Recent Advances in Membrane-Based Electrochemical Hydrogen Separation: A Review
Abstract
1. Introduction
2. Hydrogen Separation/Purification Technologies
2.1. Pressure Swing Adsorption
2.2. Cryogenic Distillation
2.3. Membrane Technologies
2.3.1. Porous Membranes
2.3.2. Dense (Non-Porous) Membranes-Diffusion Mechanism
2.3.3. Ceramic Proton-Conducting Membranes
- H2 gas diffusion to reaction sites on the surface of the feed side;
- H2 adsorption, dissociation, and charge transfer at the membrane surface;
- Proton reduction and hydrogen re-association at the membrane surfacewhere, S’, BM, S” and G is the membrane surface at the inlet, the bulk membrane, the membrane surface at the outlet, and the gas, respectively.
3. Electrochemical Hydrogen Separation
- Hydrogen, in the form of protons, is selectively transferred through the proton-conducting electrolyte;
- one-step operation provides pure hydrogen;
- the hydrogen separation rate can be controlled by the current (Faraday’s Law);
- a high hydrogen collection rate is achieved;
- simultaneous purification and compression of hydrogen is possible, in principle;
- high hydrogen separation is achieved at low cell voltages, with a high separation efficiency [156] and
- high selectivity and low permeability results in pure hydrogen (up to 99.99 vol.%) [159].
3.1. Working Principle
3.2. Current Status of Electrochemical Hydrogen Separation Technology
3.2.1. Low-Temperature EHS
“Passive” Gas Mixtures
- H2/CH4 Mixtures
- H2/Ar Mixtures
- H2/N2 Mixtures
- H2/CH4/Ar
- H2/CH4/Ethylene
“Active” Gas Mixtures
- CO and CO2-Containing Mixtures
3.2.2. High-Temperature EHS
“Passive” Gas Mixtures
- H2/CH4 Mixtures
“Active” Gas Mixtures
- CO and CO2-Containing mixtures
- H2/NH3 Mixtures
3.2.3. Review Articles
3.2.4. Overall Summary of the State of Electrochemical Hydrogen Separation
- High selectivity;
- sensitivity to catalyst deactivation (e.g., CO deactivation);
- higher tolerance to “active” impurities (e.g., CO and CO2) at higher temperatures;
- the hydrogen flux can be controlled by the current and
- simultaneous hydrogen separation and compression is possible.
4. Concluding Remarks
- Little information on component degradation, beside CO catalyst deactivation. However, the degradation studies performed on fuel cells can be used to fill this gap.
- Understanding the life cycle of an electrochemical hydrogen membrane and how an aged membrane’s performance compare to that of a membrane at the beginning of its life.
- Contribution/s of various impurities (considered separately) to the performance parameters. Impurities that commonly accompany hydrogen streams generated from various traditional hydrogen generation methods include CH4, O2, N2, CO, CO2, H2S, benzene, toluene, xylene and NH3. However, as is evident from Table 4, research articles are only available on EHS from mixtures containing CO, N2, CO2, CH4, Ar, ethylene and H2O. Furthermore, HT EHS research mainly included reformate gases. Hence, the contribution of respective impurities, separately, on the performance parameters is largely unknown.
- Further research into HT EHS. From Table 4, it is evident that more information is available on LT separation than HT separation. Further research is required to achieve a broader understanding of the expected extent of separation with respect to the various performance parameters—such as limiting currents, hydrogen recovery, selectivities and fluxes.
- One of the advantages that HT membranes present is the possibility of being able to use catalysts such as iron and cobalt. More information on this topic is required in efforts to determine how beneficial this would be—besides only focusing on the cost reduction.
- Fuel cell application. To date, no studies appear to have been conducted to verify the hydrogen purity of EHS product streams for fuel cell application. Such knowledge could be very beneficial, especially when simulated reformate streams are used from industrial hydrogen production systems.
- EHS from industrial hydrogen streams produced from fuel cells (e.g., product streams from steam methane reforming, partial oxidation and gasification of biomass and coal)
- Simultaneous EHS and electrochemical hydrogen compression, together with the process efficiency in terms of hydrogen purity, hydrogen compression, overall efficiency, etc. Specifically, the simultaneous EHS and compression from H2/CO2 streams, where both the hydrogen stream (permeate) and the carbon dioxide (retentate) is purified and compressed. Such study will be beneficial in terms of hydrogen production and CO2 sequestration (i.e., carbon capture and storage, and even carbon capture and utilization).
- Proton-conducting ceramics could be considered a new and upcoming technology and is also part of EHS. The authors suggest that future reviews be done, similar to the one presented, on this topic.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alanne, K.; Cao, S. An overview of the concept and technology of ubiquitous energy. Appl. Energy 2019, 238, 284–302. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The survey of key technologies in hydrogen energy storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Smoliński, A.; Howaniec, N. Hydrogen energy, electrolyzers and fuel cells—The future of modern energy sector. Int. J. Hydrogen Energy 2020, 45, 5607. [Google Scholar] [CrossRef]
- Lund, H. Renewable energy strategies for sustainable development. Energy 2007, 32, 912–919. [Google Scholar] [CrossRef]
- Won, W.; Kwon, H.; Han, J.H.; Kim, J. Design and operation of renewable energy sources based hydrogen supply system: Technology integration and optimization. Renew. Energy 2017, 103, 226–238. [Google Scholar] [CrossRef]
- Scamman, D.; Newborough, M. Using surplus nuclear power for hydrogen mobility and power-to-gas in France. Int. J. Hydrogen Energy 2016, 41, 10080–10089. [Google Scholar] [CrossRef]
- Khodadoost Arani, A.A.; Gharehpetian, G.B.; Abedi, M. Review on Energy Storage Systems Control Methods in Microgrids. Int. J. Electr. Power Energy Syst. 2019, 107, 745–757. [Google Scholar] [CrossRef]
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Krishan, O.; Suhag, S. An updated review of energy storage systems: Classification and applications in distributed generation power systems incorporating renewable energy resources. Int. J. Energy Res. 2019, 43, 6171–6210. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Smith, W. Role of fuel cells in energy storage. J. Power Sources 2000, 86, 74–83. [Google Scholar] [CrossRef]
- Yan, Z.; Hitt, J.L.; Turner, J.A.; Mallouk, T.E. Renewable electricity storage using electrolysis. Proc. Natl. Acad. Sci. USA 2020, 117, 12558–12563. [Google Scholar] [CrossRef] [PubMed]
- Vanhanen, J.P.; Lund, P.D.; Tolonen, J.S. Electrolyser-metal hydride-fuel cell system for seasonal energy storage. Int. J. Hydrogen Energy 1998, 23, 267–271. [Google Scholar] [CrossRef]
- Hall, P.J.; Mirzaeian, M.; Fletcher, S.I.; Sillars, F.B.; Rennie, A.J.R.; Shitta-Bey, G.O.; Wilson, G.; Cruden, A.; Carter, R. Energy storage in electrochemical capacitors: Designing functional materials to improve performance. Energy Environ. Sci. 2010, 3, 1238–1251. [Google Scholar] [CrossRef]
- Abbas, Q.; Raza, R.; Shabbir, I.; Olabi, A.G. Heteroatom doped high porosity carbon nanomaterials as electrodes for energy storage in electrochemical capacitors: A review. J. Sci. Adv. Mater. Devices 2019, 4, 341–352. [Google Scholar] [CrossRef]
- Gharehpetian, G.B.; Akhavanhejazi, M.; Arani, A.A.K.; Karami, H.; Gharehpetian, G.B.; Hejazi, A. Review of Flywheel Energy Storage Systems structures and applications in power systems and microgrids. Renew. Sustain. Energy Rev. 2016, 69, 9–18. [Google Scholar] [CrossRef]
