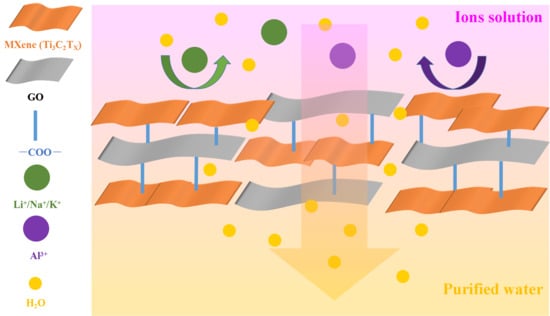
Supported MXene/GO Composite Membranes with Suppressed Swelling for Metal Ion Sieving
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Pristine MXene/GO Composite Membranes
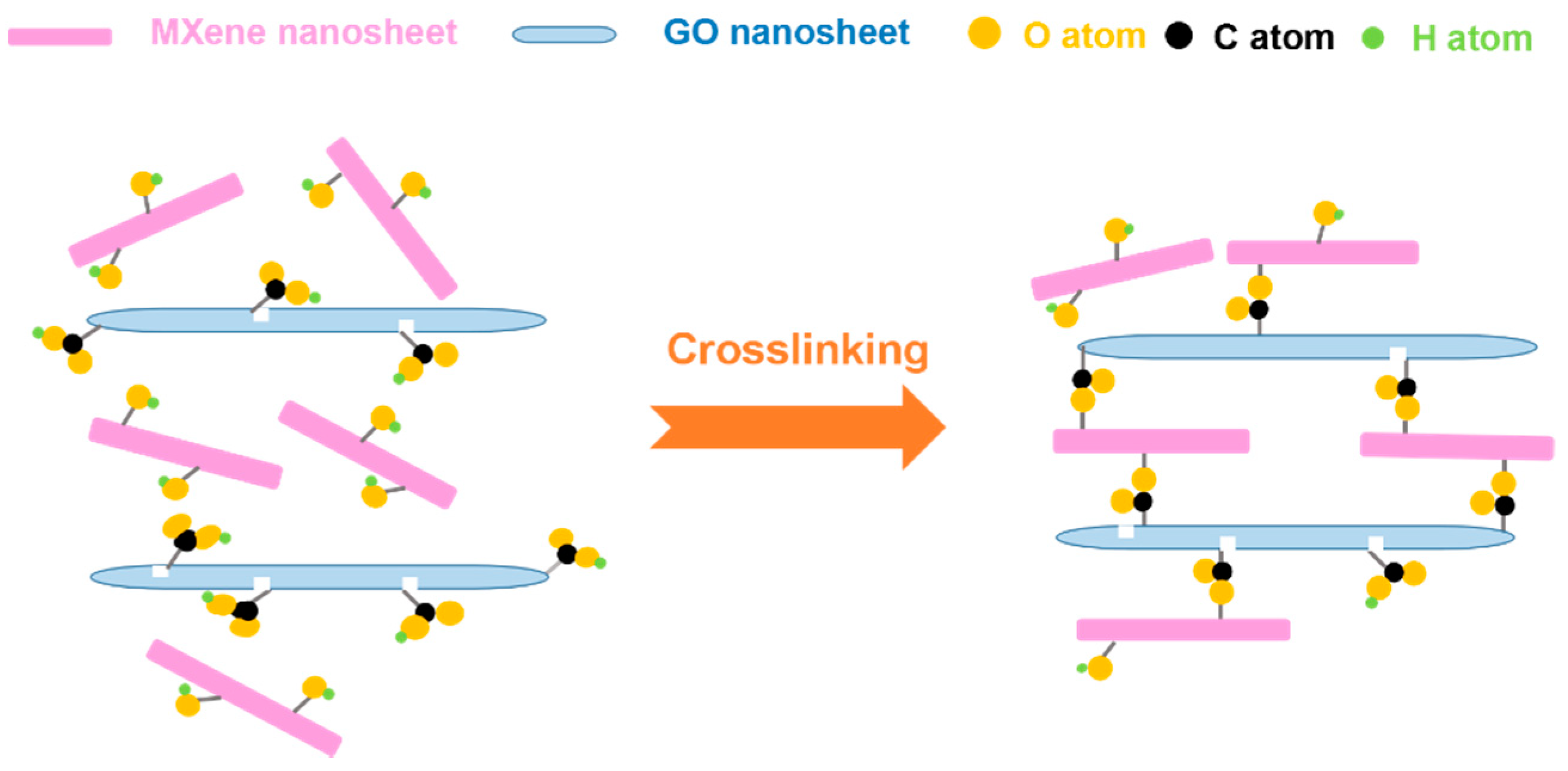
2.3. Preparation of the Cross-Linked MXene/GO Composite Membranes
2.4. Ion Permeation
2.5. Characterizations
3. Results and Discussion
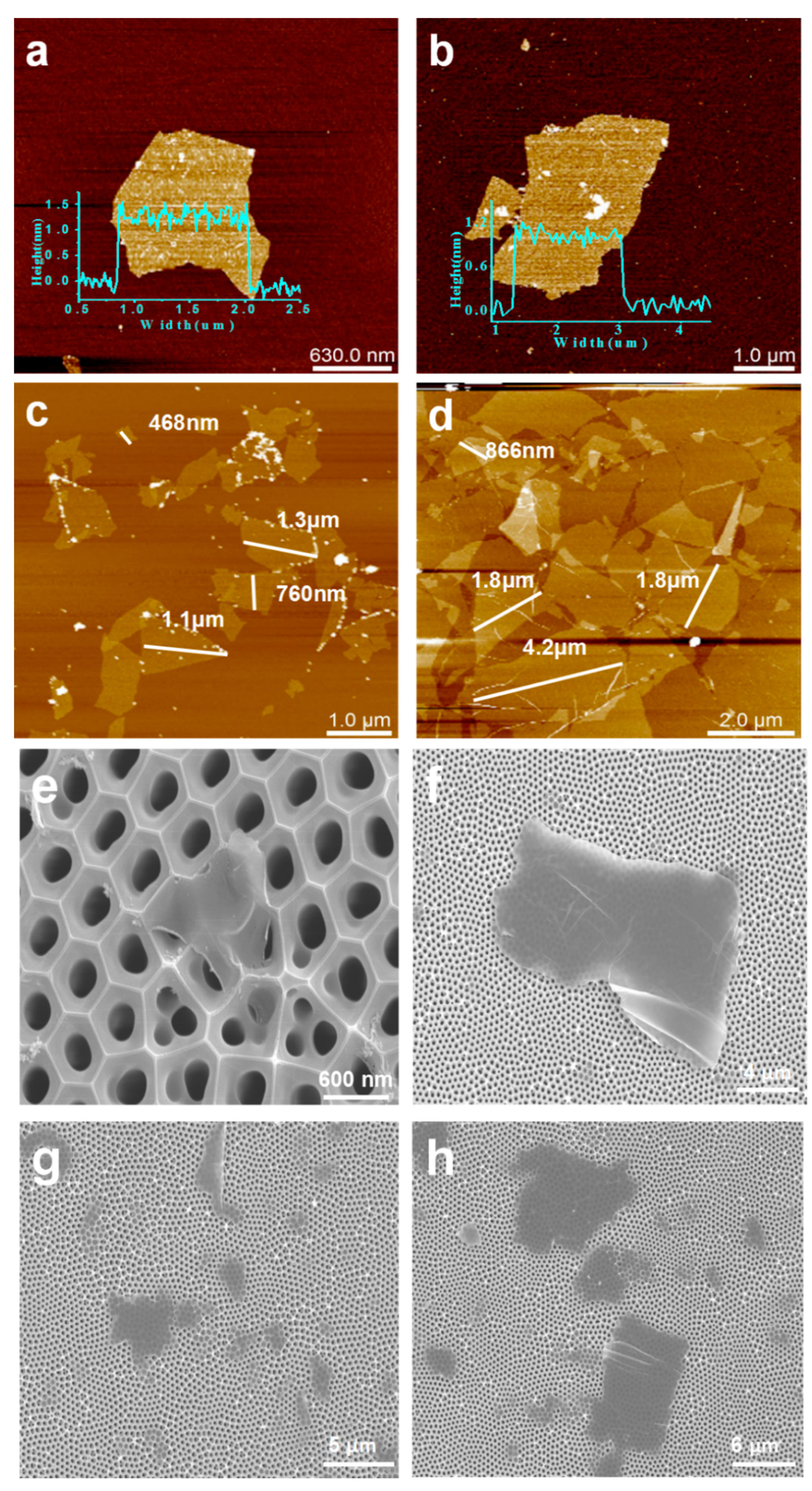
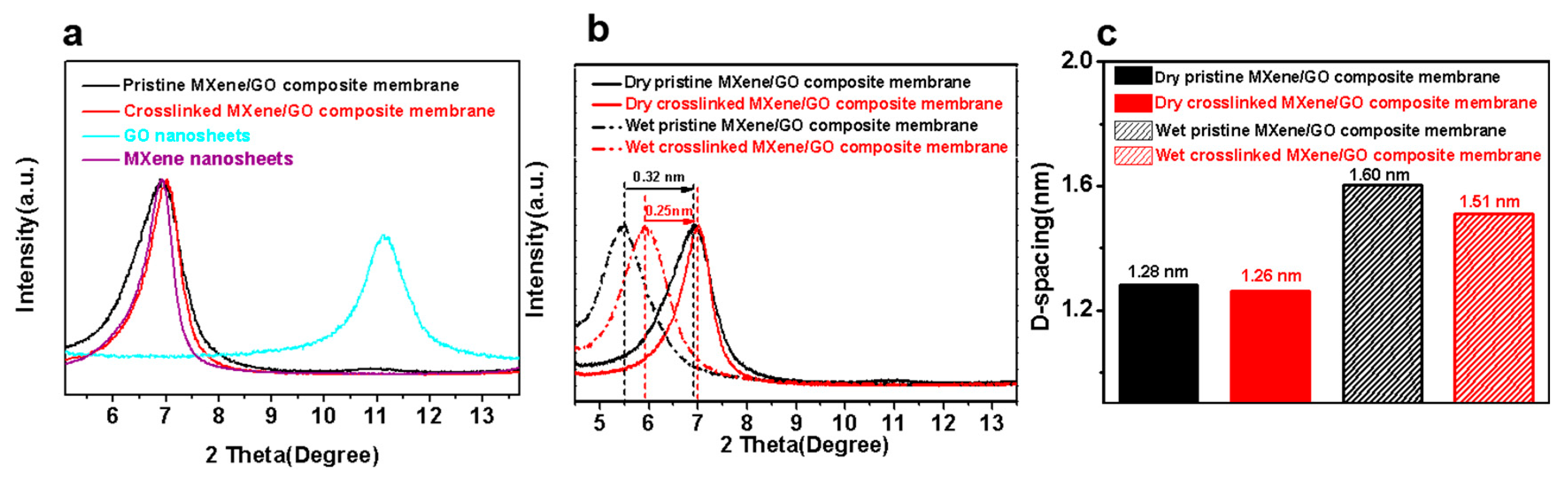
3.1. Characterization of the MXene Nanosheets and GO Nanosheets
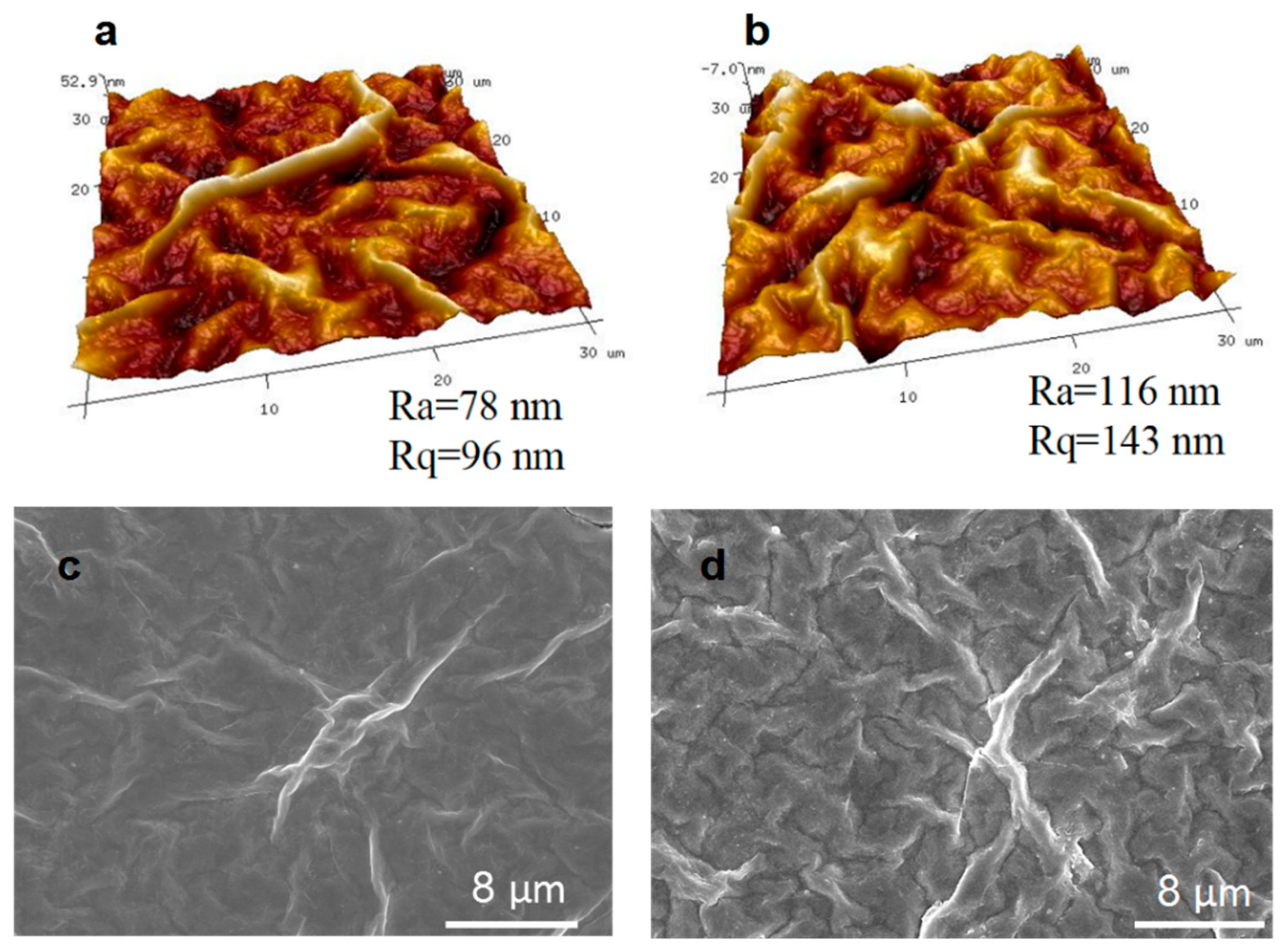
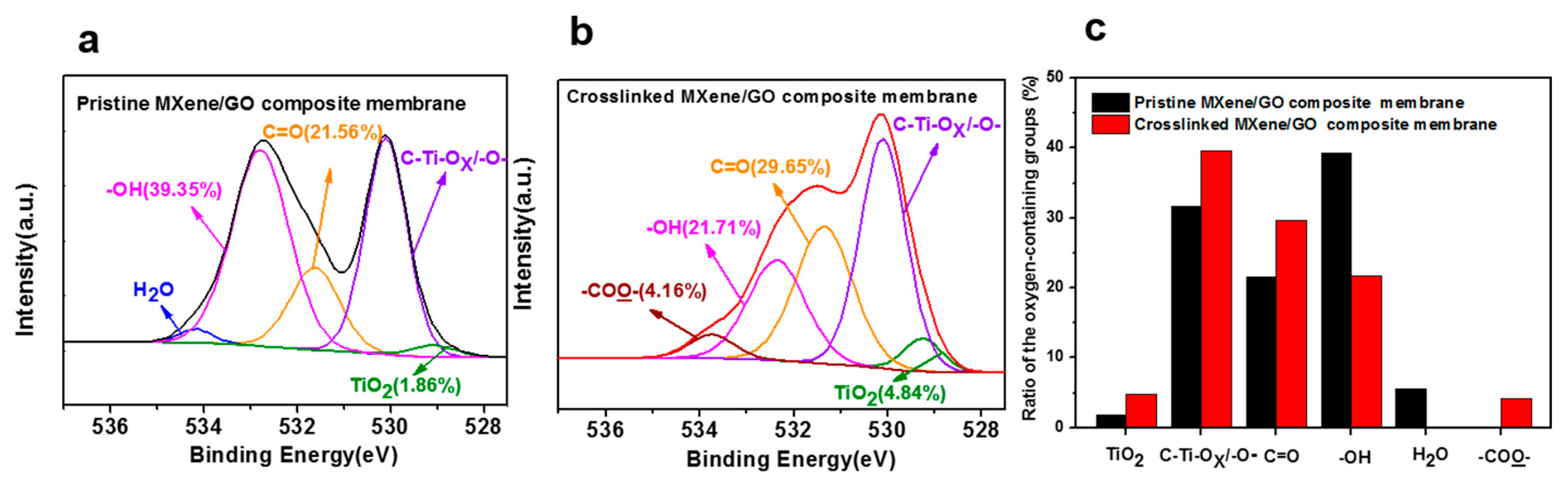
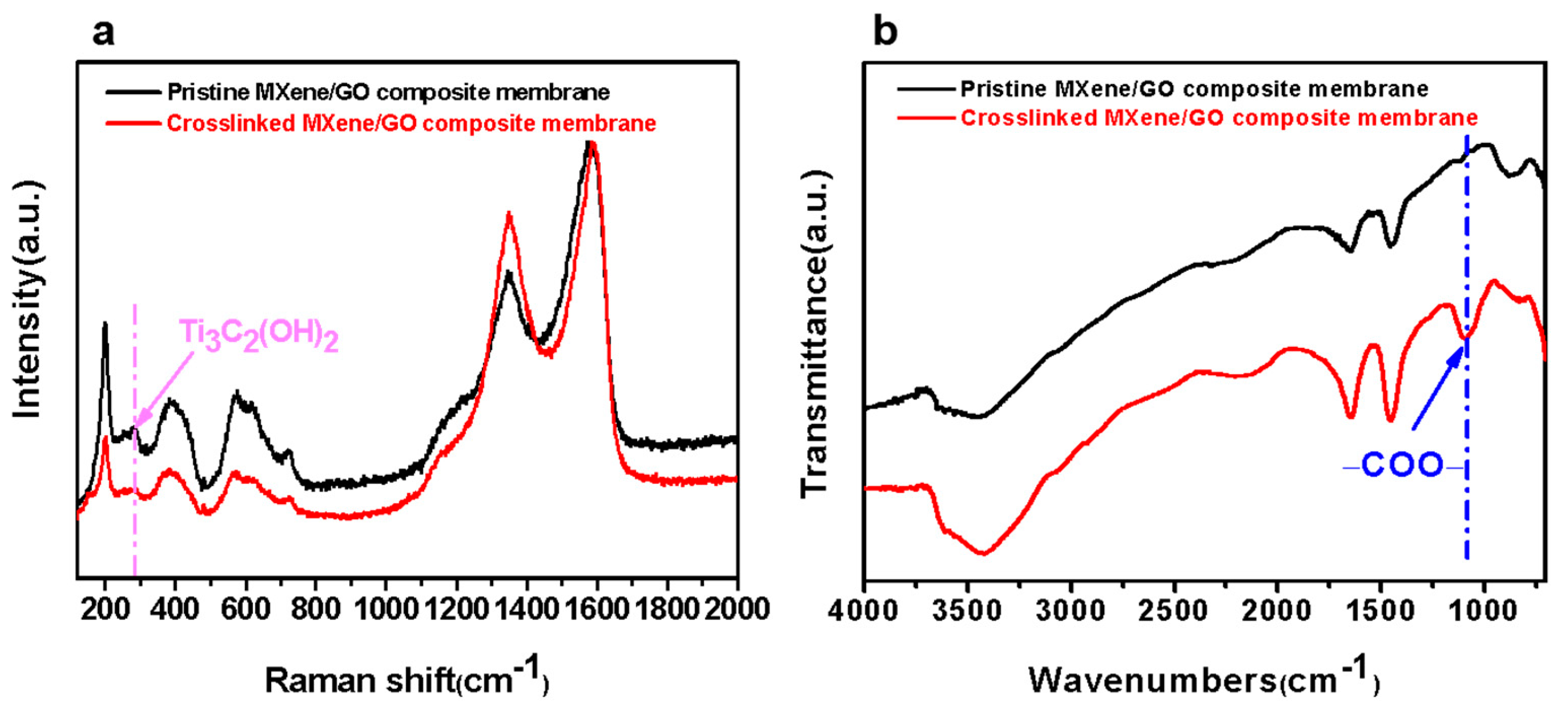
3.2. Characterization of the MXene/GO Composite Membranes
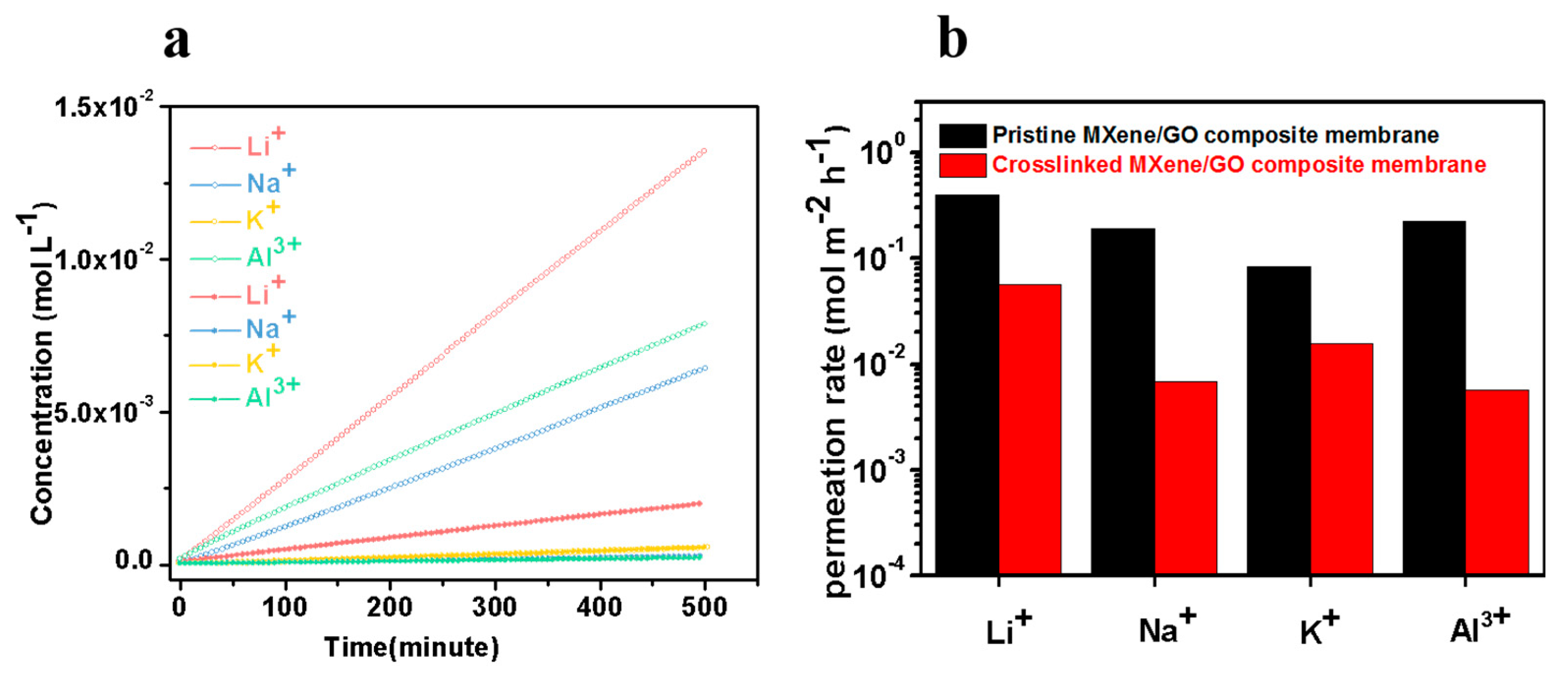
3.3. Ion Exclusion Performance of the MXene/GO Composite Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, Y.; Jiang, Z.; Zhang, Y.; Wang, T.; Zhao, P.; Zhang, Z.; Yuan, J.; Wang, H. All-poly (ionic liquid) membrane-derived porous carbon membranes: Scalable synthesis and application for photothermal conversion in seawater desalination. ACS Nano 2018, 12, 11704–11710. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-Y.; Yuan, H.-G.; Liu, Y.-Y.; Ren, D.; Su, Y.-C.; Wang, X. Metal-organic framework nanocomposite thin films with interfacial bindings and self-Standing robustness for high water flux and enhanced ion selectivity. ACS Nano 2018, 12, 9253–9265. