Membrane-Assisted Methanol Synthesis Processes and the Required Permselectivity
Abstract
:1. Introduction
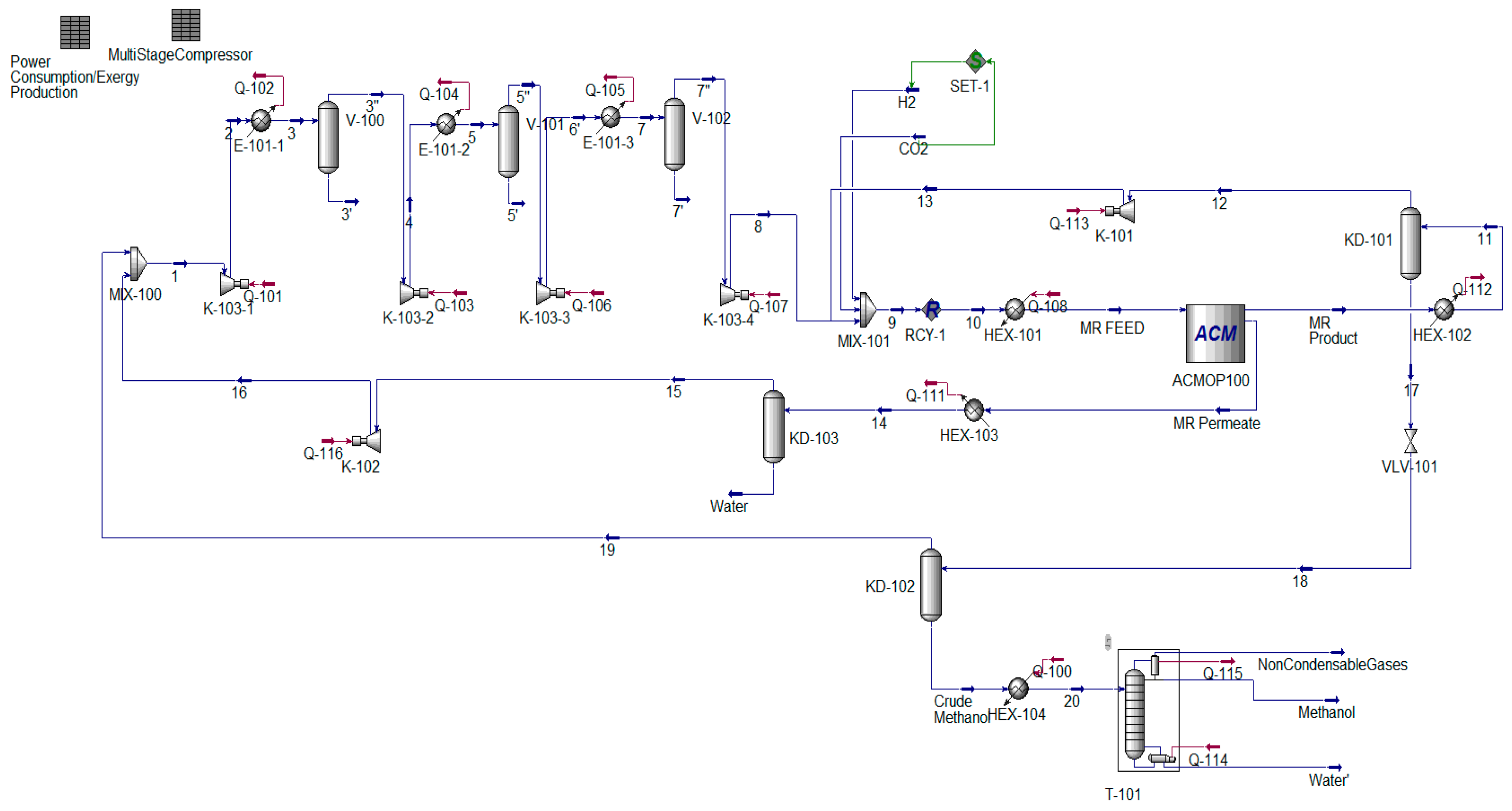
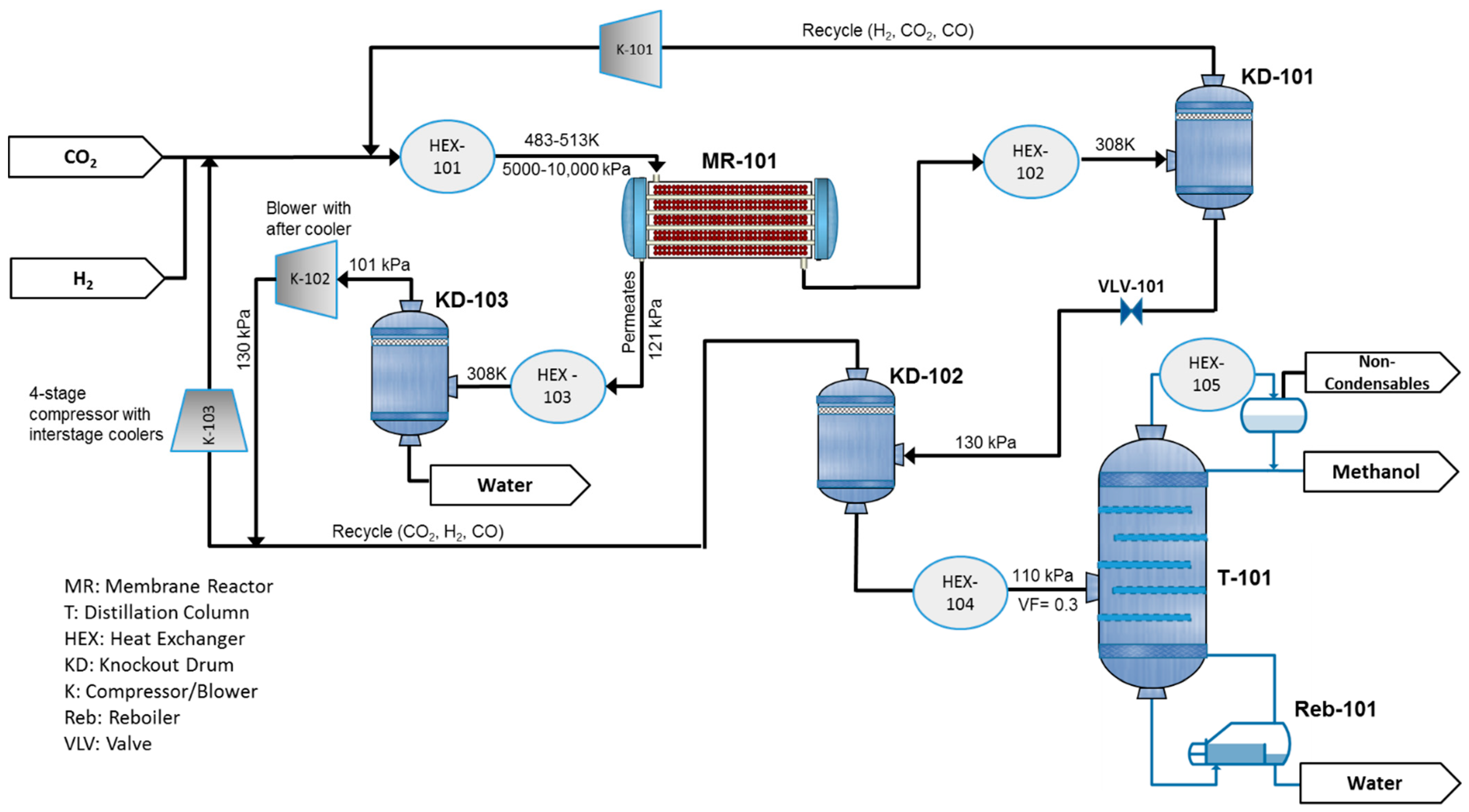
2. Process Description and Simulation
3. Reaction Kinetics
4. Membrane Properties
5. Design Data and Specifications
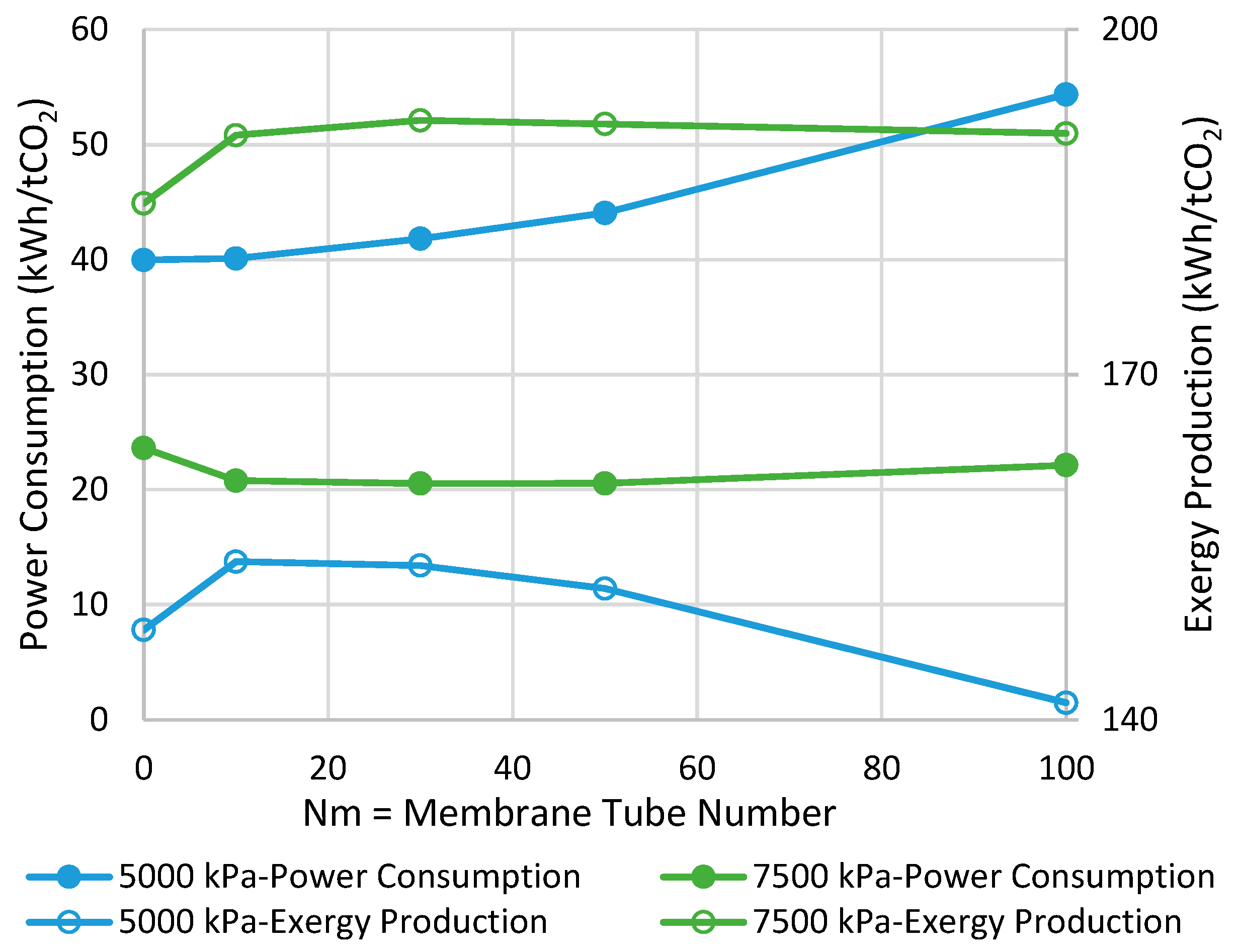
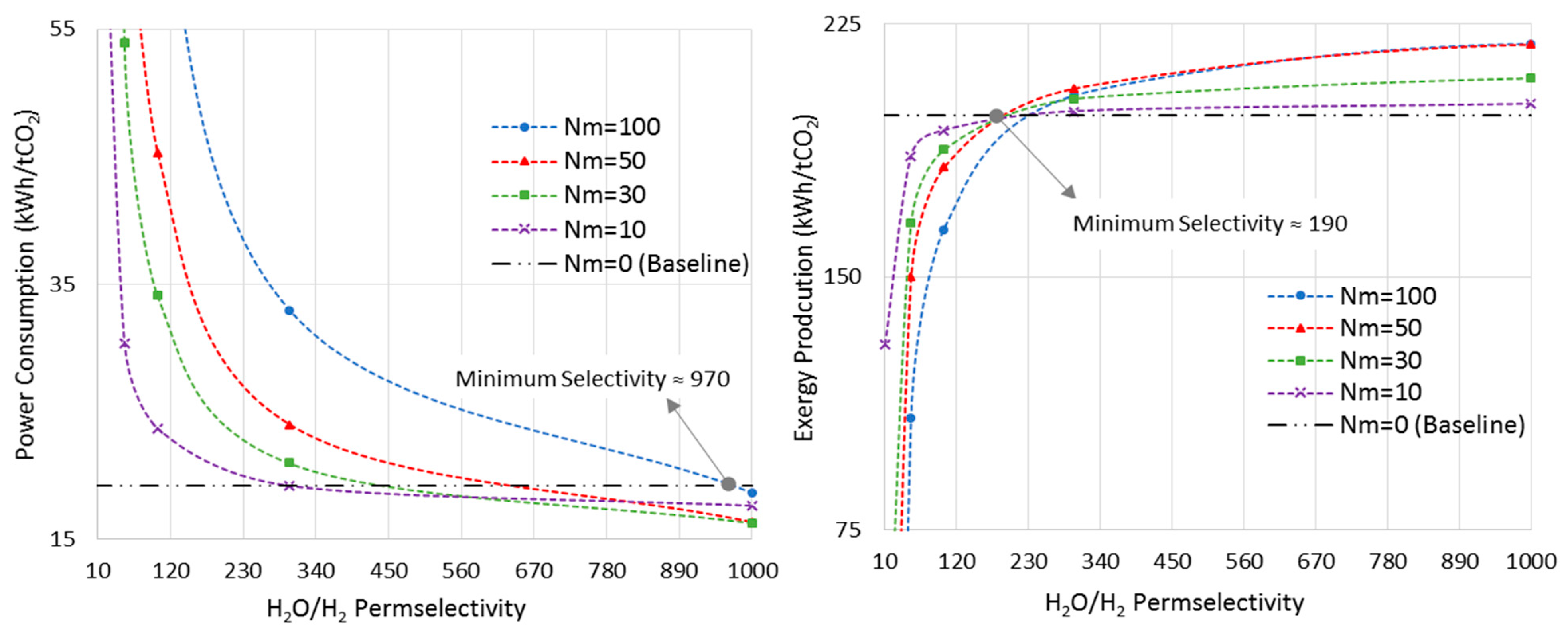
6. Results and Discussion
- (A)
- Power Consumption
- (B)
- Exergy Production
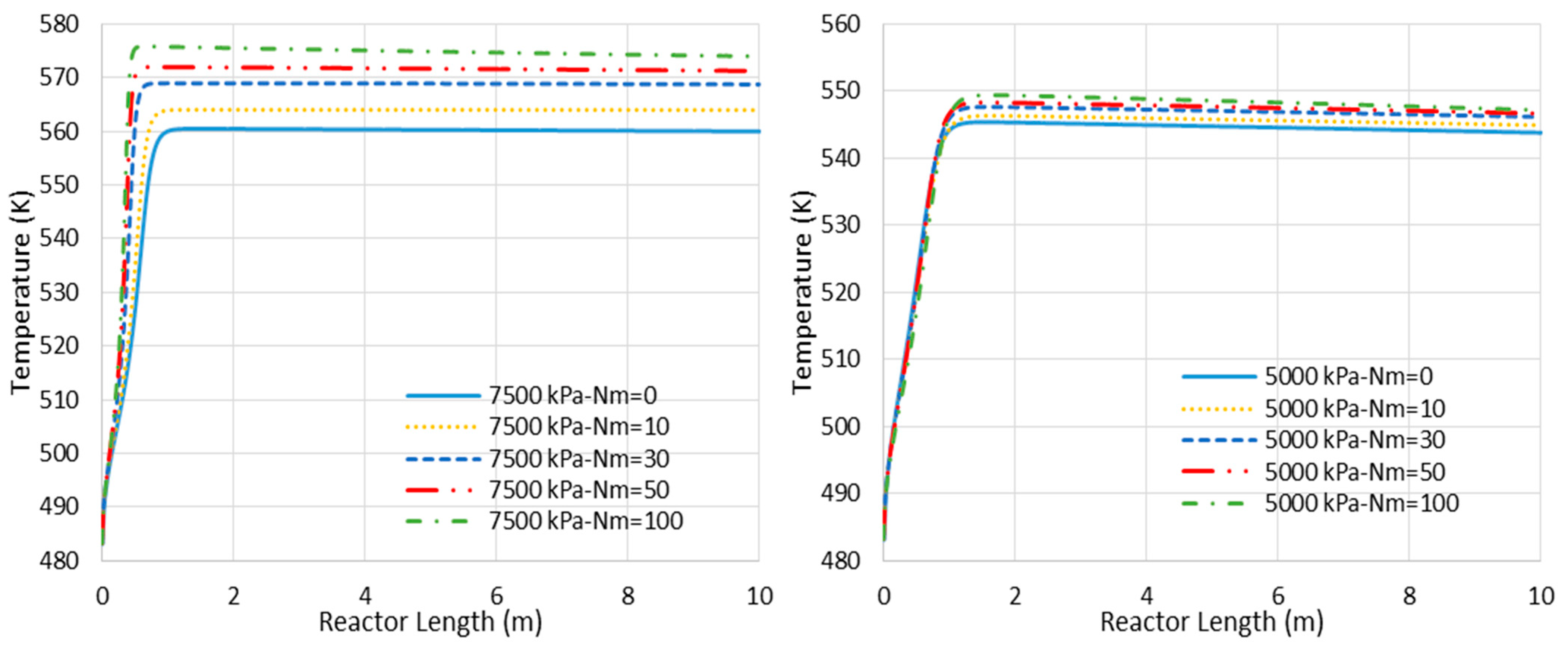
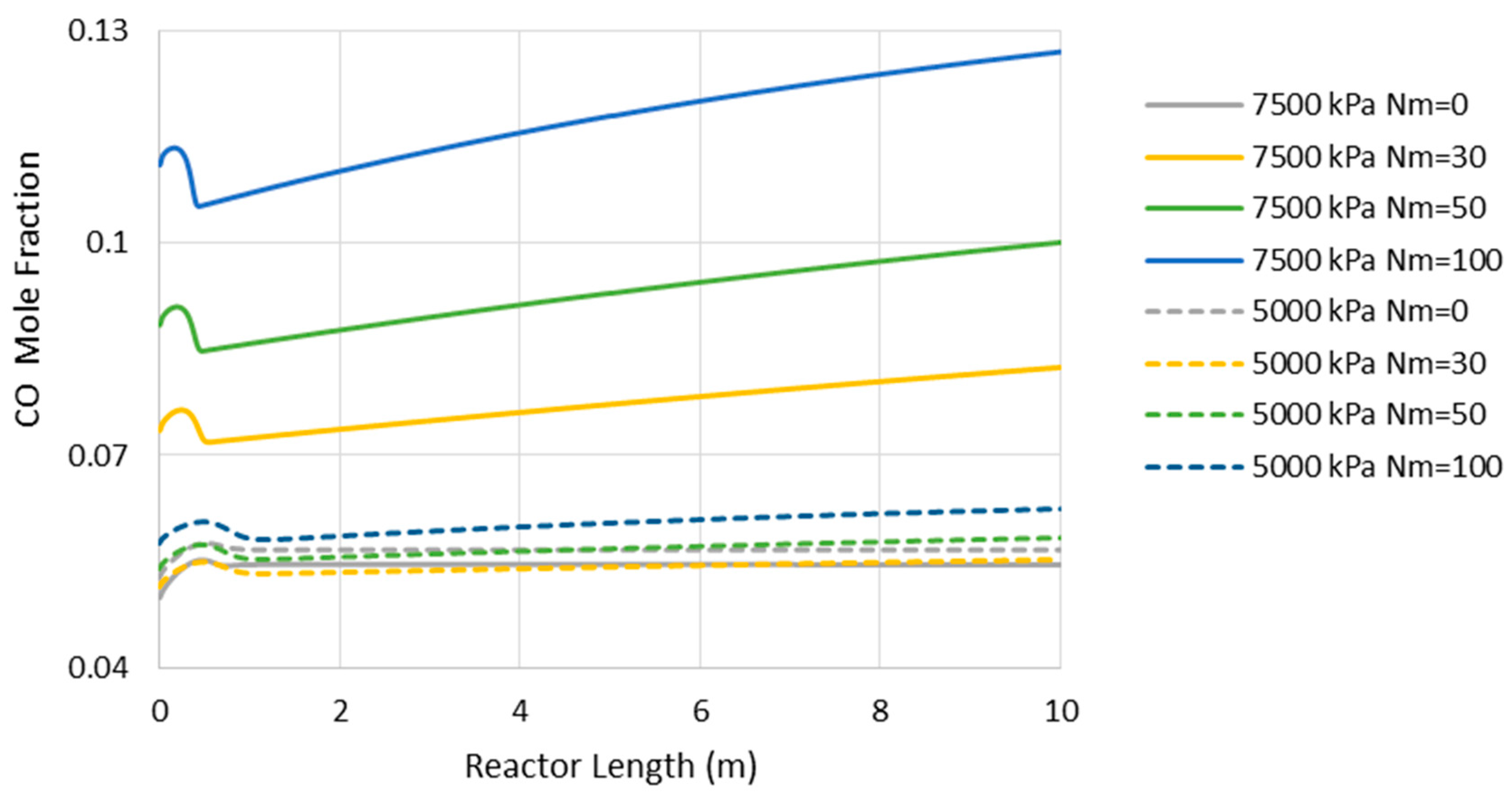
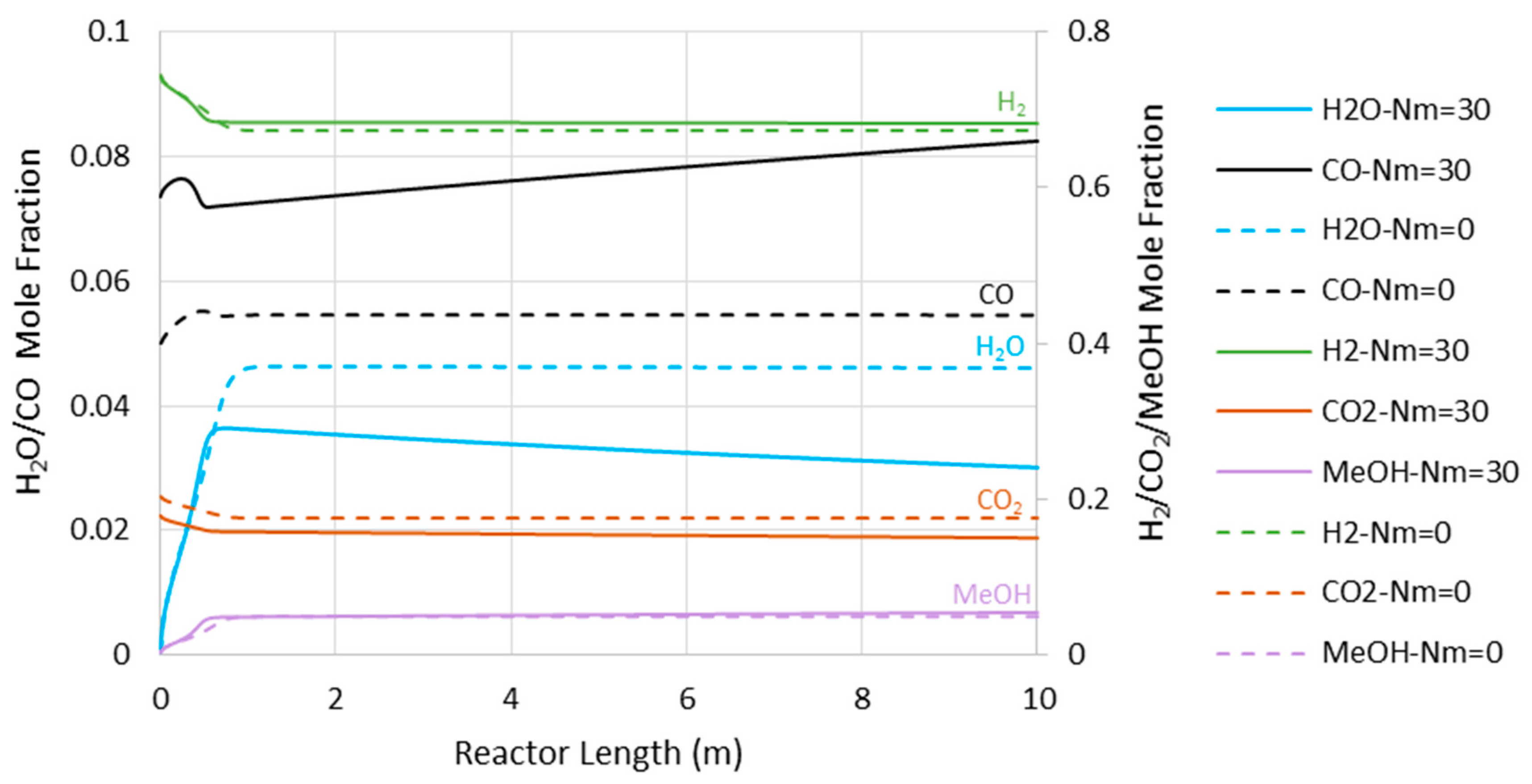
6.1. Adiabatic MR-Based Process
- (a)
- Higher loss of water from the reaction environment and the subsequent acceleration in temperature rise, as shown in Figure 4, which is not in a favor of the exothermic methanolation reaction.
- (b)
- (c)
- Higher exergy destruction of water through the isenthalpic transmembrane process.
- (d)
- Losing reaction volume to accommodate more membrane tubes.
- (e)
- A higher reactor pressure drop due to the membrane volumetric occupancy.
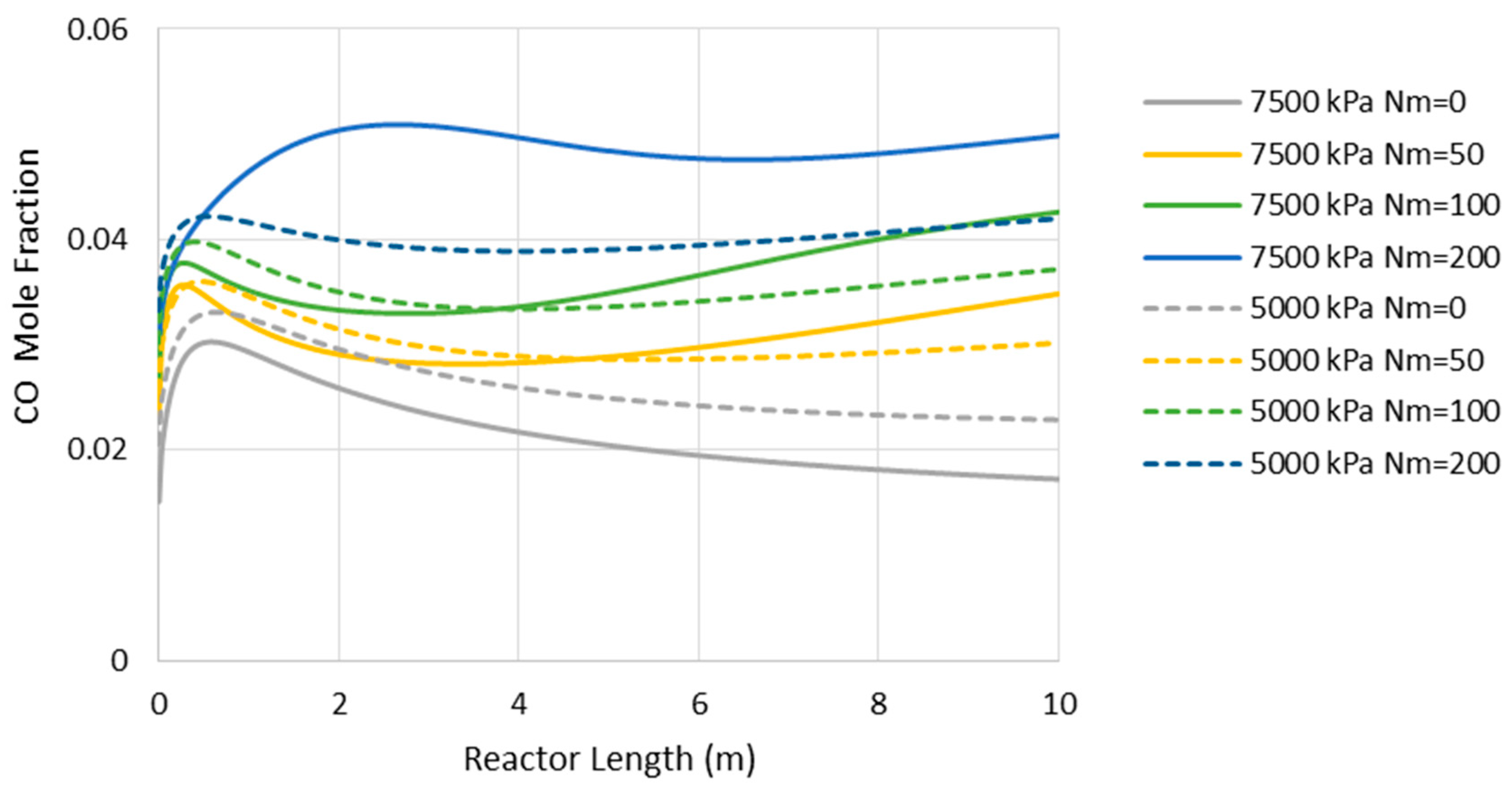
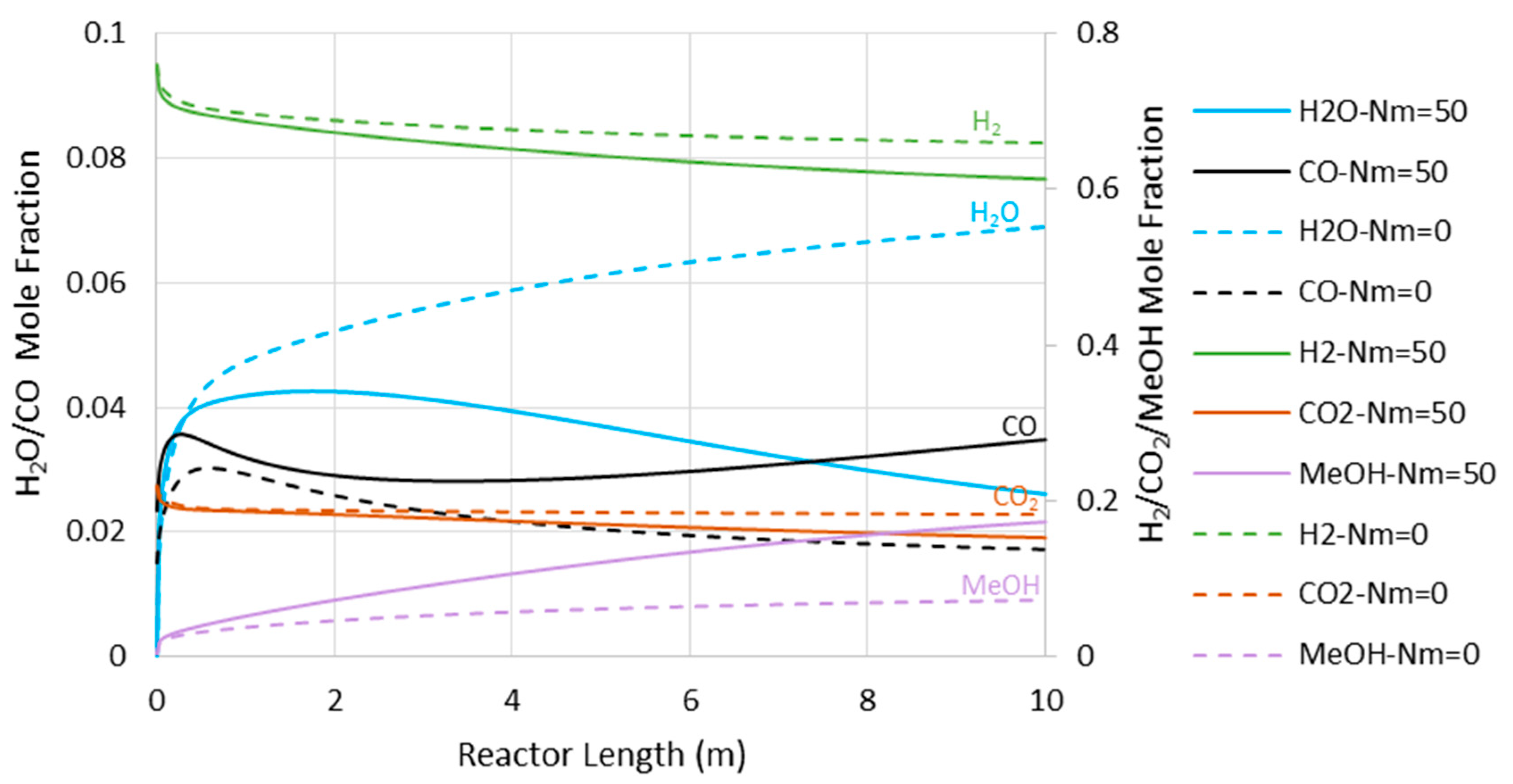
6.2. Isothermal MR-Based Process
6.3. Minimum Requirement for H2O/H2 Membrane Permselectivity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Nomenclature
| Acronyms | |
| CCU | carbon capture and utilization |
| CR | conventional reactor |
| CFMF | CO2 feed mass flowrate |
| FT | Fischer–Tropsch |
| H-SOD | hydroxy sodalite |
| LHTF | liquid hydrocarbon transportation fuels |
