Polymer–Ceramic Composite Membranes for Water Removal in Membrane Reactors
Abstract
:1. Introduction
2. Experimental System
2.1. Membrane Preparation
2.2. Membrane Characterization
3. Experimental Results
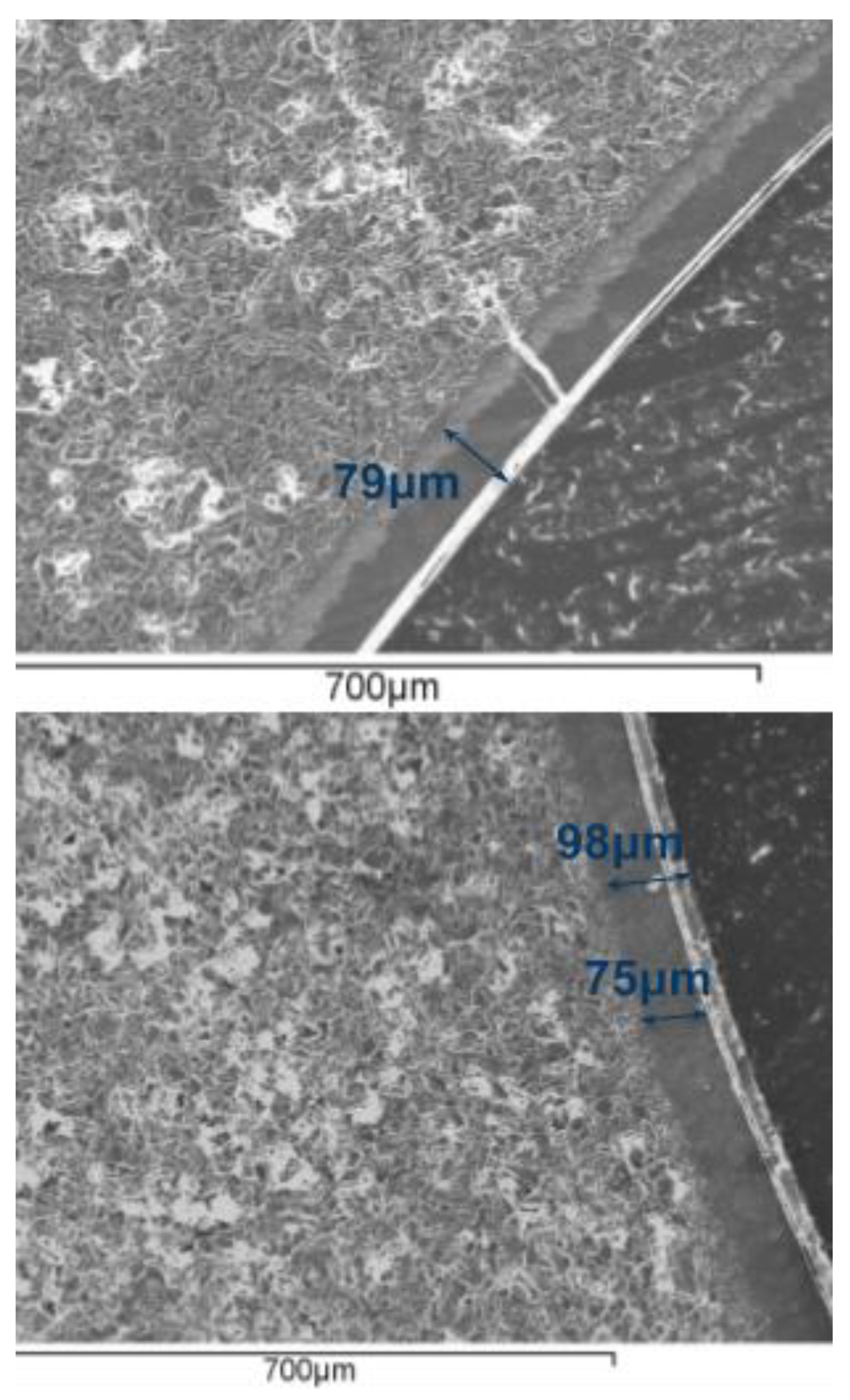
3.1. SEM Characterization
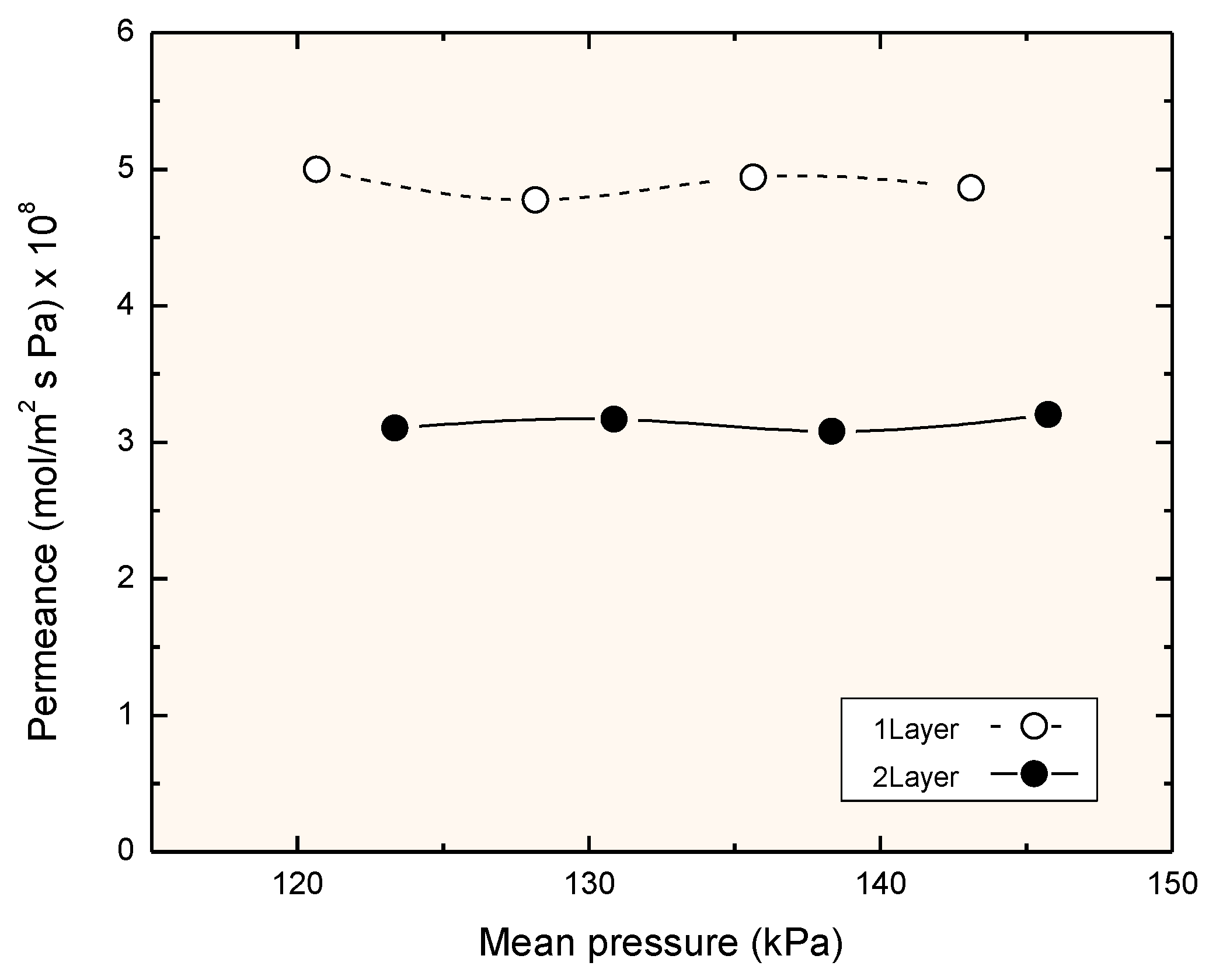
3.2. Nitrogen Permeation at Room Temperature
