Synthesis and Characterization of Titanium Dioxide Hollow Nanofiber for Photocatalytic Degradation of Methylene Blue Dye
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanofibers Template
2.3. Synthesis of THNF Photocatalysts
2.4. Characterization
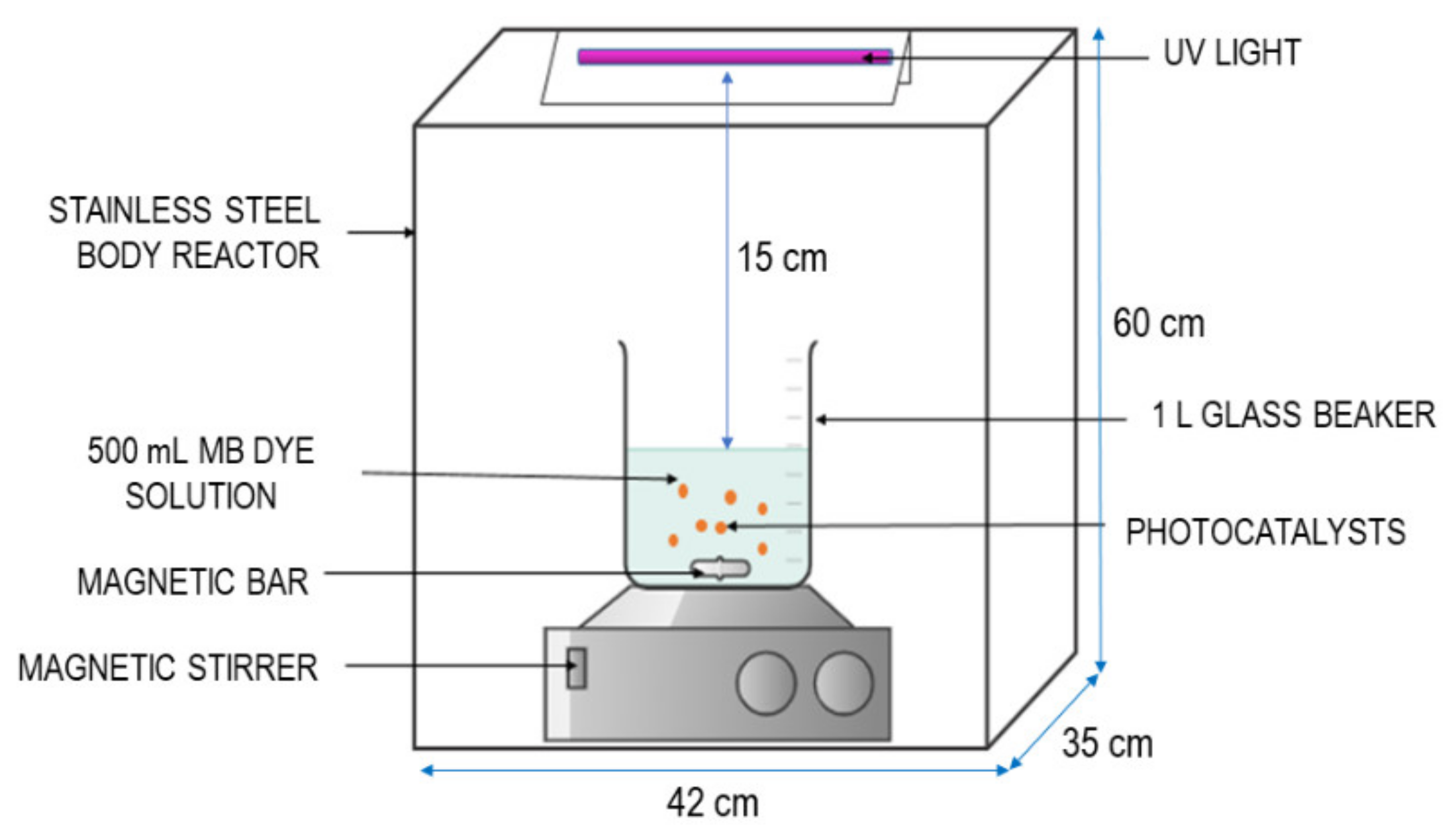
2.5. Photodegradation Experiment
3. Results
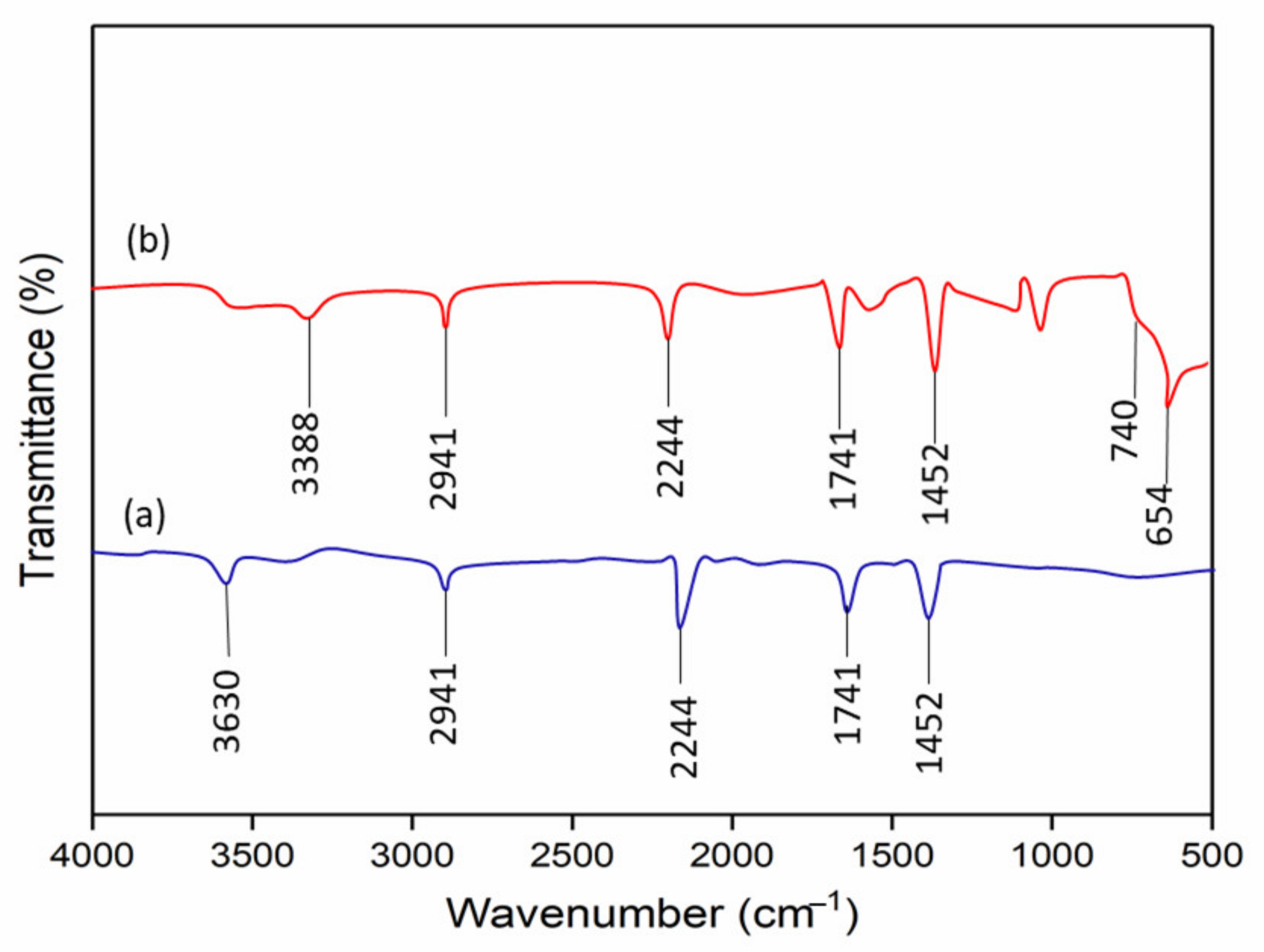
3.1. TiO2 Precursor Deposition on PAN Nanofiber
3.2. Effect of Calcination Temperature
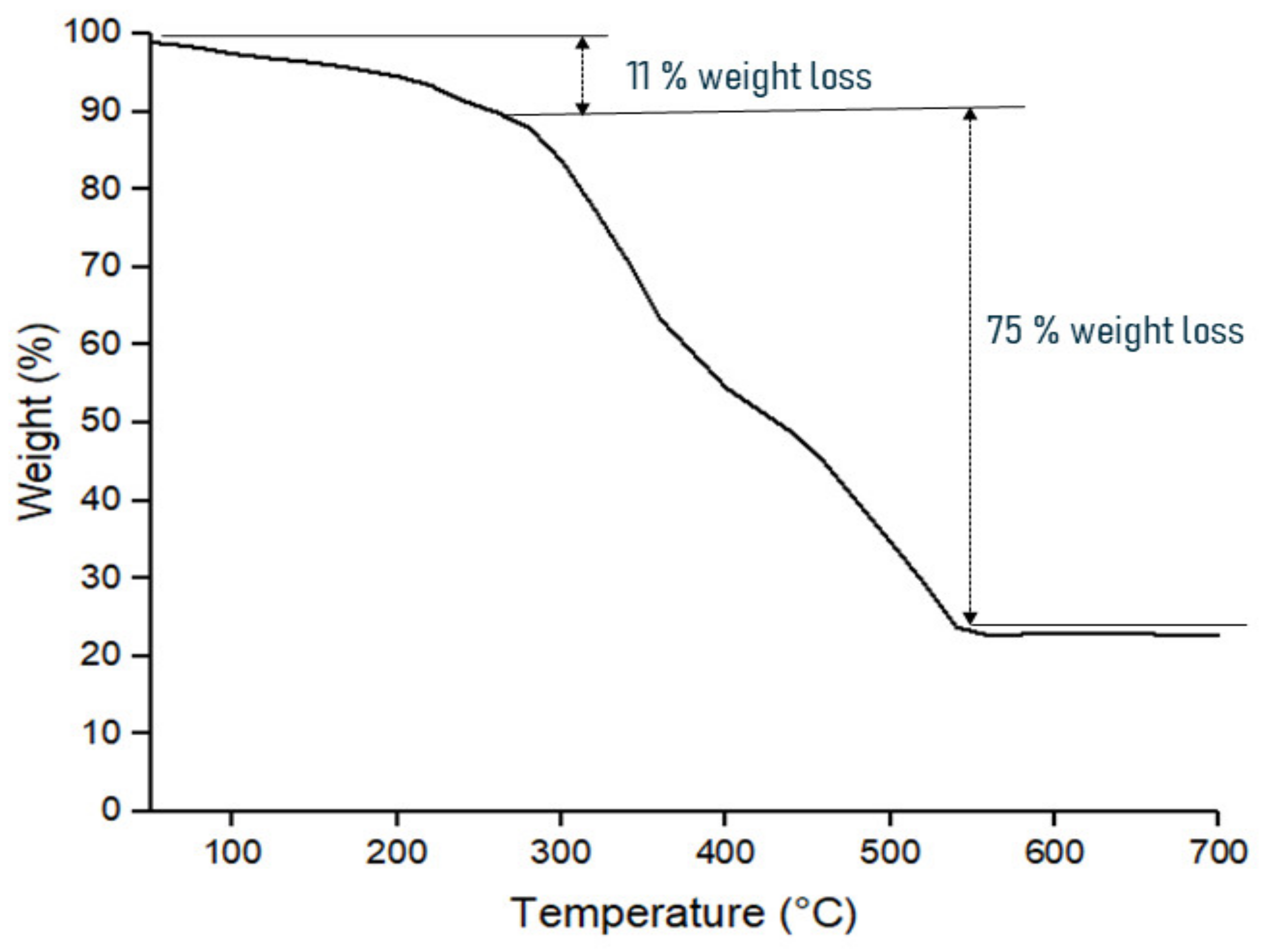
3.2.1. Thermal Decomposition Analysis
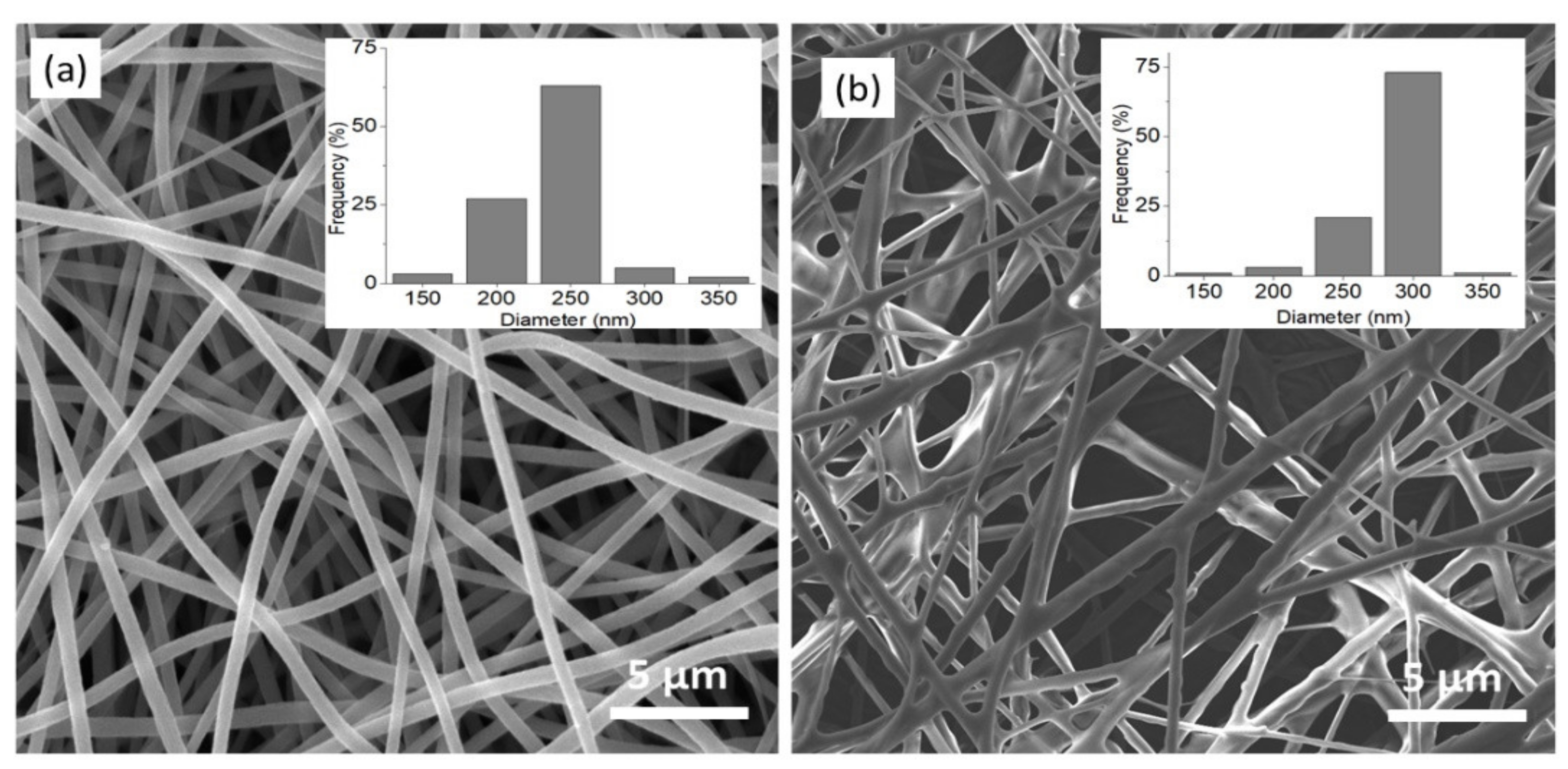
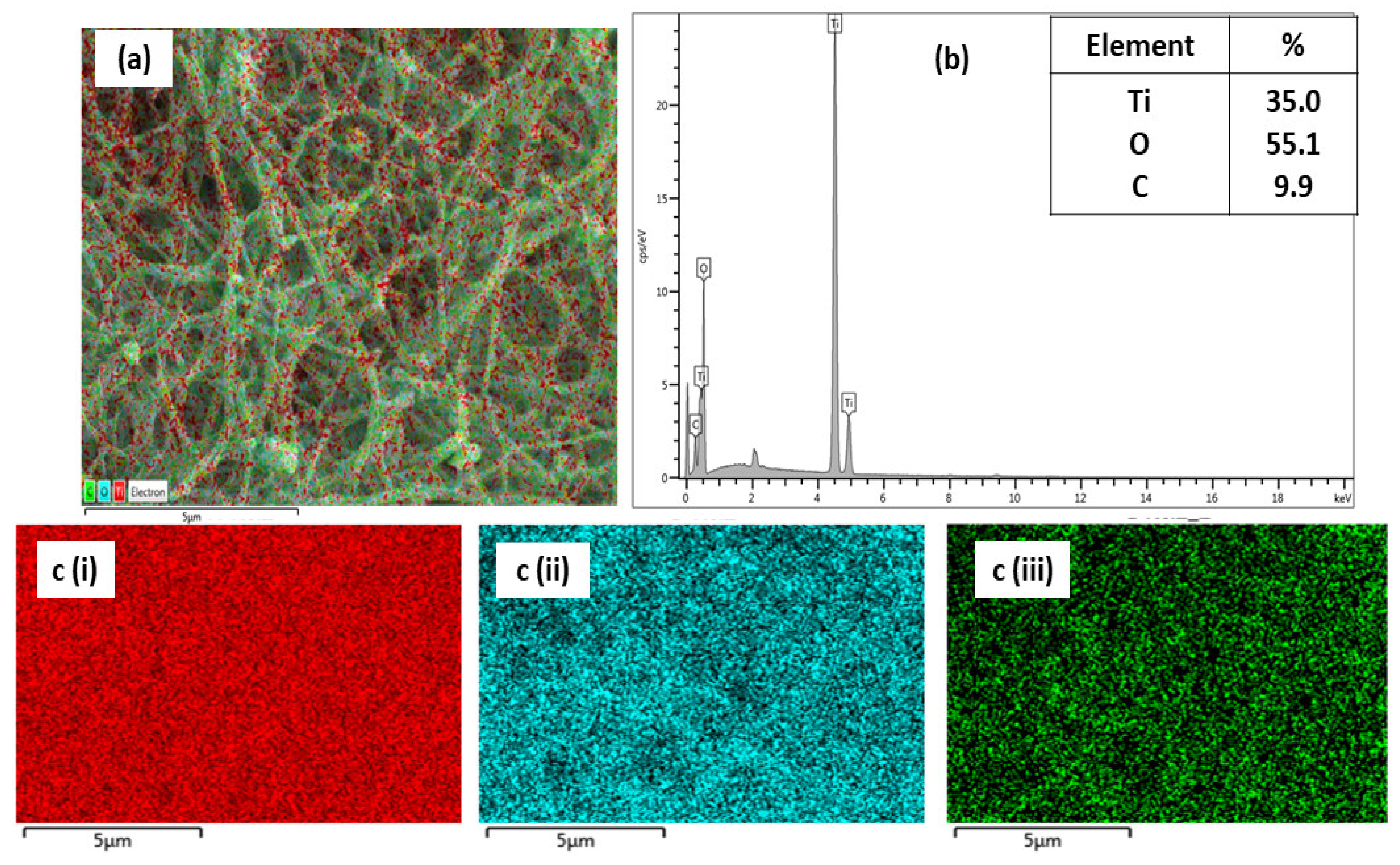
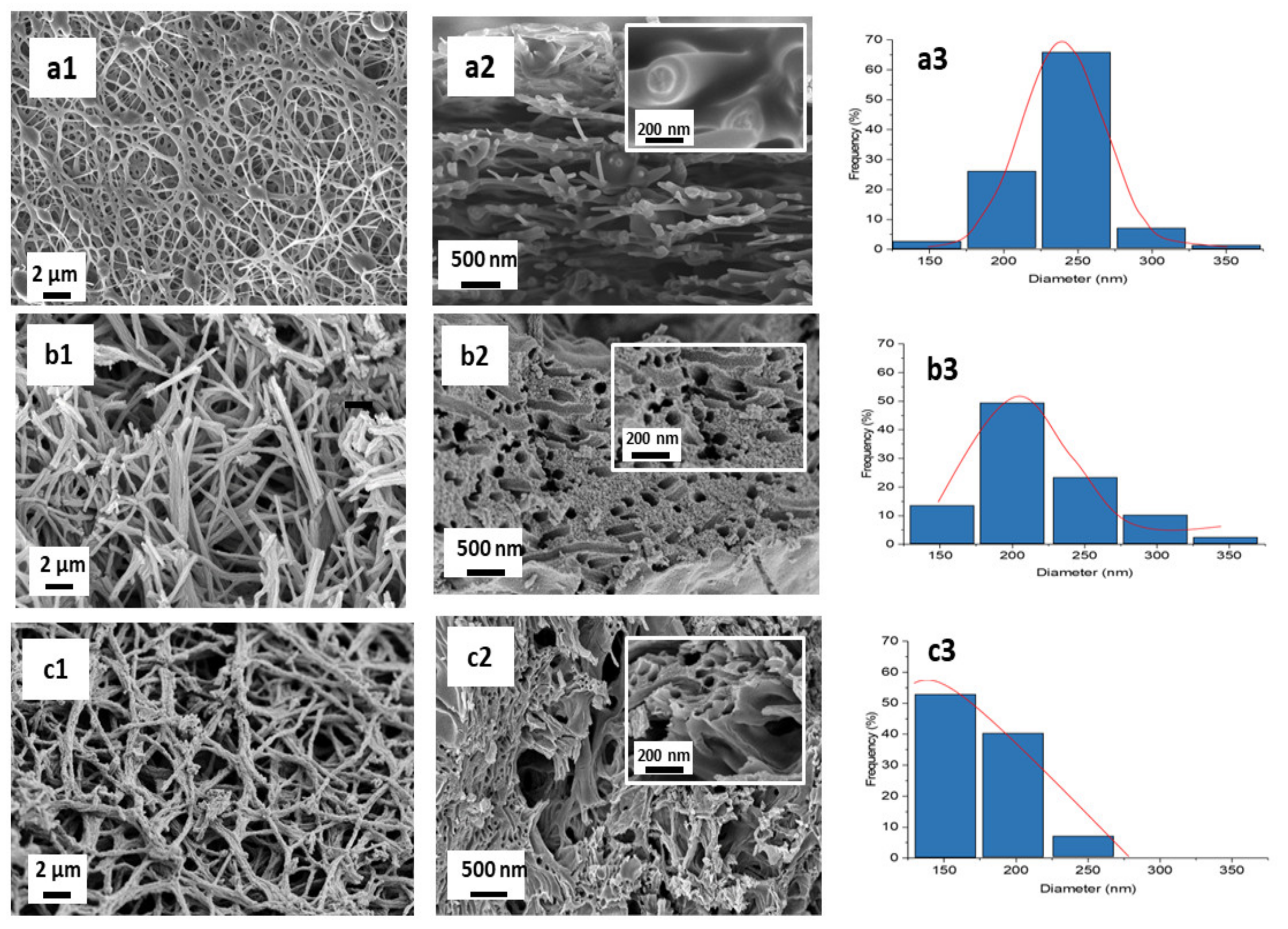
3.2.2. Morphological Analysis
3.2.3. Nitrogen-Desorption Analysis
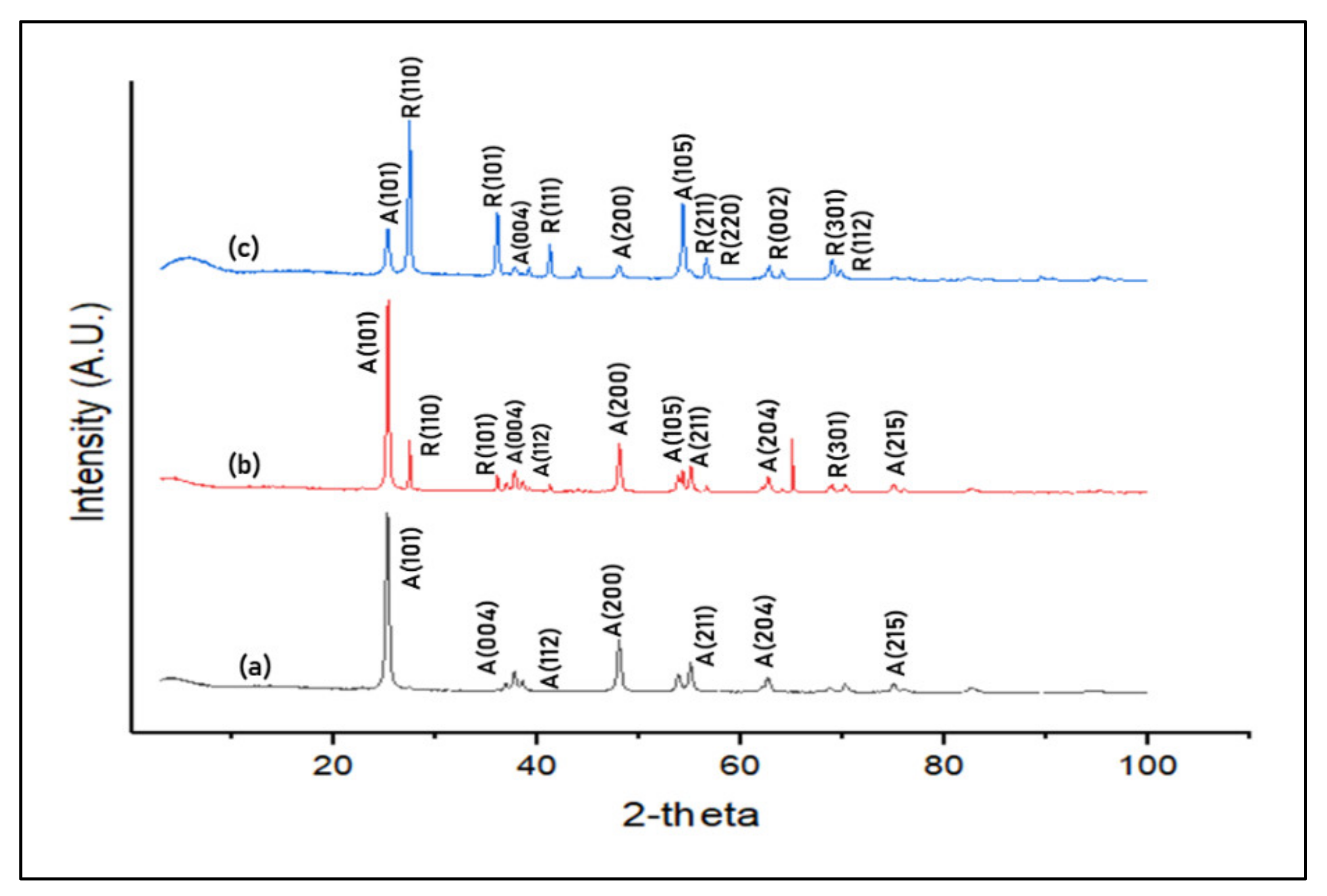
3.2.4. Crystallinity Analysis
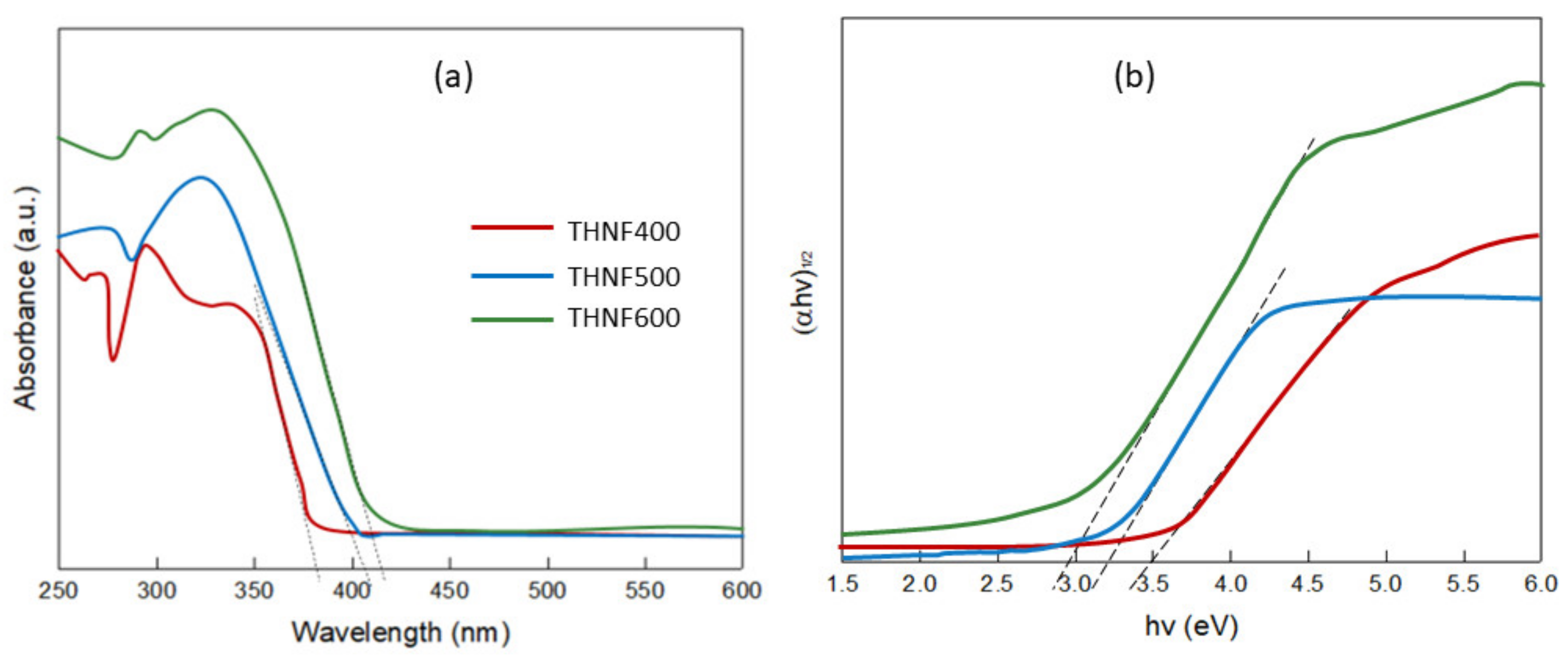
3.2.5. Optical Absorbance Analysis
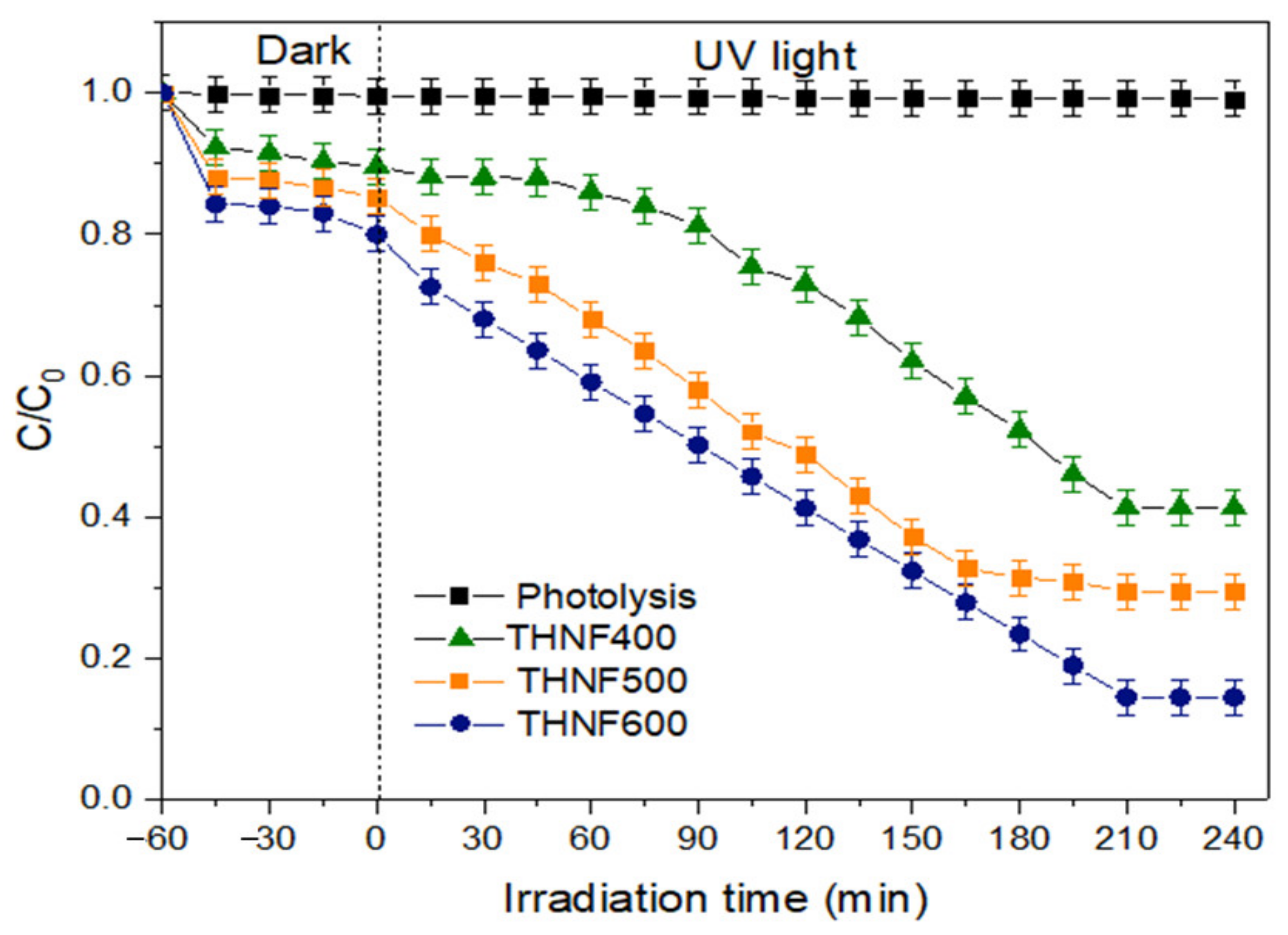
3.2.6. Photocatalytic Degradation of MB Dye
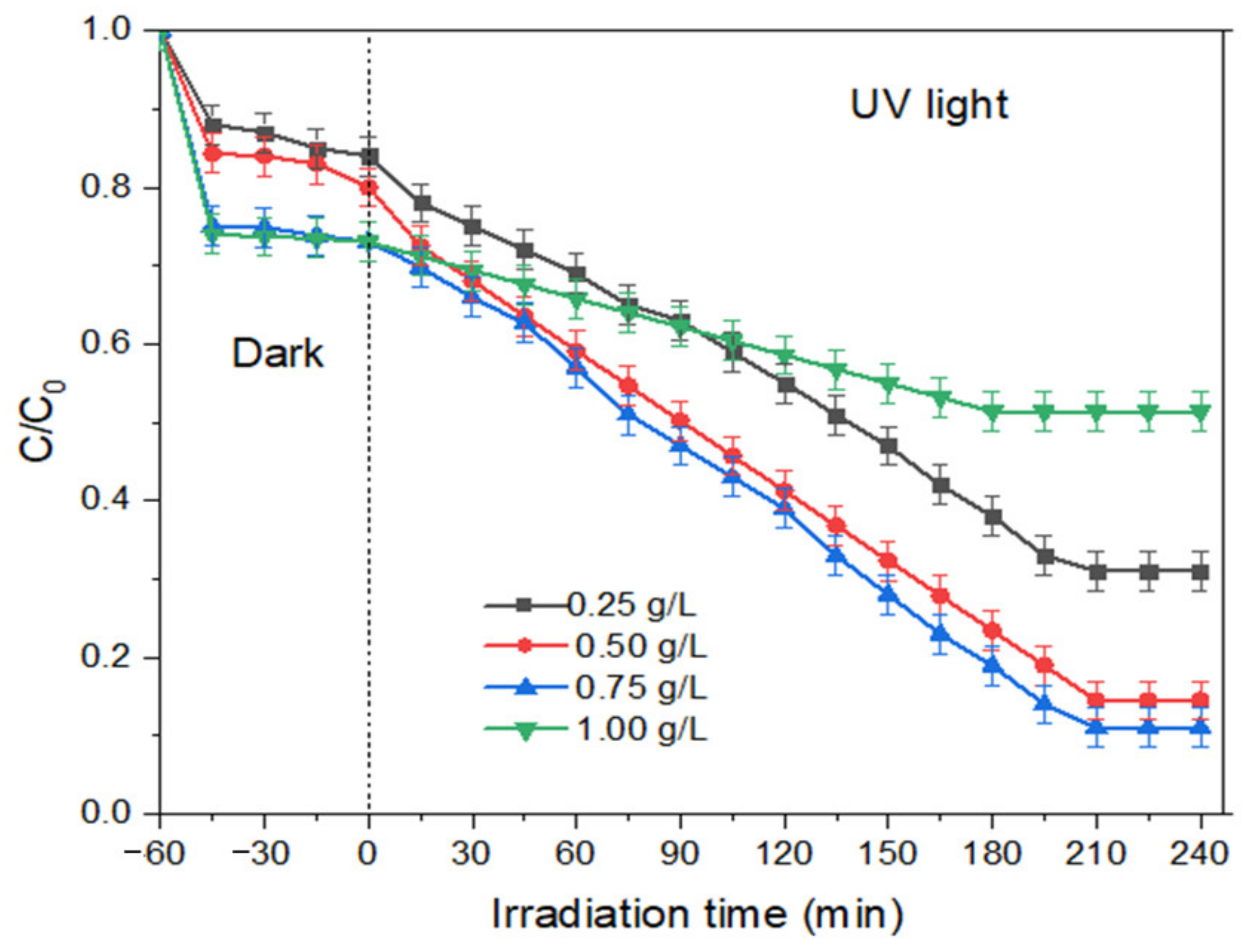
- (a)
- Effect of photocatalyst loading
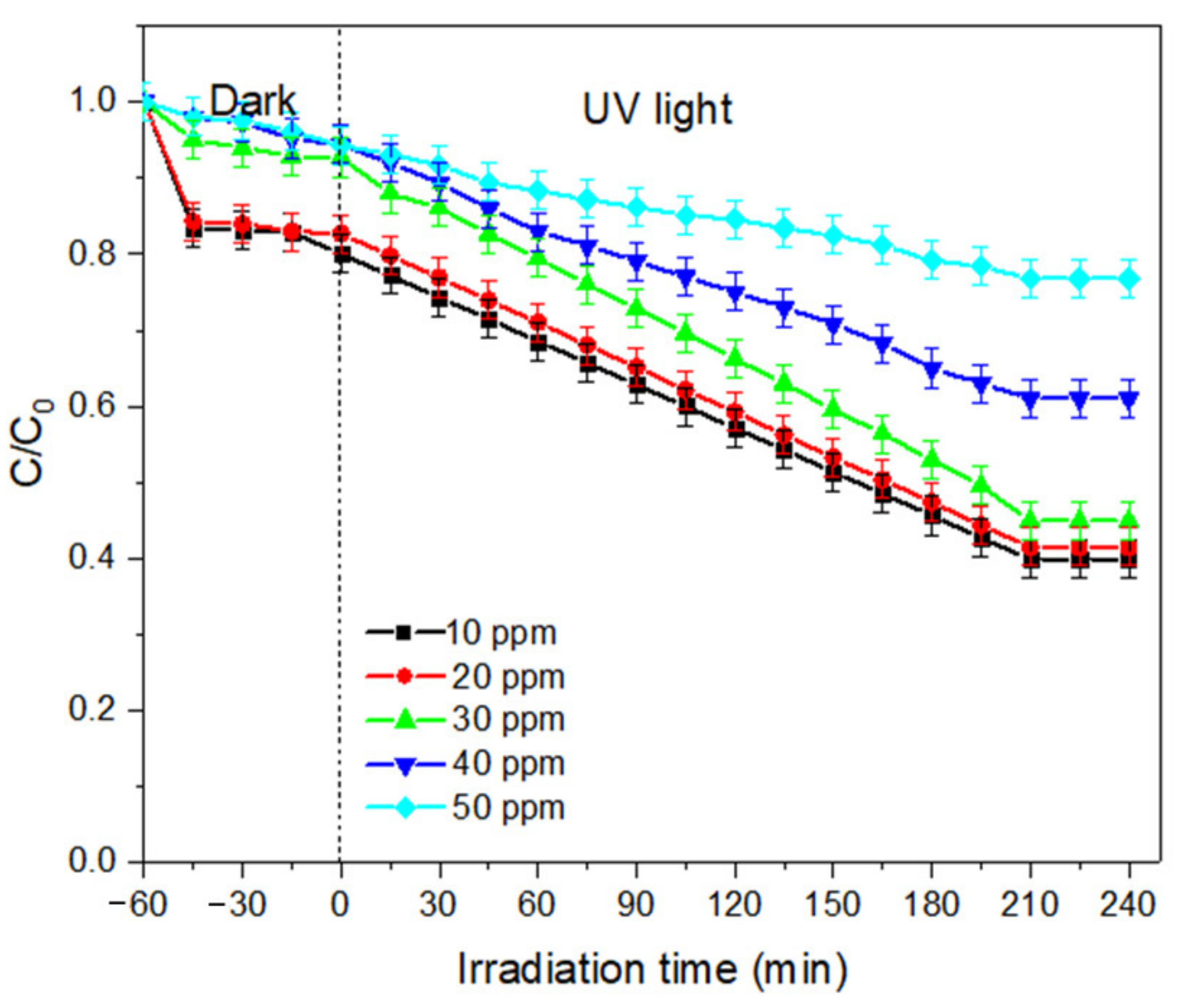
- (b)
- Effect of dye concentration
- (c)
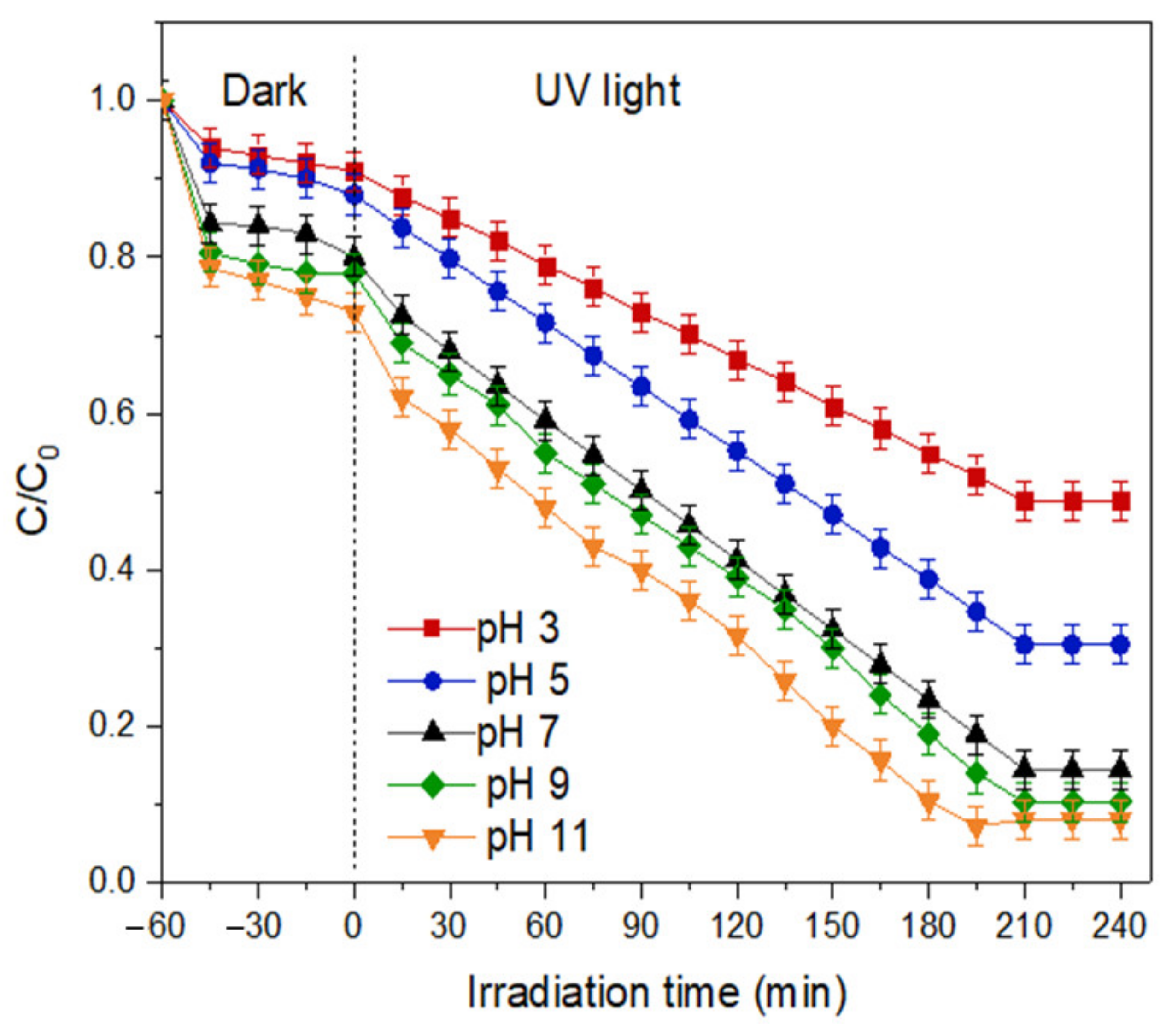
- Effect of pH of the solution
- (d)
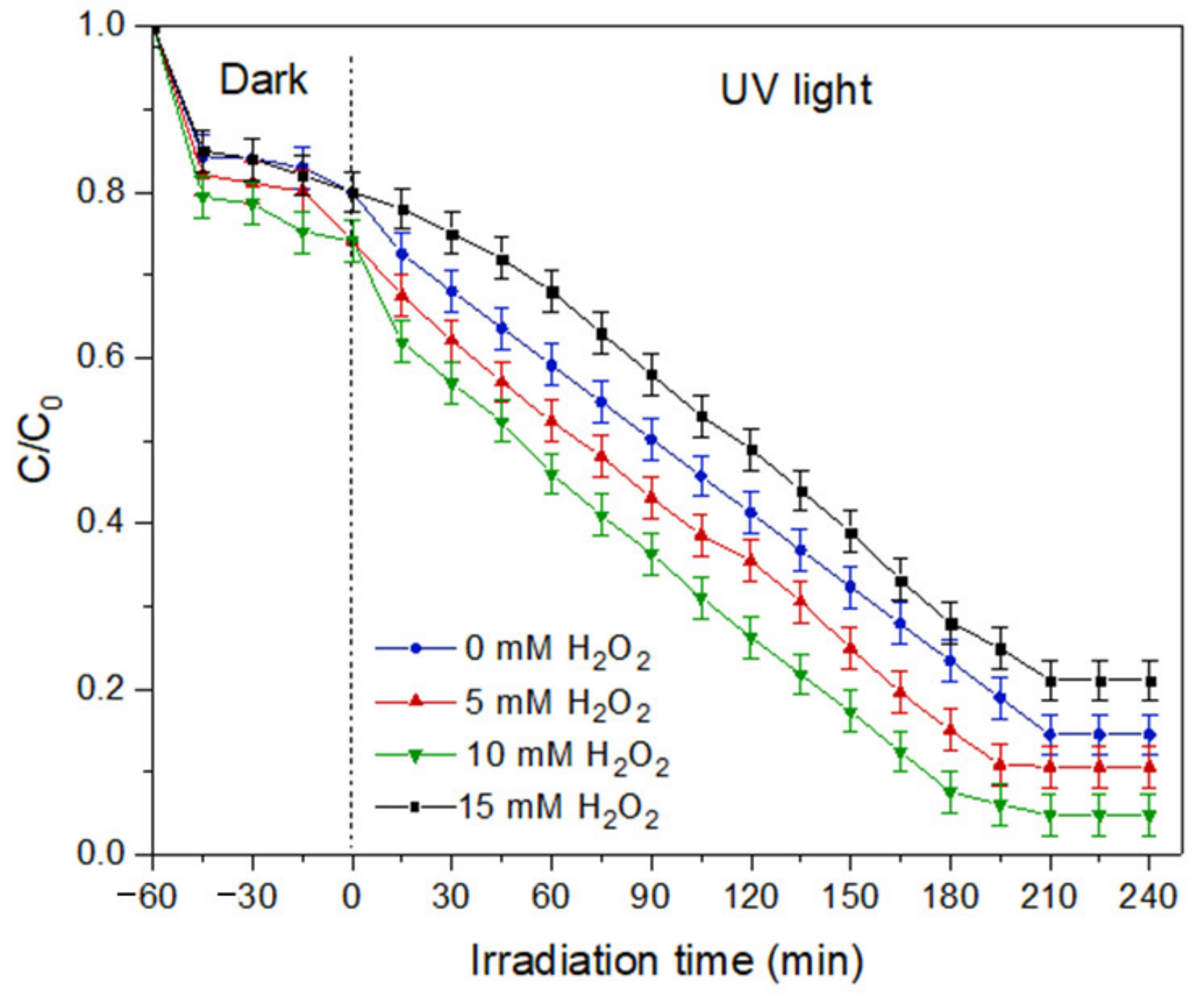
- Effect of hydrogen peroxide concentration
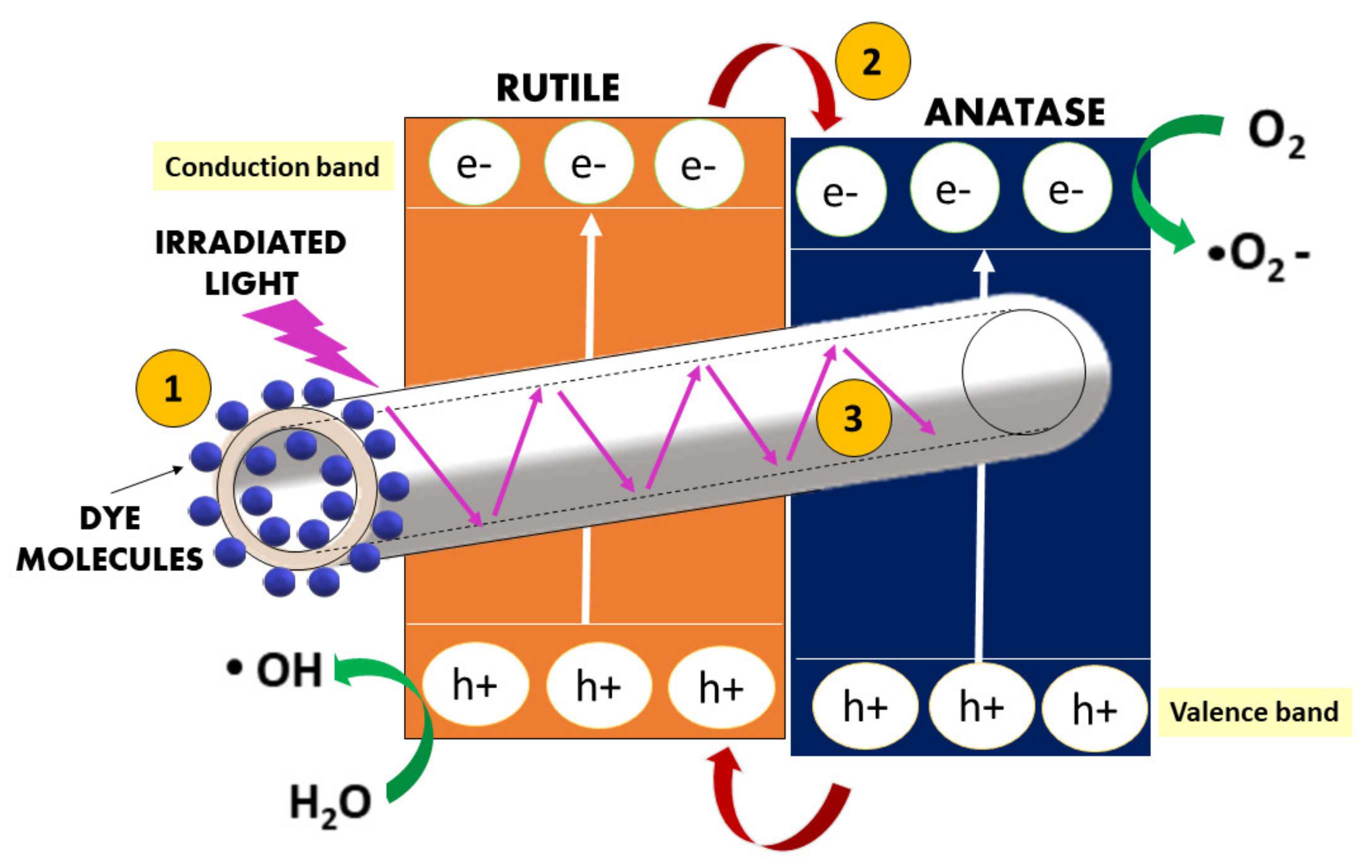
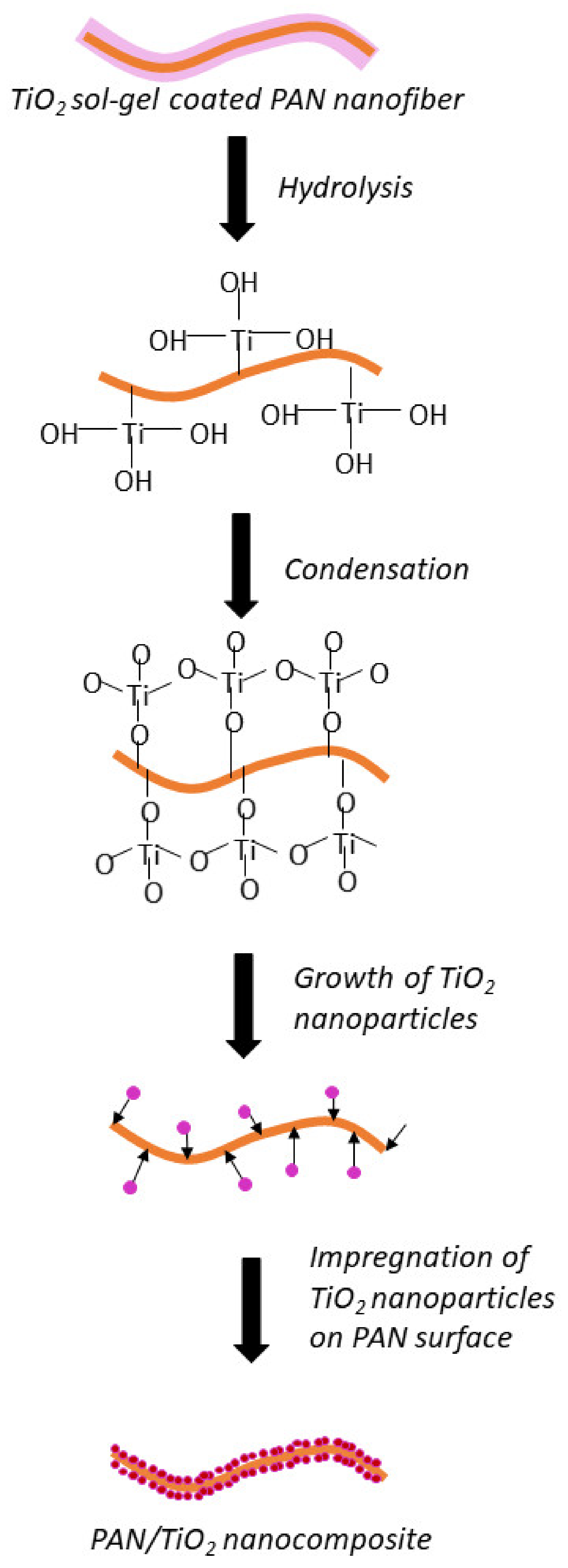
