An Experimental Performance Study of a Catalytic Membrane Reactor for Ethanol Steam Reforming over a Metal Honeycomb Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.2. Membrane Preparation
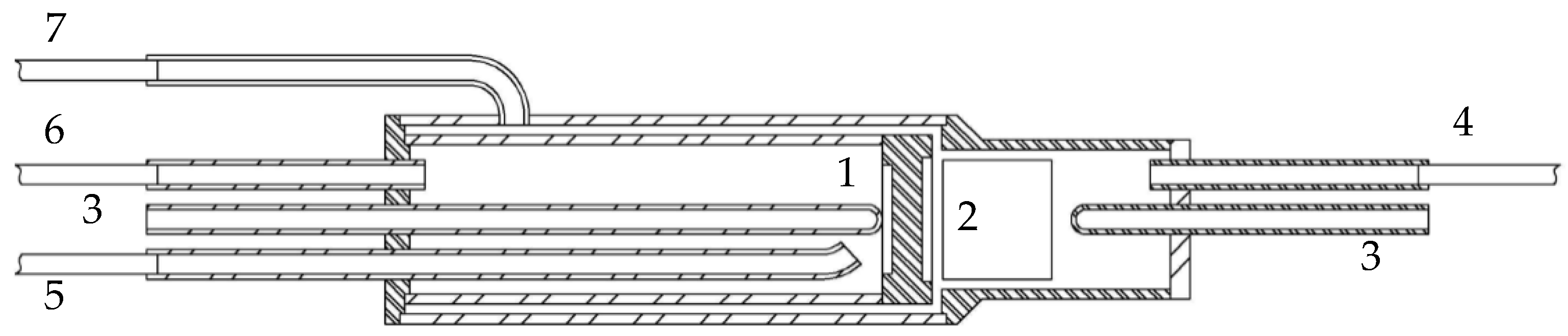
2.3. Evaluation of the Catalytic Membrane Reactor Performance
3. Results and Discussion
3.1. Performance of the Monolithic Catalyst for Steam Reforming of Ethanol for Hydrogen Generation
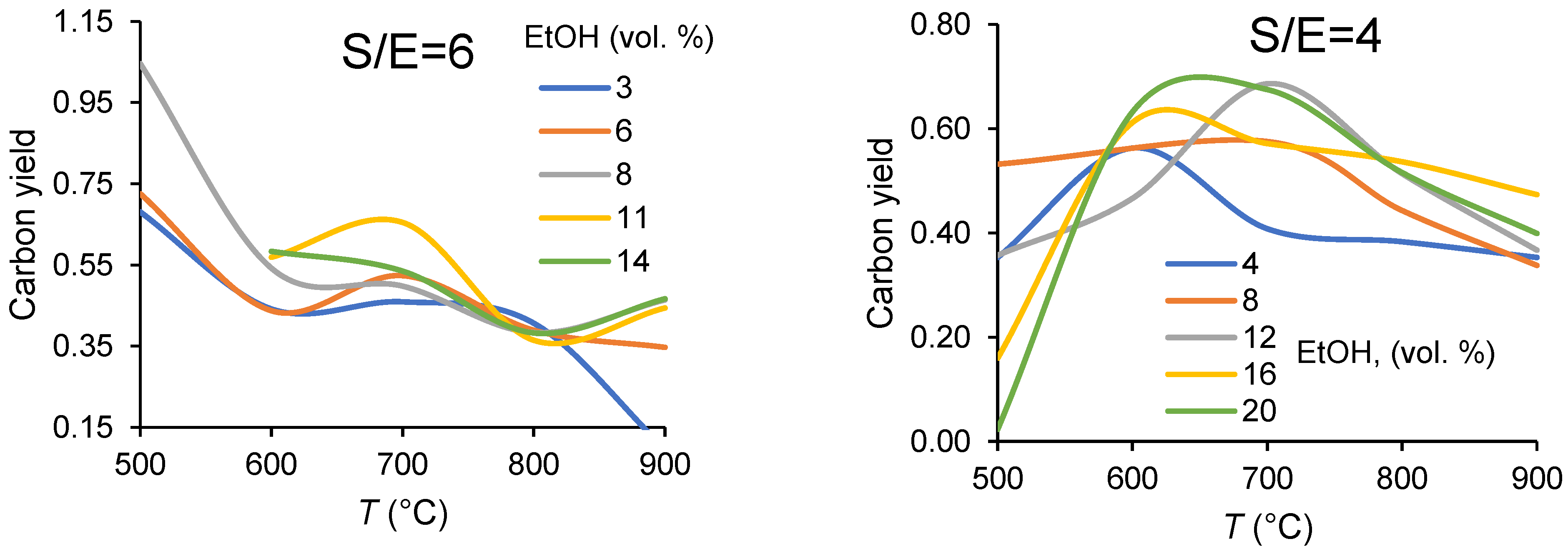
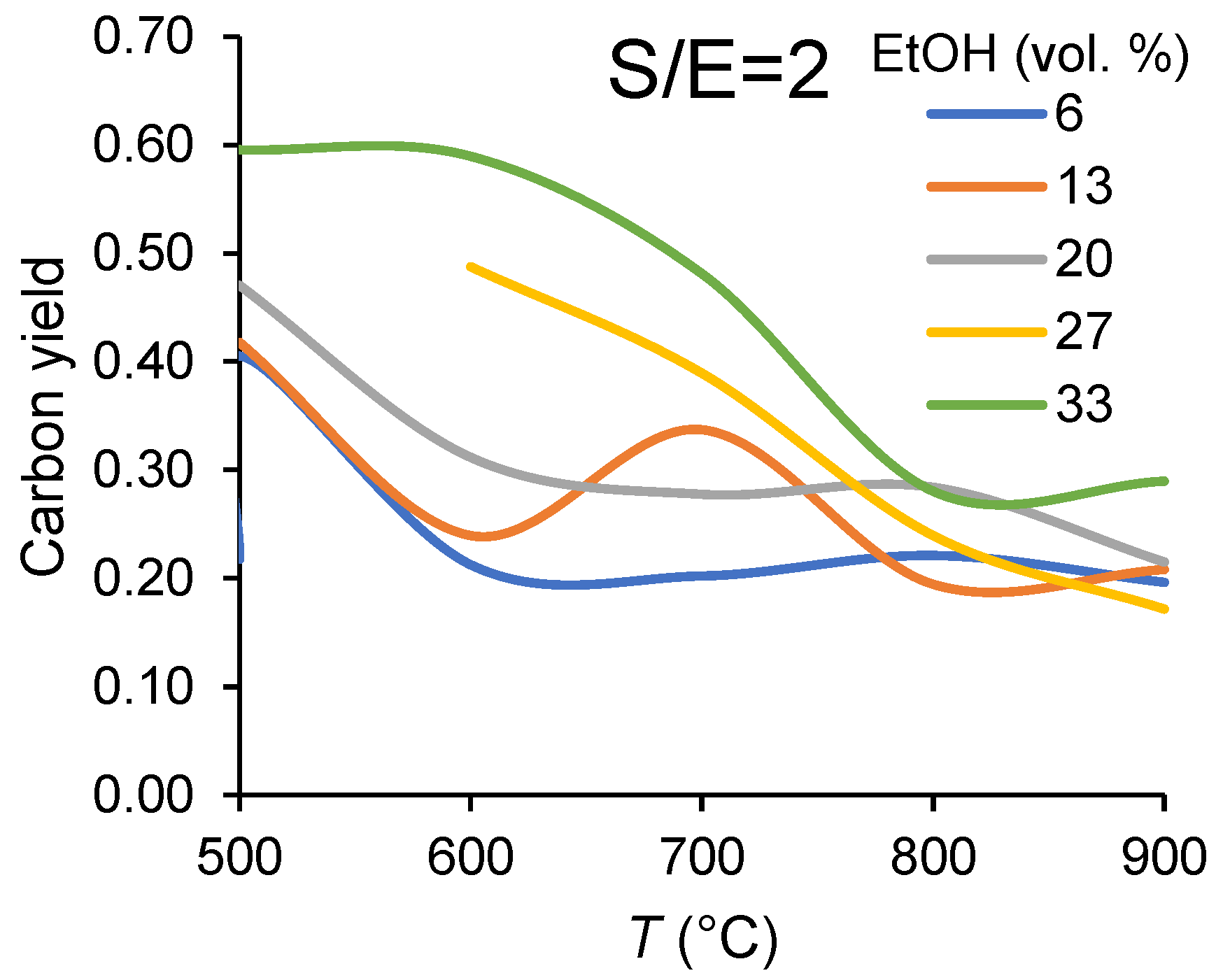
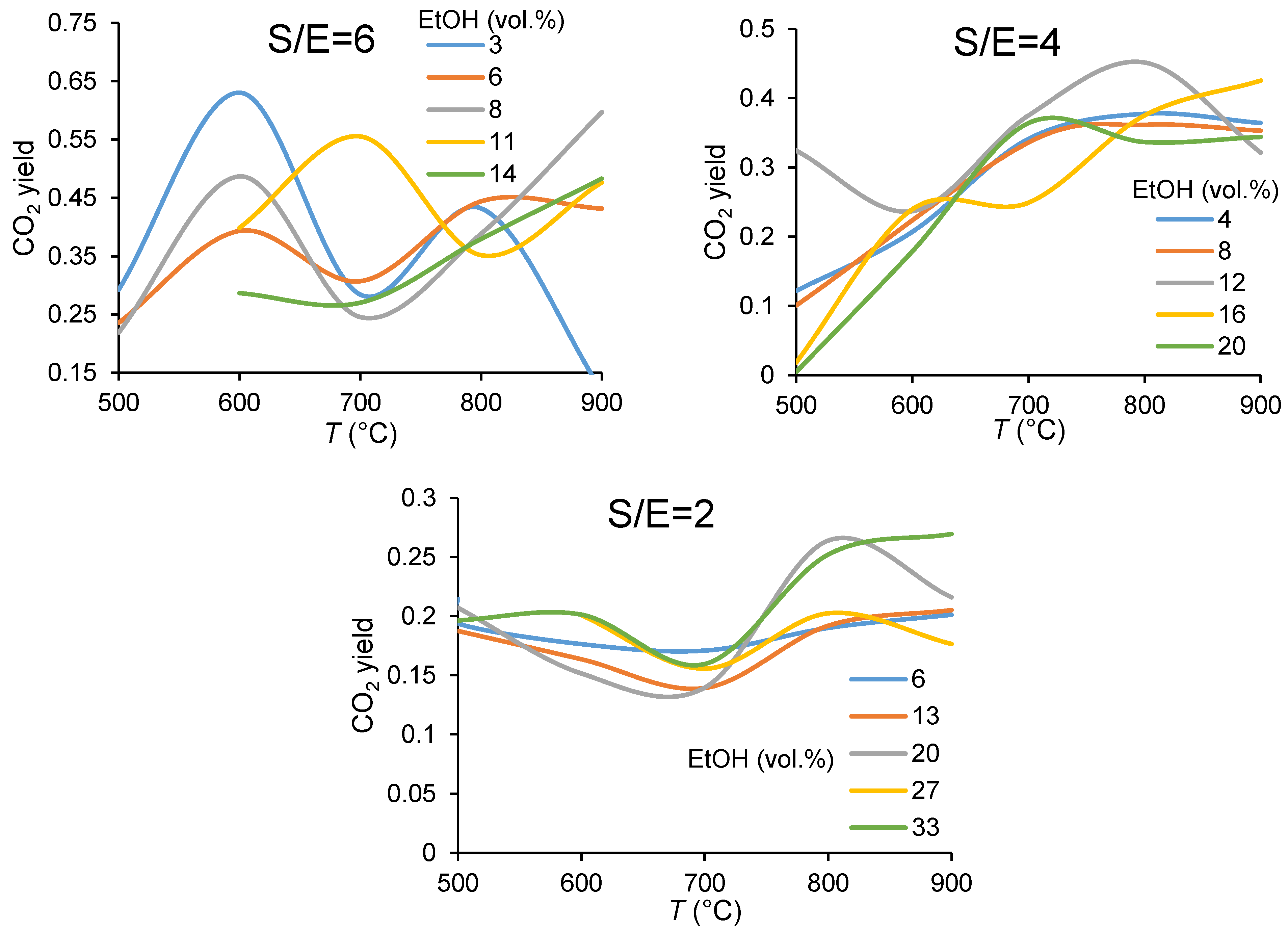
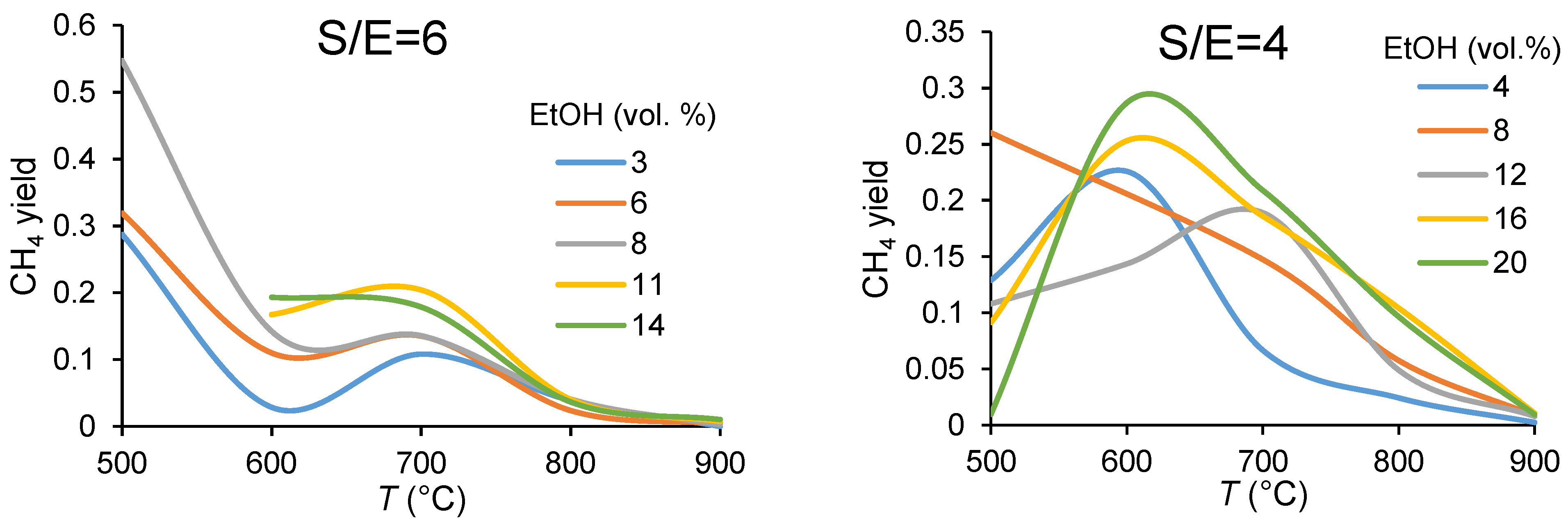
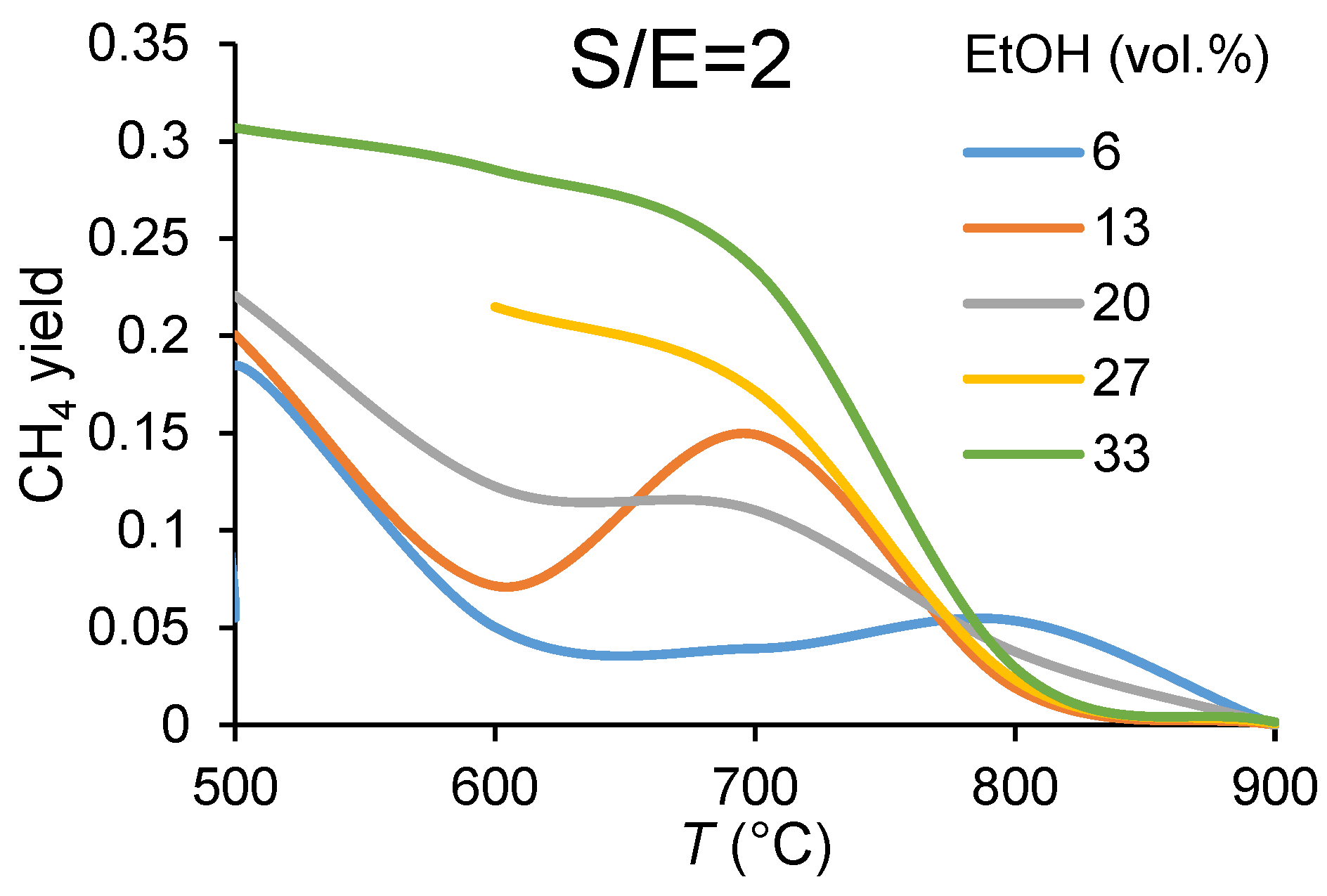
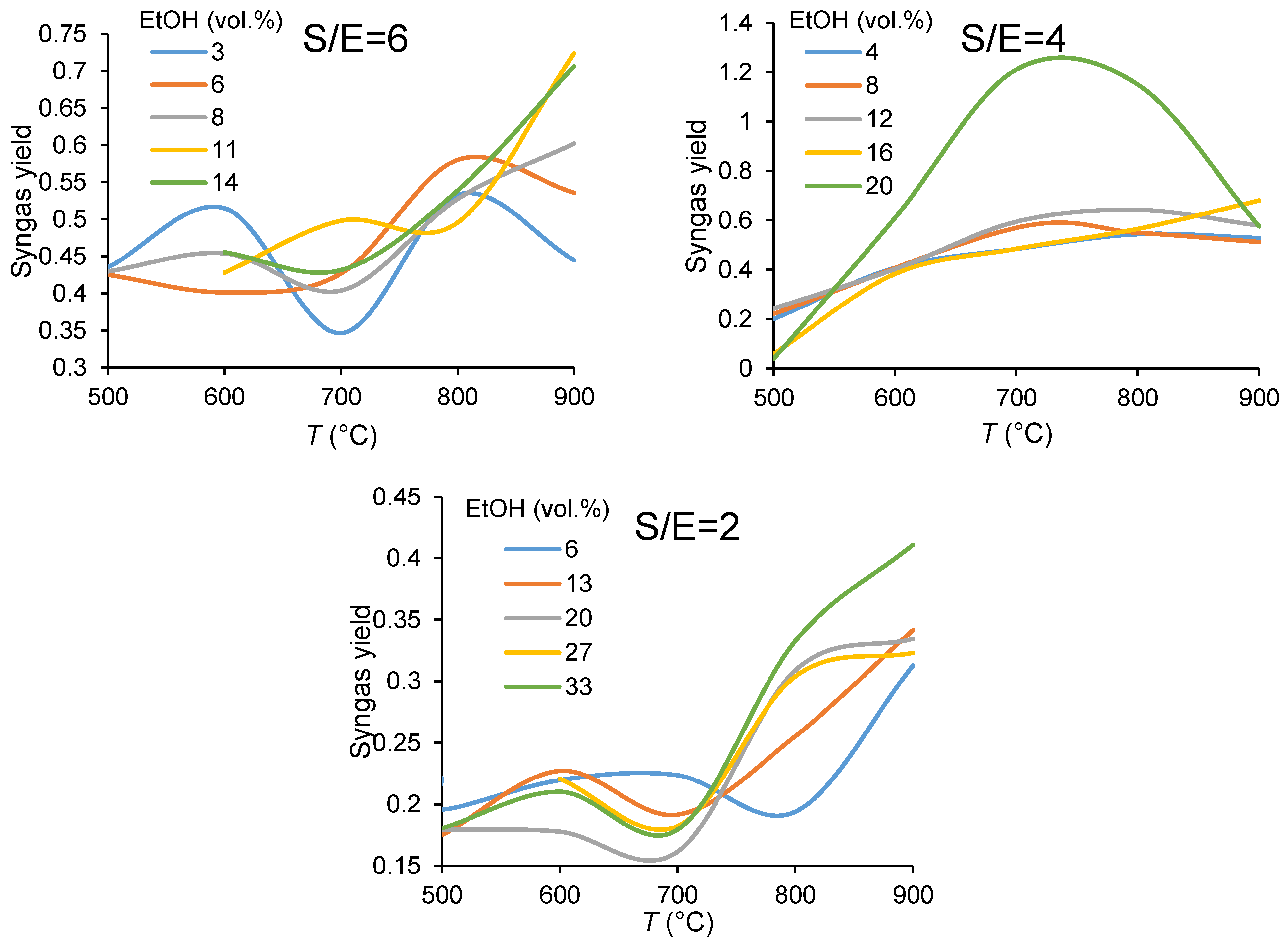
3.1.1. Carbon Balance
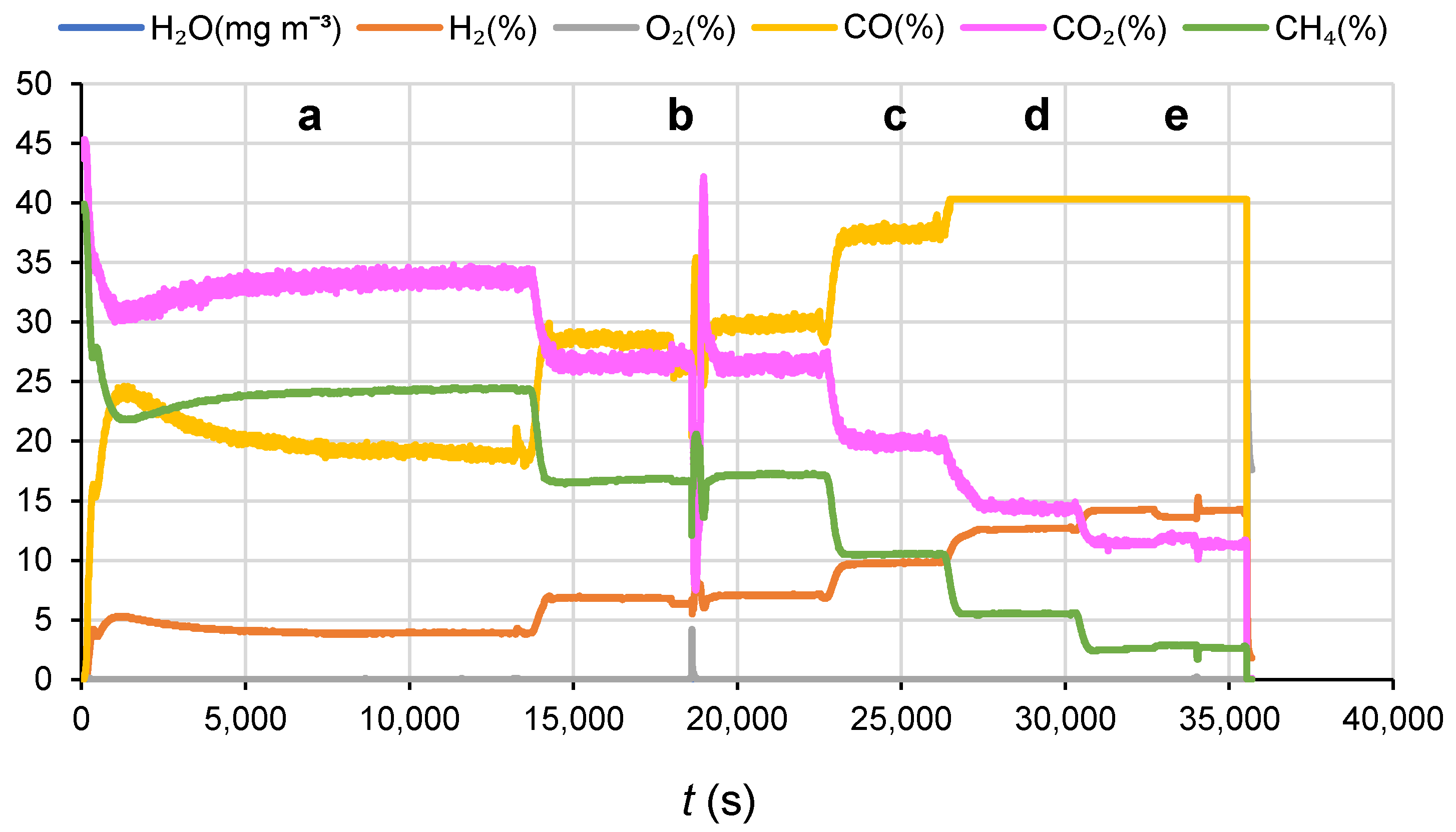
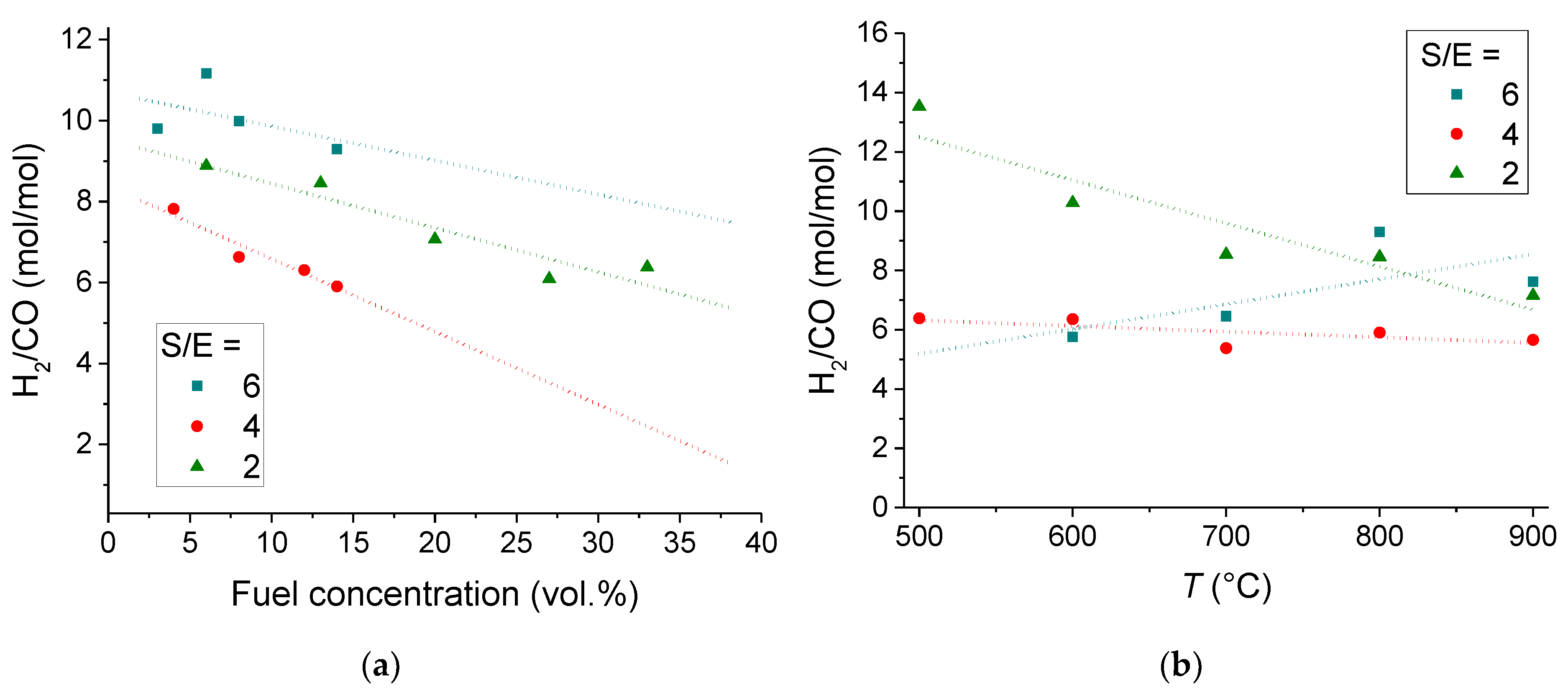
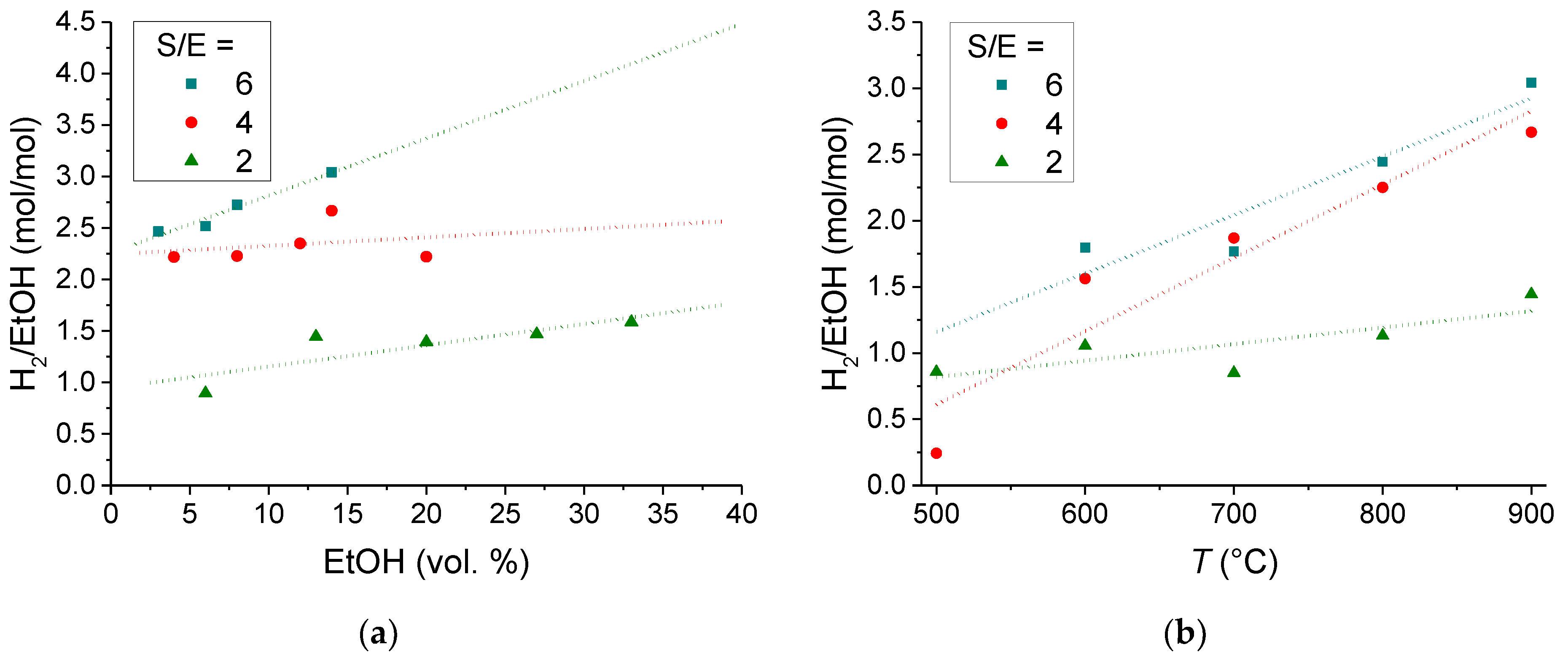
3.1.2. Catalyst Performance in Terms of Product Distribution
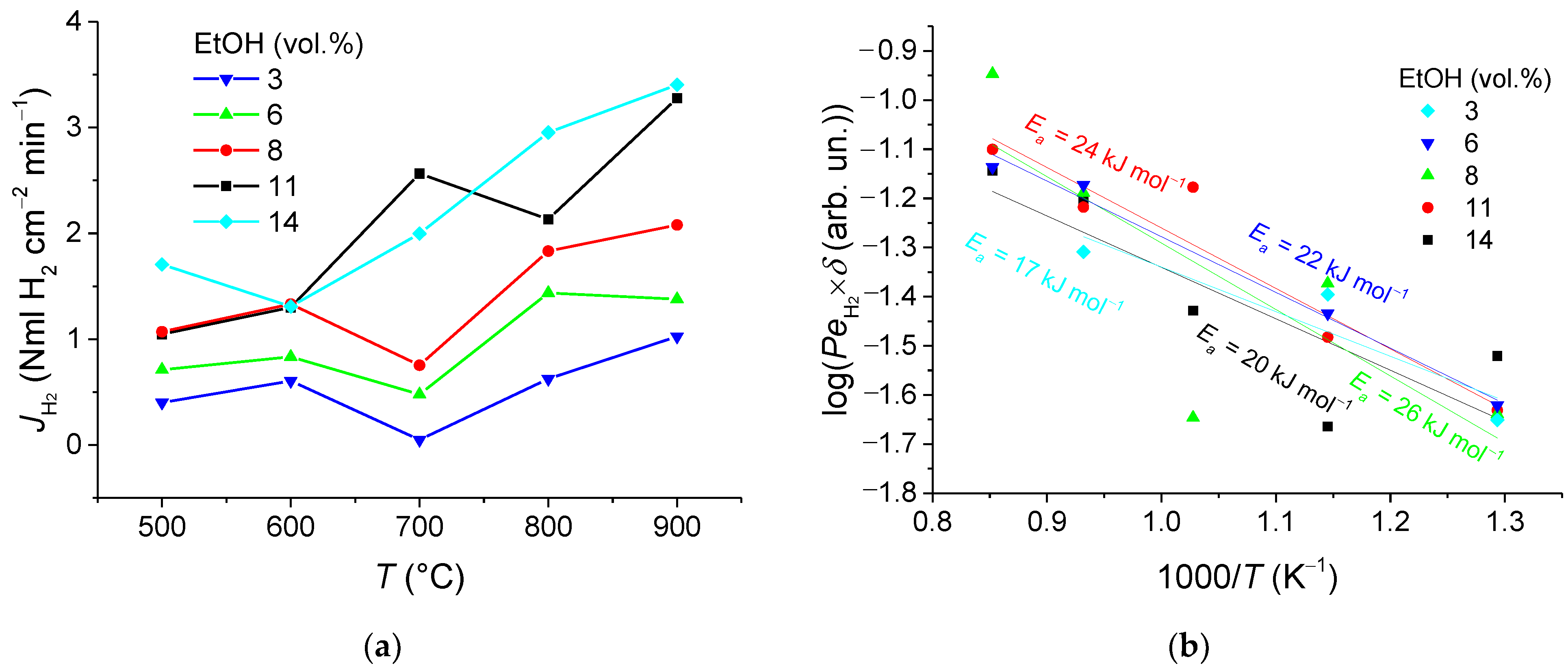
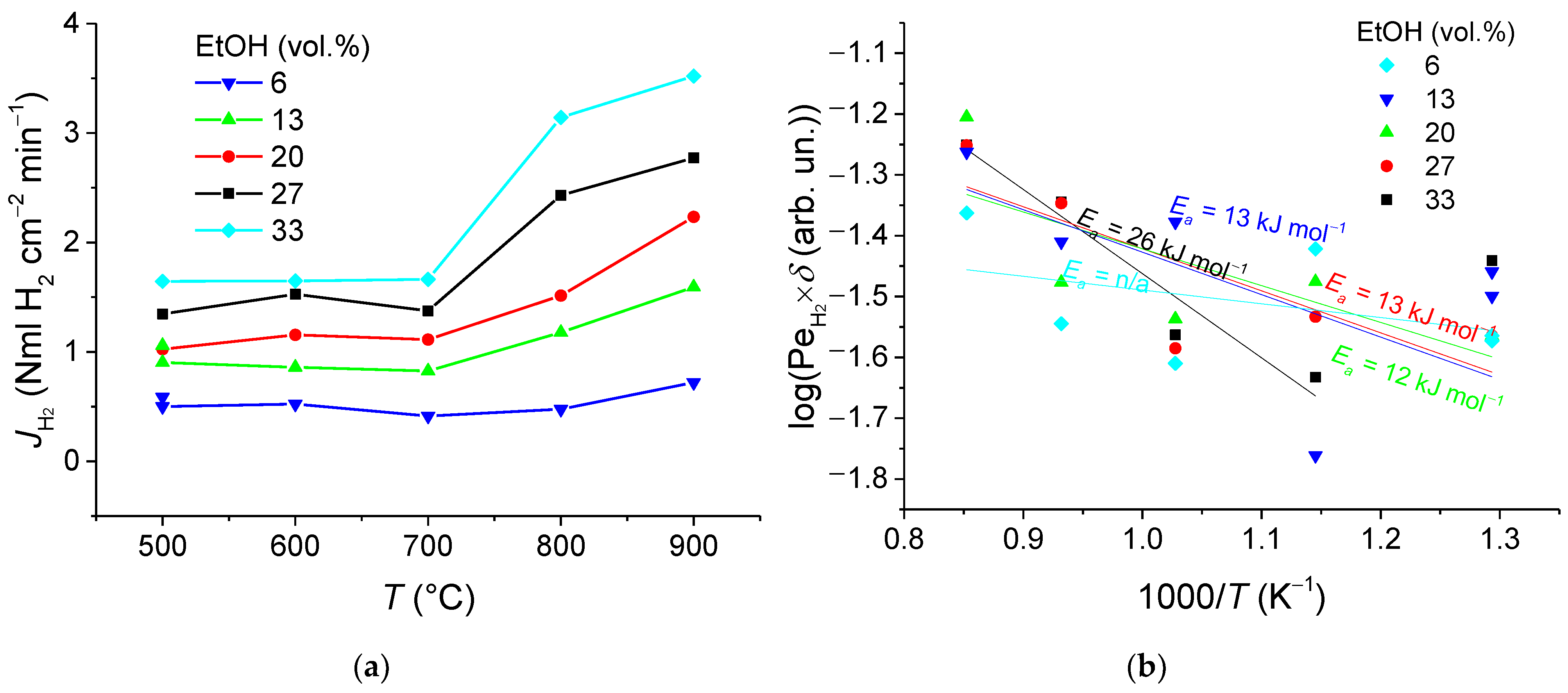
3.2. Hydrogen Permeation Flux Analysis
3.3. Reactor Performance Analysis
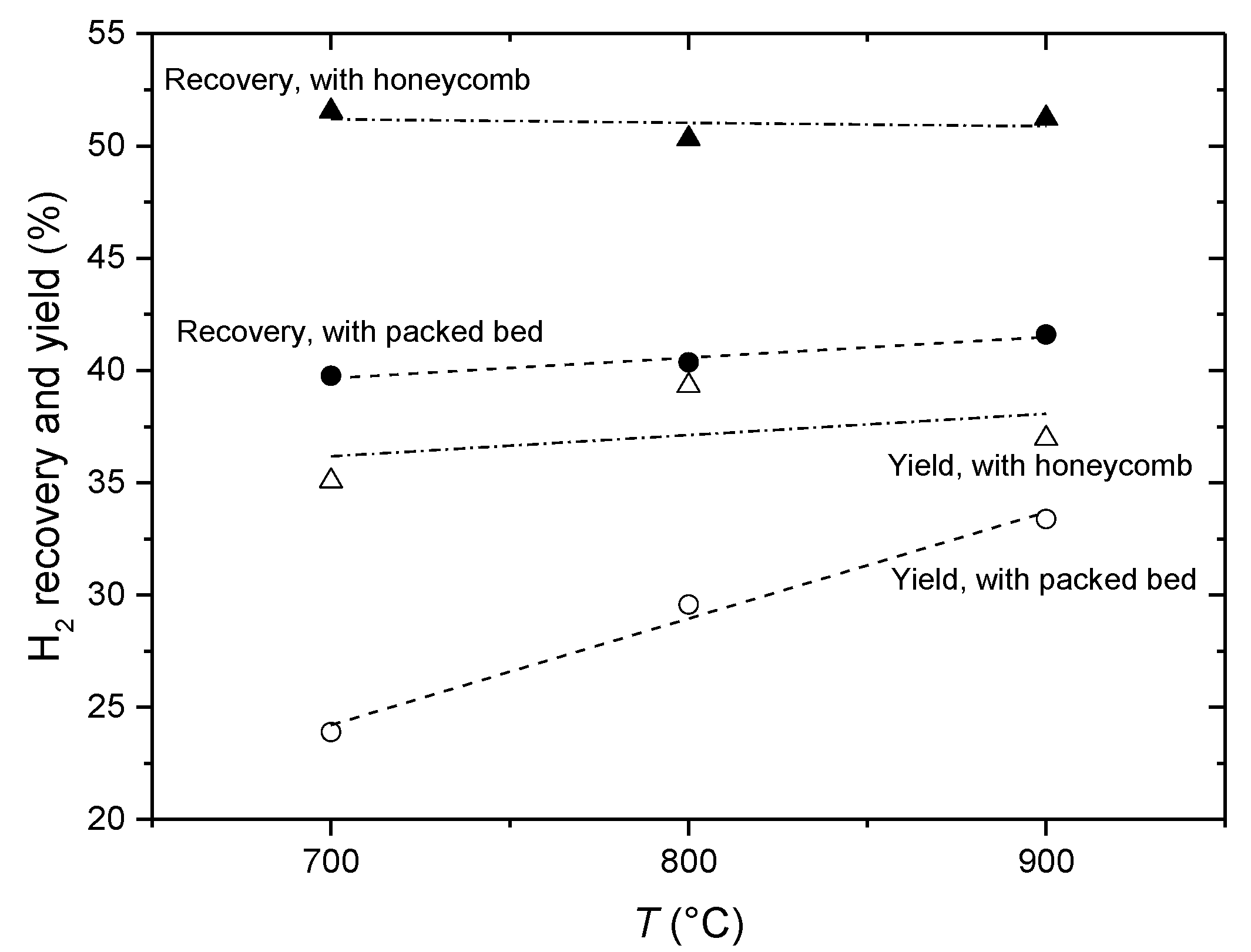
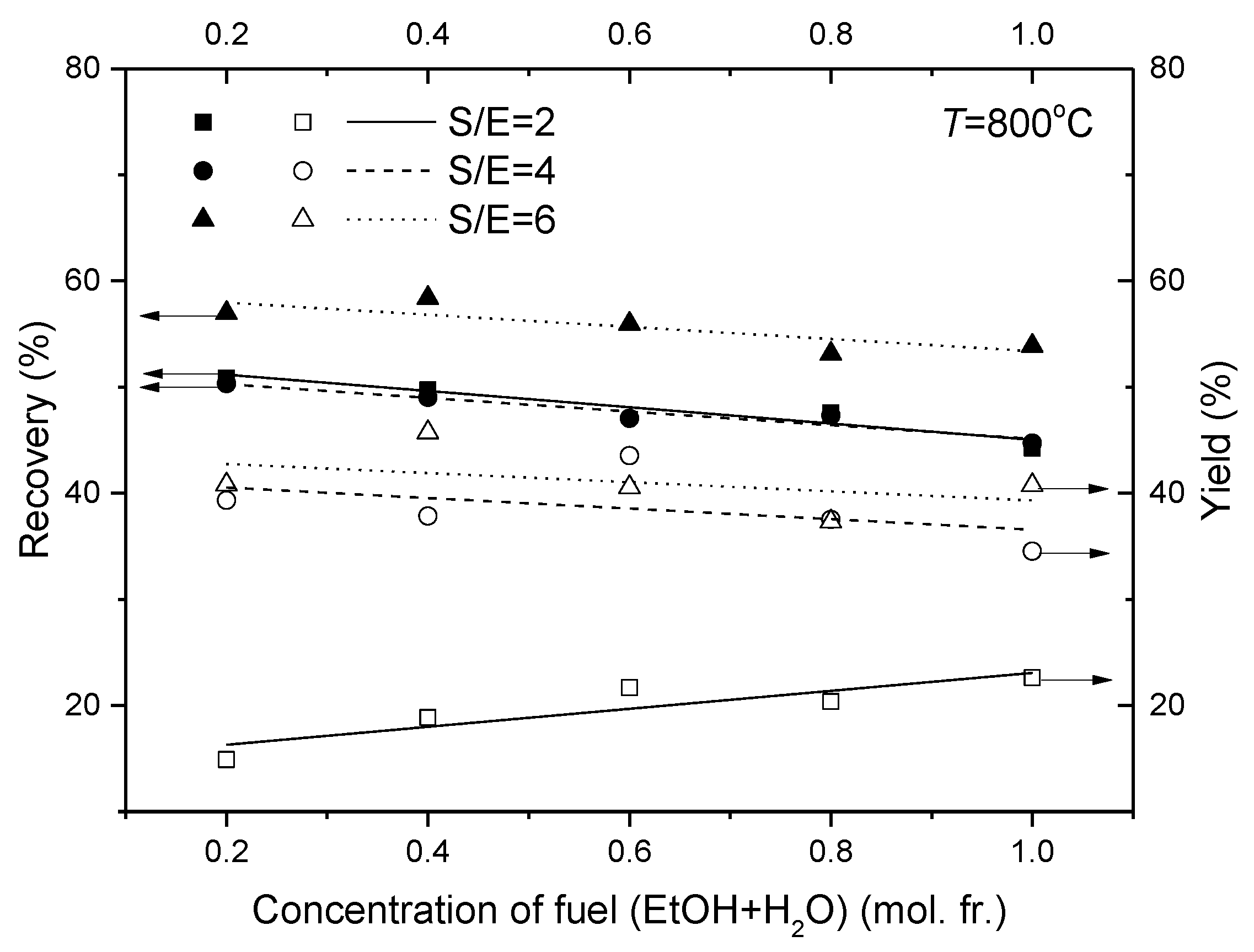
4. Conclusions
- Increase in the fuel concentration (ethanol and steam) does not result in improving reactor performance characteristics;
- The reactor operation in the temperature range of 700–900 °C with the water/ethanol molar ratio of 4 in the feed is quite sufficient in terms of ethanol transformation into syngas and CO2 as well as the amount of hydrogen produced from 1 mol of ethanol;
- A high flux of ~3.5 Nml H2 cm−2min−1 can be provided at the operating temperatures due to a high mixed protonic-electronic conductivity of NiCu alloy–Nd5.5WO11.25-δ nanocomposite as membrane material, while a possible rate-controlling step of the hydrogen permeation through the 5.65 mm thick asymmetric supported membrane is the transport in support.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| De | the equivalent/hydraulic channel diameter (m); |
| Ea | effective activation energy for permeation (kJ mol−1); |
| hydrogen permeate flux through the asymmetric membrane (mol m−2 s−1); | |
