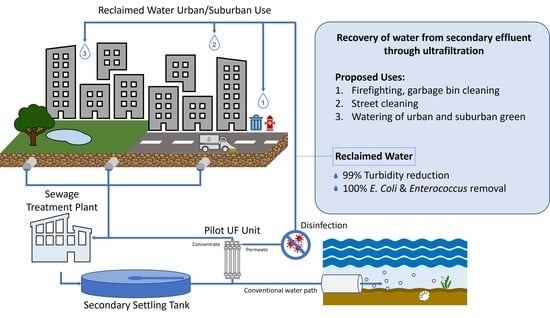
Recovery of Water from Secondary Effluent through Pilot Scale Ultrafiltration Membranes: Implementation at Patras’ Wastewater Treatment Plant
Abstract
:1. Introduction
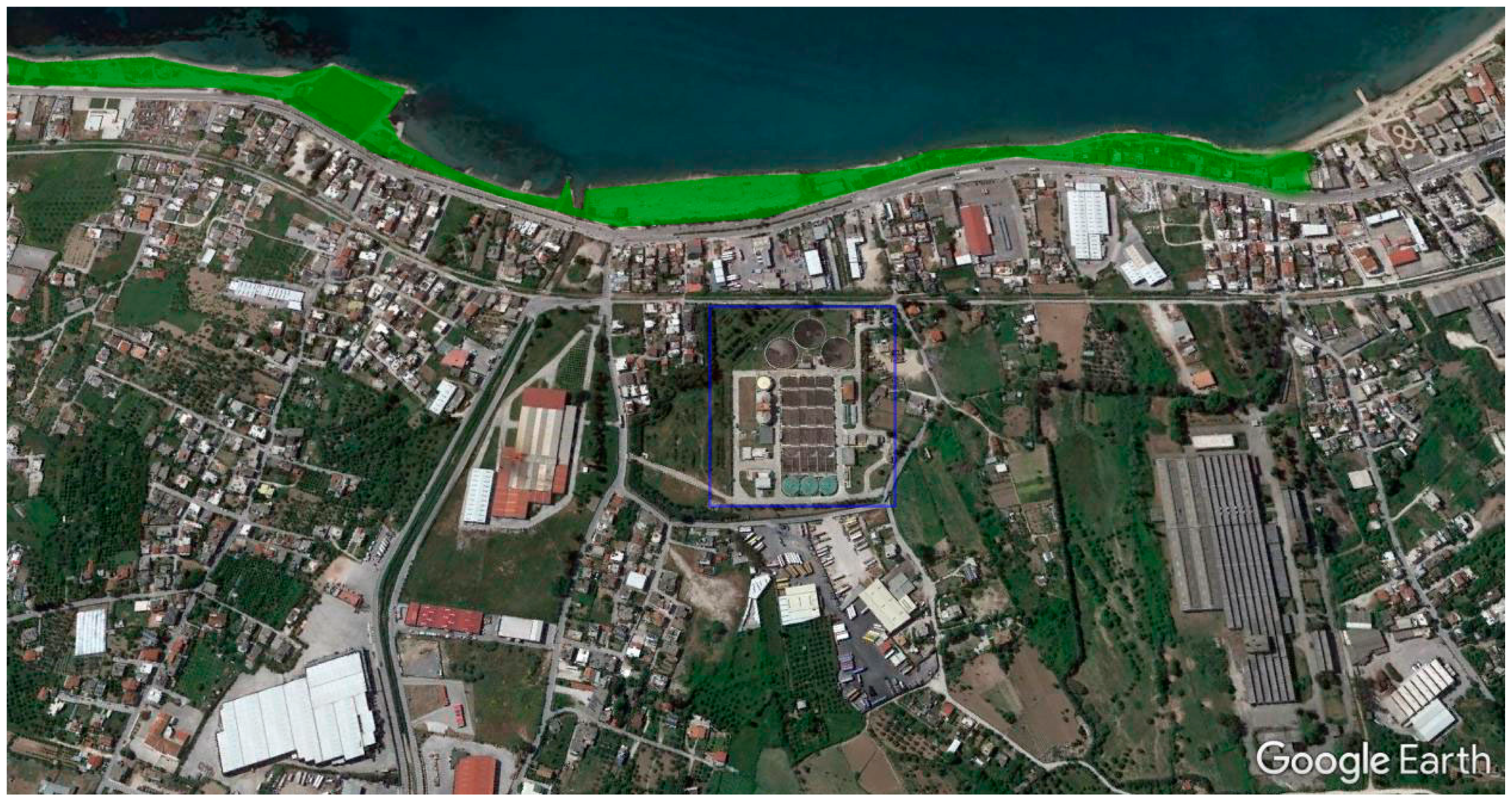
2. Materials and Methods
2.1. Analytical Techniques
2.2. Statistical Analysis
3. Results and Discussion
3.1. Membrane Performance
3.1.1. Permeate Flux
3.1.2. Membrane Operational Parameters
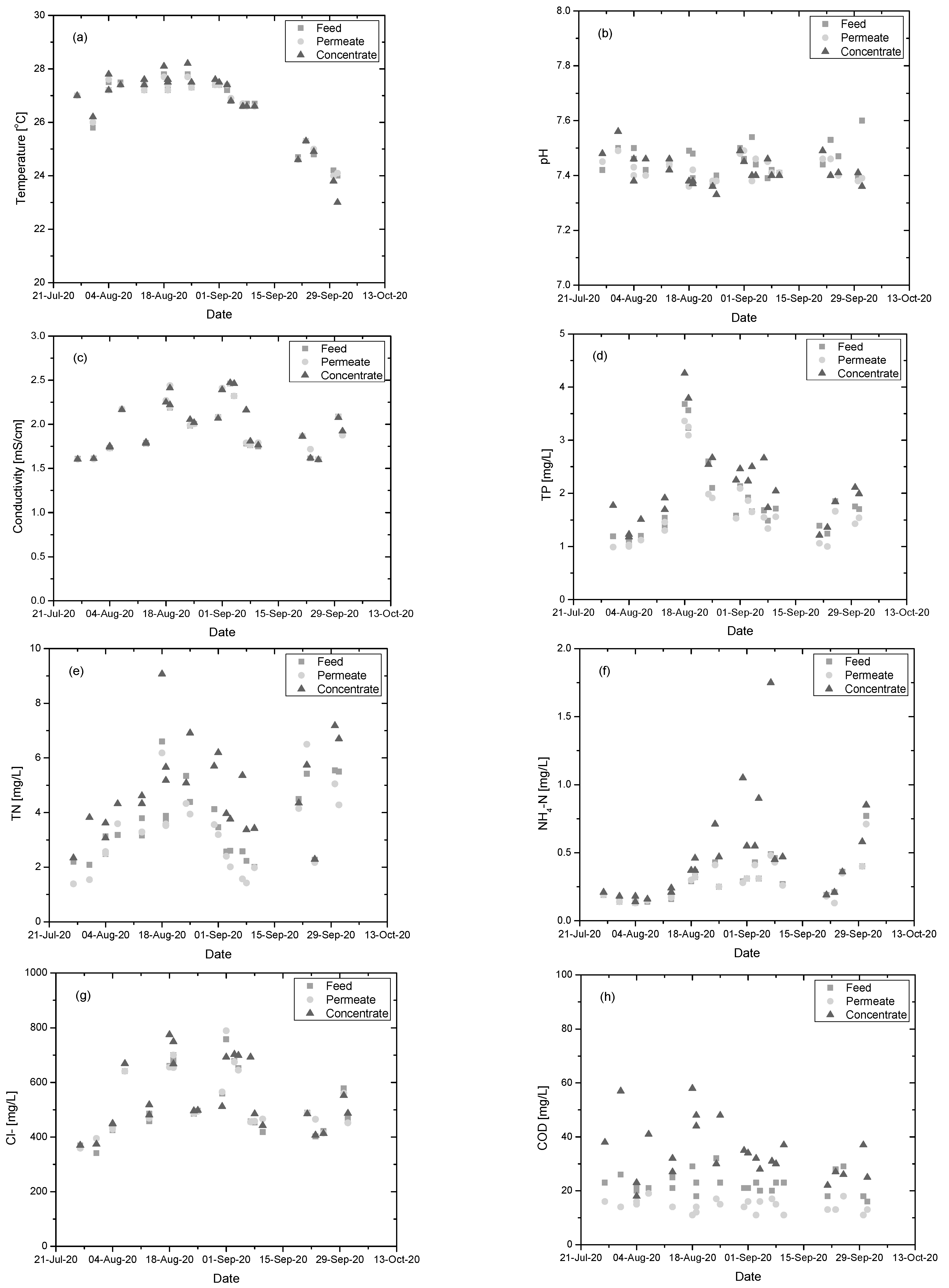
3.1.3. Physicochemical Characteristics of Feed, Permeate and Concentrate Streams
3.2. Proposed Reuse Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Operational Parameter | Type of Comparison | Values Compared | p-Value |
|---|---|---|---|
| P/F ratio | Two-tailed homoscedastic | 0.7–0.75 | 0.409 |
| 0.7–0.8 | 0.373 | ||
| 0.7–0.9 | 0.733 | ||
| 0.75–0.8 | 0.932 | ||
| 0.75–0.9 | 0.685 | ||
| 0.8–0.9 | 0.650 | ||
| Length of filtration before cleaning cycle | Two-tailed homoscedastic | 30–60 | 0.163 |
| 30–90 | 0.270 | ||
| 60–90 | 0.790 | ||
| Washing duration | Two-tailed homoscedastic | 4–8 | 0.840 |
| 4–6 | 0.102 | ||
| 6–8 | 0.361 |
| Comparison | Type of Comparison | Parameter | p-Value |
|---|---|---|---|
| Feed–Permeate | Two-tailed pairwise | Temperature | 0.575 |
| pH | 0.024 | ||
| Conductivity | 0.150 | ||
| Turbidity | <0.001 | ||
| TSS | <0.001 | ||
| TS | <0.001 | ||
| TP | <0.001 | ||
| TN | 0.001 | ||
| NH4-N | 0.037 | ||
| NO2-N | 0.255 | ||
| NO3-N | 0.501 | ||
| Cl− | 0.339 | ||
| COD | <0.001 |
References
- Weerasekara, P. The United Nations World Water Development Report 2017 Wastewater. Futur. Food J. Food Agric. Soc. 2017, 5, 80–81. [Google Scholar]