| MOGD | Mobil olefins to gasoline and distillate |
| MR | membrane reactor |
| MTG | methanol to gasoline |
| Nm | membrane tube numbers |
| ORV | overall reactor volume |
| RWGS | reverse water gas shift |
| tCO2 | tonne (metric ton) of CO2 |
| TRL | technology readiness level |
| Variables/Parameters | |
| Ex | exergy production/consumption rate per CO2 feed mass flowrate (kWh/tCO2) |
| enthalpy rate per CO2 feed mass flowrate (kWh/tCO2) | |
| reaction rate’s parameters | |
| equilibrium constants for methanol reaction, Equation (7) (1/kPa2) | |
| equilibrium constants for reverse water gas shift reaction, Equation (8) | |
| overall reactor volume (m3) | |
| p | partial pressure (kPa) |
| reaction rate (kmol/m3 h) | |
| universal gas constant (kJ/kmol K) | |
| temperature (K) | |
| ambient temperature, 298.15 (K) | |
| isothermal reactor temperature (K) | |
| compressor power per CO2 feed mass flowrate (kWh/tCO2) | |
| catalyst’s density (kg/m3) | |
| fixed bed porosity | |
| heat generation rate per CO2 feed mass flowrate for isothermal reactors (kWh/tCO2) |
Appendix A
References
- Chauvy, R.; Meunier, N.; Thomas, D.; De Weireld, G. Selecting emerging CO2 utilization products for short- to mid-term deployment. Appl. Energy 2019, 236, 662–680. [Google Scholar] [CrossRef]
- Garcia-Garcia, G.; Fernandez, M.C.; Armstrong, K.; Woolass, S.; Styring, P. Analytical review of life-cycle environmental impacts of carbon capture and utilization technologies. ChemSusChem 2021, 14, 995–1015. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.M.; Samsatli, S. Technologies and infrastructures underpinning future CO2 value chains: A comprehensive review and comparative analysis. Renew. Sustain. Energy Rev. 2018, 85, 46–68. [Google Scholar] [CrossRef]
- Vo, C.H.; Mondelli, C.; Hamedi, H.; Pérez-Ramírez, J.; Farooq, S.; Karimi, I.A. Sustainability assessment of thermocatalytic conversion of CO2 to transportation fuels, methanol, and 1-propanol. ACS Sustain. Chem. Eng. 2021. [Google Scholar] [CrossRef]
- Struis, R.P.W.J.; Stucki, S.; Wiedorn, M. A membrane reactor for methanol synthesis. J. Membr. Sci. 1996, 113, 93–100. [Google Scholar] [CrossRef]
- Barbieri, G.; Marigliano, G.; Golemme, G.; Drioli, E. Simulation of CO2 hydrogenation with ch3oh removal in a zeolite membrane reactor. Chem. Eng. J. 2002, 85, 53–59. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, Q. Methanol synthesis from CO2 using a silicone rubber/ceramic composite membrane reactor. Sep. Purif. Technol. 2004, 34, 227–237. [Google Scholar] [CrossRef]
- Gallucci, F.; Paturzo, L.; Basile, A. An experimental study of CO2 hydrogenation into methanol involving a zeolite membrane reactor. Chem. Eng. Process. Process. Intensif. 2004, 43, 1029–1036. [Google Scholar] [CrossRef]
- Raso, R.; Tovar, M.; Lasobras, J.; Herguido, J.; Kumakiri, I.; Araki, S.; Menéndez, M. Zeolite membranes: Comparison in the separation of H2O/H2/CO2 mixtures and test of a reactor for CO2 hydrogenation to methanol. Catal. Today 2020, 364, 270–275. [Google Scholar] [CrossRef]