| Sieverts permeance (mol m−2 s−1 Pa−0.5); | |
| pH2 | hydrogen partial pressure (Pa); |
| S/E | steam-to-ethanol molar ratio; |
| S(H2) | selectivity with respect to hydrogen; |
| S(CH4) | selectivity with respect to methane; |
| S(CO) | selectivity with respect to carbon monoxide; |
| S(CO2) | selectivity with respect to carbon dioxide; |
| T | operation temperature (K) or (°C); |
| x(EtOH) | ethanol conversion; |
| Greek letters | |
| δ | dense layer thickness (m); |
| Θ | permeation constant (mol m−1 s−1 Pa−0.5) |
References
- Li, X.; Li, J. Fluxes and Driving Forces in Membrane Separation Processes. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–3. [Google Scholar]
- Matsuura, T. Membrane Separation Technologies. In Wastewater Recycle, Reuse, and Reclamation; Vigneswaran, S., Ed.; EOLSS Publishers: Oxford, UK, 2009; Volume 1, pp. 98–135. [Google Scholar]
- Ockwig, N.W.; Nenoff, T.M. Membranes for hydrogen separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef] [PubMed]
- Sazali, N.; Mohamed, M.A.; Salleh, W.N.W. Membranes for hydrogen separation: A significant review. Int. J. Adv. Manuf. Syst. 2020, 107, 1859–1881. [Google Scholar] [CrossRef]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Bernardo, P.; Clarizia, G. 30 years of membrane technology for gas separation. Chem. Eng. Trans. 2013, 32, 1999–2004. [Google Scholar]
- Sadykov, V.A.; Eremeev, N.F.; Fedorova, Y.E.; Krasnov, A.V.; Bobrova, L.N.; Bespalko, Y.N.; Lukashevich, A.I.; Skriabin, P.I.; Smorygo, O.L.; Van Veen, A.C. Design and performance of asymmetric supported membranes for oxygen and hydrogen separation. Int. J. Hydrogen Energy 2021, 46, 20222–20239. [Google Scholar] [CrossRef]
- Algieri, C.; Coppola, G.; Mukherjee, D.; Shammas, M.I.; Calabro, V.; Curcio, S.; Chakraborty, S. Review: Catalytic membrane reactors: The industrial applications perspective. Catalysts 2021, 11, 691. [Google Scholar] [CrossRef]
- Caravella, A.; Scura, F.; Barbieri, G.; Drioli, E. Inhibition by CO and polarization in Pd-based membranes: A novel permeation reduction coefficient. J. Phys. Chem. B 2010, 114, 12264–12276. [Google Scholar] [CrossRef]