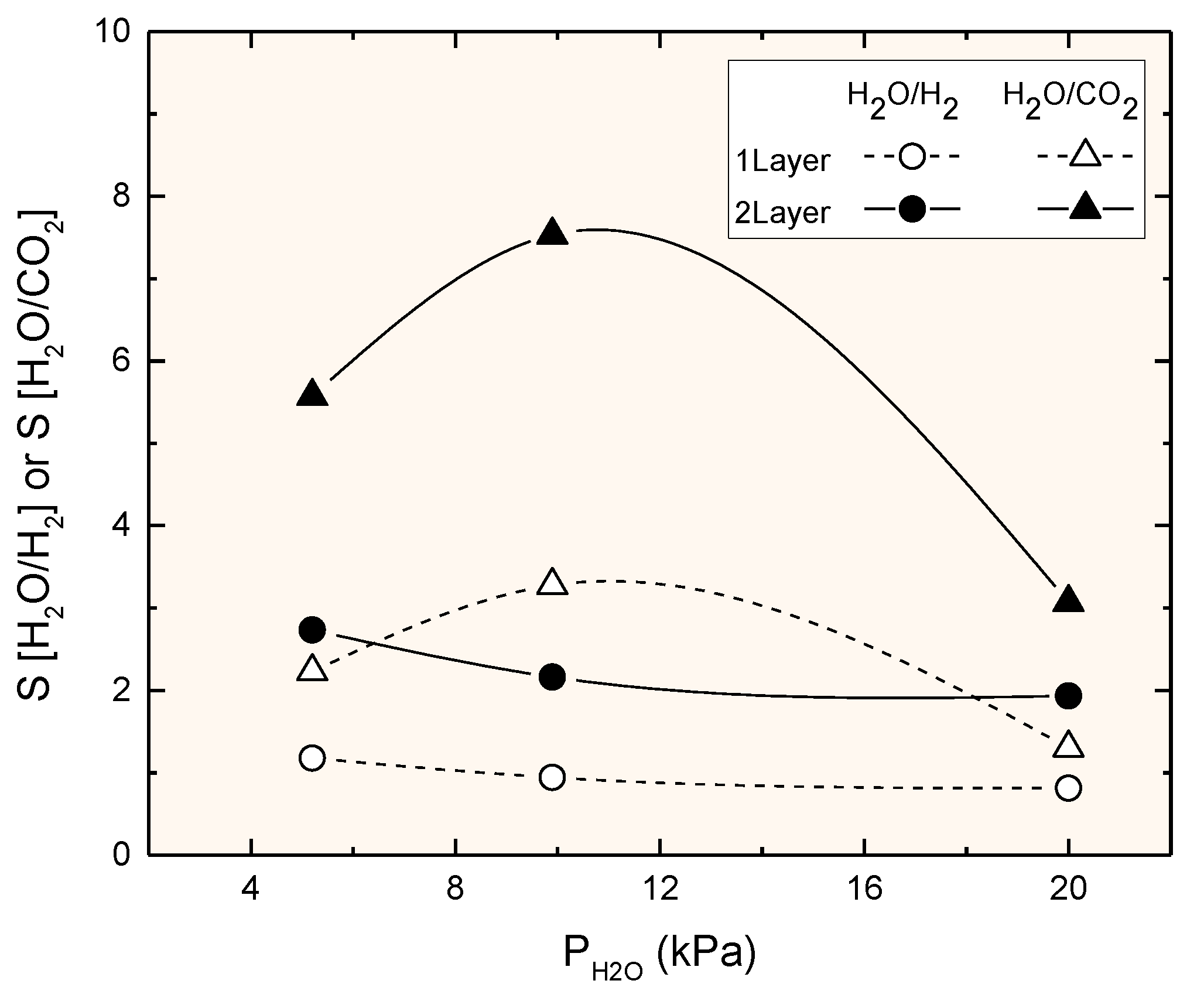
3.3. Permeation Measurements at Reaction Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olah, G.A. Beyond Oil and Gas: The Methanol Economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef] [PubMed]
- Olah, G.A. Towards Oil Independence Through Renewable Methanol Chemistry. Angew. Chem. Int. Ed. 2013, 52, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Goeppert, A.; Czaun, M.; Jones, J.-P.; Prakash, G.K.S.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products—Closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar] [CrossRef] [PubMed]
- Carbon Recycling International. Available online: https://www.carbonrecycling.is/ (accessed on 30 April 2020).