- Plevri, A.; Lytras, E.; Samios, S.; Lioumis, C.; Monokrousou, K.; Makropoulos, C. Sewer Mining as A Basis for Technological, Business and Governance Solutions for Water in the Circular Economy: The NextGen Athens Demo. Environ. Sci. Proc. 2020, 2, 54. [Google Scholar] [CrossRef]
- Rochdane, S.; Reichert, B.; Messouli, M.; Babqiqi, A.; Khebiza, M.Y. Climate change impacts on water supply and demand in Rheraya Watershed (Morocco), with potential adaptation strategies. Water 2012, 4, 28–44. [Google Scholar] [CrossRef] [Green Version]
- Gedefaw, M.; Wang, H.; Yan, D.; Qin, T.; Wang, K.; Girma, A.; Batsuren, D.; Abiyu, A. Water resources allocation systems under irrigation expansion and climate change scenario in Awash River Basin of Ethiopia. Water 2019, 11, 1966. [Google Scholar] [CrossRef] [Green Version]
- Goonetilleke, A.; Vithanage, M. Water resources management: Innovation and challenges in a changing world. Water 2017, 9, 281. [Google Scholar] [CrossRef]
- Zhou, Q. A Review of Sustainable Urban Drainage Systems Considering the Climate Change and Urbanization Impacts. Water 2014, 6, 976–992. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, B.; Asano, T. Water reclamation and reuse around the world. Water Reuse Int. Surv. Curr. Pract. Issues Needs 2008, 14, 3–26. [Google Scholar]
- Kéfi, S.; Rietkerk, M.; Alados, C.L.; Pueyo, Y.; Papanastasis, V.P.; ElAich, A.; de Ruiter, P.C. Spatial vegetation patterns and imminent desertification in Mediterranean arid ecosystems. Nature 2007, 449, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Conte, G.; Bolognesi, A.; Bragalli, C.; Branchini, S.; Carli, A.D.; Lenzi, C.; Masi, F.; Massarutto, A.; Pollastri, M.; Principi, I. Innovative Urban Water Management as a Climate Change Adaptation Strategy: Results from the Implementation of the Project “Water Against Climate Change (WATACLIC)”. Water 2012, 4, 1025–1038. [Google Scholar] [CrossRef] [Green Version]