- Sadykov, V.; Krasnov, A.; Fedorova, Y.; Lukashevich, A.; Bobrova, L.; Bespalko, Y.; Eremeev, N.; Skriabin, P.; Smorygo, O. Hydrogen separation membrane based on NiCu/Nd5.5WO11.25-δ nanocomposite. In Proceedings of the 13th European SOFC & SOE Forum 2018, Lucerne, Switzerland, 3–6 July 2019; LIBRARY: Lucerne, Switzerland, 2018; pp. 24–34. [Google Scholar]
- Bespalko, Y.; Sadykov, V.; Eremeev, N.; Skryabin, P.; Krieger, T.; Sadovskaya, E.; Bobrova, L.; Uvarov, N.; Lukashevich, A.; Krasnov, A.; et al. Synthesis of tungstates/Ni0.5Cu0.5Ox nanocomposite materials for hydrogen separation cermet membranes. Compos. Struct. 2018, 202, 1263–1274. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Bespalko, Y.N.; Krasnov, A.V.; Skriabin, P.I.; Lukashevich, A.I.; Fedorova, Y.E.; Sadovskaya, E.M.; Eremeev, N.F.; Krieger, T.A.; Ishchenko, A.V.; et al. Novel proton-conducting nanocomposites for hydrogen separation membranes. Solid State Ion. 2018, 322, 69–78. [Google Scholar] [CrossRef]
- Sadykov, V.; Krasnov, A.; Fedorova, Y.; Lukashevich, A.; Bespalko, Y.; Eremeev, N.; Skriabin, P.; Valeev, K.; Smorygo, O. Novel nanocomposite materials for oxygen and hydrogen separation membranes. Int. J. Hydrogen Energy 2020, 45, 13575–13585. [Google Scholar] [CrossRef]
- Bobrova, L.; Eremeev, N.; Vernikovskaya, N.; Sadykov, V.; Smorygo, O. Effect of asymmetric membrane structure on hydrogen transport resistance and performance of a catalytic membrane reactor for ethanol steam reforming. Membranes 2021, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Llorca, J.; Cortés Corberán, V.; Divins, J.N.; Fraile, R.O.; Taboada, E. Hydrogen from bio-ethanol. In Renewable Hydrogen Technologies, 1st ed.; Gandía, L.M., Arzamendi, G.D., Dieguez, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 135–169. [Google Scholar]
- Manzolini, G.; Tosti, S. Hydrogen production from ethanol steam reforming: Energy efficiency analysis of traditional and membrane processes. Int. J. Hydrogen Energy 2008, 33, 5571–5582. [Google Scholar] [CrossRef]