3.3. Proposed Mechanism of Hollow Nanofibers Formation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afroz, R.; Rahman, A. Health impact of river water pollution in Malaysia. Int. J. Adv. Appl. Sci. 2017, 4, 78–85. [Google Scholar] [CrossRef]
- Erfani, M.; Javanbakht, V. Methylene Blue removal from aqueous solution by a biocomposite synthesized from sodium alginate and wastes of oil extraction from almond peanut. Int. J. Biol. Macromol. 2018, 114, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Regkouzas, P.; Diamadopoulos, E. Adsorption of selected organic micro-pollutants on sewage sludge biochar. Chemosphere 2019, 224, 840–851. [Google Scholar] [CrossRef]
- Campinas, M.; Viegas, R.; Coelho, R.; Lucas, H.; Rosa, M.J. Adsorption/Coagulation/Ceramic Microfiltration for Treating Challenging Waters for Drinking Water Production. Membranes 2021, 11, 91. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal oxides as photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Hir, Z.A.M.; Moradihamedani, P.; Abdullah, A.H.; Mohamed, M.A. Immobilization of TiO2 into polyethersulfone matrix as hybrid film photocatalyst for effective degradation of methyl orange dye. Mater. Sci. Semicond. Process. 2017, 57, 157–165. [Google Scholar] [CrossRef]
- Bouanimba, N.; Laid, N.; Zouaghi, R.; Sehili, T. A Comparative Study of the Activity of TiO2 Degussa P25 and Millennium PCs in the Photocatalytic Degradation of Bromothymol Blue. Int. J. Chem. React. Eng. 2018, 16, 20170014. [Google Scholar] [CrossRef]