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Dey, K.; Pal, M.; Rout, K.C.; Kunjattu, H.S.; Das, A.; Mukherjee, R.; Kharul, U.K.; Banerjee, R. Selective molecular separation by interfacially crystallized covalent organic framework thin films. J. Am. Chem. Soc. 2017, 139, 13083–13091. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sires, I.; Scialdone, O. Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: A critical review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, P.; Li, P.; Ji, Y.; Liu, G.; Jin, W. Designing biomimic two-dimensional ionic transport channels for efficient ion sieving. ACS Nano 2021, 15, 5209–5220. [Google Scholar] [CrossRef]
- Ding, L.; Wei, Y.; Li, L.; Zhang, T.; Wang, H.; Xue, J.; Ding, L.; Wang, S.; Caro, J.; Gogotsi, Y. MXene molecular sieving membranes for highly efficient gas separation. Nat. Commun. 2018, 9, 435–676. [Google Scholar] [CrossRef]
- Deng, J.; Lu, Z.; Ding, L.; Li, Z.; Wei, Y.; Caro, J. Fast electrophoretic preparation of large-area two-dimensional titanium carbide membranes for ion sieving. Chem. Eng. J. 2021, 408, 127806. [Google Scholar] [CrossRef]
- Karan, S.; Samitsu, S.; Peng, X.; Kurashima, K.; Ichinose, I. Ultrafast viscous permeation of organic solvents through diamond-like carbon nanosheets. Science 2012, 335, 444–447. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Duan, Y.; Wei, Y.; Dong, C.; Ding, L.; Qiao, Z.; Wang, H. Selective gas diffusion in two-dimensional MXene lamellar membranes: Insights from molecular dynamics simulations. J. Mater. Chem. A 2018, 6, 11734–11742. [Google Scholar] [CrossRef]
- Wang, W.; Wei, Y.; Fan, J.; Cai, J.; Lu, Z.; Ding, L.; Wang, H. Recent progress of two-dimensional nanosheet membranes and composite membranes for separation applications. Front. Chem. Sci. Eng. 2021, 15, 793–819. [Google Scholar] [CrossRef]
- Yang, J.; Gong, D.; Li, G.; Zeng, G.; Wang, Q.; Zhang, Y.; Liu, G.; Wu, P.; Vovk, E.; Peng, Z.; et al. Self-assembly of thiourea crosslinked graphene oxide framework membranes toward separation of small molecules. Adv. Mater. 2018, 30, 1705775. [Google Scholar] [CrossRef]
- Thebo, K.H.; Qian, X.; Zhang, Q.; Chen, L.; Cheng, H.-M.; Ren, W. Highly stable graphene-oxide-based membranes with superior permeability. Nat. Commun. 2018, 9, 1486. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Wang, K.; Zhu, H. Recent developments in graphene-based membranes: Structure, mass-transport mechanism and potential applications. Adv. Mater. 2016, 28, 2287–2310. [Google Scholar] [CrossRef]