- Sun, S.; Yan, W.; Sun, P.; Chen, J. Thermodynamic analysis of ethanol reforming for hydrogen production. Energy 2012, 44, 911–924. [Google Scholar] [CrossRef]
- Chen, W.-H.; Li, S.-C.; Lim, S.; Chen, Z.-Y.; Juan, J.C. Reaction and hydrogen production phenomena of ethanol steam reforming in a catalytic membrane reactor. Energy 2021, 220, 119737. [Google Scholar] [CrossRef]
- Saidi, M.; Jahangiri, A. Theoretical study of hydrogen production by ethanol steam reforming: Technical evaluation and performance analysis of catalytic membrane reactor. Int. J. Hydrogen Energy 2018, 43, 15306–15320. [Google Scholar] [CrossRef]
- Miachon, S.; Dalmon, J.-A. Catalysis in membrane reactors: What about the catalyst? Top. Catal. 2004, 29, 59–65. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Tsuru, T.; Kang, D.-Y. Simulation and design of catalytic membrane reactor for hydrogen production via methylcyclohexane dehydrogenation. Int. J. Hydrogen Energy 2017, 42, 26296–26307. [Google Scholar] [CrossRef]
- Gozόlvez-Zafrilla, J.M.; Santafé-Moros, A.; Escolástico, S.; Serra, J.M. Fluid dynamic modeling of oxygen permeation through mixed ionic–electronic conducting membranes. J. Membr. Sci. 2011, 378, 290–300. [Google Scholar] [CrossRef]
- Hong, J.; Kirchen, P.; Ghoniem, A. Numerical simulation of ion transport membrane reactors: Oxygen permeation and transport and fuel conversion. J. Membr. Sci. 2012, 407–408, 71–85. [Google Scholar] [CrossRef]
- Montebelli, A.; Visconti, C.G.; Groppi, G.; Tronconi, E.; Cristiani, C.; Ferreira, C.; Kohler, S. Methods for the catalytic activation of metallic structured substrates. Catal. Sci. Technol. 2014, 4, 2846–2870. [Google Scholar] [CrossRef]
- Haase, S.; Murzin, D.Y.; Salmi, T. Review on hydrodynamics and mass transfer in minichannel wall reactors with gas–liquid Taylor flow. Chem. Eng. Res. Des. 2016, 113, 304–329. [Google Scholar] [CrossRef]