- Faraji, F.; Majazi, A.; Al-Haddad, K. A comprehensive review of Flywheel Energy Storage System technology. Renew. Sustain. Energy Rev. 2017, 67, 477–490. [Google Scholar] [CrossRef]
- Peña-Alzola, R.; Sebastián, R.; Quesada, J.; Colmenar, A. Review of flywheel based energy storage systems. In Proceedings of the 2011 International Conference on Power Engineering, Energy and Electrical Drives, Malaga, Spain, 11–13 May 2011. [Google Scholar] [CrossRef]
- Venkataramani, G.; Parankusam, P.; Ramalingam, V.; Wang, J. A review on compressed air energy storage—A pathway for smart grid and polygeneration. Renew. Sustain. Energy Rev. 2016, 62, 895–907. [Google Scholar] [CrossRef]
- Wang, J.; Lu, K.; Ma, L.; Wang, J.; Dooner, M.; Miao, S.; Li, J.; Wang, D. Overview of compressed air energy storage and technology development. Energies 2017, 10, 991. [Google Scholar] [CrossRef]
- Rehman, S.; Al-Hadhrami, L.M.; Alam, M.M. Pumped hydro energy storage system: A technological review. Renew. Sustain. Energy Rev. 2015, 44, 586–598. [Google Scholar] [CrossRef]
- Ma, T.; Yang, H.; Lu, L.; Peng, J. Technical feasibility study on a standalone hybrid solar-wind system with pumped hydro storage for a remote island in Hong Kong. Renew. Energy 2014, 69, 7–15. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Sarbu, I.; Dorca, A. Review on heat transfer analysis in thermal energy storage using latent heat storage systems and phase change materials. Int. J. Energy Res. 2019, 43, 29–64. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A comprehensive review of thermal energy storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Languri, E.M.; Cunningham, G. Thermal Energy Storage Systems. Lect. Notes Energy 2019, 70, 169–176. [Google Scholar] [CrossRef]
- Diaz, P. Analysis and Comparison of different types of Thermal Energy Storage Systems: A Review. J. Adv. Mech. Eng. Sci. 2016, 2, 33–46. [Google Scholar] [CrossRef]
- Mukherjee, P.; Rao, V.V. Superconducting magnetic energy storage for stabilizing grid integrated with wind power generation systems. J. Mod. Power Syst. Clean Energy 2019, 7, 400–411. [Google Scholar] [CrossRef]
- Barbir, F.; Veziroglu, T.N. Hydroger Energy System and Hydrogen Production Methods; Kluwer Academic Publishers: New York, NY, USA, 1992; pp. 277–278. [Google Scholar]
- Veziroglu, T.; Sherif, S.A.; Barbir, F. Chapter 7-Hydrogen Energy Solutions. In Environmental Solutions; Agardy, F.J., Nemerow, N.L., Eds.; Academic Press: Cambridge, MA, USA, 2005; pp. 143–180. [Google Scholar]
- Barbir, F. Future of Fuel Cells and Hydrogen; Academic Press: Waltam, MA, USA, 2013; ISBN 9780123877109. [Google Scholar]
- Züttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L. Progress and problems in hydrogen storage methods. Renew. Sustain. Energy Rev. 2005, 9, 395–408. [Google Scholar] [CrossRef]
- Kunowsky, M.; Marco-Lózar, J.P.; Linares-Solano, A. Material Demands for Storage Technologies in a Hydrogen Economy. J. Renew. Energy 2013, 2013, 878329. [Google Scholar] [CrossRef]
- Valenti, G. Hydrogen liquefaction and liquid hydrogen storage. In Compendium of Hydrogen Energy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 27–51. [Google Scholar]
- Ahluwalia, R.K.; Peng, J.-K.; Hua, T.Q. Cryo-Compressed Hydrogen Storage; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9781782423621. [Google Scholar]
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The Prospect of Hydrogen Storage Using Liquid Organic Hydrogen Carriers. Energy Fuels 2019, 33, 2778–2796. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P. Liquid Organic Hydrogen Carriers as an efficient vector for the transport and storage of renewable energy. Int. J. Hydrogen Energy 2012, 37, 18118–18132. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid organic hydrogen carriers (LOHCs): Toward a hydrogen-free hydrogen economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef]
- Makepeace, J.W.; He, T.; Weidenthaler, C.; Jensen, T.R.; Chang, F.; Vegge, T.; Ngene, P.; Kojima, Y.; de Jongh, P.E.; Chen, P.; et al. Reversible ammonia-based and liquid organic hydrogen carriers for high-density hydrogen storage: Recent progress. Int. J. Hydrogen Energy 2019, 44, 7746–7767. [Google Scholar] [CrossRef]
- Corgnale, C.; Hardy, B.J.; Anton, D.L. Structural analysis of metal hydride-based hybrid hydrogen storage systems. Int. J. Hydrogen Energy 2012, 37, 14223–14233. [Google Scholar] [CrossRef]
- Gondal, I.A. Hydrogen Transportation by Pipelines; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9781782423621. [Google Scholar]
- Liu, B.; Liu, S.; Guo, S.; Zhang, S. Economic study of a large-scale renewable hydrogen application utilizing surplus renewable energy and natural gas pipeline transportation in China. Int. J. Hydrogen Energy 2019, 45, 1385–1398. [Google Scholar] [CrossRef]
- Kim, J.W.; Boo, K.J.; Cho, J.H.; Moon, I. Key Challenges in the Development of an Infrastructure for Hydrogen Production, Delivery, Storage and Use; Woodhead Publishing Limited: Cambridge, UK, 2014; ISBN 9780857097736. [Google Scholar]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Lin, R.H.; Zhao, Y.Y.; Wu, B.D. Toward a hydrogen society: Hydrogen and smart grid integration. Int. J. Hydrogen Energy 2020, 45, 20164–20175. [Google Scholar] [CrossRef]
- Bockris, J.O.; Appleby, A.J. The hydrogen economy—An ultimate economy. Environ. This Mon. 1972, 1, 29–35. Available online: http://inis.iaea.org/Search/search.aspx?orig_q=RN:3032306 (accessed on 12 August 2020).
- Perry, K.A.; Eisman, G.A.; Benicewicz, B.C. Electrochemical hydrogen pumping using a high-temperature polybenzimidazole (PBI) membrane. J. Power Sources 2008, 177, 478–484. [Google Scholar] [CrossRef]
- Bessarabov, D.G.; Millet, P. PEM Water Electrolysis; Academic Press: Cambridge, MA, USA, 2018; Volume 1, ISBN 9780128111468. [Google Scholar]
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable hydrogen production. Science (80-) 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.; Sprik, S.; Bradley, T.H. Review of transportation hydrogen infrastructure performance and reliability. Int. J. Hydrogen Energy 2019, 44, 12010–12023. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- da Silva Veras, T.; Mozer, T.S.; da Costa Rubim Messeder dos Santos, D.; da Silva César, A. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- López Ortiz, A.; Meléndez Zaragoza, M.J.; Collins-Martínez, V. Hydrogen production research in Mexico: A review. Int. J. Hydrogen Energy 2016, 41, 23363–23379. [Google Scholar] [CrossRef]
- Steinberg, M.; Cheng, H.C. Modern and prospective technologies for hydrogen production from fossil fuels. Int. J. Hydrogen Energy 1989, 14, 797–820. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Impact assessment and efficiency evaluation of hydrogen production methods. Int. J. Energy Res. 2015, 39, 1757–1768. [Google Scholar] [CrossRef]
- Chaubey, R.; Sahu, S.; James, O.O.; Maity, S. A review on development of industrial processes and emerging techniques for production of hydrogen from renewable and sustainable sources. Renew. Sustain. Energy Rev. 2013, 23, 443–462. [Google Scholar] [CrossRef]
- Baykara, S.Z. Hydrogen: A brief overview on its sources, production and environmental impact. Int. J. Hydrogen Energy 2018, 43, 10605–10614. [Google Scholar] [CrossRef]
- Rakib, M.A.; Grace, J.R.; Lim, C.J.; Elnashaie, S.S.E.H.; Ghiasi, B. Steam reforming of propane in a fluidized bed membrane reactor for hydrogen production. Renew. Energy 2010, 35, 6276–6290. [Google Scholar] [CrossRef]
- de Campos Roseno, K.T.; de Brito Alves, R.M.; Giudici, R.; Schmal, M. Syngas Production Using Natural Gas from the Environmental Point of View. In Biofuels—State of Development; InTech: London, UK, 2018. [Google Scholar]
- Synthesis Gas Chemistry and Synthetic Fuels. Available online: https://www.syncatbeijing.com/syngaschem/ (accessed on 14 August 2020).