- Zhang, M.; Mao, Y.; Liu, G.; Liu, G.; Fan, Y.; Jin, W. Molecular bridges stabilize graphene oxide membranes in water. Angew. Chem. Int. Ed. 2020, 59, 1689–1695. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, K.; Ji, Y.; Liu, G.; Jin, W.; Xu, N. Controllable ion transport by surface-charged graphene oxide membrane. Nat. Commun. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Liu, G.; Jin, W.; Li, X. Fungal cell wall-graphene oxide microcomposite membrane for organic solvent nanofiltration. Adv. Funct. Mater. 2021, 31, 2100110. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, M.; Mao, Y.; Liu, G.; Liu, J. Theoretical study of Janus graphene oxide membrane for water transport. Front. Chem. Sci. Eng. 2021, 15, 913–921. [Google Scholar] [CrossRef]
- Hu, R.; He, Y.; Zhang, C.; Zhang, R.; Li, J.; Zhu, H. Graphene oxide embedded polyamide nanofiltration membranes for selective ion separation. J. Mater. Chem. A 2017, 5, 25632–25640. [Google Scholar] [CrossRef]
- Mi, B. Graphene oxide membranes for ionic and molecular sieving. Science 2014, 343, 740–742. [Google Scholar] [CrossRef]
- Huang, H.; Mao, Y.; Ying, Y.; Liu, Y.; Sun, L.; Peng, X. Salt concentration, pH and pressure controlled separation of small molecules through lamellar graphene oxide membranes. Chem. Commun. 2013, 49, 5963–5965. [Google Scholar] [CrossRef]