- Bespalko, Y.; Eremeev, N.; Skryabin, P.; Krieger, T.; Chesalov, Y.; Lapina, O.; Khabibulin, D.; Ulihin, A.; Uvarov, N.; Sadykov, V. Structural and transport properties of neodymium tungstates prepared via mechanochemical activation. Ceram. Int. 2019, 45, 9529–9536. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Pavlova, S.N.; Kharlamova, T.S.; Muzykantov, V.S.; Ishchenko, A.V.; Bobin, A.S.; Mezentseva, N.V.; Alikina, G.M.; Lukashevich, A.I.; Krieger, T.A.; et al. Perovskites and their nanocomposites with fluorite-like oxides as materials for solid oxide fuel cells cathodes and oxygen-conducting membranes: Mobility and reactivity of the surface/bulk oxygen as a key factor of their performance. In Perovskites: Structure, Properties and Uses; Borovski, M., Ed.; Nova Science Publishers: New York, NY, USA, 2010; pp. 67–178. [Google Scholar]
- Sadykov, V.A.; Eremeev, N.F.; Vinokurov, Z.S.; Shmakov, A.N.; Kriventsov, V.V.; Lukashevich, A.I.; Krasnov, A.V.; Ishchenko, A.V. Structural studies of Pr nickelate-cobaltite + Y-doped ceria nanocomposite. J. Ceram. Sci. Technol. 2017, 8, 129–140. [Google Scholar]
- Leonov, A.N.; Smorygo, O.L.; Sheleg, V.K. Monolithic catalyst supports with foam structure. React. Kinet. Catal. Lett. 1997, 60, 259–267. [Google Scholar] [CrossRef]
- Leonov, A.; Romashko, A. Laminar Ni-NixAl-αAl2O3 foam material with high oxidation resistance. In Proceedings of the International Conference on Cellular metals and Metal Foaming Technology, Berlin, Germany, 23–25 June 2003; Banhart, J., Fleck, N.A., Mortensen, A., Eds.; MIT-Verlag: Berlin, Germany, 2003; pp. 271–274. [Google Scholar]
- Gallucci, F.; Fernandez, E.; Corengia, P.; van Sint Annaland, M. Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. Production of hydrogen from ethanol: Review of reaction mechanism and catalyst deactivation. Chem. Rev. 2012, 112, 4094–4123. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, S.; Chen, Y.; Wang, D.; Cai, W. Hydrogen production from ethanol reforming: Catalysts and reaction mechanism. Renew. Sustain. Energy Rev. 2015, 44, 132–148. [Google Scholar] [CrossRef]