- Yue, W.; Li, Y.; Wei, W.; Jiang, J.; Caro, J.; Huang, A. Highly selective CO2 conversion to methanol in a bifunctional zeolite catalytic membrane reactor. Angew. Chem. Int. Ed. 2021, 60, 18289–18294. [Google Scholar] [CrossRef]
- Juarez, E.; Lasobras, J.; Soler, J.; Herguido, J.; Menéndez, M. Polymer–ceramic composite membranes for water removal in membrane reactors. Membranes 2021, 11, 472. [Google Scholar] [CrossRef] [PubMed]
- Diban, N.; Aguayo, A.T.; Bilbao, J.; Urtiaga, A.; Ortiz, I. Membrane reactors for in situ water removal: A review of applications. Ind. Eng. Chem. Res. 2013, 52, 10342–10354. [Google Scholar] [CrossRef]
- Dzuryk, S.; Rezaei, E. Intensification of the reverse water gas shift reaction by water-permeable packed-bed membrane reactors. Ind. Eng. Chem. Res. 2020, 59, 18907–18920. [Google Scholar] [CrossRef]
- Saeidi, S.; Najari, S.; Hessel, V.; Wilson, K.; Keil, F.J.; Concepción, P.; Suib, S.L.; Rodrigues, A.E. Recent advances in CO2 hydrogenation to value-added products—Current challenges and future directions. Prog. Energy Combust. Sci. 2021, 85, 100905. [Google Scholar] [CrossRef]
- Brunetti, A.; Migliori, M.; Cozza, D.; Catizzone, E.; Giordano, G.; Barbieri, G. Methanol conversion to dimethyl ether in catalytic zeolite membrane reactors. ACS Sustain. Chem. Eng. 2020, 8, 10471–10479. [Google Scholar] [CrossRef]
- Hamedi, H.; Brinkmann, T. Rigorous and customizable 1d simulation framework for membrane reactors to, in principle, enhance synthetic methanol production. ACS Sustain. Chem. Eng. 2021, 9, 7620–7629. [Google Scholar] [CrossRef]
