Modification of Ceramic Membranes with Carbon Compounds for Pharmaceutical Substances Removal from Water in a Filtration—Adsorption System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
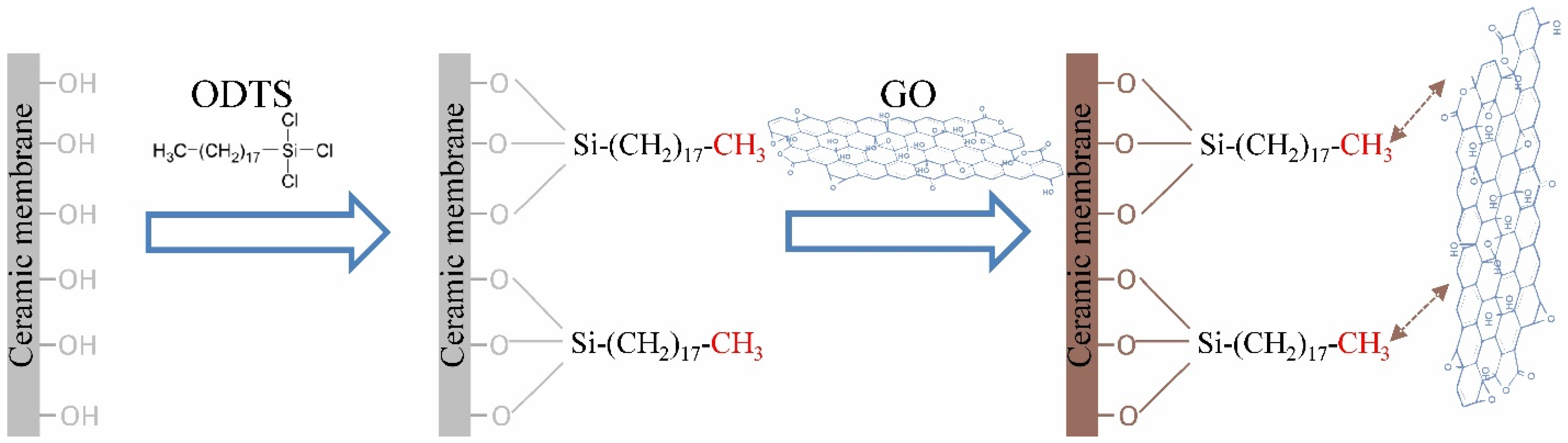
2.2. Membrane Surface Modification
2.3. Membrane Adsorption Properties
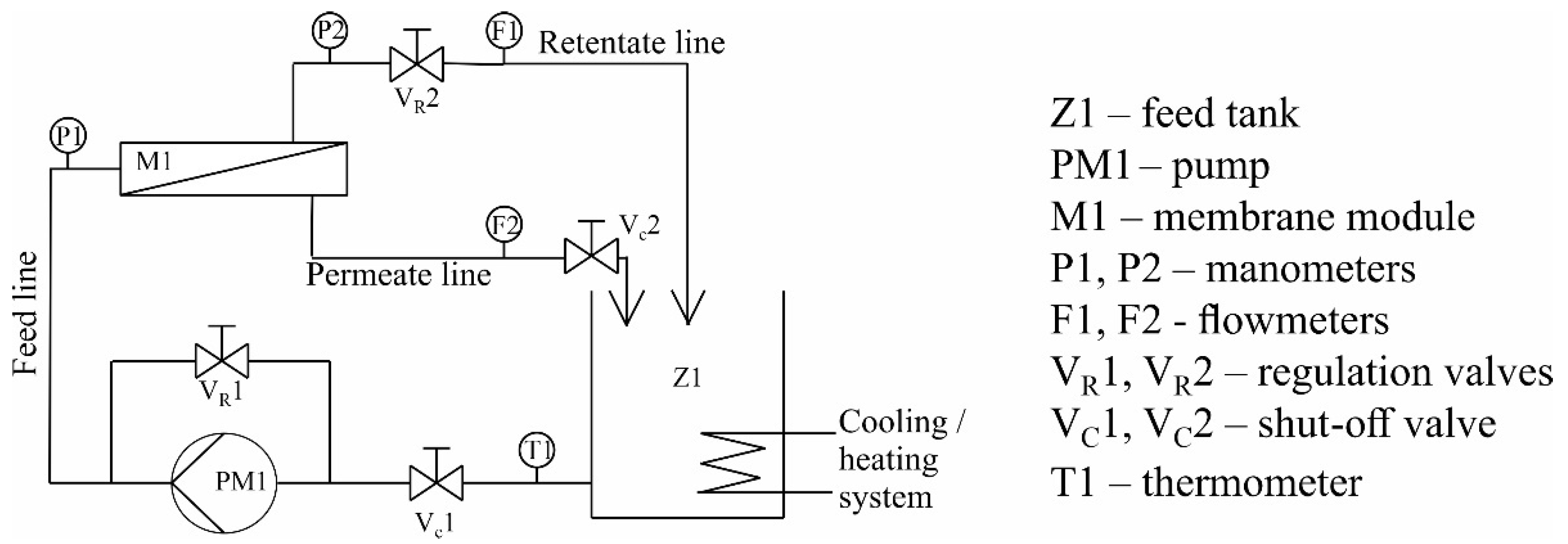
2.4. Filtration–Adsorption Setup
2.5. Examination of Membrane Surface and Structural Properties
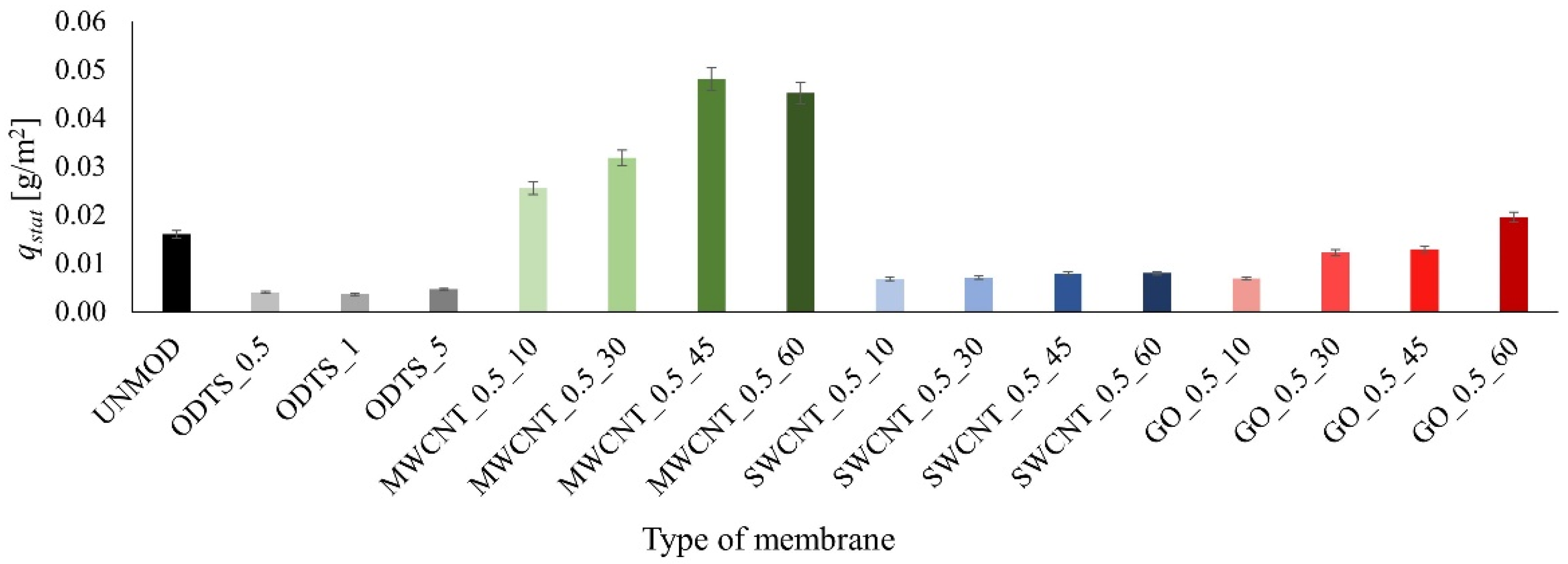
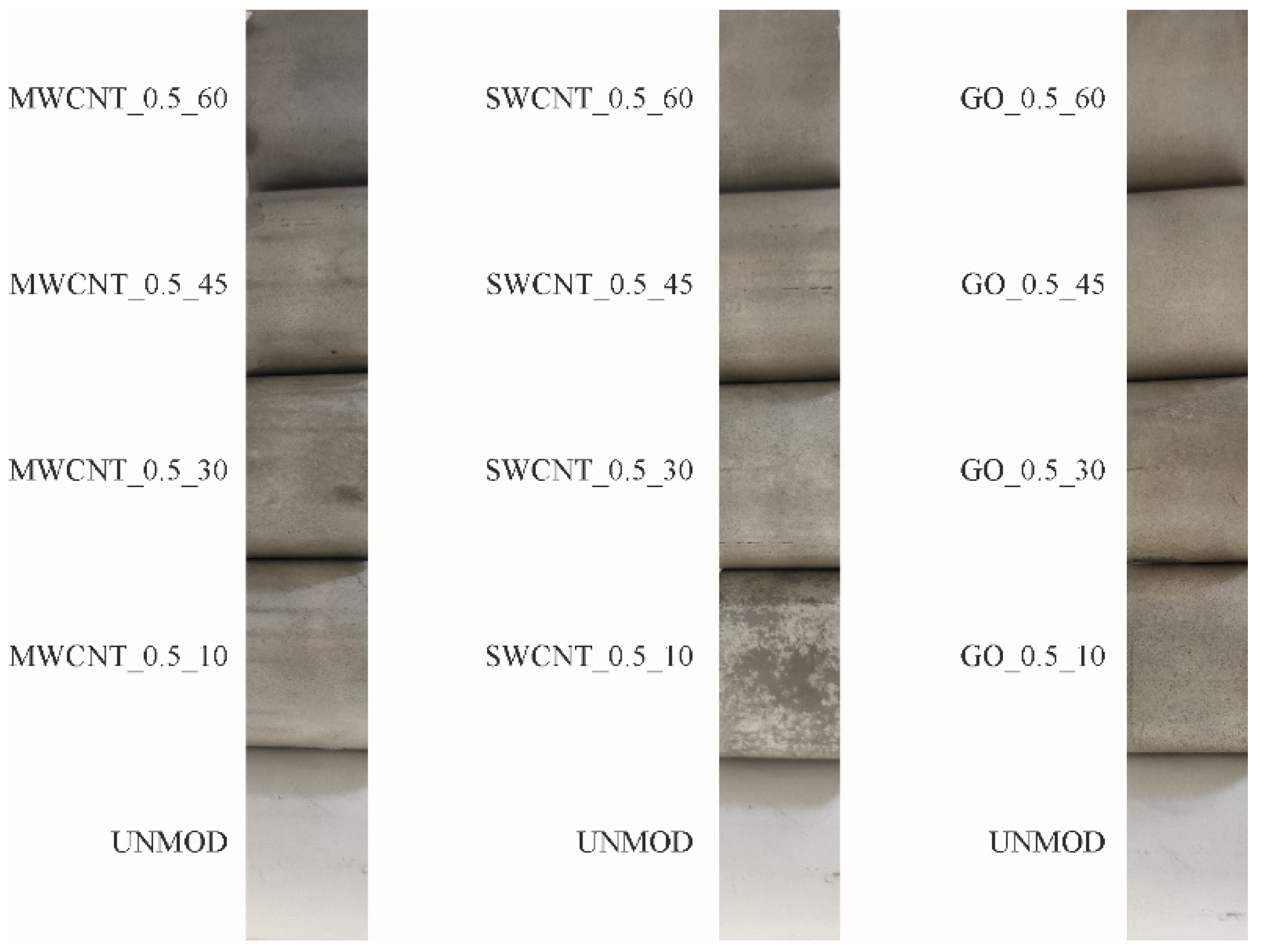
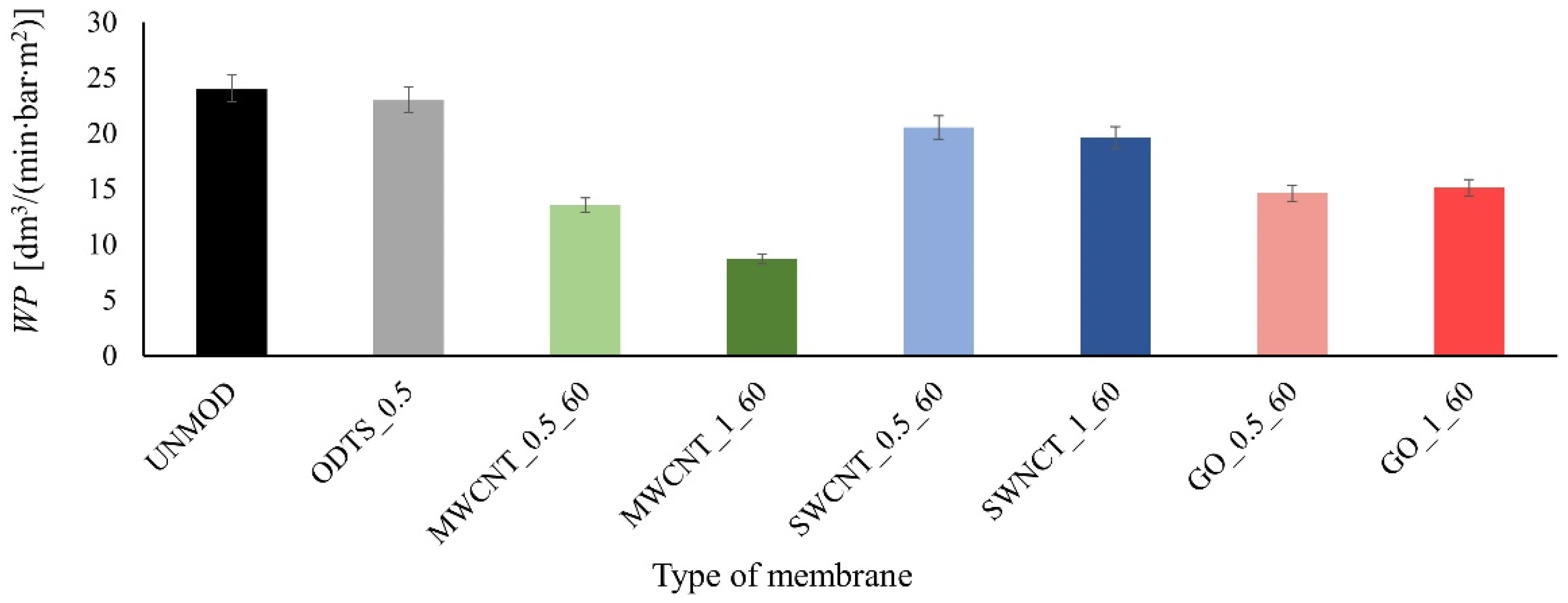
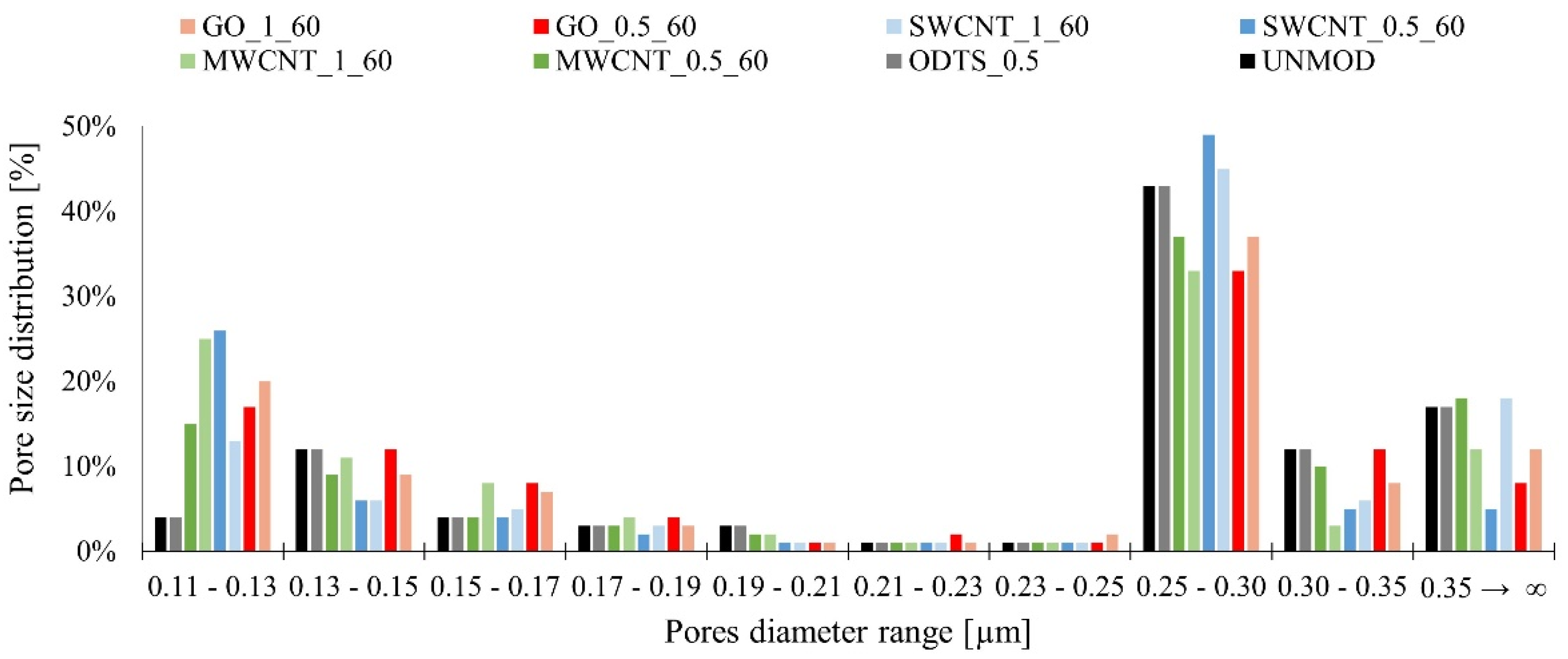
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Larsson, D.G.J.; Adolfsson-Erici, M.; Parkkonen, J.; Pettersson, M.; Berg, A.H.; Olsson, P.E.; Förlin, L. Ethinyloestradiol—An undesired fish contraceptive? Aquat. Toxicol. 1999, 45, 91–97. [Google Scholar] [CrossRef]
- Khan, A.; Wang, J.; Li, J.; Wang, X.; Chen, Z.; Alsaedi, A.; Hayat, T.; Chen, Y.; Wang, X. The role of graphene oxide and graphene oxide-based nanomaterials in the removal of pharmaceuticals from aqueous media: A review. Environ. Sci. Pollut. Res. 2017, 24, 7938–7958. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sarmah, A.K.; Padhye, L.P. Fate of pharmaceuticals and personal care products in a wastewater treatment plant with parallel secondary wastewater treatment train. J. Environ. Manag. 2019, 233, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, R.S.; Ahmed, M.; Al-Busaidi, A.; Choudri, B.S. Translocation of pharmaceuticals and personal care products (PPCPs) into plant tissues: A review. Emerg. Contam. 2017, 3, 132–137. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abdallah, M.A.E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Zhou, R.; Lu, G.; Yan, Z.; Jiang, R.; Bao, X. A review of the influences of microplastics on toxicity and transgenerational effects of pharmaceutical and personal care products in aquatic environment. Sci. Total Environ. 2020, 732, 139222. [Google Scholar] [CrossRef]
- Chopra, S.; Kumar, D. Pharmaceuticals and personal care products (PPCPs) as emerging environmental pollutants: Toxicity and risk assessment. In Advances in Animal Biotechnology and Its Applications; Springer: Singapore, 2018; pp. 337–353. [Google Scholar]
- Yin, L.; Wang, B.; Yuan, H.; Deng, S.; Huang, J.; Wang, Y.; Yu, G. Pay special attention to the transformation products of PPCPs in environment. Emerg. Contam. 2017, 3, 69–75. [Google Scholar] [CrossRef]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical industry wastewater: Review of the technologies for water treatment and reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Ren, N.; Cui, M.; Nie, S.; Hu, D. Simultaneous removal and evaluation of organic substrates and NH3-N by a novel combined process in treating chemical synthesis-based pharmaceutical wastewater. J. Hazard. Mater. 2011, 197, 49–59. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, N.; Wang, A.; Zhang, Z.P.; Shi, Y. A novel application of TPAD–MBR system to the pilot treatment of chemical synthesis-based pharmaceutical wastewater. Water Res. 2008, 42, 3385–3392. [Google Scholar] [CrossRef] [PubMed]
- Oktem, Y.A.; Ince, O.; Sallis, P.; Donnelly, T.; Ince, B.K. Anaerobic treatment of a chemical synthesis-based pharmaceutical wastewater in a hybrid upflow anaerobic sludge blanket reactor. Bioresour. Technol. 