- Das, A.; Patra, M.; Wary, R.; Nair, R. Photocatalytic performance analysis of Degussa P25 under various laboratory conditions. IOP Conf. Ser. Mater. Sci. Eng. 2018, 377, 012101. [Google Scholar] [CrossRef]
- Moztahida, M.; Lee, D.S. Photocatalytic degradation of methylene blue with P25/graphene/polyacrylamide hydrogels: Optimization using response surface methodology. J. Hazard. Mater. 2020, 400, 123314. [Google Scholar] [CrossRef]
- Tichapondwa, S.M.; Newman, J.P.; Kubheka, O. Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth Parts A/B/C 2020, 118–119, 102900. [Google Scholar] [CrossRef]
- Uddin, M.J.; Cesano, F.; Chowdhury, A.R.; Trad, T.; Cravanzola, S.; Martra, G.; Mino, L.; Zecchina, A.; Scarano, D. Surface Structure and Phase Composition of TiO2 P25 Particles After Thermal Treatments and HF Etching. Front. Mater. 2020, 7, 192. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Bao, Y.; Zhang, Y.; Wang, J.; Fu, M.; Wu, J.; Ye, D. The Applications of Morphology Controlled ZnO in Catalysis. Catalysts 2016, 6, 188. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Zhou, J.; Xi, Q.; Li, X.; Nie, E.; Piao, X.; Sun, Z. Fabricating I doped TiO2 photoelectrode for the degradation of diclofenac: Performance and mechanism study. Chem. Eng. J. 2019, 369, 968–978. [Google Scholar] [CrossRef]