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2014, 40, 11094–11111. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int. J. Hydrogen Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- David, O.C. Membrane Technologies for Hydrogen and Carbon Monoxide Recovery from Residual Gas Streams. PhD Thesis, University of Cantabria, Cantabria, Spain, 2012; pp. 1–183. [Google Scholar]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Engelbrecht, N.; Chiuta, S.; Everson, R.C.; Neomagus, H.W.J.P.; Bessarabov, D.G. Experimentation and CFD modelling of a microchannel reactor for carbon dioxide methanation. Chem. Eng. J. 2017, 313, 847–857. [Google Scholar] [CrossRef]
- Wu, H.C.; Chang, Y.C.; Wu, J.H.; Lin, J.H.; Lin, I.K.; Chen, C.S. Methanation of CO2 and reverse water gas shift reactions on Ni/SiO2 catalysts: The influence of particle size on selectivity and reaction pathway. Catal. Sci. Technol. 2015, 5, 4154–4163. [Google Scholar] [CrossRef]
- Guerra, L.; Rossi, S.; Rodrigues, J.; Gomes, J.; Puna, J.; Santos, M.T. Methane production by a combined Sabatier reaction/water electrolysis process. J. Environ. Chem. Eng. 2018, 6, 671–676. [Google Scholar] [CrossRef]
- Zhu, M.; Ge, Q.; Zhu, X. Catalytic Reduction of CO2 to CO via Reverse Water Gas Shift Reaction: Recent Advances in the Design of Active and Selective Supported Metal Catalysts. Trans. Tianjin Univ. 2020, 26, 172–187. [Google Scholar] [CrossRef]
- Abney, M.B.; Perry, J.L.; Junaedi, C.; Hawley, K.; Walsh, D.; Roychoudhury, S. Compact Lightweight Sabatier Reaction for Carbon Dioxide Reduction; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2011; pp. 1–10. [Google Scholar]
- Mayorga, S.G.; Hufton, J.R.; Sircar, S.; Gaffney, T.R. Sorption Enhanced Reaction Process for Production of Hydrogen. Phase 1 Final Report; U.S. Department of Energy: Golden, CO, USA, 1997. [Google Scholar]
- Andrews, J.W. Hydrogen production and carbon sequestration by steam methane reforming and fracking with carbon dioxide. Int. J. Hydrogen Energy 2020, 45, 9279–9284. [Google Scholar] [CrossRef]
- Ball, M.; Weeda, M. The Hydrogen Economy—Vision or Reality? The Global Energy Challenge; Woodhead Publishers: Sawston, UK, 2015. [Google Scholar] [CrossRef]
- Shoko, E.; McLellan, B.; Dicks, A.L.; da Costa, J.C.D. Hydrogen from coal: Production and utilisation technologies. Int. J. Coal Geol. 2006, 65, 213–222. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Nenoff, T.M. Membranes for hydrogen separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef] [PubMed]
- Kalamaras, C.M.; Efstathiou, A.M.; Al-Assaf, Y.; Poullikkas, A. Hydrogen Production Technologies: Current State and Future Developments. Conf. Pap. Energy 2013, 2013, 690627. [Google Scholar] [CrossRef]
- Besancon, B.M.; Hasanov, V.; Imbault-Lastapis, R.; Benesch, R.; Barrio, M.; Mølnvik, M.J. Hydrogen quality from decarbonized fossil fuels to fuel cells. Int. J. Hydrogen Energy 2009, 34, 2350–2360. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.; Xiong, X.; Tsang, D.C.; Zhang, S.; Clark, J.H.; Hu, C.; Hau Ng, Y.; Shang, J.; Sik Ok, Y. Biorenewable hydrogen production through biomass gasification: A review and future prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef]
- Shafirovich, E.; Varma, A. Underground Coal Gasification: A Brief Review of Current Status. Ind. Eng. Chem. Res. 2009, 48, 7865–7875. [Google Scholar] [CrossRef]
- Emami-Taba, L.; Faisal Irfan, M.; Ashri, W.M.; Daud, W.; Chakrabarti, M.H. Fuel blending effects on the co-gasification of coal and biomass—A review. Biomass Bioenergy 2013, 57, 249–263. [Google Scholar] [CrossRef]
- Stiegel, G.J.; Ramezan, M. Hydrogen from coal gasification: An economical pathway to a sustainable energy future. Int. J. Coal Geol. 2006, 65, 173–190. [Google Scholar] [CrossRef]
- Yusuf, N.Y.; Masdar, M.S.; Nordin, D.; Husaini, T. Challenges in Biohydrogen Technologies for Fuel Cell Application. Am. J. Chem. 2015, 5, 40–47. [Google Scholar] [CrossRef]
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into renewable hydrogen energy: Recent advances and prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Biomass-based hydrogen production: A review and analysis. Int. J. Hydrogen Energy 2009, 34, 8799–8817. [Google Scholar] [CrossRef]
- Ozbilen, A.; Dincer, I.; Rosen, M.A. Comparative environmental impact and efficiency assessment of selected hydrogen production methods. Environ. Impact Assess. Rev. 2013, 42, 1–9. [Google Scholar] [CrossRef]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life cycle assessment of hydrogen production via electrolysis—A review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Shalygin, M.G.; Abramov, S.M.; Netrusov, A.I.; Teplyakov, V.V. Membrane recovery of hydrogen from gaseous mixtures of biogenic and technogenic origin. Int. J. Hydrogen Energy 2015, 40, 3438–3451. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S. Hydrogen membrane separation techniques. Ind. Eng. Chem. Res. 2006, 45, 875–881. [Google Scholar] [CrossRef]
- Thomassen, M.; Sheridan, E.; Kvello, J. Electrochemical hydrogen separation and compression using polybenzimidazole (PBI) fuel cell technology. J. Nat. Gas Sci. Eng. 2010, 2, 229–234. [Google Scholar] [CrossRef]
- Liemberger, W.; Groß, M.; Miltner, M.; Harasek, M. Experimental analysis of membrane and pressure swing adsorption (PSA) for the hydrogen separation from natural gas. J. Clean. Prod. 2017, 167, 896–907. [Google Scholar] [CrossRef]
- Takht Ravanchi, M.; Kaghazchi, T.; Kargari, A. Application of membrane separation processes in petrochemical industry: A review. Desalination 2009, 235, 199–244. [Google Scholar] [CrossRef]
- Mores, P.L.; Arias, A.M.; Scenna, N.J.; Caballero, J.A.; Mussati, S.F.; Mussati, M.C. Membrane-Based Processes: Optimization of Hydrogen Separation by Minimization of Power, Membrane Area, and Cost. Processes 2018, 6, 221. [Google Scholar] [CrossRef]
- Schorer, L.; Schmitz, S.; Weber, A. Membrane based purification of hydrogen system (MEMPHYS). Int. J. Hydrogen Energy 2019, 44, 12708–12714. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, G.; Feng, X.; Chu, K.H.; Deng, C. Hydrogen networks synthesis considering separation performance of purifiers. Int. J. Hydrogen Energy 2014, 39, 8357–8373. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, N. Strategy of purifier selection and integration in hydrogen networks. Chem. Eng. Res. Des. 2004, 82, 1315–1330. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T.C. Purification of Hydrogen by Pressure Swing Adsorption. Sep. Sci. Technol. 2000, 35, 667–687. [Google Scholar] [CrossRef]
- Xiao, J.; Peng, Y.; Bénard, P.; Chahine, R. Thermal effects on breakthrough curves of pressure swing adsorption for hydrogen purification. Int. J. Hydrogen Energy 2016, 41, 8236–8245. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T.C. Pressure Swing Adsorption Technology for Hydrogen Production. In Hydrogen and Syngas Production and Purification Technologies; Liu, K., Song, C., Subramani, V., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 414–450. ISBN 9780471719755. [Google Scholar]
- Al-Mufachi, N.A.; Rees, N.V.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sustain. Energy Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Fahim, M.A.; Alsahhaf, T.A.; Elkilani, A. Chapter eleven: Hydrogen production. In Fundamentals of Petroleum Refining; Elsevier: Amsterdam, The Netherlands, 2010; pp. 285–302. ISBN 9780444527851. [Google Scholar]
- Grande, C.A.; Lopes, F.V.S.; Ribeiro, A.M.; Loureiro, J.M.; Rodrigues, A.E. Adsorption of Off-Gases from Steam Methane Reforming (H2, CO2, CH4, CO and N2) on Activated Carbon. Sep. Sci. Technol. 2008, 43, 1338–1364. [Google Scholar] [CrossRef]
- Marković, N.M.; Schmidt, T.J.; Grgur, B.N.; Gasteiger, H.A.; Behm, R.J.; Ross, P.N. Effect of Temperature on Surface Processes at the Pt(111)—Liquid Interface: Hydrogen Adsorption, Oxide Formation, and CO Oxidation. J. Phys. Chem. B 1999, 103, 8568–8577. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Cardoso, S.P.; Azenha, I.S.; Lin, Z.; Rodrigues, A.E.; Silva, C.M. Inorganic Membranes for Hydrogen Separation. Sep. Purif. Rev. 2018, 47, 229–266. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Qiao, Z.; Liu, Y.; Cao, X.; Li, W.; Wang, J.; Wang, S. Recent developments in membranes for efficient hydrogen purification. J. Membr. Sci. 2015, 495, 130–168. [Google Scholar] [CrossRef]
- Brinkmann, T.; Shishatskiy, S. Hydrogen Separation with Polymeric Membranes. Hydrogen Sci. Eng. Mater. Process. Syst. Technol. 2016, 1, 509–541. [Google Scholar] [CrossRef]
- Lu, G.Q.; Diniz Da Costa, J.C.; Duke, M.; Giessler, S.; Socolow, R.; Williams, R.H.; Kreutz, T. Inorganic membranes for hydrogen production and purification: A critical review and perspective. J. Colloid Interface Sci. 2007, 314, 589–603. [Google Scholar] [CrossRef]
- Escorihuela, S.; Tena, A.; Shishatskiy, S.; Escolástico, S.; Brinkmann, T.; Serra, J.M.; Abetz, V. Gas separation properties of polyimide thin films on ceramic supports for high temperature applications. Membranes 2018, 8, 16. [Google Scholar] [CrossRef]
- Hu, X.; Lee, W.H.; Bae, J.Y.; Kim, J.S.; Jung, J.T.; Wang, H.H.; Park, H.J.; Lee, Y.M. Thermally rearranged polybenzoxazole copolymers incorporating Tröger’s base for high flux gas separation membranes. J. Membr. Sci. 2020, 612, 118437. [Google Scholar] [CrossRef]
- Zornoza, B.; Casado, C.; Navajas, A. Advances in Hydrogen Separation and Purification with Membrane Technology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 245–268. [Google Scholar] [CrossRef]
- Sanchez Marcano, J.G.; Tsotsis, T.T. Catalytic Membranes and Membrane Reactors; Wiley-VCH: Weiheim, Germany, 2002; ISBN 3527302778. [Google Scholar]