- Van-Dal, É.S.; Bouallou, C. Design and simulation of a methanol production plant from CO2 hydrogenation. J. Clean. Prod. 2013, 57, 38–45. [Google Scholar] [CrossRef]
- Qadir, S.; Hussain, A.; Ahsan, M. A computational fluid dynamics approach for the modeling of gas separation in membrane modules. Processes 2019, 7, 420. [Google Scholar] [CrossRef] [Green Version]
- Giacinti Baschetti, M.; De Angelis, M.G. 8-vapour permeation modelling. In Pervaporation, Vapour Permeation and Membrane Distillation; Basile, A., Figoli, A., Khayet, M., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 203–246. [Google Scholar]
- Poto, S.; Gallucci, F.; Fernanda Neira d’Angelo, M. Direct conversion of CO2 to dimethyl ether in a fixed bed membrane reactor: Influence of membrane properties and process conditions. Fuel 2021, 302, 121080. [Google Scholar] [CrossRef]
- Bussche, K.M.V.; Froment, G.F. A steady-state kinetic model for methanol synthesis and the water gas shift reaction on a commercial cu/zno/al2o3catalyst. J. Catal. 1996, 161, 1–10. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquid via synthesis of methanol, dme or fischer–tropsch-fuels: A review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Mignard, D.; Sahibzada, M.; Duthie, J.M.; Whittington, H.W. Methanol synthesis from flue-gas CO2 and renewable electricity: A feasibility study. Int. J. Hydrogen Energy 2003, 28, 455–464. [Google Scholar] [CrossRef]