- Zhang, G.; Lan, Z.-A.; Wang, X. Conjugated Polymers: Catalysts for Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2016, 55, 15712–15727. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bi, W.; Zhang, L.; Tao, S.; Chu, W.; Zhang, Q.; Luo, Y.; Wu, C.; Xie, Y. Single-Atom Pt as Co-Catalyst for Enhanced Photocatalytic H2 Evolution. Adv. Mater. 2016, 28, 2427–2431. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Shoueir, K. Recent advances in polymer/metal/metal oxide hybrid nanostructures for catalytic applications: A review. J. Envion. Chem. Eng. 2020, 8, 104175. [Google Scholar]
- Wang, Z.; Zhao, L.; Wang, P.; Guo, L.; Yu, J. Low material density and high microwave-absorption performance of hollow strontium ferrite nanofibers prepared via coaxial electrospinning. J. Alloys Compd. 2016, 687, 541–547. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, X.; Xin, H.; Li, D.; Zhao, Y.; Shi, L.; Lin, Y.; Yu, J.; Yu, Z.; Zhu, C. Coaxial electrospinning synthesis hollow Mo2C@ C core-shell nanofibers for high-performance and long-term lithium-ion batteries. Appl. Surf. Sci. 2019, 473, 352–358. [Google Scholar] [CrossRef]
- Xu, K.; Li, S.; Yang, J.; Hu, J. Hierarchical hollow MnO2 nanofibers with enhanced supercapacitor performance. J. Colloid Interface Sci. 2018, 513, 448–454. [Google Scholar] [CrossRef]
