nanocomposites and Hybrids With Ability to Remove Hazardous Inorganic Pollutants in Water. Front. Chem. 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidoo, S.; Olaniran, A.O. Treated wastewater effluent as a source of microbial pollution of surface water resources. Int. J. Environ. Res. Public Health 2014, 11, 249–270. [Google Scholar] [CrossRef] [Green Version]
- Elliott, M. Biological pollutants and biological pollution—An increasing cause for concern. Mar. Pollut. Bull. 2003, 46, 275–280. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Tisa, F.; Raman, A.A.A.; Wan Daud, W.M.A. Applicability of fluidized bed reactor in recalcitrant compound degradation through advanced oxidation processes: A review. J. Environ. Manag. 2014, 146, 260–275. [Google Scholar] [CrossRef]
- Chabalala, M.B.; Seshabela, B.C.; Van Hulle, S.W.H.; Mamba, B.B.; Mhlanga, S.D.; Nxumalo, E.N. Cyclodextrin-Based Nanofibers and Membranes: Fabrication, Properties and Applications. In Cyclodextrin: A Versatile Ingredient; IntechOpen: London, UK, 2018; p. 29. ISBN 978-953-51-0246-5. [Google Scholar]
- Lin, T.; Wang, H.; Wang, H.; Wang, X. The charge effect of cationic surfactants on the elimination of fibre beads in the electrospinning of polystyrene. Nanotechnology 2004, 15, 1375–1381. [Google Scholar] [CrossRef]
- Kamiyama, M.; Soeda, T.; Nagajima, S.; Tanaka, K. Development and application of high-strength polyester nanofibers. Polym. J. 2012, 44, 987–994. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Wang, Z.; Sun, N.; Wang, J.; Wang, S. Performance improvement of polysulfone ultrafiltration membrane by blending with polyaniline nanofibers. J. Memb. Sci. 2008, 320, 363–371. [Google Scholar] [CrossRef]
- Liao, K.; Li, S. Interfacial characteristics of a carbon nanotube-polystyrene composite system. Appl. Phys. Lett. 2001, 79, 4225–4227. [Google Scholar] [CrossRef]
- An, H.; Shin, C.; Chase, G.G. Ion exchanger using electrospun polystyrene nanofibers. J. Memb. Sci. 2006, 283, 84–87. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, J.; Zhao, Y.; Qiu, Y.; Li, J. Room temperature ionic liquid based polystyrene nanofibers with superhydrophobicity and conductivity produced by electrospinning. Chem. Mater. 2008, 20, 3420–3424. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Dhakal, H.N.; Zhang, Z.Y.; Richardson, M.O.W. Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites. Compos. Sci. Technol. 2007, 67, 1674–1683. [Google Scholar] [CrossRef]
- Mishra, S.; Mohanty, A.K.; Drzal, L.T.; Misra, M.; Parija, S.; Nayak, S.K.; Tripathy, S.S. Studies on mechanical performance of biofibre/glass reinforced polyester hybrid composites. Compos. Sci. Technol. 2003, 63, 1377–1385. [Google Scholar] [CrossRef]
- Gopal, R.; Kaur, S.; Feng, C.Y.; Chan, C.; Ramakrishna, S.; Tabe, S.; Matsuura, T. Electrospun nanofibrous polysulfone membranes as pre-filters: Particulate removal. J. Memb. Sci. 2007, 289, 210–219. [Google Scholar] [CrossRef]
- Babaeijandaghi, F.; Shabani, I.; Seyedjafari, E.; Naraghi, Z.S.; Vasei, M.; Haddadi-Asl, V.; Hesari, K.K.; Soleimani, M. Accelerated epidermal regeneration and improved dermal reconstruction achieved by polyethersulfone nanofibers. Tissue Eng. Part A 2010, 16, 3527–3536. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Huang, X.; Deng, C.; Chen, X.; Qian, Y. Hydrothermal synthesis of ZnO nanowires and nanobelts on a large scale. Mater. Chem. Phys. 2007, 106, 58–62. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Zhang, Y.; Wei, Q.; Sun, S.; Zhao, C. Polyethersulfone nanofibers for the removal of endocrine disruptors. Desalin. Water Treat. 2012, 29, 158–163. [Google Scholar] [CrossRef]
- Ahmadiannamini, P.; Li, X.; Goyens, W.; Joseph, N.; Meesschaert, B.; Vankelecom, I.F.J. Multilayered polyelectrolyte complex based solvent resistant nanofiltration membranes prepared from weak polyacids. J. Memb. Sci. 2012, 394–395, 98–106. [Google Scholar] [CrossRef]
- Feng, C.; Xu, J.; Li, M.; Tang, Y.; Gao, C. Studies on a novel nanofiltration membrane prepared by cross-linking of polyethyleneimine on polyacrylonitrile substrate. J. Memb. Sci. 2014, 451, 103–110. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Dubey, N.C.; Subair, R.; Choudhury, S.; Stamm, M. Enhanced hydrophilic and antifouling polyacrylonitrile membrane with polydopamine modified silica nanoparticles. RSC Adv. 2016, 6, 4448–4457. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Wan, L.S.; Xu, Z.K. Structure and performance of polyacrylonitrile membranes prepared via thermally induced phase separation. J. Memb. Sci. 2012, 409–410, 355–364. [Google Scholar] [CrossRef]
- Zhi, S.H.; Deng, R.; Xu, J.; Wan, L.S.; Xu, Z.K. Composite membranes from polyacrylonitrile with poly(N,N-dimethylaminoethyl methacrylate)-grafted silica nanoparticles as additives. React. Funct. Polym. 2015, 86, 184–190. [Google Scholar] [CrossRef]
- Arshad, S.N.; Naraghi, M.; Chasiotis, I. Strong carbon nanofibers from electrospun polyacrylonitrile. Carbon 2011, 49, 1710–1719. [Google Scholar] [CrossRef]
- Wang, T.; Kumar, S. Electrospinning of polyacrylonitrile nanofibers. J. Appl. Polym. Sci. 2006, 102, 1023–1029. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Ghaee, A.; Shariaty-Niassar, M.; Barzin, J.; Matsuura, T. Effects of chitosan membrane morphology on copper ion adsorption. Chem. Eng. J. 2010, 165, 46–55. [Google Scholar] [CrossRef]
- Miao, Y.E.; Fan, W.; Chen, D.; Liu, T. High-performance supercapacitors based on hollow polyaniline nanofibers by electrospinning. ACS Appl. Mater. Interfaces 2013, 5, 4423–4428. [Google Scholar] [CrossRef]
- Lubasova, D.; Niu, H.; Zhao, X.; Lin, T. Hydrogel properties of electrospun polyvinylpyrrolidone and polyvinylpyrrolidone/poly(acrylic acid) blend nanofibers. RSC Adv. 2015, 5, 54481–54487. [Google Scholar] [CrossRef]
- Vazquez, B.; Vasquez, H.; Lozano, K. Preparation and Characterization of Polyvinylidene Fluoride Nanofibrous Membranes by Forcespinning. Polym. Eng. Sci. 2012, 52, 1–10. [Google Scholar] [CrossRef]