- Mo, W.; Zhang, Q. Energy–nutrients–water nexus: Integrated resource recovery in municipal wastewater treatment plants. J. Environ. Manag. 2013, 127, 255–267. [Google Scholar] [CrossRef]
- Zhu, Z.; Dou, J. Current status of reclaimed water in China: An overview. J. Water Reuse Desalin. 2018, 8, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Yi, L.; Jiao, W.; Chen, X.; Chen, W. An overview of reclaimed water reuse in China. J. Environ. Sci. 2011, 23, 1585–1593. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Darby, J.; Bourgeous, K.; McArdle, J.; Genest, P.; Tylla, M. Ultrafiltration as an advanced tertiary treatment process for municipal wastewater. Desalination 1998, 119, 315–321. [Google Scholar] [CrossRef]
- Alonso, E.; Santos, A.; Solis, G.J.; Riesco, P. On the feasibility of urban wastewater tertiary treatment by membranes: A comparative assessment. Desalination 2001, 141, 39–51. [Google Scholar] [CrossRef]
- Qu, F.; Wang, H.; He, J.; Fan, G.; Pan, Z.; Tian, J.; Rong, H.; Li, G.; Yu, H. Tertiary treatment of secondary effluent using ultrafiltration for wastewater reuse: Correlating membrane fouling with rejection of effluent organic matter and hydrophobic pharmaceuticals. Environ. Sci. Water Res. Technol. 2019, 5, 672–683. [Google Scholar] [CrossRef]
- Kramer, F.C.; Shang, R.; Heijman, S.G.J.; Scherrenberg, S.M.; van Lier, J.B.; Rietveld, L.C. Direct water reclamation from sewage using ceramic tight ultra- and nanofiltration. Sep. Purif. Technol. 2015, 147, 329–336. [Google Scholar] [CrossRef]
- Joint Ministerial Desicion ΚΥA 145116_2011. Establishment of measures, conditions and procedures for reuse of treated wastewater and other provisions. Greek Gov. Gaz. 2011, 354, 5229–5244. [Google Scholar]