- Borbón-Nuñez, H.A.; Dominguez, D.; Muñoz-Muñoz, F.; Lopez, J.; Romo-Herrera, J.; Soto, G.; Tiznado, H. Fabrication of hollow TiO2 nanotubes through atomic layer deposition and MWCNT templates. Powder Technol. 2017, 308, 249–257. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, Q.; Pan, X.; Jin, Y.; Lu, W.; Ding, D.; Guo, Q. Graphene oxide/polyacrylonitrile fiber hierarchical-structured membrane for ultra-fast microfiltration of oil-water emulsion. Chem. Eng. J. 2016, 307, 643–649. [Google Scholar] [CrossRef]
- Hou, J.; Yun, J.; Byun, H. Fabrication and characterization of modified graphene oxide/PAN hybrid nanofiber membrane. Membranes 2019, 9, 122. [Google Scholar] [CrossRef]
- Gao, Q.; Luo, J.; Wang, X.; Gao, C.; Ge, M. Novel hollow α-Fe2O3 nanofibers via electrospinning for dye adsorption. Nanoscale Res. Lett. 2015, 10, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, V.; Singh, I.; Chandra, A. Hollow nanostructures of metal oxides as next generation electrode materials for supercapacitors. Sci. Rep. 2018, 8, 1307. [Google Scholar] [CrossRef]
- Al-Hajji, L.A.; Ismail, A.A.; Al-Hazza, A.; Ahmed, S.A.; Alsaidi, M.; Almutawa, F.; Bumajdad, A. Impact of calcination of hydrothermally synthesized TiO2 nanowires on their photocatalytic efficiency. J. Mol. Struct. 2020, 1200, 127153. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing Methods for Calculating Nano Crystal Size of Natural Hydroxyapatite Using X-ray Diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, H.; Wang, G.; Pan, Y.; Yu, Z.; Long, H. Effect