- Leonzio, G. Mathematical modeling of a methanol reactor by using different kinetic models. J. Ind. Eng. Chem. 2020, 85, 130–140. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Huang, A.; Caro, J. Hydrophilic sod and lta membranes for membrane-supported methanol, dimethylether and dimethylcarbonate synthesis. Microporous Mesoporous Mater. 2015, 207, 33–38. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Huang, A.; Caro, J. Supported sod membrane with steam selectivity by a two-step repeated hydrothermal synthesis. Microporous Mesoporous Mater. 2014, 192, 8–13. [Google Scholar] [CrossRef]
- Sawamura, K.-I.; Shirai, T.; Takada, M.; Sekine, Y.; Kikuchi, E.; Matsukata, M. Selective permeation and separation of steam from water–methanol–hydrogen gas mixtures through mordenite membrane. Catal. Today 2008, 132, 182–187. [Google Scholar] [CrossRef]
- Rohde, M.P.; Schaub, G.; Khajavi, S.; Jansen, J.C.; Kapteijn, F. Fischer–tropsch synthesis with in situ H2O removal–Directions of membrane development. Microporous Mesoporous Mater. 2008, 115, 123–136. [Google Scholar] [CrossRef]
- Escorihuela, S.; Tena, A.; Shishatskiy, S.; Escolástico, S.; Brinkmann, T.; Serra, J.M.; Abetz, V. Gas separation properties of polyimide thin films on ceramic supports for high temperature applications. Membranes 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigelt, F.; Escorihuela, S.; Descalzo, A.; Tena, A.; Escolástico, S.; Shishatskiy, S.; Serra, J.M.; Brinkmann, T. Novel polymeric thin-film composite membranes for high-temperature gas