- Joint Ministerial Desicion ΚΥA 191002_2013. Amendment of ΚΥA 145116_2011. Greek Gov. Gaz. 2013, 2220, 31219–31222. [Google Scholar]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA: Washington, DC, USA, 2012.
- Karaolia, P.; Michael, I.; García-Fernández, I.; Agüera, A.; Malato, S.; Fernández-Ibáñez, P.; Fatta-Kassinos, D. Reduction of clarithromycin and sulfamethoxazole-resistant Enterococcus by pilot-scale solar-driven Fenton oxidation. Sci. Total Environ. 2014, 468, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Illueca-Muñoz, J.; Mendoza-Roca, J.A.; Iborra-Clar, A.; Bes-Piá, A.; Fajardo-Montañana, V.; Martínez-Francisco, F.J.; Bernácer-Bonora, I. Study of different alternatives of tertiary treatments for wastewater reclamation to optimize the water quality for irrigation reuse. Desalination 2008, 222, 222–229. [Google Scholar] [CrossRef]
- Gómez, M.; Plaza, F.; Garralón, G.; Pérez, J.; Gómez, M.A. A comparative study of tertiary wastewater treatment by physico-chemical-UV process and macrofiltration–ultrafiltration technologies. Desalination 2007, 202, 369–376. [Google Scholar] [CrossRef]
- Dialynas, E.; Diamadopoulos, E. Integration of immersed membrane ultrafiltration with coagulation and activated carbon adsorption for advanced treatment of municipal wastewater. Desalination 2008, 230, 113–127. [Google Scholar] [CrossRef]
- Al-Bastaki, N.M. Performance of advanced methods for treatment of wastewater: UV/TiO2, RO and UF. Chem. Eng. Process. Process Intensif. 2004, 43, 935–940. [Google Scholar] [CrossRef]
- University of California—Division of Agriculture and Natural Resources Landscape Water Requirement Calculators. Available online: https://ucanr.edu/sites/UrbanHort (accessed on 29 July 2021).