- Phair, J.W.; Badwal, S.P.S. Materials for separation membranes in hydrogen and oxygen production and future power generation Materials for separation membranes in hydrogen and oxygen production and future power generation. Sci. Technol. Adv. Mater. 2006, 7, 792. [Google Scholar] [CrossRef]
- Oyama, S.T.; Yamada, M.; Sugawara, T.; Takagaki, A.; Kikuchi, R. Review on mechanisms of gas permeation through inorganic membranes. J. Japan Pet. Inst. 2011, 54, 298–309. [Google Scholar] [CrossRef]
- Gilron, J.; Soffer, A. Knudsen diffusion in microporous carbon membranes with molecular sieving character. J. Membr. Sci. 2002, 209, 339–352. [Google Scholar] [CrossRef]
- Sazali, N.; Mohamed, M.A.; Norharyati, W.; Salleh, W. Membranes for hydrogen separation: A significant review. Int. J. Adv. Manuf. Technol. 2020, 107, 1859–1881. [Google Scholar] [CrossRef]
- Iulianelli, A.; Basile, A.; Li, H.; Van den Brink, R.W. Inorganic membranes for pre-combustion carbon dioxide. In Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications; Woodhead Publishing: Cambridge, UK, 2011; pp. 184–213. [Google Scholar]
- Singh, R.P.; Berchtold, K.A. H2 Selective Membranes for Precombustion Carbon Capture. In Novel Materials for Carbon Dioxide Mitigation Technology; Shi, F., Morreale, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 117–206. [Google Scholar]
- Phair, J.W.; Badwal, S.P.S. Review of proton conductors for hydrogen separation. Ionics (Kiel) 2006, 12, 103–115. [Google Scholar] [CrossRef]
- Yun, S.; Oyama, S.T. Correlations in palladium membranes for hydrogen separation: A review. J. Membr. Sci. 2011, 375, 28–45. [Google Scholar] [CrossRef]
- Uemiya, S. Brief review of steam reforming using a metal membrane reactor. Top. Catal. 2004, 29, 79–84. [Google Scholar] [CrossRef]
- Ryi, S.K.; Park, J.S.; Kim, S.H.; Cho, S.H.; Park, J.S.; Kim, D.W. Development of a new porous metal support of metallic dense membrane for hydrogen separation. J. Membr. Sci. 2006, 279, 439–445. [Google Scholar] [CrossRef]
- Teplyakov, V.; Meares, P. Correlation aspects of the selective gas permeabilities of polymeric materials and membranes. Gas Sep. Purif. 1990, 4, 66–74. [Google Scholar] [CrossRef]
- Nenoff, T.M.; Spontak, R.J.; Aberg, C.M. Membranes for hydrogen purification: An important step toward a hydrogen-based economy. MRS Bull. 2006, 31, 735–741. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Ryzhikh, V. Polymeric membrane materials for hydrogen separation. In Hydrogen Production, Separation and Purification for Energy; Basile, A., Dalena, F., Tong, J., Nejat Veziroglu, T., Eds.; Institution of Engineering and Technology: London, UK, 2017; pp. 319–341. ISBN 9781785611001. [Google Scholar]
- Yampolskii, Y. Polymeric Gas Separation Membranes. Macromolecules 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Perry, J.D.; Nagai, K.; Koros, W.J. Polymer Membranes for Hydrogen Separations. MRS Bull. 2020, 31, 745–749. [Google Scholar] [CrossRef]
- Tanaka, K.; Yoshinari, B. Hydrogen-Metal Systems: Basic Properties (2). In Encyclopedia of Materials: Science and Technology, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2001; pp. 3919–3923. [Google Scholar] [CrossRef]
- Nagy, E. Mass Transport Through a Membrane Layer. In Basic Equations of Mass Transport Through a Membrane Layer; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 21–68. [Google Scholar]
- Burggraaf, A.J. Single gas permeation of thin zeolite (MFI) membranes: Theory and analysis of experimental observations. J. Membr. Sci. 1999, 155, 45–65. [Google Scholar] [CrossRef]
- Bhandarkar, M.; Shelekhin, A.B.; Dixon, A.G.; Ma, Y.H. Adsorption, permeation, and diffusion of gases in microporous membranes. I. Adsorption of gases on microporous glass membranes. J. Membr. Sci. 1992, 75, 221–231. [Google Scholar] [CrossRef]
- Hägg, M.-B.; He, X. Chapter 15. Carbon Molecular Sieve Membranes for Gas Separation. In Membrane Engineering for the Treatment of Gases; The Royal Society of Chemistry: London, UK, 2011; pp. 162–191. [Google Scholar]
- Yin, H.; Yip, A.C.K. A review on the production and purification of biomass-derived hydrogen using emerging membrane technologies. Catalysts 2017, 7, 297. [Google Scholar] [CrossRef]
- Doong, S.J. Advanced hydrogen (H2) gas separation membrane development for power plants. In Advanced Power Plant Materials, Design and Technology; Woodhead Publishing Limited: Cambridge, UK, 2010; pp. 111–142. [Google Scholar]
- Steward, S.A. Review of Hydrogen Isotope Permeability Through Materials; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 1983. [Google Scholar] [CrossRef]
- Gallucci, F. Richardson Law. In Encyclopedia of Membrane Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Vadrucci, M.; Borgognoni, F.; Moriani, A.; Santucci, A.; Tosti, S. Hydrogen permeation through Pd-Ag membranes: Surface effects and Sieverts’ law. Int. J. Hydrogen Energy 2013, 38, 4144–4152. [Google Scholar] [CrossRef]
- Hara, S.; Ishitsuka, M.; Suda, H.; Mukaida, M.; Haraya, K. Pressure-Dependent Hydrogen Permeability Extended for Metal Membranes Not Obeying the Square-Root Law. J. Phys. Chem. B 2009, 113, 9795–9801. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Basile, A. Membrane processes for biofuel separation: An introductionNo Title. In Membranes for Clean and Renewable Power Applications; Woodhead Publishing Limited: Cambridge, UK, 2014; pp. 65–103. [Google Scholar]
- Li, W.; Cao, Z.; Cai, L.; Zhang, L.; Zhu, X.; Yang, W. H2S-tolerant oxygen-permeable ceramic membranes for hydrogen separation with a performance comparable to those of palladium-based membranes. Energy Environ. Sci. 2017, 10, 101–106. [Google Scholar] [CrossRef]
- Escolastico, S.; Solis, C.; Serra, J.M. Hydrogen separation and stability study of ceramic membranes based on the system Nd 5 LnWO 12. Int. J. Hydrogen Energy 2011, 36, 11946–11954. [Google Scholar] [CrossRef]
- Ivanova, M.E.; Serra, J.M.; Roitsch, S. Proton-Conducting Ceramic Membranes for Solid Oxide Fuel Cells and Hydrogen (H2) Processing; Woodhead Publishing: Cambridge, UK, 2011; pp. 541–567. [Google Scholar] [CrossRef]
- Hashim, S.S.; Somalu, M.R.; Loh, K.S.; Liu, S.; Zhao, W.; Sunarso, J. Perovskite-based proton conducting membranes for hydrogen separation: A review. Int. J. Hydrogen Energy 2018, 43, 15281–15305. [Google Scholar] [CrossRef]
- Iwahara, H. Hydrogen pumps using proton-conducting ceramics and their applications. Solid State Ion. 1999, 125, 271–278. [Google Scholar] [CrossRef]
- Cheng, S.; Gupta, V.K.; Lin, J.Y.S. Synthesis and hydrogen permeation properties of asymmetric proton-conducting ceramic membranes. Solid State Ion. 2005, 176, 2653–2662. [Google Scholar] [CrossRef]
- Tong, Y.; Meng, X.; Luo, T.; Cui, C.; Wang, Y.; Wang, S.; Peng, R.; Xie, B.; Chen, C.; Zhan, Z. Protonic Ceramic Electrochemical Cell for Efficient Separation of Hydrogen. Appl. Mater. Interfaces 2020, 12, 25809–25817. [Google Scholar] [CrossRef]
- Leonard, K.; Deibert, W.; Ivanova, M.E.; Meulenberg, W.A.; Ishihara, T.; Matsumoto, H. Processing Ceramic Proton Conductor Membranes for Use in Steam Electrolysis. Membranes 2020, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Yan, L.; Qiao, J.; Wang, B.; Zhang, L.; Zhang, J. A review of advanced proton-conducting materials for hydrogen separation. Prog. Mater. Sci. 2015, 74, 1–50. [Google Scholar] [CrossRef]
- Norby, T.; Haugsrud, R. Dense Ceramic Membranes for Hydrogen Separation Oxide thermoelectric materials View project Metal supported proton conducting electrolyser cell for renewable hydrogen production (METALLICA) View project. In Nonporous Inorganic Membranes: For Chemical Processing; Sammells, A.F., Mundschau, M.V., Eds.; Wiley-VCH: Weinheim, Germany, 2014; pp. 1–48. ISBN 3527313427. [Google Scholar]
- Kreuer, K.D. On the complexity of proton conduction phenomena. Solid State Ion. 2000, 136–137, 149–160. [Google Scholar] [CrossRef]
- Fontaine, M.L.; Norby, T.; Larring, Y.; Grande, T.; Bredesen, R. Oxygen and Hydrogen Separation Membranes Based on Dense Ceramic Conductors. Membr. Sci. Technol. 2008, 13, 401–458. [Google Scholar] [CrossRef]
- Gallucci, F.; Fernandez, E.; Corengia, P.; van Sint Annaland, M. Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Gardner, C.L.; Ternan, M. Electrochemical separation of hydrogen from reformate using PEM fuel cell technology. J. Power Sources 2007, 171, 835–841. [Google Scholar] [CrossRef]
- Bessarrabov, D. Electrochemically-aided membrane separation and catalytic processes. Membr. Technol. 1998, 93, 8–11. [Google Scholar] [CrossRef]
- Sakai, T.; Matsumoto, H.; Kudo, T.; Yamamoto, R.; Niwa, E.; Okada, S.; Hashimoto, S.; Sasaki, K.; Ishihara, T. High performance of electroless-plated platinum electrode for electrochemical hydrogen pumps using strontium-zirconate-based proton conductors. Electrochim. Acta 2008, 53, 8172–8177. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Grigoriev, S.A.; Kalinnikov, A.A.; Filippov, A.A.; Millet, P.; Fateev, V.N. Characterisation of a electrochemical hydrogen pump using electrochemical impedance spectroscopy. J. Appl. Electrochem. 2011, 41, 1033–1042. [Google Scholar] [CrossRef]
- Wu, X.; Benziger, J.; He, G. Comparison of Pt and Pd catalysts for hydrogen pump separation from reformate. J. Power Sources 2012, 218, 424–434. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Shtatniy, I.G.; Millet, P.; Porembsky, V.I.; Fateev, V.N. Description and characterization of an electrochemical hydrogen compressor/concentrator based on solid polymer electrolyte technology. Int. J. Hydrogen Energy 2011, 36, 4148–4155. [Google Scholar] [CrossRef]
- Hydrogen Mobility Europe. Available online: https://h2me.eu/ (accessed on 21 April 2020).