- Wójcik, A. Haldor Topsoe Joins Ambitious Sustainable Fuel Project in Denmark. Available online: https://blog.topsoe.com/ (accessed on 26 April 2020).
- Tullo, A.H. Sumitomo eyes new methanol process in Singapore. Chem. Eng. News 2021, 99, 8. [Google Scholar] [CrossRef]
- Araya, S.S.; Liso, V.; Cui, X.; Li, N.; Zhu, J.; Sahlin, S.L.; Jensen, S.H.; Nielsen, M.P.; Kær, S.K. A Review of The Methanol Economy: The Fuel Cell Route. Energies 2020, 13, 596. [Google Scholar] [CrossRef] [Green Version]
- Razali, N.A.M.; Lee, K.T.; Bhatia, S.; Mohamed, A.R. Heterogeneous catalysts for production of chemicals using carbon dioxide as raw material: A review. Renew. Sustain. Energy Rev. 2012, 16, 4951–4964. [Google Scholar] [CrossRef]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Fierro, J.; Navarro, R.M. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials 2019, 12, 3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, S.; Yang, H.; Gao, P.; Wang, H.; Li, X.; Wei, W.; Sun, Y. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation. Catal. Today 2019, 330, 61–75. [Google Scholar] [CrossRef]
- Stankiewicz, A. Reactive separations for process intensification: An industrial perspective. Chem. Eng. Process. Process. Intensif. 2003, 42, 137–144. [Google Scholar] [CrossRef]
- Struis, R.; Stucki, S.; Wiedorn, M. A membrane reactor for methanol synthesis. J. Membr. Sci. 1996, 113, 93–100. [Google Scholar] [CrossRef]
- Struis, R.; Stucki, S. Verification of the membrane reactor concept for the methanol synthesis. Appl. Catal. A: Gen. 2001, 216, 117–129. [Google Scholar] [CrossRef]
- Menéndez, M.; Piera, E.; Coronas, J.; Santamaría, J. Reactor de membrana zeolítica para la obtención de metanol y otros alcoholes a partir de gas de síntesis. Spanish Patent 2,164,544 B1, 1 May 2003. [Google Scholar]
- Gallucci, F.; Paturzo, L.; Basile, A. An experimental study of CO2 hydrogenation into methanol involving a zeolite membrane reactor. Chem. Eng. Process. Process. Intensif. 2004, 43, 1029–1036. [Google Scholar] [CrossRef]
- Barbieri, G.; Marigliano, G.; Golemme, G.; Drioli, E. Simulation of CO2 hydrogenation with CH3OH removal in a zeolite membrane reactor. Chem. Eng. J. 2002, 85, 53–59. [Google Scholar] [CrossRef]
- Gallucci, F.F.; Basile, A. A theoretical analysis of methanol synthesis from CO2 and H2 in a ceramic membrane reactor. Int. J. Hydrogen Energy 2007, 32, 5050–5058. [Google Scholar] [CrossRef]
- Gorbe, J.; Lasobras, J.; Francés, E.; Herguido, J.; Menéndez, M.; Kumakiri, I.; Kita, H. Preliminary study on the feasibility of using a zeolite A membrane in a membrane reactor for methanol production. Sep. Purif. Technol. 2018, 200, 164–168. [Google Scholar] [CrossRef] [Green Version]
- Raso, R.; Tovar, M.; Lasobras, J.; Herguido, J.; Kumakiri, I.; Araki, S.; Menéndez, M. Zeolite membranes: Comparison in the separation of H2O/H2/CO2 mixtures and test of a reactor for CO2 hydrogenation to methanol. Catal. Today 2021, 364, 270–275. [Google Scholar] [CrossRef]