- Sinha, M.K.; Purkait, M.K. Use of CS-PAA nanoparticles as an alternative to metal oxide nanoparticles and their effect on fouling mitigation of a PSF ultrafiltration membrane. RSC Adv. 2015, 5, 66109–66121. [Google Scholar] [CrossRef]
- Adout, A.; Kang, S.; Asatekin, A.; Mayes, A.M.; Elimelech, M. Ultrafiltration membranes incorporating amphiphilic comb copolymer additives prevent irreversible adhesion of bacteria. Environ. Sci. Technol. 2010, 44, 2406–2411. [Google Scholar] [CrossRef] [PubMed]
- Yar, A.; Haspulat, B.; Ustun, T.; Eskizeybek, V.; Avcı, A.; Kamış, H.; Achour, S. Electrospun TiO2/ZnO/PAN hybrid nanofiber membranes with efficient photocatalytic activity. RSC Adv. 2017, 7, 29806–29814. [Google Scholar] [CrossRef] [Green Version]
- Reddy, A.V.R.; Patel, H.R. Chemically treated polyethersulfone/polyacrylonitrile blend ultrafiltration membranes for better fouling resistance. Desalination 2008, 221, 318–323. [Google Scholar] [CrossRef]
- Mandal, T.; Maity, S.; Dasgupta, D.; Datta, S. Advanced oxidation process and biotreatment: Their roles in combined industrial wastewater treatment. Desalination 2010, 250, 87–94. [Google Scholar] [CrossRef]
- Yoon, K.; Kim, K.; Wang, X.; Fang, D.; Hsiao, B.S.; Chu, B. High flux ultrafiltration membranes based on electrospun nanofibrous PAN scaffolds and chitosan coating. Polymer 2006, 47, 2434–2441. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Yao, M.; Ren, J. Electrospinning for Membrane Fabrication: Strategies and Applications. In Comprehensive Membrane Science and Engineering; Elsevier: Oxford, UK, 2017; ISBN 9780124095472. [Google Scholar]
- Xu, Y.; Lin, W.; Wang, H.; Guo, J.; Yuan, D.; Bao, J.; Sun, S.; Zhao, W.; Zhao, C. Dual-functional polyethersulfone composite nanofibrous membranes with synergistic adsorption and photocatalytic degradation for organic dyes. Compos. Sci. Technol. 2020, 199, 108353–108363. [Google Scholar] [CrossRef]
- Anwar, S.; Nabeela, A.; Sundarrajan, S.; Abdulrahim, S.; Nizar, S.; Balamurugan, R.; Ramakrishna, S. Advancement in electrospun nanofibrous membranes modification and their application in water treatment. Membranes 2013, 3, 266–284. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous Photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds: A review. Catal. Today 2017, 281, 144–164. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Khumalo, N.; Dlamini, D.; Mamba, B.B. Polysulfone/N, Pd co-doped TiO2 composite membranes for photocatalytic dye degradation. Sep. Purif. Technol. 2018, 191, 122–133. [Google Scholar] [CrossRef]
- Fernández-ramos, C.; Šatínský, D.; Šmídová, B.; Solich, P. Trends in Analytical Chemistry Analysis of trace organic compounds in environmental, food and biological matrices using large-volume sample injection in column- switching liquid chromatography. Trends Anal. Chem. 2014, 62, 69–85. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.K.; Diniz, J.C. Recent progresses on fabrication of photocatalytic membranes for water treatment. Catal. Today 2014, 230, 47–54. [Google Scholar] [CrossRef]
- Liu, Z.; Miao, Y.; Liu, M.; Ding, Q.; Weei, W.; Cui, X.; Liu, T. Flexible polyaniline-coated TiO2/SiO2 nanofiber membranes with enhanced visible-light photocatalytic degradation performance. J. Colloid Interface Sci. 2014, 424, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Wan, L.S.; Liu, Z.M.; Huang, X.J.; Xu, Z.K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 For Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Georgieva, J.; Valova, E.; Armyanov, S.; Philippidis, N.; Poulios, I.; Sotiropoulos, S. Bi-component semiconductor oxide photoanodes for the photoelectrocatalytic oxidation of organic solutes and vapours: A short review with emphasis to TiO2-WO3 photoanodes. J. Hazard. Mater. 2012, 211–212, 30–46. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Do, S.; Avrutin, V. A comprehensive review of ZnO materials and devices. Appl. Phys. Rev. 2018, 98, 54–84. [Google Scholar] [CrossRef] [Green Version]
- Boon, C.; Yong, L.; Wahab, A. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, S.; Wen, T.; Gu, P.; Li, L.; Zhao, G.; Niu, F. A critical review on visible-light-response CeO2 -based photocatalysts with enhanced photooxidation of organic pollutants. Catal. Today 2019, 335, 20–30. [Google Scholar] [CrossRef]
- Kubiak, A.; Siwinska-Ciesielczyk, K.; Jesionowski, T. Titania-Based Hybrid Materials with ZnO, ZrO2 and MoS2: A Review. Materials 2018, 11, 2295. [Google Scholar] [CrossRef] [Green Version]
- Reddy, B.M.; Khan, A. Recent Advances on TiO2-ZrO2 Mixed Oxides as Catalysts and Catalyst Supports. Catal. Rev. 2007, 4940. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. Progress in Materials Science SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Xiong, L.; Guo, Y.; Wen, J.; Liu, H.; Yang, G.; Qin, P. Review on the Application of SnO2 in Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1802757. [Google Scholar] [CrossRef]
- Varela, A.; Longo, E.; Bueno, P.R. SnO2, ZnO and related polycrystalline compound semiconductors: An overview and review on the voltage-dependent resistance (non-ohmic) feature. J. Eur. Ceram. Soc. 2008, 28, 505–529. [Google Scholar] [CrossRef]
- Buch, V.R.; Kumar, A.; Rawal, S.K. Review on electrochromic property for WO3 thin films usingdifferent deposition techniques. Mater. Today Proc. 2016, 3, 1429–1437. [Google Scholar] [CrossRef]
- Kalanoor, B.S.; Seo, H.; Kalanur, S.S. Recent developments in photoelectrochemical water-splitting using WO3/BiVO4 heterojunction photoanode: A review. Mater. Sci. Energy Technol. 2018, 1, 49–62. [Google Scholar] [CrossRef]
- Zhou, F.; Zeng, L.; Guo, W. Enhanced performance of alpha—Fe2O3 nanoparticles with optimized graphene coated layer as anodes for lithium-ion batteries. Energy Res. 2019, 7095–7106. [Google Scholar] [CrossRef]
- Ahmad, W.R.W.; Mamat, M.H.; Zoolfakar, A.S.; Khusaimi, Z.; Rusop, M. A Review on Hematite α-Fe2O3 Focusing on Nanostructures, Synthesis Methods and Applications. In Proceedings of the 2016 IEEE Student Conference on Research and Development (SCOReD), Kuala Lumpur, Malaysia, 13–14 December 2016; pp. 1–6. [Google Scholar]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar] [CrossRef]