- Speers, P. Hydrogen Mobility Europe (H2ME): Vehicle and hydrogen refuelling station deployment results. World Electr. Veh. J. 2018, 9, 2. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2001; pp. 261–304. ISBN 978-0-471-04372-0. Available online: https://books.google.com.mx/books?id=kv56QgAACAAJ (accessed on 13 January 2020).
- Barbir, F.; Görgün, H. Electrochemical hydrogen pump for recirculation of hydrogen in a fuel cell stack. J. Appl. Electrochem. 2007, 37, 359–365. [Google Scholar] [CrossRef]
- Lee, H.K.; Choi, H.Y.; Choi, K.H.; Park, J.H.; Lee, T.H. Hydrogen separation using electrochemical method. J. Power Sources 2004, 132, 92–98. [Google Scholar] [CrossRef]
- Granite, E.J.; O’Brien, T. Review of novel methods for carbon dioxide separation from flue and fuel gases. Fuel Process. Technol. 2005, 86, 1423–1434. [Google Scholar] [CrossRef]
- Ibeh, B.; Gardner, C.; Ternan, M. Separation of hydrogen from a hydrogen/methane mixture using a PEM fuel cell. Int. J. Hydrogen Energy 2007, 32, 908–914. [Google Scholar] [CrossRef]
- Onda, K.; Ichihara, K.; Nagahama, M.; Minamoto, Y.; Araki, T. Separation and compression characteristics of hydrogen by use of proton exchange membrane. J. Power Sources 2007, 164, 1–8. [Google Scholar] [CrossRef]
- Casati, C.; Longhi, P.; Zanderighi, L.; Bianchi, F. Some fundamental aspects in electrochemical hydrogen purification/compression. J. Power Sources 2008, 180, 103–113. [Google Scholar] [CrossRef]
- Doucet, R.; Gardner, C.L.; Ternan, M. Separation of hydrogen from hydrogen/ethylene mixtures using PEM fuel cell technology. Int. J. Hydrogen Energy 2009, 34, 998–1007. [Google Scholar] [CrossRef]
- Onda, K.; Araki, T.; Ichihara, K.; Nagahama, M. Treatment of low concentration hydrogen by electrochemical pump or proton exchange membrane fuel cell. J. Power Sources 2009, 188, 1–7. [Google Scholar] [CrossRef]
- Abdulla, A.; Laney, K.; Padilla, M.; Sundaresan, S.; Benziger, J. Efficiency of hydrogen recovery from reformate with a polymer electrolyte hydrogen pump. Am. Inst. Chem. Eng. J. 2011, 57, 1767–1779. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, B.S.; Ahn, S.H.; Han, J.Y.; Park, H.Y.; Kim, S.H.; Yoo, S.J.; Kim, H.J.; Cho, E.; Henkensmeier, D.; et al. Characterizations of polybenzimidazole based electrochemical hydrogen pumps with various Pt loadings for H2/CO2 gas separation. Int. J. Hydrogen Energy 2013, 38, 14816–14823. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lai, W.H.; Chen, Y.K.; Su, S.S. Characteristic studies of a PBI/H3PO4 high temperature membrane PEMFC under simulated reformate gases. Int. J. Hydrogen Energy 2014, 39, 13757–13762. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, H.Y.; Ahn, S.H.; Lee, B.S.; Kim, H.J.; Cho, E.A.; Henkensmeier, D.; Nam, S.W.; Kim, S.H.; Yoo, S.J.; et al. Highly active and CO2 tolerant Ir nanocatalysts for H2/CO2 separation in electrochemical hydrogen pumps. Appl. Catal. B Environ. 2014, 158–159, 348–354. [Google Scholar] [CrossRef]
- Bouwman, P.J. Advances in Electrochemical Hydrogen Compression and Purification. ECS Trans. 2016, 75, 503–510. [Google Scholar] [CrossRef]
- Huang, S.; Wang, T.; Wu, X.; Xiao, W.; Yu, M.; Chen, W.; Zhang, F.; He, G. Coupling hydrogen separation with butanone hydrogenation in an electrochemical hydrogen pump with sulfonated poly (phthalazinone ether sulfone ketone) membrane. J. Power Sources 2016, 327, 178–186. [Google Scholar] [CrossRef]
- Ru, F.Y.; Zulkefli, N.N.; Yusra, N.; Yusuf, M.; Masdar, M.S. Effect of Operating Parameter on H2/CO2 Gas Separation using Electrochemical Cell. Int. J. Appl. Eng. Res. 2018, 13, 505–510. [Google Scholar]
- Nordio, M.; Rizzi, F.; Manzolini, G.; Mulder, M.; Raymakers, L.; Van Sint Annaland, M.; Gallucci, F. Experimental and modelling study of an electrochemical hydrogen compressor. Chem. Eng. J. 2019, 369, 432–442. [Google Scholar] [CrossRef]
- Nordio, M.; Eguaras Barain, M.; Raymakers, L.; Van Sint Annaland, M.; Mulder, M.; Gallucci, F. Effect of CO2 on the performance of an electrochemical hydrogen compressor. Chem. Eng. J. 2019, 392, 123647. [Google Scholar] [CrossRef]
- Huang, F.; Pingitore, A.T.; Benicewicz, B.C. Electrochemical Hydrogen Separation from Reformate Using High-Temperature Polybenzimidazole (PBI) Membranes: The Role of Chemistry. ACS Sustain. Chem. Eng. 2020, 8, 6234–6242. [Google Scholar] [CrossRef]
- Jackson, C.; Raymakers, L.F.J.M.; Mulder, M.J.J.; Kucernak, A.R.J. Assessing electrocatalyst hydrogen activity and CO tolerance: Comparison of performance obtained using the high mass transport ‘floating electrode’ technique and in electrochemical hydrogen pumps. Appl. Catal. B Environ. 2020, 268, 118734. [Google Scholar] [CrossRef]
- Jackson, C.; Raymakers, L.F.J.M.; Mulder, M.J.J.; Kucernak, A.R.J. Poison mitigation strategies for the use of impure hydrogen in electrochemical hydrogen pumps and fuel cells. J. Power Sources 2020, 472, 228476. [Google Scholar] [CrossRef]
- Ohs, B.; Abduly, L.; Krödel, M.; Wessling, M. Combining electrochemical hydrogen separation and temperature vacuum swing adsorption for the separation of N2, H2 and CO2. Int. J. Hydrogen Energy 2020, 45, 9811–9820. [Google Scholar] [CrossRef]
- Rhandi, M.; Trégaro, M.; Druart, F.; Deseure, J.; Chatenet, M. Electrochemical hydrogen compression and purification versus competing technologies: Part I. Pros and cons. Chinese J. Catal. 2020, 41, 756–769. [Google Scholar] [CrossRef]
- Trégaro, M.; Rhandi, M.; Druart, F.; Deseure, J.; Chatenet, M. Electrochemical hydrogen compression and purification versus competing technologies: Part II. Challenges in electrocatalysis. Chinese J. Catal. 2020, 41, 770–782. [Google Scholar] [CrossRef]
- Vermaak, L.; Neomagus, H.W.J.P.; Bessarabov, D.G. Hydrogen Separation and Purification from Various Gas Mixtures by Means of Electrochemical Membrane Technology in the Temperature Range 100–160 °C, 2021; Unpublished.