of Calcination Temperature on the Activation Performance and Reaction Mechanism of Ce–Mn–Ru/TiO2 Catalysts for Selective Catalytic Reduction of NO with NH3. ACS Omega 2020, 5, 33357–33371. [Google Scholar] [CrossRef]
- Hossain, M.; Pervez, M.F.; Uddin, J.; Tayyaba, E.D.S.; Mia, M.N.; Bashar, M.; Jewel, M.; Haque, M.; Hakim, M.; Khan, M. Influence of natural dye adsorption on the structural, morphological and optical properties of TiO2 based photoanode of dye-sensitized solar cell. Mater. Sci. 2018, 36, 93–101. [Google Scholar] [CrossRef]
- Paul, S.; Choudhury, A. Investigation of the optical property and photocatalytic activity of mixed phase nanocrystalline titania. Appl. Nanosci. 2014, 4, 839–847. [Google Scholar] [CrossRef]
- Kacprzyńska-Gołacka, J.; Łożyńska, M.; Barszcz, W.; Sowa, S.; Wieciński, P.; Woskowicz, E.; Życki, M. Influence of Deposition Parameters of TiO2 + CuO Coating on the Membranes Surface Used in the Filtration Process of Dairy Wastewater on Their Functional Properties. Membranes 2021, 11, 290. [Google Scholar] [CrossRef]
- Elahifard, M.; Sadrian, M.R.; Mirzanejad, A.; Behjatmanesh-Ardakani, R.; Ahmadvand, S. Dispersion of defects in TiO2 semiconductor: Oxygen vacancies in the bulk and surface of rutile and anatase. Catalysts 2020, 10, 397. [Google Scholar] [CrossRef]