- Tan, H.L.; Amal, R. Alternative strategies in improving the photocatalytic and photoelectrochemical activities of visible light-driven BiVO4: A review. J. Mater. Chem. A 2017, 16498–16521. [Google Scholar] [CrossRef]
- Lee, B.; Chin, P.; Lai, W.; Ching, J.; Loke, P.; Wei, S.; Chen, H. A review of synthesis and morphology of SrTiO3 for energy and other applications. Energy Res. 2019, 5151–5174. [Google Scholar] [CrossRef]
- Hayward, S.A.; Salje, E.K.H. Cubic-tetragonal phase transition in SrTiO3 revisited: Landau theory and transition mechanism. Phase Transit. 2006, 1594. [Google Scholar] [CrossRef]
- Christensen, D.V.; Trier, F.; Niu, W.; Gan, Y.; Zhang, Y.; Jespersen, T.S.; Chen, Y.; Pryds, N. Stimulating Oxide Heterostructures: A Review on Controlling SrTiO3 -Based Heterointerfaces with External Stimuli. Adv. Mater. Interfaces 2019, 1900772, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Dai, Y.; Wang, X. Methods and mechanism for improvement of photocatalytic activity and stability of Ag3PO4: A review. J. Alloys Compd. 2015, 649, 910–932. [Google Scholar] [CrossRef]
- Al, M.; Sen, S.; Chakrabortty, D. Ag3PO4-based nanocomposites and their applications in photodegradation of toxic organic dye contaminated wastewater: Review on material design to performance enhancement. J. Saudi Chem. Soc. 2019, 24, 20–41. [Google Scholar] [CrossRef]
- Ibrahim, I.; Lim, H.N.; Zawawi, R.M.; Tajudin, A.A.; Ng, Y.H.; Guo, H.; Huang, N.M. A review on visible-light induced photoelectrochemical sensors based on CdS nanoparticles. J. Mater. Chem. B 2018, 4551–4568. [Google Scholar] [CrossRef]
- Ye, S.; Wang, R.; Wu, M.; Yuan, Y. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4 -based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Raghava, K.; Aminabhavi, T.M. Graphitic carbon nitride (g-C3N4) based metal-free photocatalysts for water splitting: A review. Carbon 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Bai, S.; Gao, C.; Low, J.; Xiong, Y. Crystal phase engineering on photocatalytic materials for energy and environmental applications. Nano Res. 2019, 12, 2031–2054. [Google Scholar] [CrossRef]
- Ren, H.; Koshy, P.; Chen, W.; Qi, S.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef]
- Park, J.A.; Moon, J.; Lee, S.J.; Lim, S.C.; Zyung, T. Fabrication and characterization of ZnO nanofibers by electrospinning. Curr. Appl. Phys. 2009, 9, S210–S212. [Google Scholar] [CrossRef]
- Yang, X.; Shao, C.; Guan, H.; Li, X.; Gong, J. Preparation and characterization of ZnO nanofibers by using electrospun PVA/zinc acetate composite fiber as precursor. Inorg. Chem. Commun. 2004, 7, 176–178. [Google Scholar] [CrossRef]
- Di, A.; Zimbone, M.; Elena, M.; Impellizzeri, G. Synthesis of ZnO nano fi bers by the electrospinning process. Mater. Sci. Semicond. Process. 2016, 42, 98–101. [Google Scholar] [CrossRef]
- Chuangchote, S.; Jitputti, J.; Sagawa, T.; Yoshikawa, S. Photocatalytic activity for hydrogen evolution of electrospun TiO2 nanofibers. ACS Appl. Mater. Interfaces 2009, 1, 1140–1143. [Google Scholar] [CrossRef]
- Li, L.; Sun, X.; Yang, Y.; Guan, N.; Zhang, F. Synthesis of anatase TiO2 nanoparticles with β-cyclodextrin as a supramolecular shell. Chem. Asian J. 2006, 1, 664–668. [Google Scholar] [CrossRef]
- Wang, H.Y.; Yang, Y.; Li, X.; Li, L.J.; Wang, C. Preparation and characterization of porous TiO2/ZnO composite nanofibers via electrospinning. Chin. Chem. Lett. 2010, 21, 1119–1123. [Google Scholar] [CrossRef]
- Hwang, S.H.; Song, J.; Jung, Y.; Kweon, O.Y.; Song, H.; Jang, J. Electrospun ZnO/TiO2 composite nanofibers as a bactericidal agent. Chem. Commun. 2011, 47, 9164–9166. [Google Scholar] [CrossRef]
- Manaf, O.; Anjana, K.; Prasanth, R.; Reshmi, C.R.; Juraij, K.; Rajesh, P.; Chingakham, Ch.; Sajith, V.; Sujith, A. ZnO decorated anti-bacterial electrspun ABS nanocomposite membrane for oil-water separation. Mater. Let. 2019, 256, 126626–126630. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Molinari, R.; Pirillo, F.; Falco, M.; Loddo, V.; Palmisano, L. Photocatalytic degradation of dyes by using a membrane reactor. Chem. Eng. Process. Process. Intesification 2004, 43, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Mendret, J.; Hatat-fraile, M.; Rivallin, M.; Brosillon, S. Hydrophilic composite membranes for simultaneous separation and photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2013, 111, 9–19. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Baker, M.A.; Grilli, R.; Lozzi, L.; Di, D.; Mamane, H. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: Influence of physical parameters. J. Hazard. Mater. 2016, 310, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Wang, F.; Qian, K.; Bao, H.; Jiang, Z.; Huang, W. Bifunctional N-Doped mesoporous TiO2 photocatalysts. J. Phys. Chem. C 2008, 112, 18150–18156. [Google Scholar] [CrossRef]
- Mohamed, A.; Ghobara, M.M.; Abdelmaksoud, M.K.; Mohamed, G.G. A novel and highly efficient photocatalytic degradation of malachite green dye via surface modified polyacrylonitrile nanofibers/biogenic silica composite nano fi bers. Sep. Purif. Technol. 2019, 210, 935–942. [Google Scholar] [CrossRef]
- Khalil, A.; Aboamera, N.M.; Nasser, W.S.; Mahmoud, W.H.; Mohamed, G.G. Photodegradation of organic dyes by PAN/SiO2-TiO2-NH2 nanofiber membrane under visible light. Sep. Purif. Technol. 2019, 224, 509–514. [Google Scholar] [CrossRef]
- Lei, J.; Wang, W.; Song, M.; Dong, B.; Li, Z.; Wang, C.; Li, L. Ag/AgCl coated polyacrylonitrile nanofiber membranes: Synthesis and photocatalytic properties. React. Funct. Polym. 2011, 71, 1071–1076. [Google Scholar] [CrossRef]
- Chen, Y.; Kuo, C.; Chen, B.; Chiu, P.; Tsai, P. Multifunctional polyacrylonitrile-ZnO/Ag electrospun nanofiber membranes with various ZnO morphologies for photocatalytic, UV-shielding, and antibacterial applications. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 262–269. [Google Scholar] [CrossRef]