- Zhang, J. Investigation of CO Tolerance in Proton Exchange Membrane Fuel Cells. Ph.D. Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2004; pp. 1–219. [Google Scholar]
- Uribe, F.A.; Gottesfeld, S.; Zawodzinski, T.A. Effect of Ammonia as Potential Fuel Impurity on Proton Exchange Membrane Fuel Cell Performance. J. Electrochem. Soc. 2002, 149, A293. [Google Scholar] [CrossRef]
- Halseid, R.; Vie, P.J.S.; Tunold, R. Influence of Ammonium on Conductivity and Water Content of Nafion 117 Membranes. J. Electrochem. Soc. 2004, 151, A381. [Google Scholar] [CrossRef]
- Kirsten, W.; Krüger, A.; Neomagus, H.; Bessarabov, D. Effect of Relative Humidity and Temperature on the Mechanical Properties of PFSA NafionTM-cation-exchanged membranes for Electrochemical Applications. Int. J. Electrochem. Sci. 2017, 12, 2573–2582. [Google Scholar] [CrossRef]
- Friend, P.J. Modelling and experimental characterization of an ionic polymer metal composite actuator PJ Friend Supervisor. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2018. [Google Scholar]
- Bessarabov, D. Chapter 8: Other Polymer Membrane Electrolysis Processes. In RSC Energy and Environment Series; Royal Society of Chemistry: London, UK, 2020; pp. 286–305. [Google Scholar]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Dafalla, A.M.; Jiang, F. Stresses and their impacts on proton exchange membrane fuel cells: A review. Int. J. Hydrogen Energy 2018, 43, 2327–2348. [Google Scholar] [CrossRef]
- Taymaz, I.; Benli, M. Numerical study of assembly pressure effect on the performance of proton exchange membrane fuel cell. Energy 2010, 35, 2134–2140. [Google Scholar] [CrossRef]
- Bouwman, P. Fundamental of Electrochemical Hydrogen Compression. In PEM Electrolysis for Hydrog. Production: Principles and Applications; Bessarabov, D., Wang, H., Li, H., Zhao, N., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 269–299. [Google Scholar]
- Haque, M.A.; Sulong, A.B.; Loh, K.S.; Majlan, E.H.; Husaini, T.; Rosli, R.E. Acid doped polybenzimidazoles based membrane electrode assembly for high temperature proton exchange membrane fuel cell: A review. Int. J. Hydrogen Energy 2017, 42, 9156–9179. [Google Scholar] [CrossRef]
- Gao, D.; Cai, F.; Xu, Q.; Wang, G.; Pan, X.; Bao, X. Gas-phase electrocatalytic reduction of carbon dioxide using electrolytic cell based on phosphoric acid-doped polybenzimidazole membrane. J. Energy Chem. 2014, 23, 674–700. [Google Scholar] [CrossRef]
- Tao, Y.; Hwang, Y.; Wang, C.; Radermacher, R. The Integration of Ammonia Electrochemical Compressor in Vapor Compression System; In Proceedings of the 12th IEA Heat Pump Conference, Rotterdam, The Netherlands, 15–18 May 2017; Volume 4.
- Tao, Y.; Gibbons, W.; Hwang, Y.; Radermacher, R.; Wang, C. Electrochemical ammonia compression. Chem. Commun. 2017, 53, 5637. [Google Scholar] [CrossRef]
- Tao, Y.; Hwang, Y.; Radermacher, R.; Wang, C. Experimental study on electrochemical compression of ammonia and carbon dioxide for vapor compression refrigeration system. Int. J. Refrig. 2019, 104, 180–188. [Google Scholar] [CrossRef]