separations. Membranes 2019, 9, 51. [Google Scholar] [CrossRef] [Green Version]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Application of hydroxy sodalite films as novel water selective membranes. J. Membr. Sci. 2009, 326, 153–160. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Mirvakili, A.; Paymooni, K. A novel water perm-selective membrane dual-type reactor concept for fischer–tropsch synthesis of gtl (gas to liquid) technology. Energy 2011, 36, 1223–1235. [Google Scholar] [CrossRef]
- El Sibai, A.; Rihko Struckmann, L.K.; Sundmacher, K. Model-based optimal sabatier reactor design for power-to-gas applications. Energy Technol. 2017, 5, 911–921. [Google Scholar] [CrossRef]
- Hamedi, H.; Karimi, I.A.; Gundersen, T. Simulation-based approach for integrating work within heat exchange networks for sub-ambient processes. Energy Convers. Manag. 2020, 203, 112276. [Google Scholar] [CrossRef]


| Parameters | Value |
|---|---|
| A1, kmol/(h kPa2 kgcat) | 3.852 × 10−4 |
| B1, kJ/kmol | 40,000 |
| A2 | 3453.38 |
| B2, kJ/kmol | 0 |
| A3, kmol/(h kPa0.5 kgcat) | 4.99 × 10−2 |
| B3, kJ/kmol | 17,197 |
| A4, kmol/(h kPa kgcat) | 6.62 × 10−13 |
| B4, kJ/kmol | 124,119 |
| A5, kmol/(h kPa kgcat) | 4.392 × 108 |
| B5, kJ/kmol | −98,084 |
| Parameters | Value |
|---|---|
| CO2 feed flowrate, kmol/h | 50 |
| H2/CO2 feed ratio | 2.974 |
| H2/CO2 feed temperature, K | 308 |
| H2/CO2 feed pressure, kPa | 5020 and 7520 |
| reactor feed pressure, kPa | 5000 and 7500 |
| reactor’s inlet temperature for adiabatic reactors, K | 483.15 |