2008, 99, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Cyr, P.J.; Suri, R.P.; Helmig, E.D. A pilot scale evaluation of removal of mercury from pharmaceutical wastewater using granular activated carbon. Water Res. 2002, 36, 4725–4734. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Esparza, M. HPLC-fluorescence detection and adsorption of bisphenol A, 17β-estradiol, and 17α-ethynyl estradiol on powdered activated carbon. Water Res. 2003, 37, 3530–3537. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.F.; Carvalho, A.P. Activated carbons for the adsorption of ibuprofen. Carbon 2007, 45, 1979–1988. [Google Scholar] [CrossRef]
- Reyes, C.; Fernandez, J.; Freer, J.; Mondaca, M.A.; Zaror, C.; Malato, S.; Mansilla, H.D. Degradation and inactivation of tetracycline by TiO2 photocatalysis. J. Photochem. Photobiol. A 2006, 184, 141–146. [Google Scholar] [CrossRef]
- Wang, G.; Wang, D.; Xu, X.; Liu, L.; Yang, F. Wet air oxidation of pretreatment of pharmaceutical wastewater by Cu2+ and [PxWmOy] q− co-catalyst system. J. Hazard. Mater. 2012, 217, 366–373. [Google Scholar] [CrossRef]
- Dalrymple, O.K.; Yeh, D.H.; Trotz, M.A. Removing pharmaceuticals and endocrine-disrupting compounds from wastewater by photocatalysis. J. Chem. Technol. Biotechnol. 2007, 82, 121–134. [Google Scholar] [CrossRef]
- Iliuta, I.; Larachi, F. Wet air oxidation solid catalysis analysis of fixed and sparged three-phase reactors. Chem. Eng. Process. 2001, 40, 175–185. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, P.; Wang, Q.; Wang, X.; Yu, K.; Xue, T.; Wen, X. Influences of multi influent matrices on the retention of PPCPs by nanofiltration membranes. Sep. Purif. Technol. 2019, 212, 299–306. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lee, C.H. Elucidating the rejection mechanisms of PPCPs by nanofiltration and reverse osmosis membranes. Ind. Eng. Chem. Res. 2014, 53, 6798–6806. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Z.L.; Wang, X.C.; Shen, J.M.; Xu, H. PPCPs removal by aerobic granular sludge membrane bioreactor. Appl. Microbiol. Biotechnol. 2014, 98, 9843–9848. [Google Scholar] [CrossRef] [PubMed]
- Darowna, D.; Grondzewska, S.; Morawski, A.W.; Mozia, S. Removal of non-steroidal anti-inflammatory drugs from primary and secondary effluents in a photocatalytic membrane reactor. J. Chem. Technol. Biotechnol. 2014, 89, 1265–1273. [Google Scholar] [CrossRef]
- Fernández, R.L.; McDonald, J.A.; Khan, S.J.; Le-Clech, P. Removal of pharmaceuticals and endocrine disrupting chemicals by a submerged membrane photocatalysis reactor (MPR). Sep. Purif. Technol. 2014, 127, 131–139. [Google Scholar] [CrossRef]
- Rana, D.; Scheier, B.; Narbaitz, R.M.; Matsuura, T.; Tabe, S.; Jasim, S.Y.; Khulbe, K.C. Comparison of cellulose acetate (CA) membrane and novel CA membranes containing surface modifying macromolecules to remove pharmaceutical and personal care product micropollutants from drinking water. J. Membr. Sci. 2012, 409, 346–354. [Google Scholar] [CrossRef]
- Narbaitz, R.M.; Rana, D.; Dang, H.T.; Morrissette, J.; Matsuura, T.; Jasim, S.Y.; Yang, P. Pharmaceutical and personal care products removal from drinking water by modified cellulose acetate membrane: Field testing. Chem. Eng. J. 2013, 225, 848–856. [Google Scholar] [CrossRef]
- Rana, D.; Narbaitz, R.M.; Garand-Sheridan, A.M.; Westgate, A.; Matsuura, T.; Tabe, S.; Jasim, S.Y. Development of novel charged surface modifying macromolecule blended PES membranes to remove EDCs and PPCPs from drinking water sources. J. Mater. Chem. A 2014, 2, 10059–10072. [Google Scholar] [CrossRef]