- Zhang, G.; Sheng, H.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Hierarchical titanium dioxide nanowire/metal–organic framework/carbon nanofiber membranes for highly efficient photocatalytic degradation of hydrogen sulfide. Chem. Eur. J. 2018, 24, 15019–15025. [Google Scholar] [CrossRef]
- Sun, K.; Wang, L.; Wu, C.; Deng, J.; Pan, K. Fabrication of α -Fe2O3@rGO/PAN Nanofiber Composite Membrane for Photocatalytic Degradation of Organic Dyes. Adv. Mater. Interfaces 2017, 1700845, 2–8. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Bai, H.; Sun, D.D. Concurrent filtration and solar photocatalytic disinfection/degradation using high-performance Ag/TiO2 nanofiber membrane. Water Res. 2011, 46, 1101–1112. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, S.; Han, G. Fabrication and photocatalytic activity of TiO2 nanofiber membrane. Mater. Lett. 2009, 63, 1761–1763. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, S.; Wan, D.; Zhang, P.; Zhang, Z. Multilevel polarization- fi elds enhanced capture and photocatalytic conversion of particulate matter over flexible schottky-junction nano fi ber membranes. J. Hazard. Mater. 2020, 395, 1–10. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, L.; Li, H.; Wang, H.; Lang, J. One-pot growth of free-standing CNTs/TiO2 nanofiber membrane for enhanced photocatalysis. Mater. Lett. 2013, 95, 13–16. [Google Scholar] [CrossRef]
- Kayaci, F.; Cagla Ozgit-Akgun, N.B. and T.U. Surface-decorated ZnO nanoparticles and ZnO nanocoating on electrospun polymeric nanofibers by atomic layer deposition for flexible photocatalytic nanofibrous membranes. RSC Adv. 2013, 90, 6817–6820. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, C.; Gao, F.; Mailhot, G.; Pan, G. Algae decorated TiO2/Ag hybrid nanofiber membrane with enhanced photocatalytic activity for Cr (VI) removal under visible light. Chem. Eng. J. 2017, 314, 622–630. [Google Scholar] [CrossRef] [Green Version]
- Zinadini, S.; Rostami, S.; Vatanpour, V.; Jalilian, E. Preparation of antibiofouling polyethersulfone mixed matrix NF membrane using photocatalytic activity of ZnO/MWCNTs nanocomposite. J. Memb. Sci. 2017, 529, 133–141. [Google Scholar] [CrossRef]
- Hassanpour, A.; Nahar, S.; Tong, X.; Zhang, G.; Gauthier, M.A.; Sun, S. Photocatalytic interlayer spacing adjustment of a graphene oxide/zinc oxide hybrid membrane for efficient water filtration. Desalination 2020, 475, 114174. [Google Scholar] [CrossRef]
- Bai, H.; Zan, X.; Zhang, L.; Delai, D. Multi-functional CNT/ZnO/TiO2 nanocomposite membrane for concurrent filtration and photocatalytic degradation. Sep. Purif. Technol. 2015, 156, 922–930. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 1847–1868. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Yan, J.; Yu, J.; Sun, G.; Ding, B. Soft Zr-doped TiO2 nanofibrous membranes with enhanced photocatalytic activity for water purification. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ding, Q.; Miao, Y.; Liu, T. Morphology and photocatalytic property of hierarchical polyimide/ZnO fibers prepared via a direct ion-exchange process. ACS Appl. Mater. Interfaces 2013, 5, 5617–5622. [Google Scholar] [CrossRef]
- Rajeswari, A.; Vismaiya, S.; Pius, A. Preparation, characterization of nano ZnO-blended cellulose acetate-polyurethane membrane for photocatalytic degradation of dyes from water. Chem. Eng. J. 2017, 313, 928–937. [Google Scholar] [CrossRef]
- Wu, H.; Inaba, T.; Wang, Z.M.; Endo, T. Photocatalytic TiO2@CS-embedded cellulose nanofiber mixed matrix membrane. Appl. Catal. B Environ. 2020, 276, 119111. [Google Scholar] [CrossRef]
- Kanjwal, M.A.; Barakat, N.A.M.; Chronakis, I.S. Photocatalytic degradation of dairy effluent using AgTiO2 nanostructures/polyurethane nanofiber membrane. Ceram. Int. 2015, 41, 9615–9621. [Google Scholar] [CrossRef]
- Alias, N.H.; Jaafar, J.; Samitsu, S.; Matsuura, T.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Othman, N.H.; Abdullah, N.; Paiman, S.H.; et al. Photocatalytic nanofiber-coated alumina hollow fiber membranes for highly efficient oilfield produced water treatment. Chem. Eng. J. 2019, 360, 1437–1446. [Google Scholar] [CrossRef]
- An, S.; Lee, M.W.; Joshi, B.N.; Jo, A.; Jung, J.; Yoon, S.S. Water purification and toxicity control of chlorophenols by 3D nanofiber membranes decorated with photocatalytic titania nanoparticles. Ceram. Int. 2014, 40, 3305–3313. [Google Scholar] [CrossRef]
- Pan, T.; Liu, Y.; Li, Z.; Fan, J.; Wang, L.; Liu, J.; Shou, W. A Sm-doped Egeria-densa-like ZnO nanowires@PVDF nanofiber membrane for high-efficiency water clean. Sci. Total Environ. 2020, 737, 139818. [Google Scholar] [CrossRef]
- Sun, F.; Huang, S.Y.; Ren, H.T.; Li, T.T.; Zhang, Y.; Lou, C.W.; Lin, J.H. Core-sheath structured TiO2@PVDF/PAN electrospun membranes for photocatalysis and oil-water separation. Polym. Compos. 2020, 41, 1013–1023. [Google Scholar] [CrossRef]
- Pachepsky, Y.; Shelton, Ã.D.R.; Mclain, Ã.J.E.T.; Patel, J.; Mandrell, R.E. Irrigation Waters as a Source of Pathogenic Microorganisms in Produce: A Review; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 113, ISBN 9780123864734. [Google Scholar]
- Leclerc, H.; Schwartzbrod, L.; Leclerc, H.; Schwartzbrod, L. Microbial agents associated with waterborne diseases. Crit. Rev. Microbiol. 2008, 7828. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.R.; Colford, J.M.; Lechevallier, M.W.; Binder, S.; Berger, P.S.; Chapman, L. Waterborne Diseases Xenotransplantation: Benefits and Risks. Emerg. Infecious Dis. 2001, 7, 544–545. [Google Scholar] [CrossRef] [Green Version]