- Fujiwara, N.; Nagase, H.; Tada, S.; Kikuchi, R. Hydrogen Production by Steam Electrolysis in Solid Acid Electrolysis Cells Naoya. Chem. Sustain. Energy Mater. 2020, 14, 417–427. [Google Scholar] [CrossRef]
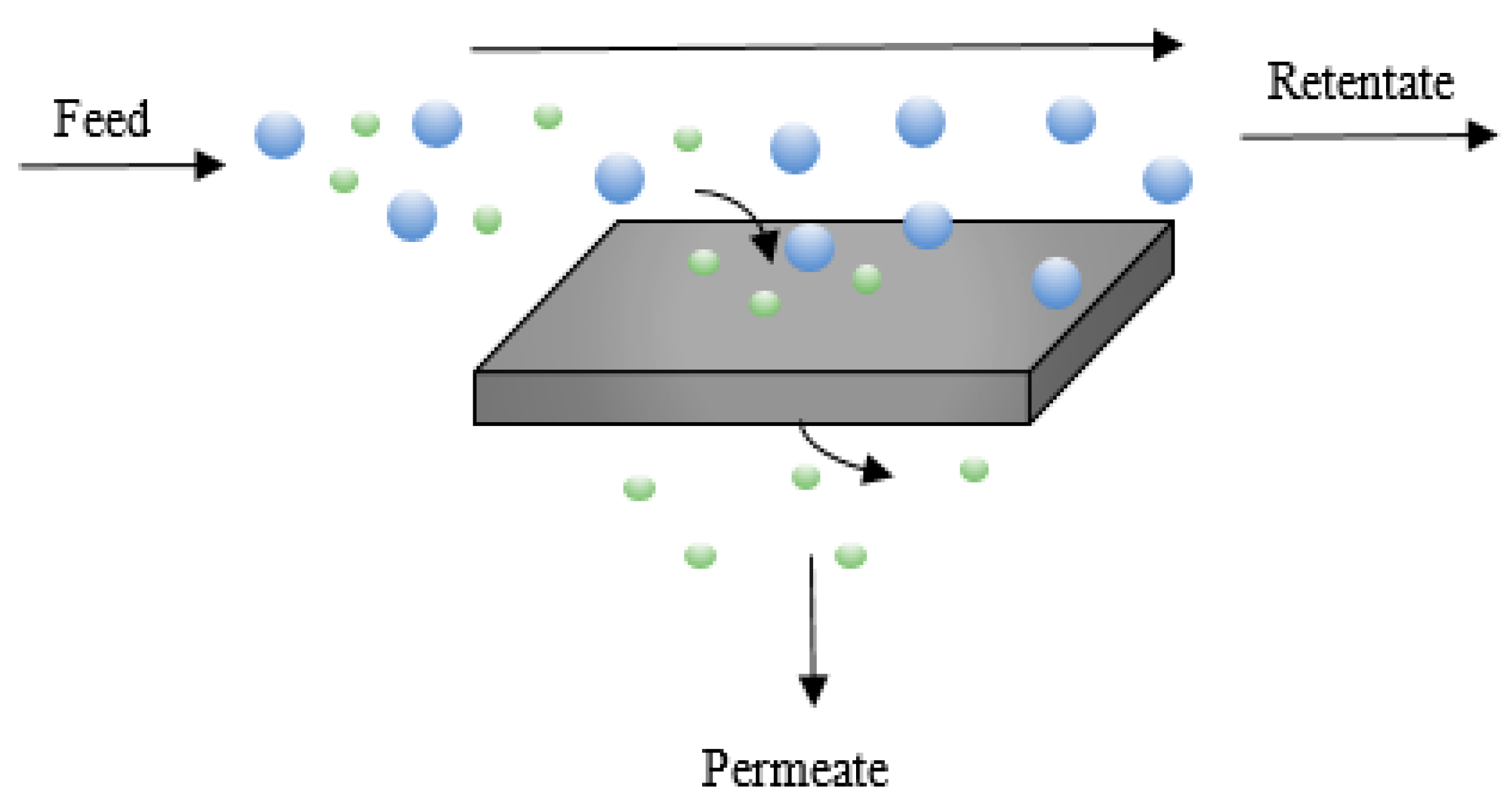
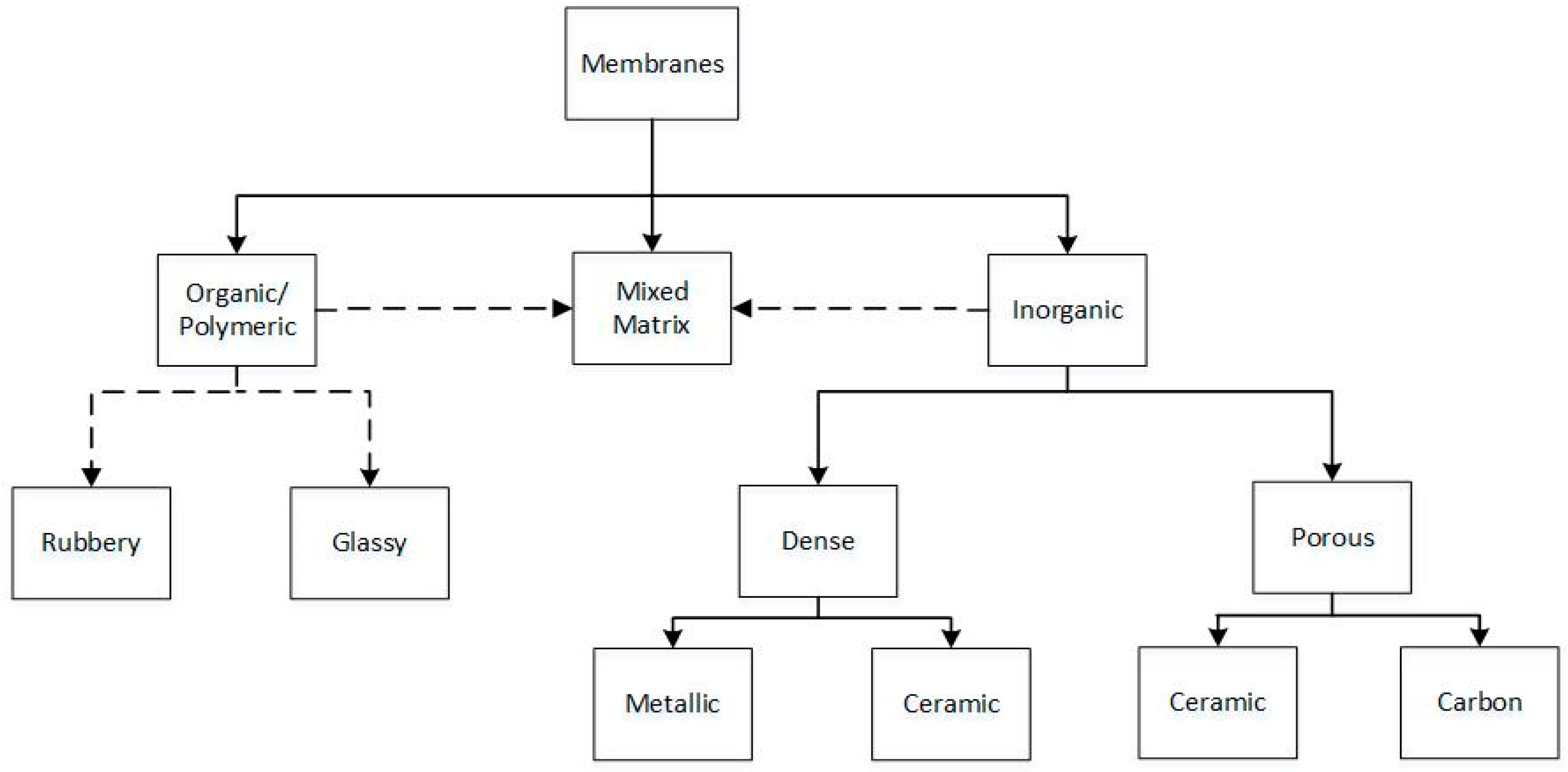
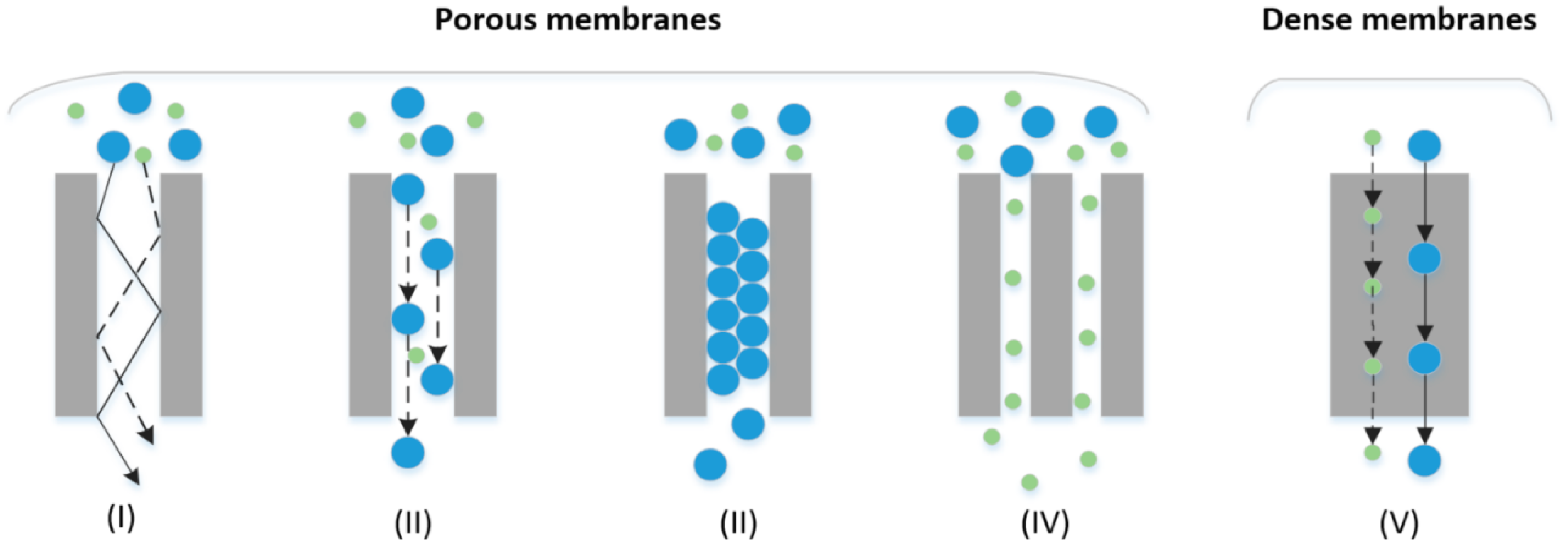
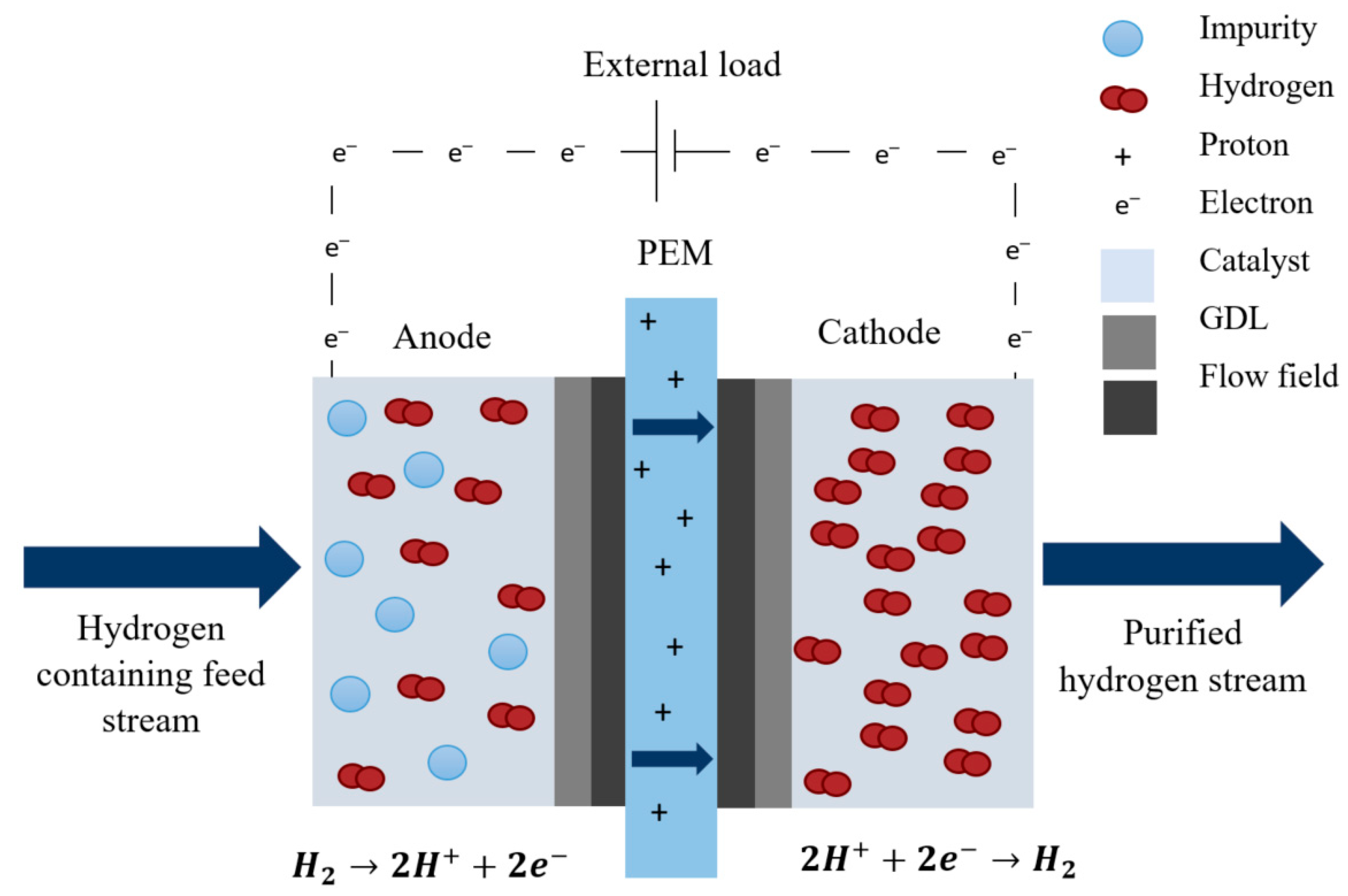

| Method | Advantages | Disadvantages | TML * | PE ** (%) | Cleanness *** | Impurities | References |
|---|---|---|---|---|---|---|---|
| Reforming: | |||||||
| SMR a | Most developed industrial process, lowest cost, existing infrastructure, high efficiency, best H2/CO ratio | Highest air emissions, system is complex, system is sensitive to natural gas quantities. Capital, operation, and maintenance cost. Fossil fuel feedstock. | 10 | 65–75 | NC/CCS | CO2, CO, CH4, N2 | [2,3,46,55,59,60,61,65,68,75,76,77,78,79] |
| POX c | Well-established. Variety of fuels, reduced desulphurization requirement, no catalyst required | Complex handling process, high operating temperature, low H2/CO ratio. Fossil fuel feedstock | 7–9 | 50 | NC | CO, CO2, H2O, CH4, H2S, COS and sometimes CH4 | [46,60,61,65,66,78,80] |
| ATR b | Lower temperatures than POX c, Requires less oxygen than POX c | Limited commercial application, required air or oxygen. Fossil fuel feedstock. | 6–8 | 60–75 | NC | CO, CO2, N2, CH4 and sometimes Ar | [60,81] |
| Gasification: | |||||||
| Coal | Abundant and affordable, Low-cost synthetic fuel in addition to H2 | Reactor costs, system efficiency, feedstock impurities, significant carbon footprint unless CCS is used. Separation and purification of gas products are difficult [82]. Fossil fuel feedstock (coal gasification). Season limitations and heterogeneity (biomass) | 10 | 74–85 | NC/CCS | N2, CO2, CO, CH4, H2S | [79,83,84,85] |
| Biomass | 3 (R&D) | 35–50 | NC/CCS | COx, SOx and CH4 | [2,78,84,86,87] | ||
| Electrolysis: | |||||||
| Water electrolysis | Simplicity of process design, compactness, renewable feedstock, cost effective way to produce hydrogen locally. Does not involve moving parts. Silent operation. | Energy input is required and it is more costly than fossil-fuel alternatives. | 9–10 | 62–82 | C | H2O | [2,66,67] |
| Constituent | Limits (μmol·mol−1 Unless Stated Otherwise) | Minimum Analytical Detection Limit |
|---|---|---|
| Hydrogen fuel index | >99.97% | |
| Water a | 5 | 0.12 |
| Total hydrocarbons b (C1 basis) | 2 | 0.1 |
| Oxygen | 5 | 1 |
| Helium | 300 | 100 |
| Nitrogen, Argon | 100 | 5 |
| Carbon dioxide | 2 | 0.1 |
| Carbon monoxide | 0.2 | 0.01 |
| Total sulphur c | 0.004 | 0.00002 |
| Formaldehyde | 0.01 | 0.01 |
| Formic acid | 0.2 | 0.02 |
| Ammonia | 0.1 | 0.02 |
| Total halogenates d | 0.05 | 0.01 |
| Particulate concentration | 1 mg·kg−1 | 0.005 mg·kg−1 |
| Properties | PSA | Membranes | Cryogenic |
|---|---|---|---|
| Min. feed purity (vol.%) | >40 | >25 | 15–80 |
| Product purity (vol.%) | 98–99.999 | >98 | 95–99.8 |
| Hydrogen recovery (%) | Up to 90 | Up to 99 | Up to 98 |
| Year | Type | Membrane | Catalyst * | Impurities | Temp. (°C) | Refs. |
|---|---|---|---|---|---|---|
| 2004 | Experimental | Nafion | Pt (B) | N2, CO2 | 30–70 | [166] |
| 2005 | Review | N/A | N/A | N/A | N/A | [167] |