- Lou, Y.; Tan, F.J.; Zeng, R.; Wang, M.; Li, P.; Xia, S. Preparation of cross-linked graphene oxide on polyethersulfone membrane for pharmaceuticals and personal care products removal. Polymers 2020, 12, 1921. [Google Scholar] [CrossRef]
- Lawler, J. Incorporation of graphene-related carbon nanosheets in membrane fabrication for water treatment: A review. Membranes 2016, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, N.; Zhao, Y.; Xia, S. Graphene oxide modified semi-aromatic polyamide thin film composite membranes for PPCPs removal. Desalin. Water Treat. 2017, 66, 166–175. [Google Scholar] [CrossRef]
- Yang, G.C.; Chen, Y.C.; Yang, H.X.; Yen, C.H. Performance and mechanisms for the removal of phthalates and pharmaceuticals from aqueous solution by graphene-containing ceramic composite tubular membrane coupled with the simultaneous electrocoagulation and electrofiltration process. Chemosphere 2016, 155, 274–282. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Koutahzadeh, N. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Polak, D.; Szwast, M.; Fabianowski, W.; Rosinski, M. Ceramic Membranes Modified by Carbon used for Laundry Wastewater Treatment. Chem. Eng. Trans. 2019, 74, 931–936. [Google Scholar]
- Polak, D.; Tonecka, I.; Fabianowski, W.; Szwast, M. Development of graphene oxide-coated membranes to support the process of removing pharmacological agents from water. Desalin. Water Treat. 2021, 214, 49–55. [Google Scholar] [CrossRef]