- Thomas, V.; Mcdonnell, G.; Denyer, S.P.; Maillard, J.Y. Free-living amoebae and their intracellular pathogenicmicroorganisms: Risks for water quality. FEMS Microbiol. Rev. 2010, 34, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Yu, Z.; Pan, Y.; Zeng, G.; Shi, H. Enhancing the photocatalytic and antibacterial property of polyvinylidene fluoride membrane by blending Ag–TiO2 nanocomposites. J. Mater. Sci. Mater. Electron. 2017, 28, 3865–3874. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Zhang, Y.; Tian, M.; He, T.; Liu, J.; Chen, V. Polymeric antimicrobial membranes enabled by nanomaterials for water treatment. J. Memb. Sci. 2018, 550, 173–197. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospinning of nanofibers from non-polymeric systems: Polymer-free nanofibers from cyclodextrin derivatives. Nanoscale 2012, 4, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Yang, F.; Yang, X. Excellent antimicrobial properties of mesoporous anatase TiO2 and Ag/TiO2 composite films. Microporous Mesoporous Mater. 2008, 114, 431–439. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Wang, W.; Wang, Y.; Gao, B.; Wang, Z. Enhanced antifouling and antimicrobial thin film nanocomposite membranes with incorporation of Palygorskite/titanium dioxide hybrid material. J. Colloid Interface Sci. 2019, 537, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hee, J.; Kumar, M.; Lee, J.; Hee, C.; Sang, C. Polydopamine-assisted immobilization of hierarchical zinc oxide nanostructures on electrospun nanofibrous membrane for photocatalysis and antimicrobial activity. J. Colloid Interface Sci. 2018, 513, 566–574. [Google Scholar] [CrossRef]
- Panthi, G.; Park, S.; Chae, S.; Kim, T.; Chung, H.; Hong, S.; Park, M.; Kim, H. Immobilization of Ag3PO4 nanoparticles on electrospun PAN nano fi bers via surface oximation: Bifunctional composite membrane with enhanced photocatalytic and antimicrobial activities. J. Ind. Eng. Chem. 2017, 45, 277–286. [Google Scholar] [CrossRef]
- Xu, Z.; Ye, S.; Zhang, G.; Li, W.; Gao, C.; Shen, C. Antimicrobial polysulfone blended ultra fi ltration membranes prepared with Ag/Cu2O hybrid nanowires. J. Membr. Sci. 2016, 509, 83–93. [Google Scholar] [CrossRef]
- Damodar, R.A.; You, S.; Chou, H. Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes. J. Hazard. Mater. 2009, 172, 1321–1328. [Google Scholar] [CrossRef]
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antimicrobial and antibiofilm efficacy of self-cleaning surfaces functionalized by TiO2 photocatalytic nanoparticles against Staphylococcus aureus and Pseudomonas putida. J. Hazard. Mater. 2017, 340, 160–170. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef]
- Bodaghi, H.; Mosto, Y.; Oromiehie, A.; Zamani, Z.; Ghanbarzadeh, B.; Costa, C.; Conte, A.; Alessandro, M.; Nobile, D. LWT-Food Science and Technology Evaluation of the photocatalytic antimicrobial effects of a TiO2 nanocomposite food packaging film by in vitro and in vivo tests. LWT-Food Sci. Technol. 2013, 50, 702–706. [Google Scholar] [CrossRef]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Luo, J.; Menkhaus, T.J.; Varadaraju, H.; Sun, Y.; Fong, H. Antimicrobial nano-fibrous membranes developed from electrospun polyacrylonitrile nanofibers. J. Memb. Sci. 2011, 369, 499–505. [Google Scholar] [CrossRef]
- Chaudhary, A.; Gupta, A.; Mathur, R.B.; Dhakate, S.R. Effective antimicrobial filter from electrospun polyacrylonitrile-silver composite nanofibers membrane for conducive environment. Adv. Mater. 2014, 10, 562–568. [Google Scholar] [CrossRef]
- Pan, S.; Ke, X.; Wang, T.; Liu, Q.; Zhong, L. Synthesis of Silver Nanoparticles Embedded Electrospun PAN Nanofiber Thin-Film Composite Forward Osmosis Membrane to Enhance Performance and Antimicrobial Activity. Ind. Eng. Chem. Res. 2019, 58, 984–993. [Google Scholar] [CrossRef]
- Cremar, L.; Gutierrez, J.; Martinez, J.; Materon, L.A.; Gilkerson, R.; Xu, F.; Lozano, K. Development of antimicrobial chitosan based nanofiber dressings for wound healing applications. Nanomed. J. 2020, 5, 6–14. [Google Scholar] [CrossRef]
- Mocanu, A.; Rusen, E.; Diacon, A.; Isopencu, G.; Must, G.; Raluca, Ş.; Dinescu, A. Antimicrobial properties of polysulfone membranes modified with carbon nanofibers and silver nanoparticles. Mater. Chem. Phys. 2019, 223, 39–45. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, S.; Hu, Q.; Xue, G.; Ni, Q.; Jiang, Q.; Qiu, Y. Antimicrobial three dimensional woven filters containing silver nanoparticle doped nanofibers in a membrane bioreactor for wastewater treatment. Sep. Purif. Technol. 2017, 175, 130–139. [Google Scholar] [CrossRef]
- Kwon, H.; Cha, J.; Lee, C.W. Preparation and Characterization of Antimicrobial Bilayer Electrospun Nanofiber Membrane for Oily Wastewater Treatment. J. Korean Phys. Soc. 2020, 76, 34–43. [Google Scholar] [CrossRef]
- Hashmi, M.; Ullah, S.; Kim, I.S. Copper oxide (CuO) loaded polyacrylonitrile (PAN) nano fi ber membranes for antimicrobial breath mask applications. Curr. Res. Biotechnol. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Dilamian, M.; Montazer, M.; Masoumi, J. Antimicrobial electrospun membranes of chitosan/poly (ethylene oxide) incorporating poly (hexamethylene biguanide) hydrochloride. Carbohydr. Polym. 2013, 94, 364–371. [Google Scholar] [CrossRef]
- Shi, R.; Geng, H.; Gong, M.; Ye, J.; Wu, C.; Hu, X.; Zhang, L. Long-acting and broad-spectrum antimicrobial electrospun poly(e-caprolactone)/gelatin micro/anofibers for wound dressing. J. Colloid Interface Sci. 2018, 509, 275–284. [Google Scholar] [CrossRef]
- Han, C.; Cai, N.; Chan, V.; Liu, M.; Feng, X.; Yu, F. Enhanced drug delivery, mechanical properties and antimicrobial activities in poly (lactic acid) nanofiber with mesoporous Fe3O4-COOH nanoparticles. Colloids Surfaces A 2018, 559, 104–114. [Google Scholar] [CrossRef]
- Han, D.; Sherman, S.; Filocamo, S.; Steckl, A.J. Long-term antimicrobial effect of nisin released from electrospun triaxial fiber membranes. Acta Biomater. 2017, 53, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, T.; Gao, Y.; Xu, Z.; Huang, C.; Yao, G.; Jiang, L.; Tang, Y.; Wang, X. Antimicrobial electrospun nanofibers of cellulose acetate and polyester urethane composite for wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100 B, 1556–1565. [Google Scholar] [CrossRef]