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge-and size-selective ion sieving through Ti3C2Tx MXene membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031. [Google Scholar] [CrossRef]
- Ding, L.; Wei, Y.; Wang, Y.; Chen, H.; Caro, J.; Wang, H. A Two-dimensional lamellar membrane: MXene nanosheet stacks. Angew. Chem. Int. Ed. 2017, 56, 1825–1829. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, G.-F.; Ding, L.; Chen, X.; Ding, L.-X.; Wang, H. Efficient electrocatalytic N2 fixation with MXene under ambient conditions. Joule 2019, 3, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Fan, Y.; Wei, L.; Meng, X.; Zhang, W.; Yang, N.; Jin, Y.; Wang, X.; Zhao, M.; Liu, S. An unprecedented high-temperature tolerance 2D laminar MXene membrane for ultrafast hydrogen sieving. J. Membr. Sci. 2019, 569, 117–123. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Li, L.; Liu, Y.; Wu, Y.; Lu, Z.; Deng, J.; Wei, Y.; Caro, J.; Wang, H. Effective ion sieving with Ti3C2Tx MXene membranes for production of drinking water from seawater. Nat. Sustain. 2020, 3, 296–302. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Ji, Y.; Liu, Q.; Cheng, L.; Guan, K.; Zhang, M.; Liu, G. 2D MXene nanofilms with tunable gas transport channels. Adv. Funct. Mater. 2018, 28, 1801511. [Google Scholar] [CrossRef]