| 2007 | Experimental | Nafion | Pt (A), Ru (C) | CO, CO2 | 20 | [156] |
| Experimental/modelling | Nafion | Pt/Pt-Ru (B) | Ar, CH4 | 20–70 | [168] | |
| Simulation | N/S ** | N/S ** | N2 | 25, 60 | [169] | |
| 2008 | Experimental | Nafion | Pt (B) | N2 | 25, 60 | [170] |
| Experimental | PBI | Pt (B) | CO, CO2, N2 | 120–160 | [49] | |
| 2009 | Experimental/modelling | Nafion | Pt (B) | Ar/C2H4 | 25 | [171] |
| Experimental | N/S ** | N/S ** | N2/CO2 | 60 | [172] | |
| 2010 | Experimental | PBI | N/S ** | N2; CO2, CO, CH4; N2,CO2, CO | 160–180 | [93] |
| 2011 | Experimental | Nafion | Pt/C (B) | CO2, H2O | 50–70 | [173] |
| Experimental | Nafion | Pt (B) | N2 | 35, 55, 75 | [161] | |
| Experimental | Nafion | Pt (B) | Ar | 20–70 | [159] | |
| 2012 | Experimental | Nafion | Pt/C, Pd/C (B) | CO2 reformate | 30–50 | [160] |
| 2013 | Experimental | PBI | Pt(B) | CO2 | 80, 160 | [174] |
| 2014 | Experimental | PBI | Pt (B) | Simulated reformate: N2, CO | 140–160 | [175] |
| Experimental | Nafion | Ir/C (B) | Ar, CO2 | 25, 70 | [176] | |
| 2016 | Experimental | Nafion | N/S ** | CO2, CO, CH4 | 25–75 | [177] |
| Experimental | SPPESK, Nafion | Pt (B) | CO2 | 20–60 | [178] | |
| 2018 | Experimental | Nafion | Pt-Ru (A), Pt (C) | CO2 | 25, 50 | [179] |
| 2019 | Experimental/modelling | Nafion | N/S ** | N2, CH4, He, CO2 | 28 | [180] |
| Experimental | Nafion | N/S ** | N2, CO2 and air | 15–22.5 | [181] | |
| Experimental case study: MEMPHYS (Membrane based purification of hydrogen system) system | N/S ** | N/S ** | N2 | 35 | [97] | |
| 2020 | Experimental | PBI | Pt (B) | N2, CO | 160–200 | [182] |
| Experimental/modelling | Nafion | Pt/C (B), Pt-Ru/C (A) and Pt/C (C), Pt-Ni/C (A) and Pt/C (C) | CO/Ar; N2 | 35 | [183] | |
| Experimental/modelling | Nafion | Pt/C (A), Pt-Ru/C (A) and Pt-Ru(C) | CO; CO2, CH4, CO, H2S | 35 | [184] | |
| Case study/modelling | N/A | N/A | H2,N2 and CO2 | N/A | [185] | |
| Review | N/A | N/A | N/A | N/A | [186] | |
| Review | N/A | N/A | N/A | N/A | [187] | |
| 2021 | Experimental | TPS | Pt-Co/C (A) and Pt/C (C) | CH4, CO2, and NH3 | 120–160 | [188] |
| Gas | Composition [%] | |||
|---|---|---|---|---|
| SPPESK-0.71 | Nafion 115 | Nafion 212 | Nafion/PTFE | |
| H2 | >99.99 | >99.99 | 99.79 | 99.25 |
| CO2 | <0.01 | <0.01 | 0.21 | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vermaak, L.; Neomagus, H.W.J.P.; Bessarabov, D.G. Recent Advances in Membrane-Based Electrochemical Hydrogen Separation: A Review. Membranes 2021, 11, 127. https://doi.org/10.3390/membranes11020127
Vermaak L, Neomagus HWJP, Bessarabov DG. Recent Advances in Membrane-Based Electrochemical Hydrogen Separation: A Review. Membranes. 2021; 11(2):127. https://doi.org/10.3390/membranes11020127
Chicago/Turabian StyleVermaak, Leandri, Hein W. J. P. Neomagus, and Dmitri G. Bessarabov. 2021. "Recent Advances in Membrane-Based Electrochemical Hydrogen Separation: A Review" Membranes 11, no. 2: 127. https://doi.org/10.3390/membranes11020127
APA StyleVermaak, L., Neomagus, H. W. J. P., & Bessarabov, D. G. (2021). Recent Advances in Membrane-Based Electrochemical Hydrogen Separation: A Review. Membranes, 11(2), 127. https://doi.org/10.3390/membranes11020127