- Xue, J.; Niu, Y.; Gong, M.; Shi, R.; Chen, D.; Zhang, L.; Lvov, Y.; Materials, B.; Composites, I.; Technology, C.; et al. Electrospun Microfiber Membranes Embedded with Drug-Loaded Clay Nanotubes for Sustained Antimicrobial. ACS Nano 2015, 9, 1600–1612. [Google Scholar] [CrossRef]
- Woong, D.; Hwan, S.; Hwan, H.; Hoon, K.; Seok, C.; Hwan, Y. Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: Implications for wound healing. Acta Biomater. 2016, 39, 146–155. [Google Scholar] [CrossRef]
- Huang, Y.; Dan, N.; Dan, W.; Zhao, W.; Bai, Z.; Chen, Y.; Yang, C. Bilayered Antimicrobial Nano fiber Membranes for Wound Dressings via in Situ Cross-Linking Polymerization and Electrospinning. Ind. Eng. Chem. Res. 2018, 57, 17048–17057. [Google Scholar] [CrossRef]
- Tan, K.; Obendorf, S.K. Fabrication and evaluation of electrospun nanofibrous antimicrobial nylon 6 membranes. J. Memb. Sci. 2007, 305, 287–298. [Google Scholar] [CrossRef]
- Su, Y.; Mainardi, V.L.; Wang, H.; Mccarthy, A.; Zhang, Y.S.; Chen, S.; John, J.V.; Wong, S.L.; Hollins, R.R.; Wang, G.; et al. Dissolvable microneedles coupled with nanfiber dressings eradicate biofilms via effectively delivering a database designed antimicrobial peptite. ACS Nano 2020, 14, 11775–11786. [Google Scholar] [CrossRef]
- Kaur, S.; Sundarrajan, S.; Rana, D. Review: The characterization of electrospun nanofibrous liquid filtration membranes. J. Mater. Sci. 2014, 49, 6143–6159. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Romanos, G.E.; Katsaros, F.K.; Kordatos, K.; Likodimos, V.; Falaras, P. Very efficient composite titania membranes in hybrid ultrafiltration/photocatalysis water treatment processes. J. Memb. Sci. 2012, 392–393, 192–203. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Y.; Quan, X.; Fan, X.; Zhao, H. Performing a microfiltration integrated with photocatalysis using an Ag-TiO2/HAP/Al2O3 composite membrane for water treatment: Evaluating effectiveness for humic acid removal and anti-fouling properties. Water Res. 2010, 44, 6104–6114. [Google Scholar] [CrossRef]
- Zhang, X.; Jianhong, A.; Lee, P.; Delai, D.; Leckie, J.O. TiO2 nanowire membrane for concurrent filtration and photocatalytic oxidation of humic acid in water. J. Membr. Sci. 2008, 313, 44–51. [Google Scholar] [CrossRef]
- Moustakas, N.G.; Katsaros, F.K.; Kontos, A.G.; Romanos, G.E.; Dionysiou, D.D.; Falaras, P. Visible light active TiO2 photocatalytic filtration membranes with improved permeability and low energy consumption. Catal. Today 2014, 224, 56–69. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Morales-torres, S.; Likodimos, V.; Em, G.; Pastrana-martinez, L.M.; Falaras, P.; Dionysiou, D.D.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T. Prototype composite membranes of partially reduced graphene oxide/TiO2 for photocatalytic ultrafiltration water treatment under visible light. Appl. Catal. B Environ. 2014, 158–159, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-torres, S.; Pastrana-martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T.; Dona-rodriguez, J.M.; Em, G.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B Environ. 2015, 178, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Nyamutswa, L.T.; Zhu, B.; Navaratna, D.; Collins, S.; Duke, M.C. Proof of concept for light conducting membrane substrate for UV-activated photocatalysis as an alternative to chemical cleaning. Membranes 2018, 8, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Shao, J.; He, Y.; Liu, B.; Zhong, X. Natural organic matter removal and flux decline with PEG–TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. J. Memb. Sci. 2012, 405–406, 48–56. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z.; Sun, D.D. Nano gives the answer: Breaking the bottleneck of internal concentration polarization with a nanofiber composite forward osmosis membrane for a high water production rate. Adv. Mater. 2011, 23, 3256–3260. [Google Scholar] [CrossRef]
- Xu, Z.; Greiner, A. Preparation and performance assessment of low-pressure affinity membranes based on functionalized, electrospun polyacrylates for gold nanoparticle filtration. ACS Appl. Mater. Interfaces 2021, 13, 15659–15667. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, M.; Mi, B. Membrane surface modification with TiO2–graphene oxide for enhanced photocatalytic performance. J. Membr. Sci. 2014, 455, 349–356. [Google Scholar] [CrossRef]
- Liu, T.; Li, B.; Hao, Y.; Yao, Z. photocatalyst for wastewater treatment. Chem. Eng. J. 2014, 244, 382–390. [Google Scholar] [CrossRef]
- Molinari, R.; Pirillo, F.; Loddo, V.; Palmisano, L. Heterogeneous photocatalytic degradation of pharmaceuticals in water by using polycrystalline TiO2 and a nanofiltration membrane reactor. Catal. Today 2006, 118, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.; Zhang, C.; He, A.; Yang, S.; Wu, G.; Darling, S.B. Photocatalytic Nanofiltration Membranes with Self-Cleaning Property for Wastewater Treatment. Adv. Funct. Mater. 2017, 27, 1700251. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.; Zhang, Q.; Zhang, W.; Zhang, Q. Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Maleki, A.; Hayati, B.; Najafi, F.; Gharibi, F.; Joo, S.W. Heavy metal adsorption from industrial wastewater by PAMAM/TiO2 nanohybrid: Preparation, characterization and adsorption studies. J. Mol. Liq. 2016, 224, 95–104. [Google Scholar] [CrossRef]
- Huang, F.; Xu, Y.; Liao, S.; Yang, D.; Hsieh, Y.L.; Wei, Q. Preparation of amidoxime polyacrylonitrile chelating nanofibers and their application for adsorption of metal ions. Materials 2013, 6, 969–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foroozmehr, F.; Borhani, S.; Hosseini, S.A. Removal of Reactive Dyes from Wastewater using Cyclodextrin Functionalized Polyacrylonitrile Nanofibrous Membranes. J. Text. Polym. 2016, 4, 45–52. [Google Scholar]
- Georgieva, V.G.; Tavlieva, M.P.; Genieva, S.D.; Vlaev, L.T. Adsorption kinetics of Cr(VI) ions from aqueous solutions onto black rice husk ash. J. Mol. Liq. 2015, 208, 219–226. [Google Scholar] [CrossRef]
- Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon—A critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef]