- Moraes, T.S.; Borges, L.E.P.; Farrauto, R.; Noronha, F.B. Steam reforming of ethanol on Rh/SiCeO2 washcoated monolith catalyst: Stable catalyst performance. Int. J. Hydrogen Energy 2018, 43, 115–126. [Google Scholar] [CrossRef]
- Raskó, J.; Hancz, A.; Erdӧhelyi, A. Surface species and gas phase products in steam reforming of ethanol on TiO2 and Rh/TiO2. Appl. Catal. A Gen. 2004, 269, 13–25. [Google Scholar] [CrossRef]
- Trimm, D.L. Catalysts for the control of coking during steam reforming. Catal. Today 1999, 49, 3–10. [Google Scholar] [CrossRef]
- Chen, J.; Xu, D. Hydrogen production by the steam reforming of bio-ethanol over nickel-based catalysts for fuel cell applications. Int. J. Sustain. Green Energy 2017, 6, 28–38. [Google Scholar] [CrossRef][Green Version]
- Vicente, J.; Ereña, J.; Montero, C.; Azkoiti, M.J.; Bilbao, J.; Gayubo, A.G. Reaction pathway for ethanol steam reforming on a Ni/SiO2 catalyst including coke formation. Int. J. Hydrogen Energy 2014, 39, 18820–18834. [Google Scholar] [CrossRef]
- Zanchet, D.; Santos, J.B.O.; Damyanova, S.; Gallo, J.M.R.; Bueno, J.M.C. Toward understanding metal-catalyzed ethanol reforming. ACS Catal. 2015, 5, 3841–3863. [Google Scholar] [CrossRef]
- Mas, V.; Bergamini, M.L.; Baronetti, G.; Amadeo, N.; Laborde, M. A kinetic study of ethanol steam reforming using a nickel based catalyst. Top. Catal. 2008, 51, 39–48. [Google Scholar] [CrossRef]
- De Lima, S.M.; da Cruz, I.O.; Jacobs, G.; Davis, B.H.; Mattos, L.V.; Noronha, F.B. Steam reforming, partial oxidation, and oxidative steam reforming of ethanol over Pt/CeZrO2 catalyst. J. Catal. 2008, 257, 356–368. [Google Scholar] [CrossRef]
- De Lima, S.M.; da Silva, A.M.; da Costa, L.O.O.; Graham, U.M.; Jacobs, G.; Davis, B.H.; Mattos, L.V.; Noronha, F.B. Study of catalyst deactivation and reaction mechanism of steam reforming, partial oxidation, and oxidative steam reforming of ethanol over Co/CeO2 catalyst. J. Catal. 2009, 268, 268–281. [Google Scholar] [CrossRef]