- Lazar, P.; Karlicky, F.; Jurecka, P.; Kocman, M.; Otyepková, E.; Šafářová, K.; Otyepka, M. Adsorption of small organic molecules on grapheme. J. Am. Chem. Soc. 2013, 135, 6372–6377. [Google Scholar] [CrossRef]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans. 2013, 42, 5682–5689. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F.; Zhao, J.; Wang, S.; Du, P.; Xing, B. Effects of solution chemistry on adsorption of selected pharmaceuticals and personal care products (PPCPs) by graphenes and carbon nanotubes. Environ. Sci. Technol. 2014, 48, 13197–13206. [Google Scholar] [CrossRef]
- Masud, A.; Soria NG, C.; Aga, D.S.; Aich, N. Adsorption and advanced oxidation of diverse pharmaceuticals and personal care products (PPCPs) from water using highly efficient rGO–nZVI nanohybrids. Environ. Sci. Water Res. Technol. 2020, 6, 2223–2238. [Google Scholar] [CrossRef]
- Ersan, G.; Apul, O.G.; Perreault, F.; Karanfil, T. Adsorption of organic contaminants by graphene nanosheets: A review. Water Res. 2017, 126, 385–398. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Huang, H.; Cho, H.H. Carbon nanotube composite membranes for microfiltration of pharmaceuticals and personal care products: Capabilities and potential mechanisms. J. Membr. Sci. 2015, 479, 165–174. [Google Scholar] [CrossRef]
- Rodriguez, E.; Campinas, M.; Acero, J.L.; Rosa, M.J. Investigating PPCP removal from wastewater by powdered activated carbon/ultrafiltration. Water Air Soil Pollut. 2016, 227, 177. [Google Scholar] [CrossRef]
- Ai, Y.; Liu, Y.; Huo, Y.; Zhao, C.; Sun, L.; Han, B.; Cao, X.; Wang, X. Insights into the adsorption mechanism and dynamic behavior of tetracycline antibiotics on reduced graphene oxide (RGO) and graphene oxide (GO) materials. Environ. Sci. Nano 2019, 6, 3336–3348. [Google Scholar] [CrossRef]