- Kim, J.; Bruggen, B. Van Der The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef]
- Yang, X.; Yi, H.; Tang, X.; Zhao, S.; Yang, Z.; Ma, Y. Behaviors and kinetics of toluene adsorption-desorption on activated carbons with varying pore structure. J. Environ. Sci. 2017, 67, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Seredych, M.; Lison, J.; Jans, U.; Bandosz, T.J. Textural and chemical factors affecting adsorption capacity of activated carbon in highly efficient desulfurization of diesel fuel. Carbon 2009, 47, 2491–2500. [Google Scholar] [CrossRef]
- Verona, D.A.; Antizar-Ladislao, B.; Semiao, A. Harversting Microalgae with Ultrafiltration—Membrane Fouling and Flux Decline. Available online: https://sites.google.com/site/algaeultrafiltration/current-issues/fouling-and-flux-optimisation?tmpl=%2Fsystem%2Fapp%2Ftemplates%2Fprint%2F&showPrintDialog=1 (accessed on 26 June 2021).
- Zhang, X.; Fang, X.; Li, J.; Pan, S.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Zhao, S. Developing new adsorptive membranes by modification of support layer with iron oxide microspheres for arsenic removal. J. Colloid Interface Sci. 2018, 514, 760–768. [Google Scholar] [CrossRef]
- Balta, S.; Sotto, A.; Luis, P.; Benea, L.; Van Der Bruggen, B.; Kim, J. A new outlook on membrane enhancement with nanoparticles: The alternative of ZnO. J. Memb. Sci. 2012, 389, 155–161. [Google Scholar] [CrossRef]
- Koushkbaghi, S.; Zakialamdari, A.; Pishnamazi, M. Aminated-Fe3O4 nanoparticles fi lled chitosan/PVA/PES dual layers nano fi brous membrane for the removal of Cr (VI) and Pb (II) ions from aqueous solutions in adsorption and membrane processes. Chem. Eng. J. 2018, 337, 169–182. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Mudhoo, A.; Bhatnagar, A.; Rantalankila, M.; Srivastava, V.; Sillanpää, M. Endosulfan removal through bioremediation, photocatalytic degradation, adsorption and membrane separation processes: A review. Chem. Eng. J. 2019, 360, 912–928. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, J.; Sun, P.; Liu, J.; Ji, Y.; Nakada, N.; Qiao, Z.; Tanaka, H.; Yang, Y. Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: Systematic review and bibliometric analysis. Sci. Total Environ. 2018, 627, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions—A Review. Int. J. Hydrog. Energy 2014, 40, 256–274. [Google Scholar] [CrossRef]
- Yuan, N.; Jiang, Q.; Li, J.; Tang, J. A review on non-noble metal based electrocatalysis for the oxygen evolution reaction. Arab. J. Chem. 2020, 13, 4294–4309. [Google Scholar] [CrossRef]
- Tang, C.; Titirici, M.; Zhang, Q. A review of nanocarbons in energy electrocatalysis: Multifunctional substrates and highly active sites. J. Energy Chem. 2017, 26, 1077–1093. [Google Scholar] [CrossRef] [Green Version]
- Tarasevich, M.R.; Korchagin, O.V. Electrocatalysis and pH (a Review). Electrochim. Acta 2013, 49, 600–618. [Google Scholar] [CrossRef]
- Lu, L. Nanoporous noble metal-based alloys: A review on synthesis and applications to electrocatalysis and electrochemical sensing. Microchim. Acta 2019, 186, 664. [Google Scholar] [CrossRef]
- Griese, S.; Kampouris, D.K.; Kadara, R.O.; Banks, C.E. A Critical Review of the Electrocatalysis Reported at C 60 Modified Electrodes. Electroanalysis 2008, 20, 1507–1512. [Google Scholar] [CrossRef]
- Koper, M.T.M. Electrocatalysis on bimetallic and alloy surfaces. Inorg. Mater. Catal. 2004, 548, 1–3. [Google Scholar] [CrossRef]
- Zeradjanin, A.R.; Grote, J.; Polymeros, G.; Mayrhofer, K.J.J. A Critical Review on Hydrogen Evolution Electrocatalysis: Re-exploring the Volcano-relationship. Electroanalysis 2016, 28, 2256–2269. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Weststrate, C.J.K.; Fredriksson, H.O.A.; Niemantsverdriet, J.W.H. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef] [Green Version]

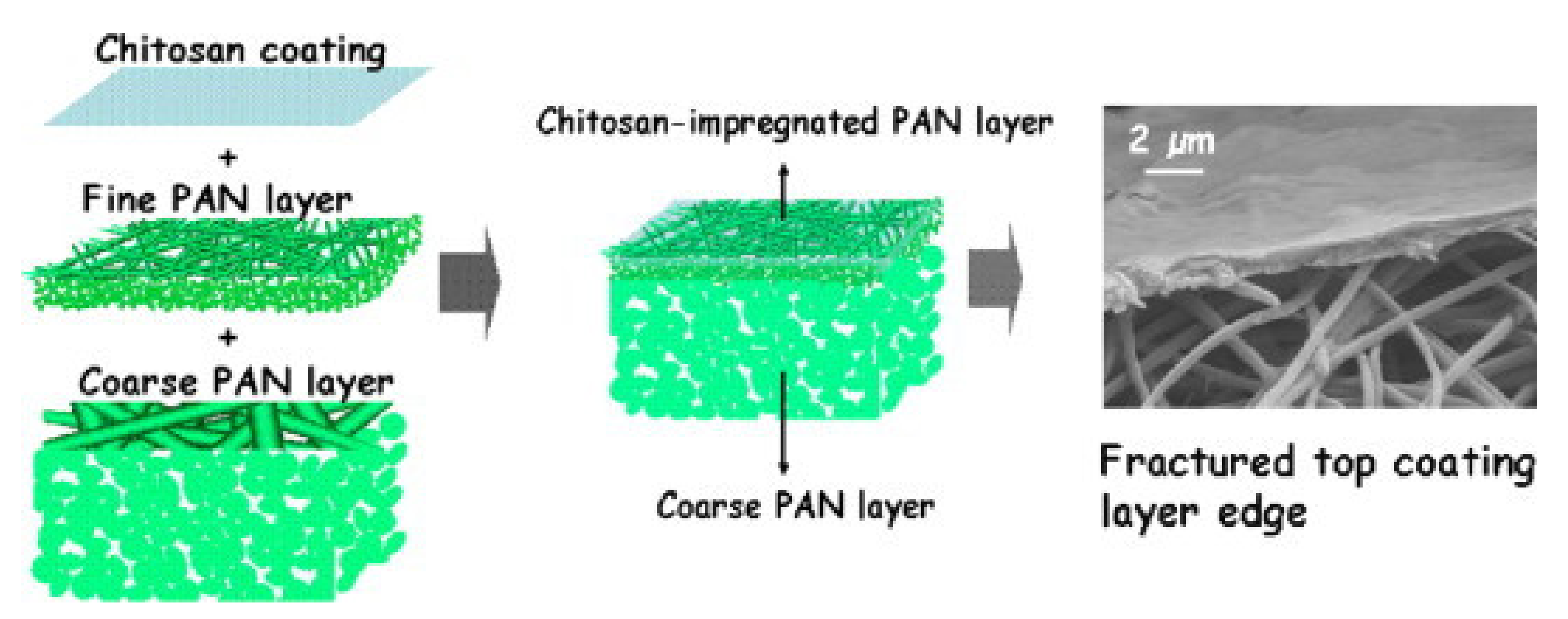
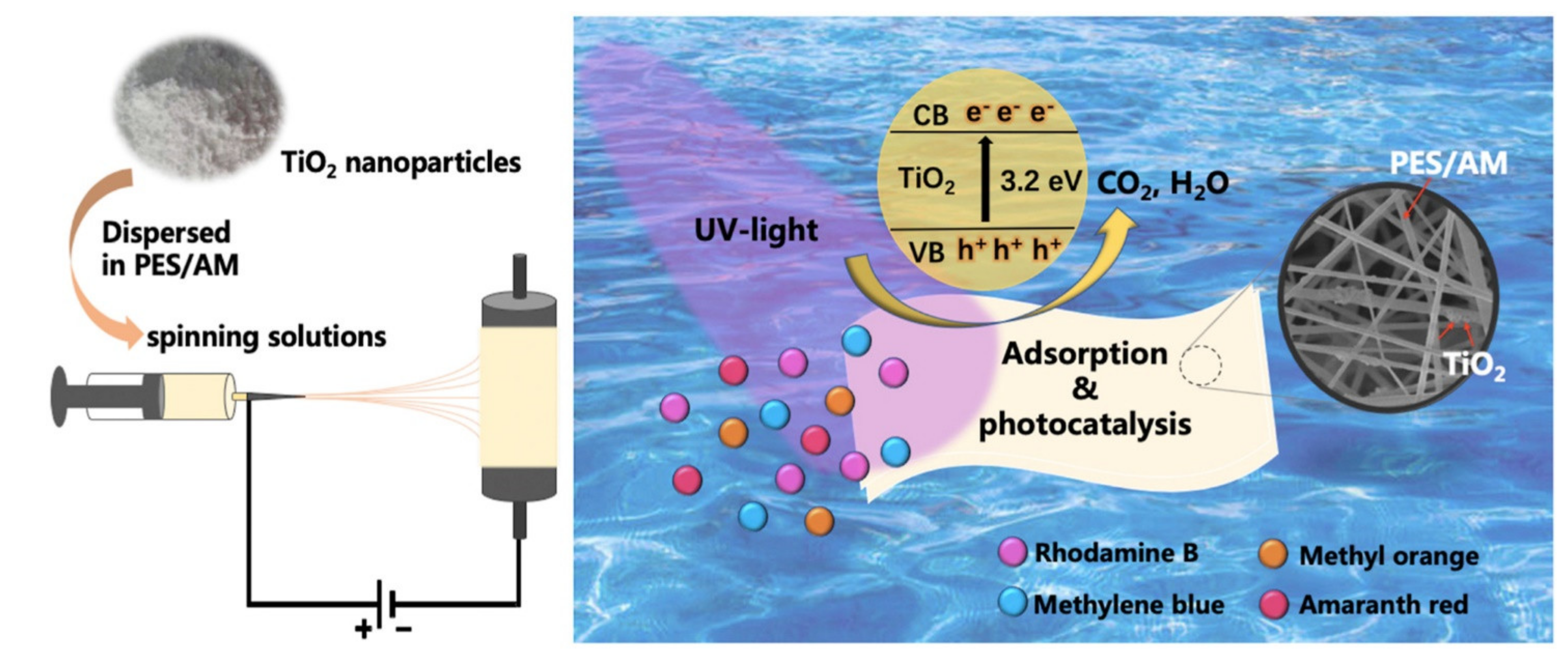
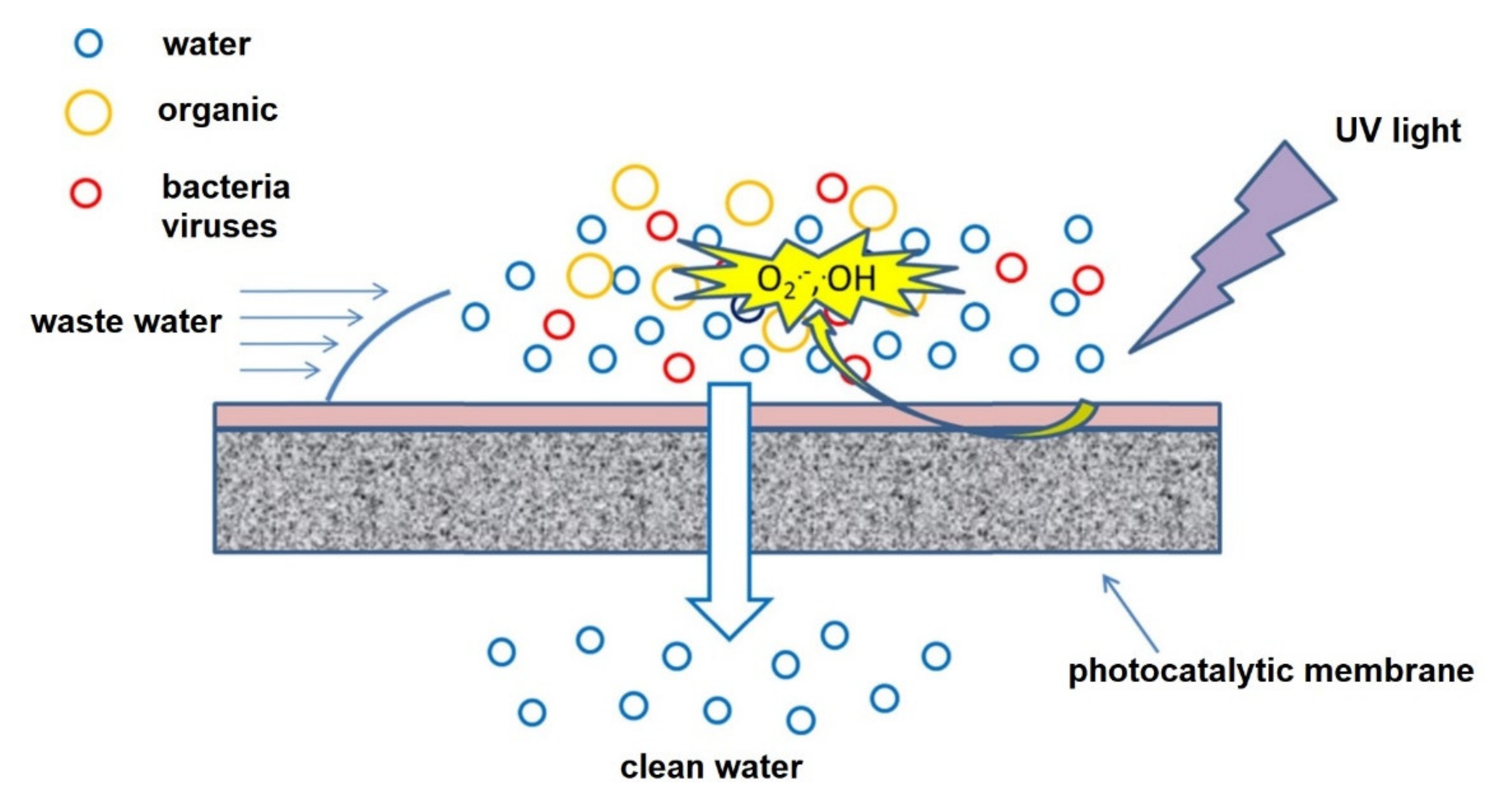
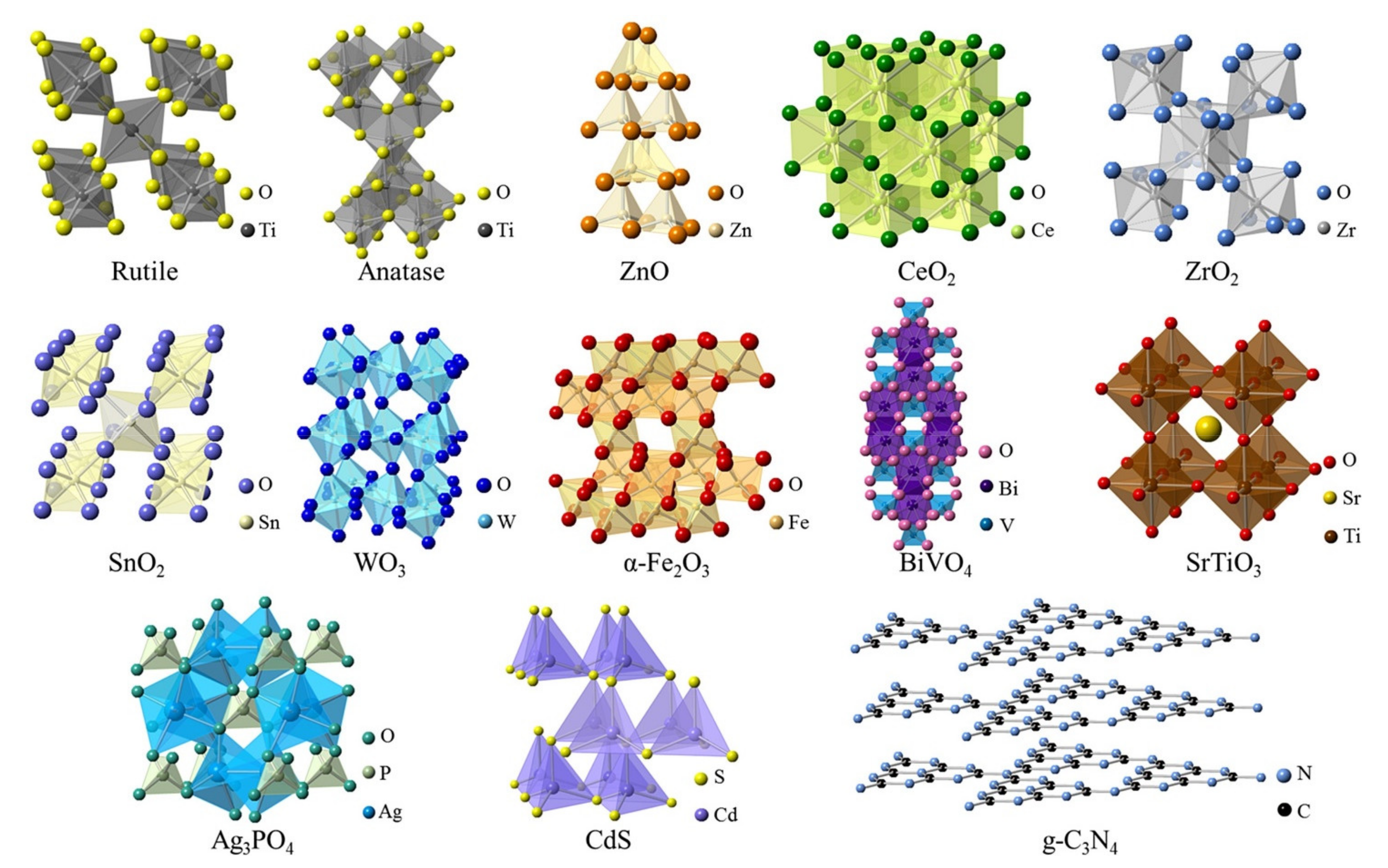
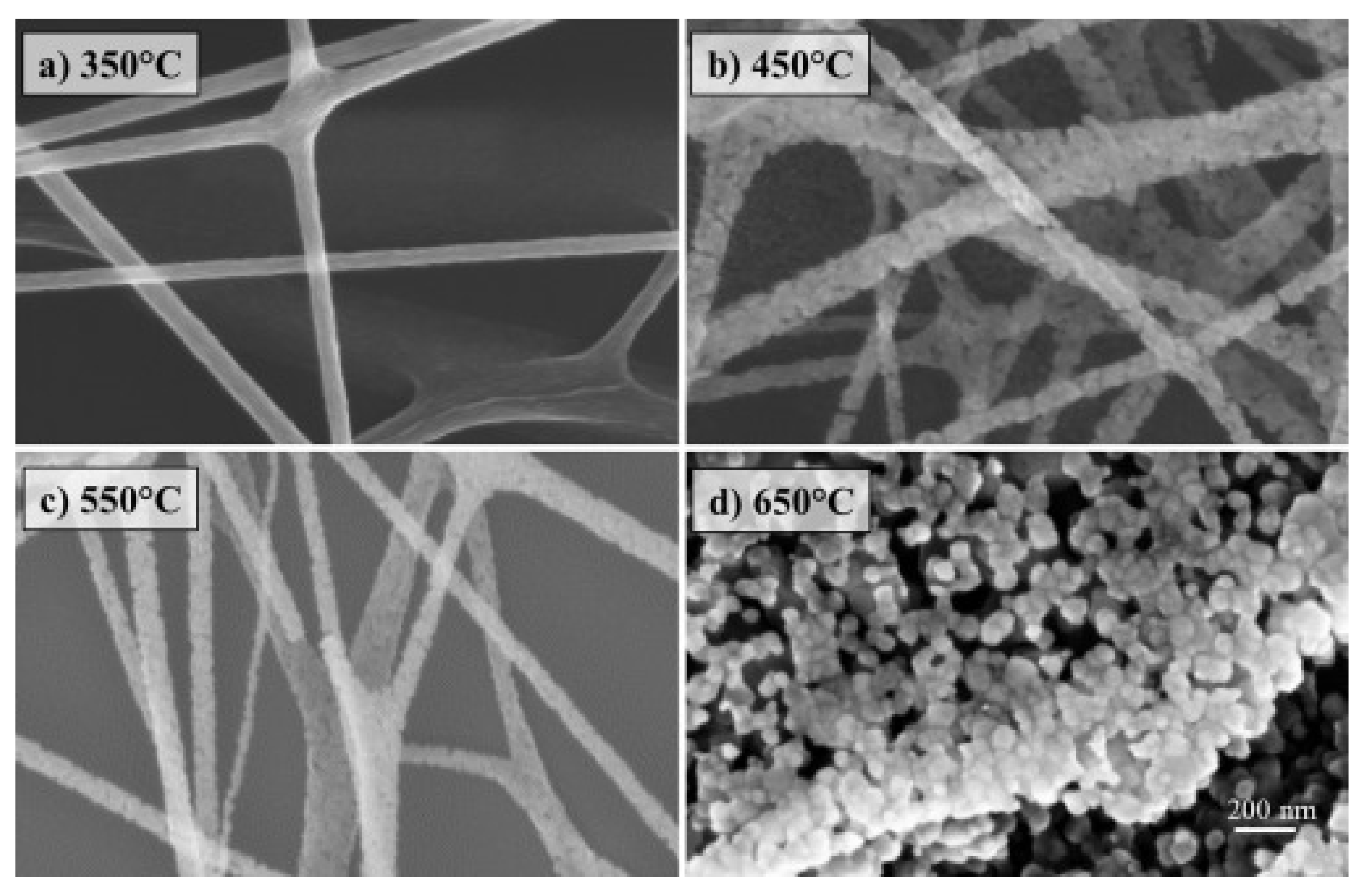
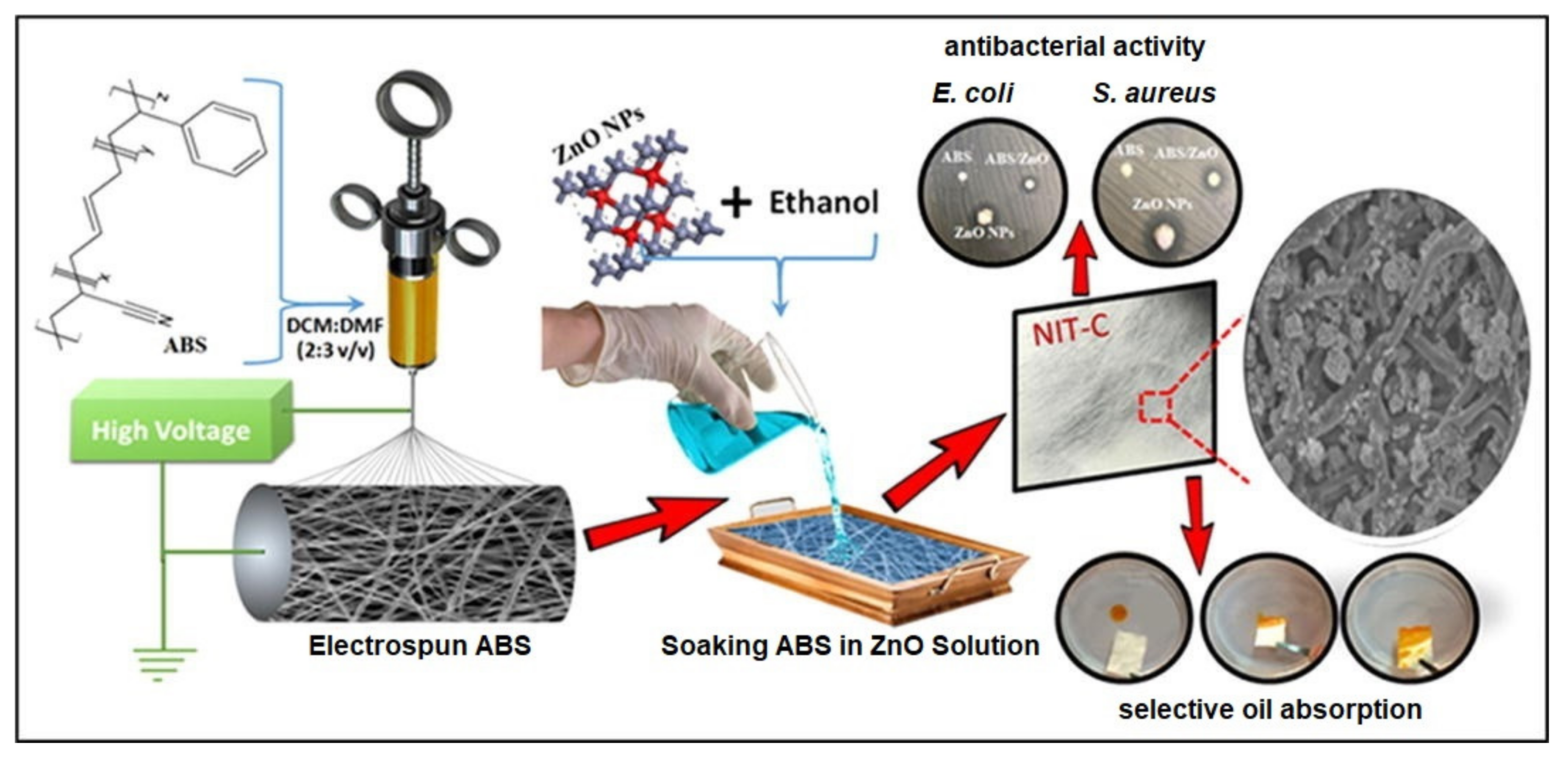
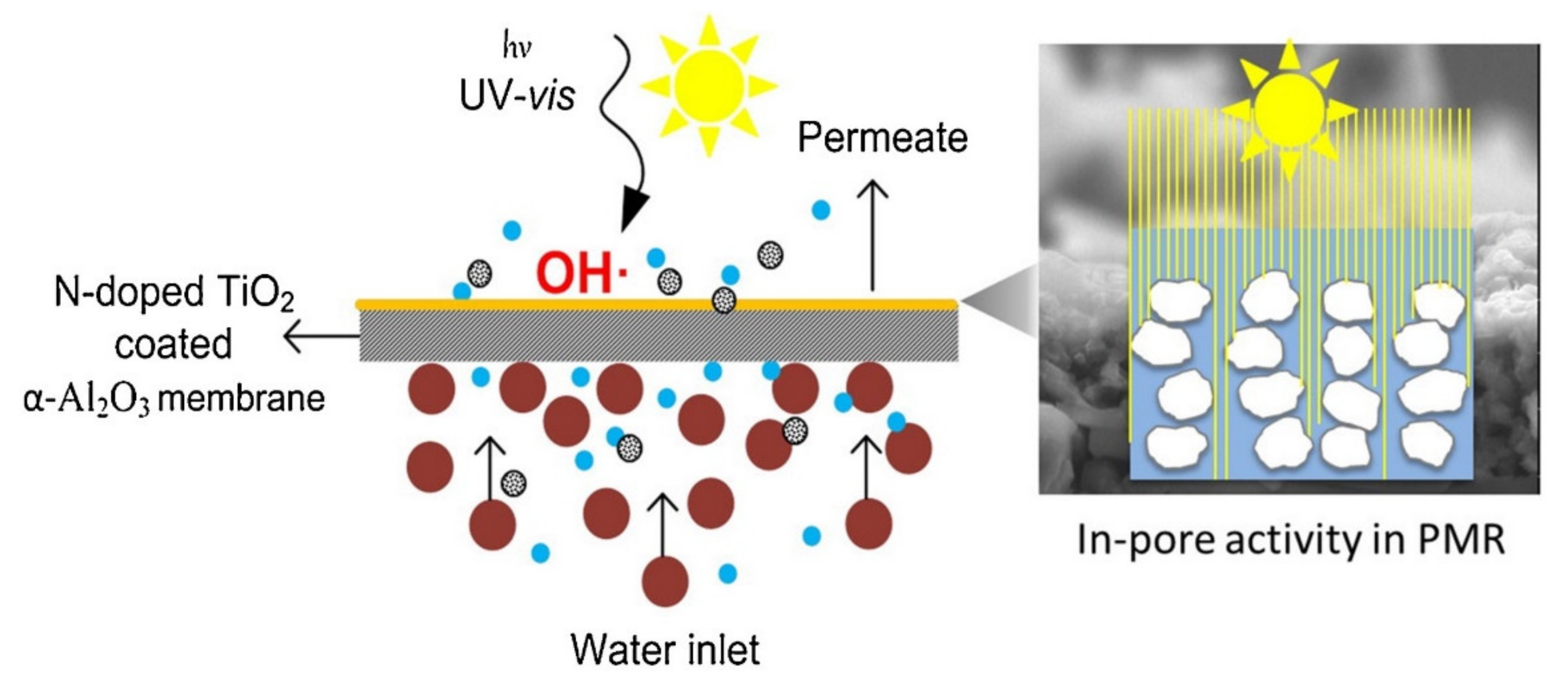

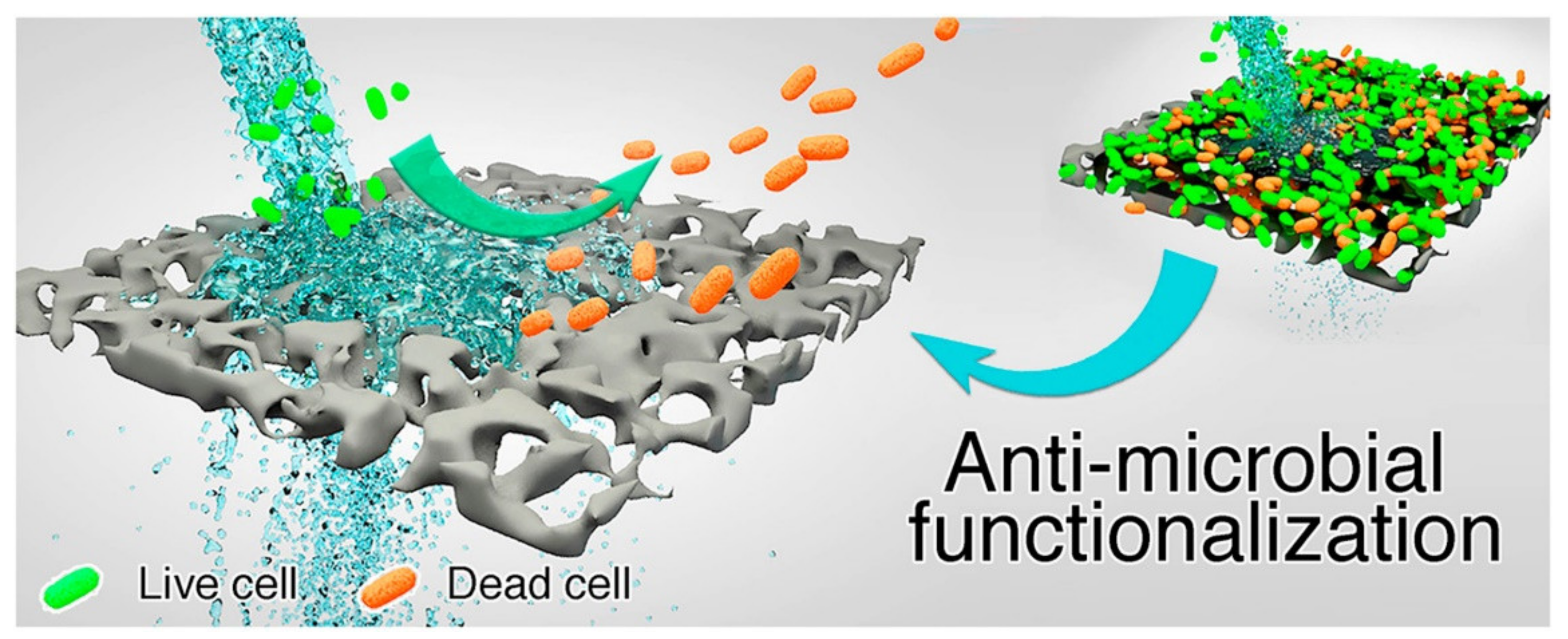

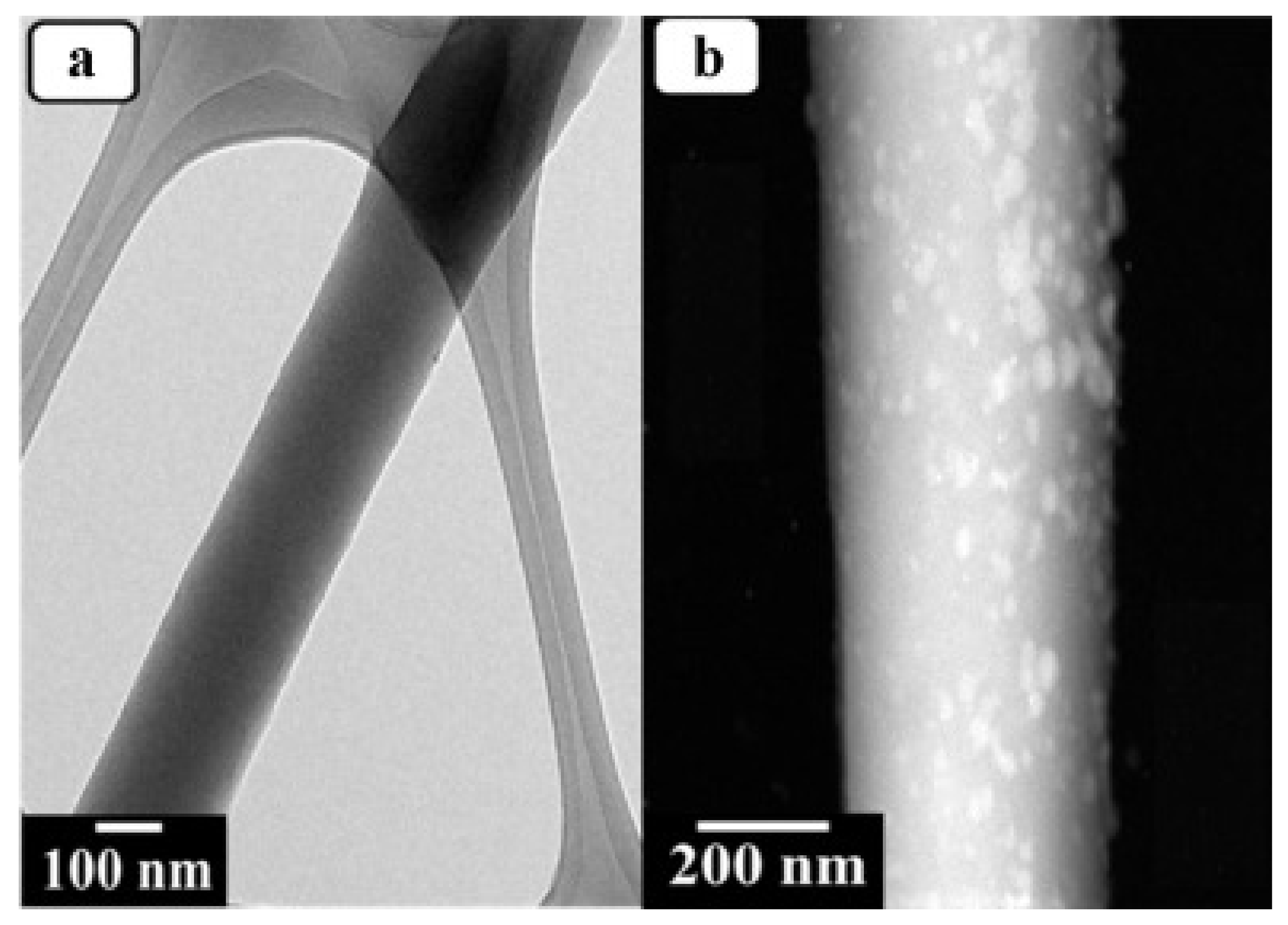

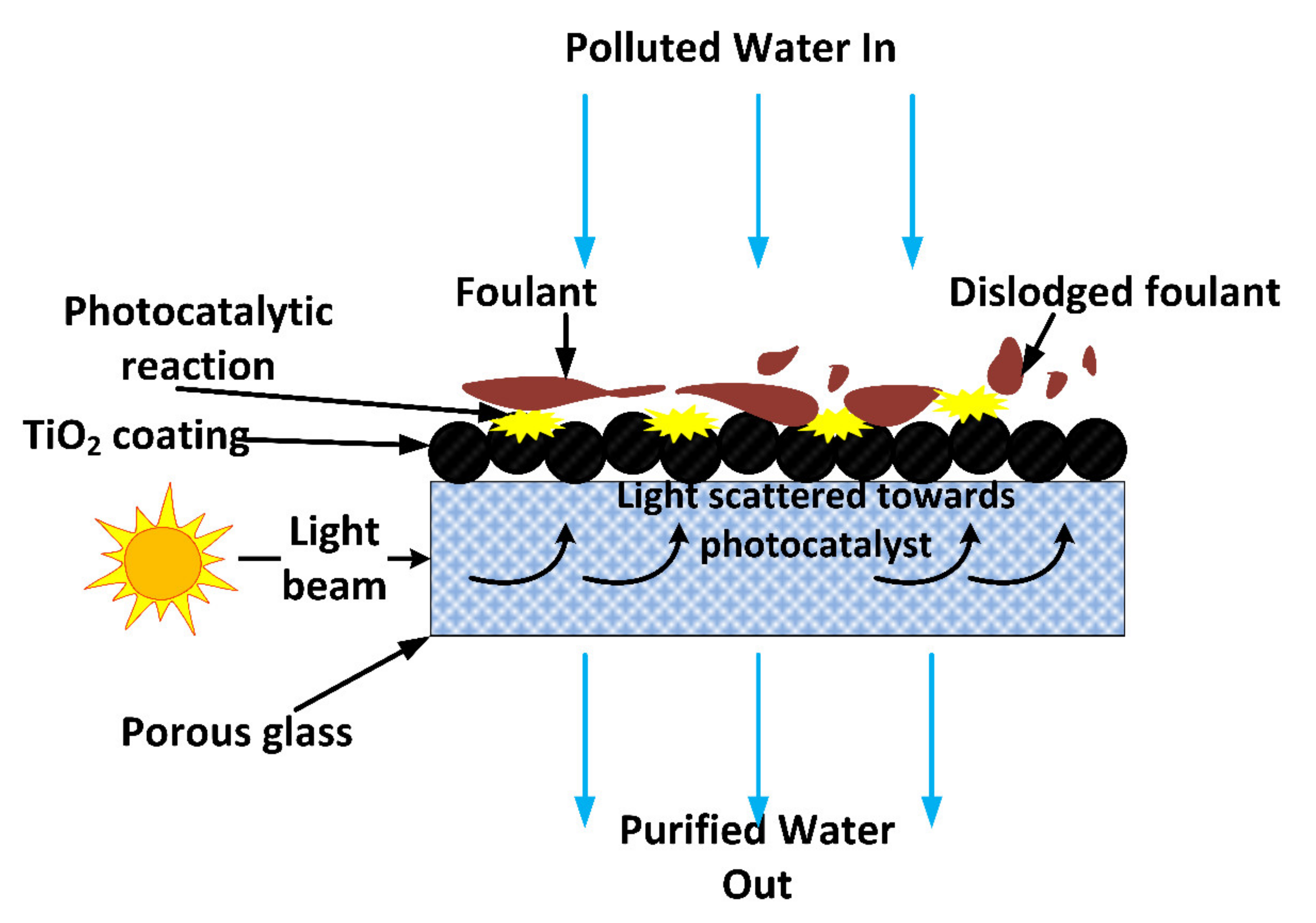


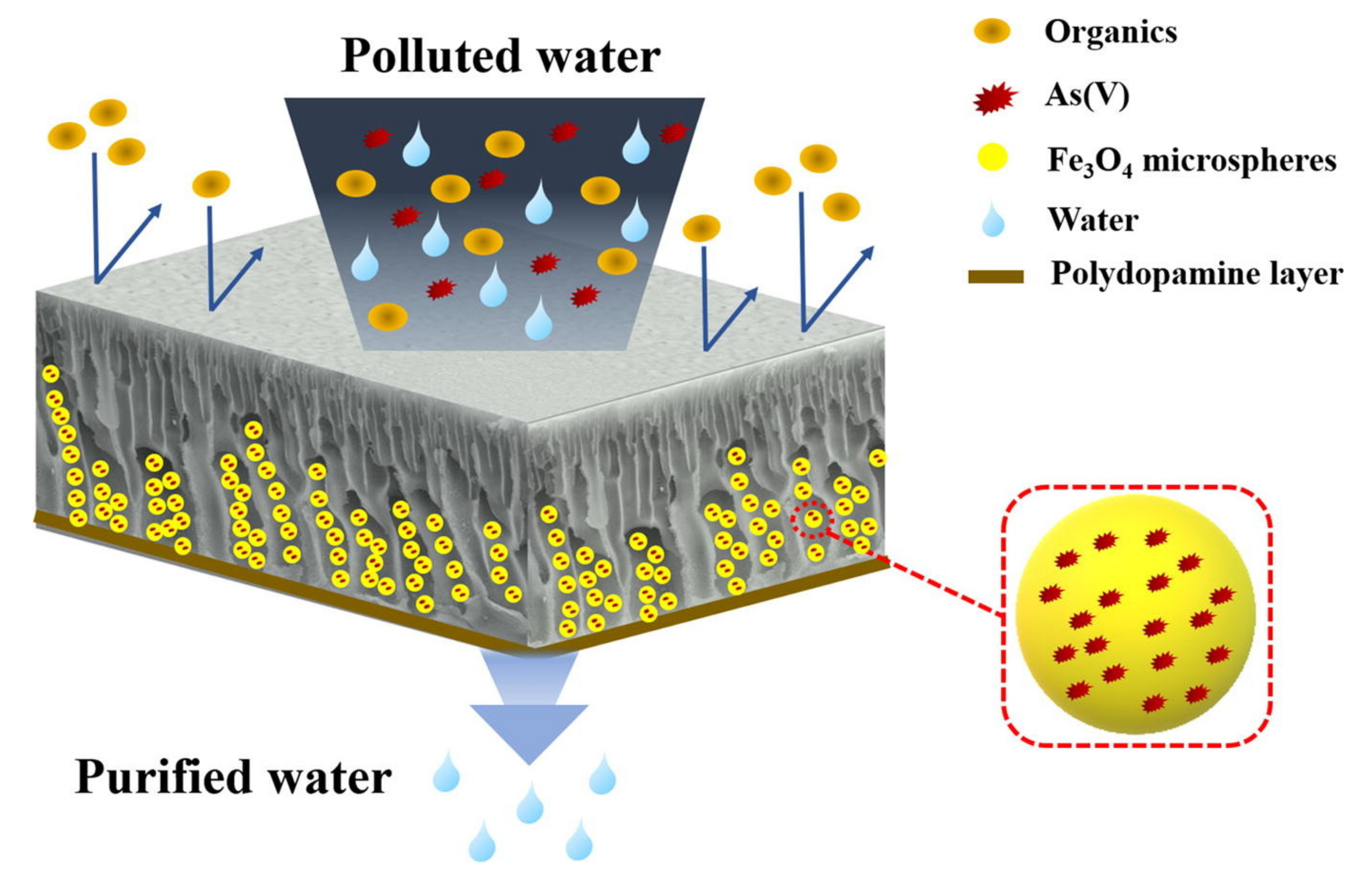


| Polymer/Membrane | Additive | Method | Application(s) | Photocatalytic Efficiency (%) | Ref. |
|---|---|---|---|---|---|
| Polyvinylpyrrolidone | Ag/TiO2 | Co/Blend-Electrospinning | Filtration Photodegradation (Methylene blue) Antimicrobial (E. coli) | 80 | [100] |
| Polyvinylpyrrolidone | TiO2 | Electrospinning | Photodegradation (Rhodamine b) | 72 | [101] |
| Polyvinylpyrrolidone | TiO2/C | Electrospinning | Capture and photocatalytic conversion of particulate matter | 99.92 | [102] |
| Polyacrylonitrile | ZnO/TiO2 | Electrospinning | Photodegradation (Malachite green) | 99 | [39] |
| Polyacrylonitrile | biogenetic silica | Electrospinning | Photodegradation (malachite green) | 100 | [94] |
| Polyacrylonitrile | SiO2-TiO2-NH2 | Electrospinning | Photodegradation (Malachite green and Acid red 27) | 100 | [95] |
| Polyacrylonitrile | Ag/AgCl | Electrospinning | Filtration Photodegradation (Methyl orange) | 85 | [96] |
| Polyacrylonitrile | ZnO/Ag | Electrospinning—reflux | UV-shielding Photocatalysis (Methylene blue) Antimicrobial (S. aureus) | 99 | [97] |
| Polyacrylonitrile | TiO2/MOF/CNT | Electrospinning—self assembly | Photodegradation (Hydrogen sulphide) | 93.5 | [98] |
| Polyacrylonitrile | α-Fe2O3/rGO | Hydrothermal vacuum filtration | Photodegradation (Methylene blue) | 98.5 | [99] |
| Polyaniline | TiO2/SiO2 | Electrospinning | Photodegradation (Methyl orange) | 87 | [51] |
| Free standing | CNTs/TiO2 | Chemical vapor deposition | Photodegradation (Methylene blue) | Not specified | [103] |
| Free standing | CNT/ZnO/TiO2 | Hydrothermal | Filtration Photodegradation (Acid Orange 7) Adsorption (Acid Orange 7) | 100 | [108] |