- Yunus, N.N.; Hamzah, F.; So’aib, M.S.; Krishnan, J. Effect of Catalyst Loading on Photocatalytic Degradation of Phenol by Using N, S Co-doped TiO2. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012092. [Google Scholar] [CrossRef]
- Siddique, M.; Khan, R.; Khan, A.; Farooq, R. Improved Photocatalytic Activity of TiO2 Coupling Ultrasound for Reactive Blue 19 Degradation. J. Chem. Soc. Pak. 2014, 38, 37–43. [Google Scholar]
- Ramirez, L.; Ramseier Gentile, S.; Zimmermann, S.; Stoll, S. Behavior of TiO2 and CeO2 Nanoparticles and Polystyrene Nanoplastics in Bottled Mineral, Drinking and Lake Geneva Waters. Impact of Water Hardness and Natural Organic Matter on Nanoparticle Surface Properties and Aggregation. Water 2019, 11, 721. [Google Scholar] [CrossRef]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 7104. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, A.; Khoshghadam-Pireyousefan, M.; Shokrianfard-Ravasjan, B.; Azadbeh, M.; Rashedi, H.; Dibazar, M.; Mostafaei, A. Synergetic photocatalytic effect of high purity ZnO pod shaped nanostructures with H2O2 on methylene blue dye degradation. J. Alloys Compd. 2020, 845, 156333. [Google Scholar] [CrossRef]
| Sample | Specific Surface Area (m2/g) | Specific Pore Volume (cm3/g) |
|---|---|---|
| THNF400 | 13.3216 | 0.06354 |
| THNF500 | 43.4085 | 0.23490 |