- Ji, L.; Shao, Y.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of monoaromatic compounds and pharmaceutical antibiotics on carbon nanotubes activated by KOH etching. Environ. Sci. Technol. 2010, 44, 6429–6436. [Google Scholar] [CrossRef] [PubMed]


| Membrane Type | ODTS Solution Concentration [%] | Modification Time in Carbon Compound Suspension [min] |
|---|---|---|
| UNMOD | 0 | 0 |
| ODTS_0.5 | 0.5 | 0 |
| ODTS_1 | 1 | 0 |
| ODTS_5 | 5 | 0 |
| MWCNT_0.5_10 | 0.5 | 10 |
| MWCNT_0.5_30 | 0.5 | 30 |
| MWCNT_0.5_45 | 0.5 | 45 |
| MWCNT_0.5_60 | 0.5 | 60 |
| SWCNT_0.5_10 | 0.5 | 10 |
| SWCNT_0.5_30 | 0.5 | 30 |
| SWCNT_0.5_45 | 0.5 | 45 |
| SWCNT_0.5_60 | 0.5 | 60 |
| GO_0.5_10 | 0.5 | 10 |
| GO_0.5_30 | 0.5 | 30 |
| GO_0.5_45 | 0.5 | 45 |
| GO_0.5_60 | 0.5 | 60 |
| Membrane Type | ODTS Solution Concentration [%] | Modification Time in Carbon Compound Suspension [min] |
|---|---|---|
| UNMOD | 0 | 0 |
| ODTS_0.5 | 0.5 | 0 |
| MWCNT_0.5_60 | 0.5 | 60 |
| MWCNT_1_60 | 1 | 60 |
| SWCNT_0.5_60 | 0.5 | 60 |
| SWCNT_1_60 | 1 | 60 |
| GO_0.5_60 | 0.5 | 60 |
| GO_1_60 | 1 | 60 |
| Process Time | UNMOD | MWCNT_1_60 | GO_1_60 | |||
|---|---|---|---|---|---|---|
| [min] | cN [g/dm3] | cP [g/dm3] | cN [g/dm3] | cP [g/dm3] | cN [g/dm3] | cP [g/dm3] |
| 0 | 0.033 | 0.033 | 0.032 | 0.032 | 0.033 | 0.033 |
| 1 | 0.033 | 0.033 | 0.032 | 0.032 | 0.033 | 0.033 |
| 5 | 0.032 | 0.031 | 0.029 | 0.025 | 0.030 | 0.028 |
| 10 | 0.030 | 0.031 | 0.026 | 0.025 | 0.029 | 0.028 |
| 15 | 0.029 | 0.030 | 0.024 | 0.024 | 0.028 | 0.027 |
| 30 | 0.027 | 0.029 | 0.021 | 0.023 | 0.026 | 0.026 |
| 60 | 0.027 | 0.027 | 0.021 | 0.020 | 0.025 | 0.025 |
| 90 | 0.026 | 0.026 | 0.019 | 0.018 | 0.024 | 0.023 |
| Membrane Material | Membrane Process | PPCP | Flux L·h−1·m−2·bar−1 | Retention % | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Al2O3 + MWCNT | MF | Tetracycline | 520 | 45.4 | Adsorption/filtration | This work |