| Free standing | Zr-TiO2 | Electrospinning | Photodegradation (Methylene blue) | 95.4 | [110] |
| Nylon | ZnO | Electrospinning—atomic layer deposition | Photodegradation (Rhodamine b) | 99 | [104] |
| Chitosan | Algae-TiO2/Ag | Electrospinning | Photocatalytic reduction of Cr(VI) | 91 | [105] |
| Graphene oxide | ZnO | Vacuum filtration | Filtration Anti-biofouling via photodegradation (Powder milk and direct red 16 dye) | 90.5 | [107] |
| Polyether sulfone | ZnO/MWCNTs | Non-solvent induced phase inversion | Filtration Antimicrobial (E. coli) Photodegradation rejection (Rhodamine b) | 99.6 | [106] |
| Polyimide | ZnO | Electrospinning | Photodegradation (Methylene blue) | 98 | [111] |
| Cellulose acetate/polyurethane | ZnO | Solution dispersion blending | Photodegradation (Reactive Red 11 and Reactive Orange 84) | 100 95 | [112] |
| Cellulose | TiO2-coreshell | Vacuum filtration | Photodegradation (methyl orange) | 100 | [113] |
| Polyurethane | Ag-TiO2 | Electrospinning | Photodegradation (Dairy effluents) | 95 | [114] |
| PAN-Alumina hollow fiber | GCN | Electrospinning | Oilfield produced water treatment. | 99 | [115] |
| Nylon-6 | TiO2 | Electrospraying and electrospinning | Photodegradation (Methylene blue) Toxicity control of chlorophenols | 100 | [116] |
| Polyvinylidene fluoride | Sm-ZnO | Electrospinning | Photodegradation (Reactive golden yellow and Rhodamine b) | 100 | [117] |
| Polyvinylidene fluoride—Polyacrylonitrile | TiO2 | Electrospinning | Photodegradation (Rhodamine b) Oil-water separation | 97 99 | [118] |
| Polymer | Antimicrobial Agent | Method | Application | Antimicrobial Activity | Ref. |
|---|---|---|---|---|---|
| Polyurethane | Polydopamine-ZnO | Electrospinning | Antimicrobial (E. coli) Photodegradation (Methylene blue) | Active | [128] |
| Polyacrylonitrile | Ag3PO4 | Electrospinning | Antimicrobial (E. coli and S. aureus) Photodegradation (Methylene blue) | Active | [129] |
| Polyacrylonitrile | Ag nanoparticles | Electrospinning | Antimicrobial (E. coli and S. aureus) | Active | [136] |
| Polyacrylonitrile | Ag nanoparticles | Electrospinning | Antimicrobial (E. coli and S. aureus) | Active | [137] |
| Polyacrylonitrile | Ag nanoparticles | Electrospinning | Antimicrobial (E. coli and S. aureus) Forward osmosis | Active | [138] |
| Chitosan | Ag nanoparticles | Centrifugal spinning | Antimicrobial (S. aureus) Wound healing | Active | [139] |
| Polysulfone | CNT/Ag | Radical solution polymerization and wet-phase inversion | Antimicrobial (E. coli and B. subtilis) | Active | [140] |
| 3D woven fabric filters | Ag nanoparticles | Electrospinning | Antimicrobial (S. aureus) Water treatment | Active | [141] |
| Polyvinyl alcohol | Polyimide-Ag | Electrospinning | Antimicrobial (E. coli and S. aureus) Oily wastewater treatment | Active | [142] |
| Polyacrylonitrile | CuO | Electrospinning | Antimicrobial (E. coli and B. subtilis) for breath masks Drug release | Active | [143] |
| Chitosan/poly(ethylene oxide) | Poly(hexamethylene biguanide) hydrochloride | Electrospinning | Antimicrobial (E. coli and S. aureus) | Active | [144] |
| Poly(ε-caprolactone) and gelatine | Octadecyldimethyl[3 -(trimethoxysilyl)propyl]ammonium chloride | Electrospinning | Antimicrobial (S. aureus and P. aeruginosa) Wound dressing | Active | [145] |
| Polylactic acid | Fe3O4-COOH | Electrospinning | Antimicrobial (E. coli and S. aureus) Drug delivery | Active | [146] |
| Triaxial | Nisin | Electrospinning | Antimicrobial (S. aureus) | Active | [147] |
| Cellulose acetate/polyester urethane | Polyhexamethylene biguanide | Electrospinning | Antimicrobial (E. coli) Cytotoxicity Wound healing | Active | [148] |
| Polycaprolactone/gelatine | Metronidazole | Electrospinning | Antimicrobial (F. nucleatum) Cytotoxicity (L929 Cells) Drug delivery | Active | [149] |
| Silk fibroin | Peptide motif | Electrospinning | Antimicrobial (S. aureus, E. coli, S. epidermidis and P. aeruginosa) Wound dressing | Active | [150] |
| Polycaprolactone | 2-(Methacryloyloxy) ethyl trimethylammonium/polycaprolactone | Cross-linking polymerization and electrospinning | Antimicrobial (E. coli and S. aureus) Wound dressing | Active | [151] |
| Nylon 6 | N-Halamine | Electrospinning | Antimicrobial (E. coli and S. aureus) | Active | [152] |
| Polycaprolactone | Peptide dissolved micro needles | Coaxial electrospinning and electrospray deposition | Antimicrobial (S. aureus, K. pneumoniae, A. baumannii, and P. aeruginosa) Chronic wound dressing | Active | [153] |
| Electrolysis | Overall Reaction at Anode | Overall Reaction at Cathode |
|---|---|---|
| Alkaline | 4OH− → 2H2O + 4e− + O2 | 4H2O + 4e− → 4OH− + 2H2 |
| Polymer-electrolyte membrane | 2H2O → 4H+ +4e− + O2 | 4H+ + 4e− → 2H2 |
| Solid oxide | O2 → ½ O2 + 2e− | H2O + 2e− → H2 + O2− |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chabalala, M.B.; Gumbi, N.N.; Mamba, B.B.; Al-Abri, M.Z.; Nxumalo, E.N. Photocatalytic Nanofiber Membranes for the Degradation of Micropollutants and Their Antimicrobial Activity: Recent Advances and Future Prospects. Membranes 2021, 11, 678. https://doi.org/10.3390/membranes11090678
Chabalala MB, Gumbi NN, Mamba BB, Al-Abri MZ, Nxumalo EN. Photocatalytic Nanofiber Membranes for the Degradation of Micropollutants and Their Antimicrobial Activity: Recent Advances and Future Prospects. Membranes. 2021; 11(9):678. https://doi.org/10.3390/membranes11090678
Chicago/Turabian StyleChabalala, Mandla B., Nozipho N. Gumbi, Bhekie B. Mamba, Mohammed Z. Al-Abri, and Edward N. Nxumalo. 2021. "Photocatalytic Nanofiber Membranes for the Degradation of Micropollutants and Their Antimicrobial Activity: Recent Advances and Future Prospects" Membranes 11, no. 9: 678. https://doi.org/10.3390/membranes11090678
APA StyleChabalala, M. B., Gumbi, N. N., Mamba, B. B., Al-Abri, M. Z., & Nxumalo, E. N. (2021). Photocatalytic Nanofiber Membranes for the Degradation of Micropollutants and Their Antimicrobial Activity: Recent Advances and Future Prospects. Membranes, 11(9), 678. https://doi.org/10.3390/membranes11090678