| reactor’s inlet temperature for nonadiabatic reactors, K | 513.15 |
| heat exchanger pressure drop, kPa | 20 |
| separator pressure drop, kPa | 0 |
| water product purity % | ≥99.0 |
| methanol product purity % | 99.0 |
| reactor length, m | 10.0 |
| reactor diameter, m | 1.0 |
| membrane water permeance, mol/(s m2 Pa) | 10−6 |
| membrane tube diameter, m | 0.05 |
| catalyst particle diameter, m | 0.002 |
| apparent catalyst density, kgcat/m3cat | 1775 |
| porosity | 0.4 |
| sphericity | 1.0 |
| number of distillation column trays | 20 |
| feed tray number for distillation column | 10 |
| ambient temperature, K | 298.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamedi, H.; Brinkmann, T.; Shishatskiy, S. Membrane-Assisted Methanol Synthesis Processes and the Required Permselectivity. Membranes 2021, 11, 596. https://doi.org/10.3390/membranes11080596
Hamedi H, Brinkmann T, Shishatskiy S. Membrane-Assisted Methanol Synthesis Processes and the Required Permselectivity. Membranes. 2021; 11(8):596. https://doi.org/10.3390/membranes11080596
Chicago/Turabian StyleHamedi, Homa, Torsten Brinkmann, and Sergey Shishatskiy. 2021. "Membrane-Assisted Methanol Synthesis Processes and the Required Permselectivity" Membranes 11, no. 8: 596. https://doi.org/10.3390/membranes11080596
APA StyleHamedi, H., Brinkmann, T., & Shishatskiy, S. (2021). Membrane-Assisted Methanol Synthesis Processes and the Required Permselectivity. Membranes, 11(8), 596. https://doi.org/10.3390/membranes11080596