| PES + CSMM | UF | Ibuprofen | 29.0 | 12.57 | Adsorption/filtration | [28] |
| PES + GO | UF | Ibuprofen | 5.27 | 44.9 | Adsorption/filtration | [29] |
| PA + GO | NF | Norfloxacin | 12.78 | 53.32 | Filtration/adsorption | [31] |
| CA | NF | Sulfamethazine | 2.87 | 85.2 | Filtration | [26] |
| CA + CSMM | NF | Sulfamethazine | 1.85 | 84.1 | Filtration | [26] |
| CA + LSMM | NF | Sulfamethazine | 1.54 | 78.6 | Filtration | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polak, D.; Zielińska, I.; Szwast, M.; Kogut, I.; Małolepszy, A. Modification of Ceramic Membranes with Carbon Compounds for Pharmaceutical Substances Removal from Water in a Filtration—Adsorption System. Membranes 2021, 11, 481. https://doi.org/10.3390/membranes11070481
Polak D, Zielińska I, Szwast M, Kogut I, Małolepszy A. Modification of Ceramic Membranes with Carbon Compounds for Pharmaceutical Substances Removal from Water in a Filtration—Adsorption System. Membranes. 2021; 11(7):481. https://doi.org/10.3390/membranes11070481
Chicago/Turabian StylePolak, Daniel, Izabela Zielińska, Maciej Szwast, Igor Kogut, and Artur Małolepszy. 2021. "Modification of Ceramic Membranes with Carbon Compounds for Pharmaceutical Substances Removal from Water in a Filtration—Adsorption System" Membranes 11, no. 7: 481. https://doi.org/10.3390/membranes11070481
APA StylePolak, D., Zielińska, I., Szwast, M., Kogut, I., & Małolepszy, A. (2021). Modification of Ceramic Membranes with Carbon Compounds for Pharmaceutical Substances Removal from Water in a Filtration—Adsorption System. Membranes, 11(7), 481. https://doi.org/10.3390/membranes11070481