| Parameter | (a) | (b) | (c) |
|---|---|---|---|
| Escherichia coli (CFU/100 mL) | <200 | <5 | N.A. |
| Total coliform bacteria (CFU/100 mL) | N.A. | N.A. | <2 |
| BOD5 (mg/L) | <25 | <10 | <10 |
| TSS (mg/L) | <35 | <10 | <2 |
| Residual chlorine | Cont. monitoring | Cont. monitoring | Cont. monitoring |
| Turbidity (NTU) | N.A. | <2 | <2 |
| Total nitrogen (mg/L) | <15 * | <15 * | <15 * |
| Total phosphorus (mg/L) | <2 * | <2 * | <2 * |
| Minimum treatment required | Secondary treatment and disinfection | Secondary, tertiary treatment, and disinfection | Secondary, advanced treatment (ultrafiltration or equal treatment), and disinfection |
| Parameter | Value |
|---|---|
| Number of parallel treatment lines | 2 |
| Membrane modules | 8 UF membrane modules (hollow fiber) LH3-1060 |
| Membrane material | PVC |
| Filtration mode | Cross flow |
| Operational temperature | 5–38 °C |
| Operational pH | 2–13 |
| Reverse flow cleaning frequency | 20–60 min |
| Membrane pore size | 0.01 μm |
| Membrane MWCO | 50,000 Da |
| Permeate suspended solids | <2 mg/L |
| Feed capacity | 400 m3/d |
| Permeate flow rate | 280–360 m3/d |
| Concentrate flow rate | 40–120 m3/d |
| Feeding pumps | 2 × 2.2 kW |
| Parameter | Unit | Number of Experiments | Membrane Feed | Membrane Permeate | Membrane Concentrate |
|---|---|---|---|---|---|
| pH | 24 | 7.45 ± 0.06 | 7.42 ± 0.04 | 7.42 ± 0.05 | |
| Conductivity | mS/cm | 24 | 1.95 ± 0.28 | 1.96 ± 0.28 | 1.98 ± 0.29 |
| Turbidity | NTU | 24 | 89.29 ± 15.56 | <1 | 31.15 ± 11.04 |
| TSS | mg/L | 24 | 16.45 ± 7.19 | <1 | 26.68 ± 1.38 |
| TS | mg/L | 17 | 831 ± 159 | 597 ± 132 | 979 ± 198 |
| TP | mg/L | 23 | 1.86 ± 0.74 | 1.69 ± 0.69 | 2.21 ± 0.83 |
| TN | mg/L | 24 | 3.62 ± 1.32 | 3.25 ± 1.4 | 4.83 ± 1.63 |
| NH4+-N | mg/L | 24 | 0.30 ± 0.15 | 0.29 ± 0.14 | 0.48 ± 0.37 |
| NO2−-N | mg/L | 24 | 0.13 ± 0.07 | 0.13 ± 0.07 | 0.17 ± 0.07 |
| NO3−-N | mg/L | 24 | 1.18 ± 0.88 | 1.15 ± 0.93 | 1.47 ± 0.97 |
| Cl− | mg/L | 24 | 521 ± 118 | 526 ± 113 | 545 ± 126 |
| COD | mg/L | 24 | 22.63 ± 3.93 | 14.38 ± 2.26 | 34.50 ± 10.48 |
| BOD | mg/L | 9 | 6.67 ± 3.2 | 4.78 ± 3.19 | 8.45 ± 4.37 |
| Na | mg/L | 1 | 86.70 | 92.80 | 94.25 |
| K | mg/L | 1 | 16.15 | 19.66 | 18.35 |
| Fe | μg/L | 1 | 87.00 | 63.00 | 107.00 |
| Mn | μg/L | 1 | 63.00 | 56.00 | 67.00 |
| E. coli | CFU/100 mL | 4 | (6.12 ± 7.78) × 104 | N.D. | (1.67 ± 1.59) × 105 |
| Enterococcus | CFU/100 mL | 4 | (1.87 ± 2.85) × 105 | N.D. | (5.27 ± 4.48) × 105 |
| Other Coliforms | CFU/100 mL | 4 | (5.05 ± 4.47) × 104 | (5.35 ± 2.6) × 103 | (8.60 ± 3.68) × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagklis, D.; Katrivesis, F.K.; Sygouni, V.; Tsarouchi, L.; Tsigkou, K.; Kornaros, M.; Paraskeva, C.A. Recovery of Water from Secondary Effluent through Pilot Scale Ultrafiltration Membranes: Implementation at Patras’ Wastewater Treatment Plant. Membranes 2021, 11, 663. https://doi.org/10.3390/membranes11090663
Zagklis D, Katrivesis FK, Sygouni V, Tsarouchi L, Tsigkou K, Kornaros M, Paraskeva CA. Recovery of Water from Secondary Effluent through Pilot Scale Ultrafiltration Membranes: Implementation at Patras’ Wastewater Treatment Plant. Membranes. 2021; 11(9):663. https://doi.org/10.3390/membranes11090663
Chicago/Turabian StyleZagklis, Dimitris, Fotios K. Katrivesis, Varvara Sygouni, Lamprini Tsarouchi, Konstantina Tsigkou, Michael Kornaros, and Christakis A. Paraskeva. 2021. "Recovery of Water from Secondary Effluent through Pilot Scale Ultrafiltration Membranes: Implementation at Patras’ Wastewater Treatment Plant" Membranes 11, no. 9: 663. https://doi.org/10.3390/membranes11090663
APA StyleZagklis, D., Katrivesis, F. K., Sygouni, V., Tsarouchi, L., Tsigkou, K., Kornaros, M., & Paraskeva, C. A. (2021). Recovery of Water from Secondary Effluent through Pilot Scale Ultrafiltration Membranes: Implementation at Patras’ Wastewater Treatment Plant. Membranes, 11(9), 663. https://doi.org/10.3390/membranes11090663