| THNF600 | 81.2776 | 0.32716 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad Jafri, N.N.; Jaafar, J.; Alias, N.H.; Samitsu, S.; Aziz, F.; Wan Salleh, W.N.; Mohd Yusop, M.Z.; Othman, M.H.D.; Rahman, M.A.; Ismail, A.F.; et al. Synthesis and Characterization of Titanium Dioxide Hollow Nanofiber for Photocatalytic Degradation of Methylene Blue Dye. Membranes 2021, 11, 581. https://doi.org/10.3390/membranes11080581
Mohammad Jafri NN, Jaafar J, Alias NH, Samitsu S, Aziz F, Wan Salleh WN, Mohd Yusop MZ, Othman MHD, Rahman MA, Ismail AF, et al. Synthesis and Characterization of Titanium Dioxide Hollow Nanofiber for Photocatalytic Degradation of Methylene Blue Dye. Membranes. 2021; 11(8):581. https://doi.org/10.3390/membranes11080581
Chicago/Turabian StyleMohammad Jafri, Nurul Natasha, Juhana Jaafar, Nur Hashimah Alias, Sadaki Samitsu, Farhana Aziz, Wan Norharyati Wan Salleh, Mohd Zamri Mohd Yusop, Mohd Hafiz Dzarfan Othman, Mukhlis A Rahman, Ahmad Fauzi Ismail, and et al. 2021. "Synthesis and Characterization of Titanium Dioxide Hollow Nanofiber for Photocatalytic Degradation of Methylene Blue Dye" Membranes 11, no. 8: 581. https://doi.org/10.3390/membranes11080581
APA StyleMohammad Jafri, N. N., Jaafar, J., Alias, N. H., Samitsu, S., Aziz, F., Wan Salleh, W. N., Mohd Yusop, M. Z., Othman, M. H. D., Rahman, M. A., Ismail, A. F., Matsuura, T., & Isloor, A. M. (2021). Synthesis and Characterization of Titanium Dioxide Hollow Nanofiber for Photocatalytic Degradation of Methylene Blue Dye. Membranes, 11(8), 581. https://doi.org/10.3390/membranes11080581