- Liu, G.; Liu, S.; Ma, K.; Wang, H.; Wang, X.; Liu, G.; Jin, W. Polyelectrolytes functionalized Ti2CTx MXene membranes for pervaporation dehydration of isopropanol/water mixtures. Ind. Eng. Chem. Res. 2020, 59, 4732–4741. [Google Scholar] [CrossRef]
- Surwade, S.P.; Smirnov, S.N.; Vlassiouk, I.V.; Unocic, R.R.; Veith, G.M.; Dai, S.; Mahurin, S.M. Water desalination using nanoporous single-layer graphene. Nat. Nanotechnol. 2015, 10, 459–464. [Google Scholar] [CrossRef]
- Yeh, C.-N.; Raidongia, K.; Shao, J.; Yang, Q.-H.; Huang, J. On the origin of the stability of graphene oxide membranes in water. Nat. Chem. 2015, 7, 166. [Google Scholar] [CrossRef]
- Zheng, S.; Tu, Q.; Urban, J.J.; Li, S.; Mi, B. Swelling of graphene oxide membranes in aqueous solution: Characterization of interlayer spacing and insight into water transport mechanisms. ACS Nano 2017, 11, 6440–6450. [Google Scholar] [CrossRef]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 2017, 12, 546. [Google Scholar] [CrossRef]
- He, J.; Lin, X.M.; Chan, H.; Vukovic, L.; Král, P.; Jaeger, H.M. Diffusion and filtration properties of self-assembled gold nanocrystal membranes. Nano Lett. 2011, 11, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, C.; Hung, W.S.; Lu, X.; Lee, K.R. One-step constructed ultrathin Janus polyamide nanofilm with opposite charges for highly efficient nanofiltration. J. Mater. Chem. A 2017, 5, 22988–22996. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Gao, C. High-flux graphene oxide nanofiltration membrane intercalated by carbon nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 8147–8155. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, Y.; Xue, J.; Wang, H. Enhanced anti-pressure ability through graphene oxide membrane by intercalating g-C3N4 nanosheets for water purification. AIChE J. 2019, 65, e16699. [Google Scholar] [CrossRef]
- Goh, K.; Jiang, W.; Karahan, H.E.; Zhai, S.; Wei, L.; Yu, D.; Fane, A.G.; Wang, R.; Chen, Y. All-carbon nanoarchitectures as high performance separation membranes with superior stability. Adv. Funct. Mater. 2015, 25, 7348–7359. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Enabling graphene oxide nanosheets as water separation membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Chung, T.-S. Nanometric graphene oxide framework membranes with enhanced heavy metal removal via nanofiltration. Environ. Sci. Technol. 2015, 49, 10235–10242. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z.; et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, J.; Guo, D.; Li, Z.; Su, Y. Ti3C2Tx MXene/Graphene nanocomposites: Synthesis and application in electrochemical energy storage. J. Alloys Compd. 2019, 815, 152403. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, Y.; Deng, J.; Ding, L.; Li, Z.; Wang, H. Self-crosslinked MXene (Ti3C2Tx) membranes with good antiswelling property for monovalent metal ion exclusion. ACS Nano 2019, 13, 10535–10544. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Z.; Lu, Z.; Xu, Y.; Zhang, Y.; He, L.; Li, P.; Xiong, L.; Ding, L.; Wei, Y.; Wang, H. Supported MXene/GO Composite Membranes with Suppressed Swelling for Metal Ion Sieving. Membranes 2021, 11, 621. https://doi.org/10.3390/membranes11080621
Yin Z, Lu Z, Xu Y, Zhang Y, He L, Li P, Xiong L, Ding L, Wei Y, Wang H. Supported MXene/GO Composite Membranes with Suppressed Swelling for Metal Ion Sieving. Membranes. 2021; 11(8):621. https://doi.org/10.3390/membranes11080621
Chicago/Turabian StyleYin, Zongjie, Zong Lu, Yanyan Xu, Yonghong Zhang, Liliang He, Peishan Li, Lei Xiong, Li Ding, Yanying Wei, and Haihui Wang. 2021. "Supported MXene/GO Composite Membranes with Suppressed Swelling for Metal Ion Sieving" Membranes 11, no. 8: 621. https://doi.org/10.3390/membranes11080621
APA StyleYin, Z., Lu, Z., Xu, Y., Zhang, Y., He, L., Li, P., Xiong, L., Ding, L., Wei, Y., & Wang, H. (2021). Supported MXene/GO Composite Membranes with Suppressed Swelling for Metal Ion Sieving. Membranes, 11(8), 621. https://doi.org/10.3390/membranes11080621