- Li, H.; Qiu, C.; Ren, S.; Dong, Q.; Zhang, S.; Zhou, F.; Liang, X.; Wang, J.; Li, S.; Yu, M. Na+-gated water-conducting nanochannels for boosting CO2 conversion to liquid fuels. Science 2020, 367, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wei, W.; Jin, W.; Xu, N. Polymer/Ceramic Composite Membranes and Their Application in Pervaporation Process. Chin. J. Chem. Eng. 2012, 20, 62–70. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, Q. Methanol synthesis from CO2 using a silicone rubber/ceramic composite membrane reactor. Sep. Purif. Technol. 2004, 34, 227–237. [Google Scholar] [CrossRef]
- Téllez, C.; Menéndez, M. Zeolite membrane reactors. In Membranes for Membrane Reactors: Preparation, Optimization and Selection; Basile, A., Gallucci, F., Eds.; Wiley: Chichester, UK, 2011; pp. 243–274. [Google Scholar]
- Menéndez, M. Inorganic membrane reactors for energy applications. In Nanoporous materials for Energy and the Environment; Rios, G., Kanellopoulos, N., Centi, G., Eds.; Pan Stanford Pub, CRC Press: Boca Raton, FL, USA, 2012; pp. 283–294. [Google Scholar]
- Diban, N.; Aguayo, A.T.; Bilbao, J.; Urtiaga, A.; Ortiz, I. Membrane Reactors for in Situ Water Removal: A Review of Applications. Ind. Eng. Chem. Res. 2013, 52, 10342–10354. [Google Scholar] [CrossRef]


| T (°C) | PH2O (kPa) | J (10−7 mol/m2 s Pa) | |||
|---|---|---|---|---|---|
| H2 | N2 | CO2 | H2O | ||
| 180 | 9.9 | 3.10 | 1.02 | 1.34 | 3.87 |
| 200 | 9.9 | 3.37 | 0.93 | 1.47 | 3.18 |
| 220 | 9.9 | 3.40 | 0.96 | 1.44 | 3.82 |
| 200 | 5.2 | 3.32 | 0.87 | 1.43 | 3.91 |
| 200 | 20 | 3.24 | 0.75 | 1.36 | 2.63 |
| T (°C) | PH2O (kPa) | J (10−7 mol/m2 s Pa) | |||
|---|---|---|---|---|---|
| H2 | N2 | CO2 | H2O | ||
| 180 | 9.9 | 0.35 | 0.17 | 0.16 | 0.57 |
| 200 | 9.9 | 0.35 | 0.16 | 0.15 | 1.13 |
| 220 | 9.9 | 0.29 | 0.13 | 0.12 | 1.40 |
| 200 | 5.2 | 0.35 | 0.17 | 0.14 | 0.78 |
| 200 | 20 | 0.33 | 0.17 | 0.14 | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juarez, E.; Lasobras, J.; Soler, J.; Herguido, J.; Menéndez, M. Polymer–Ceramic Composite Membranes for Water Removal in Membrane Reactors. Membranes 2021, 11, 472. https://doi.org/10.3390/membranes11070472
Juarez E, Lasobras J, Soler J, Herguido J, Menéndez M. Polymer–Ceramic Composite Membranes for Water Removal in Membrane Reactors. Membranes. 2021; 11(7):472. https://doi.org/10.3390/membranes11070472
Chicago/Turabian StyleJuarez, Ester, Javier Lasobras, Jaime Soler, Javier Herguido, and Miguel Menéndez. 2021. "Polymer–Ceramic Composite Membranes for Water Removal in Membrane Reactors" Membranes 11, no. 7: 472. https://doi.org/10.3390/membranes11070472
APA StyleJuarez, E., Lasobras, J., Soler, J., Herguido, J., & Menéndez, M. (2021). Polymer–Ceramic Composite Membranes for Water Removal in Membrane Reactors. Membranes, 11(7), 472. https://doi.org/10.3390/membranes11070472