- Silva, A.M.; Costa, L.O.O.; Barandas, A.P.M.G.; Borges, L.E.P.; Mattos, L.V.; Noronha, F.B. Effect of the metal nature on the reaction mechanism of the partial oxidation of ethanol over CeO2-supported Pt and Rh catalysts. Catal. Today 2008, 133–135, 755–761. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Chub, O.V.; Chesalov, Y.A.; Mezentseva, N.V.; Pavlova, S.N.; Arapova, M.V.; Rogov, V.A.; Simonov, M.N.; Roger, A.-C.; Parkhomenko, K.V.; et al. Mechanism of ethanol steam reforming over Pt/(Ni + Ru)-promoted oxides by FTIRS in situ. Top. Catal. 2016, 59, 1332–1342. [Google Scholar] [CrossRef]
- Song, H.; Ozkan, U.S. Changing the oxygen mobility in Co/ceria catalysts by Ca incorporation: Implications for ethanol steam reforming. J. Phys. Chem. A 2010, 114, 3796–3801. [Google Scholar] [CrossRef]
- López, E.; Divins, N.J.; Anzola, A.; Schbib, S.; Borio, D.; Llorca, J. Ethanol steam reforming for hydrogen generation over structured catalysts. Int. J. Hydrogen Energy 2013, 38, 4418–4428. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Simonov, M.N.; Bespalko, Y.N.; Bobrova, L.N.; Eremeev, N.F.; Arapova, M.V.; Smal’, E.A.; Mezentseva, N.V.; Pavlova, S.N. Design and characterization of nanocomposite catalysts for biofuel conversion into syngas and hydrogen in structured reactors and membranes. Kinet. Catal. 2019, 60, 582–605. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Arapova, M.V.; Smal, E.A.; Pavlova, S.N.; Bobrova, L.N.; Eremeev, N.F.; Mezentseva, N.V.; Simonov, M.N. Nanocomposite Catalysts for Transformation of Biofuels Into Syngas and Hydrogen: Fundamentals of Design and Performance, Application in Structured Reactors and Catalytic Membranes. In Catalysis; Spivey, J., Han, Y.-F., Shekhawat, D., Eds.; RSC Publishing: London, UK, 2019; Volume 31, pp. 216–241. [Google Scholar]
- Song, H.; Zhang, L.; Ozkan, U.S. Investigation of the reaction network in ethanol steam reforming over supported cobalt catalysts. Ind. Eng. Chem. Res. 2010, 49, 8984–8989. [Google Scholar] [CrossRef]
- Uvarov, N.F.; Ulihin, A.C.; Bespalko, Y.N.; Eremeeev, N.F.; Krasnov, A.V.; Skriabin, P.I.; Sadykov, V.A. Study of transport properties of composite metal-ceramic membrane materials for selective separation of hydrogen. ISJAEE 2017, 31–36, 24–35. [Google Scholar]
- Uvarov, N.F.; Ulihin, A.C.; Bespalko, Y.N.; Eremeeev, N.F.; Krasnov, A.V.; Skriabin, P.I.; Sadykov, V.A. Study of proton conductivity of composite metal-ceramic materials based on neodymium tungstates using a four-electrode technique with ionic probes. Int. J. Hydrogen Energy 2018, 43, 19521–19527. [Google Scholar] [CrossRef]
- Nordio, M.; Soresi, S.; Manzolini, G.; Melendez, J.; Van Sint Annaland, M.; Tanaka, A.P.; Gallucci, F. Effect of sweep gas on hydrogen permeation of supported Pd membranes: Experimental and modeling. Int. J. Hydrogen Energy 2019, 44, 4228–4239. [Google Scholar] [CrossRef]



| Length | 22 | mm |
| Diameter | 24 | mm |
| Cross section area | 452.16 | mm2 |
| Channel wall thickness | 125 | μm |
| Equivalent channel diameter | 0.6924 | mm |
| Porosity | 0.58 | |
| Specific geometric surface area | 3355 | m−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eremeev, N.; Krasnov, A.; Bespalko, Y.; Bobrova, L.; Smorygo, O.; Sadykov, V. An Experimental Performance Study of a Catalytic Membrane Reactor for Ethanol Steam Reforming over a Metal Honeycomb Catalyst. Membranes 2021, 11, 790. https://doi.org/10.3390/membranes11100790
Eremeev N, Krasnov A, Bespalko Y, Bobrova L, Smorygo O, Sadykov V. An Experimental Performance Study of a Catalytic Membrane Reactor for Ethanol Steam Reforming over a Metal Honeycomb Catalyst. Membranes. 2021; 11(10):790. https://doi.org/10.3390/membranes11100790
Chicago/Turabian StyleEremeev, Nikita, Alexey Krasnov, Yuliya Bespalko, Ludmilla Bobrova, Oleg Smorygo, and Vladislav Sadykov. 2021. "An Experimental Performance Study of a Catalytic Membrane Reactor for Ethanol Steam Reforming over a Metal Honeycomb Catalyst" Membranes 11, no. 10: 790. https://doi.org/10.3390/membranes11100790
APA StyleEremeev, N., Krasnov, A., Bespalko, Y., Bobrova, L., Smorygo, O., & Sadykov, V. (2021). An Experimental Performance Study of a Catalytic Membrane Reactor for Ethanol Steam Reforming over a Metal Honeycomb Catalyst. Membranes, 11(10), 790. https://doi.org/10.3390/membranes11100790








