Antiviral Nanomaterials for Designing Mixed Matrix Membranes
Abstract
:1. Introduction
1.1. Virus Removal Mechanisms of Membrane Filters
1.2. Addition of Virucidal Capability to Membrane Filters
1.3. Scope of This Review
2. Antiviral Nanomaterials
2.1. Silver Nanomaterials (AgNMs)
2.2. Gold Nanomaterials (AuNMs)
2.3. Copper Nanomaterials (CuNMs)
2.4. Zinc Oxide Nanomaterials
2.5. Titanium Oxide Nanomaterials (TiO2NMs)
2.6. Carbon-Based Nanomaterials
2.7. Silica Nanomaterials
2.8. Tin Oxide Nanomaterials (SnO2NMs)
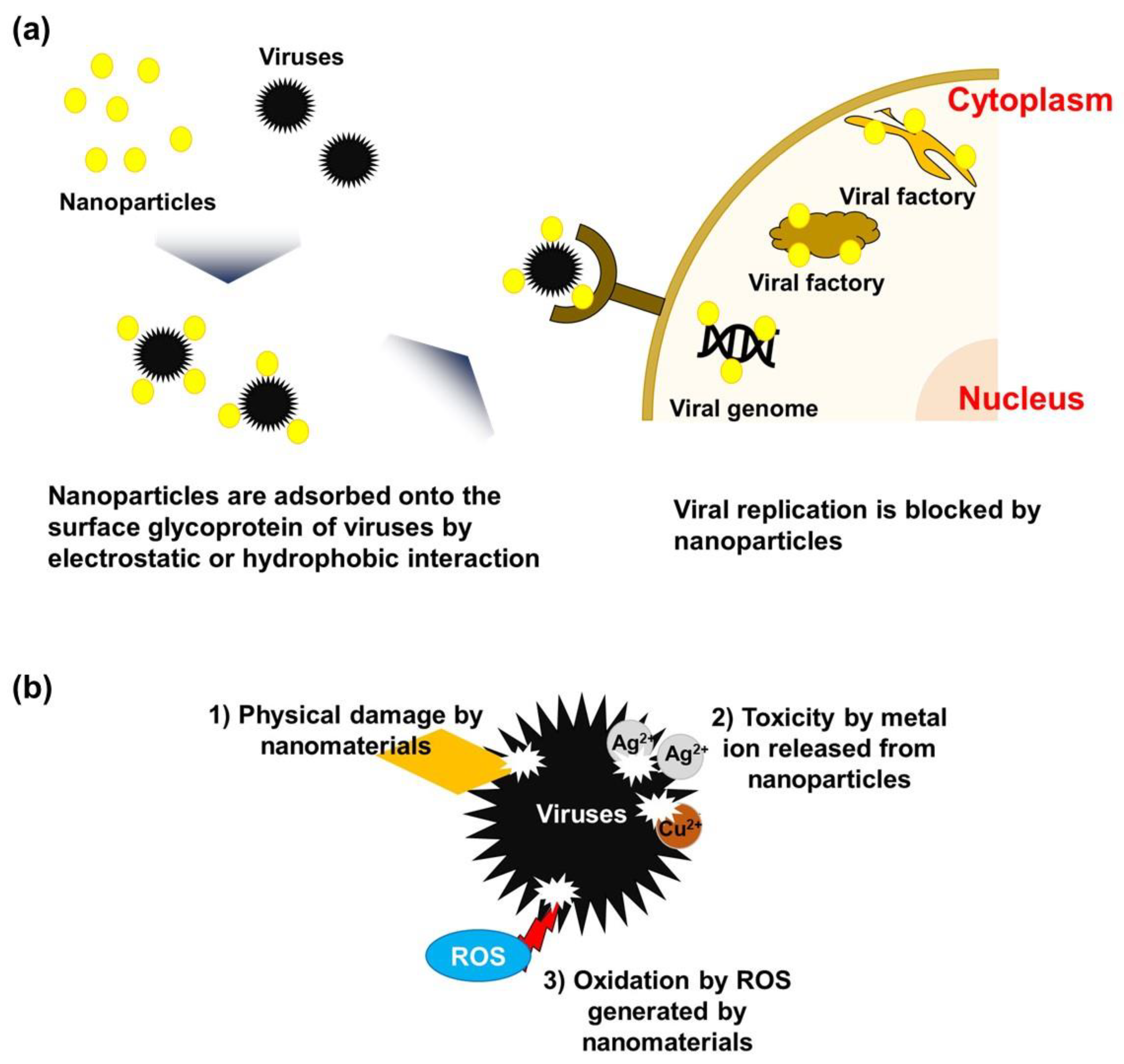
3. Mechanisms of the Antiviral Activity of Nanomaterials
4. Development of Antiviral MMMs
5. Current Application of Antiviral MMMs
5.1. Air
Antifouling and Antibacterial in Air Purification
5.2. Water
5.2.1. Water Sanitation
5.2.2. Virus Purification
5.3. Another Application: Medical Skin Patch
6. Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, N.J.; Glenn, J.S. Materials science approaches in the development of broad-spectrum antiviral therapies. Nat. Mater. 2020, 19, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Vecitis, C.D. Antiviral-nanoparticle interactions and reactions. Environ. Sci. Nano 2021, 8, 11–19. [Google Scholar] [CrossRef]
- Monto, A.S. Vaccines and Antiviral Drugs in Pandemic Preparedness. Emerg. Infect. Dis. 2006, 12, 55–60. [Google Scholar] [CrossRef]
- Li, R.; Cui, L.; Chen, M.; Huang, Y. Nanomaterials for Airborne Virus Inactivation: A Short Review. Aerosol Sci. Eng. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- Nardell, E.A. Air Disinfection for Airborne Infection Control with a Focus on COVID-19: Why Germicidal UV is Essential†. Photochem. Photobiol. 2021, 97, 493–497. [Google Scholar] [CrossRef]
- Chu, W.; Fang, C.; Deng, Y.; Xu, Z. Intensified Disinfection Amid COVID-19 Pandemic Poses Potential Risks to Water Quality and Safety. Environ. Sci. Technol. 2021, 55, 4084–4086. [Google Scholar] [CrossRef]
- Goswami, K.P.; Pugazhenthi, G. Credibility of polymeric and ceramic membrane filtration in the removal of bacteria and virus from water: A review. J. Environ. Manag. 2020, 268, 110583. [Google Scholar] [CrossRef] [PubMed]
- Padaki, M.; Murali, R.S.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation: A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Woo, M.-H.; Lee, J.-H.; Rho, S.-G.; Ulmer, K.; Welch, J.C.; Wu, C.-Y.; Song, L.; Baney, R.H. Evaluation of the Performance of Dialdehyde Cellulose Filters against Airborne and Waterborne Bacteria and Viruses. Ind. Eng. Chem. Res. 2011, 50, 11636–11643. [Google Scholar] [CrossRef]
- Liu, C.; Hsu, P.-C.; Lee, H.-W.; Ye, M.; Zheng, G.; Liu, N.; Li, W.; Cui, Y. Transparent air filter for high-efficiency PM2.5 capture. Nat. Commun. 2015, 6, 6205. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Chen, R.; Li, Y.-Y.; Sano, D. Virus removal by membrane bioreactors: A review of mechanism investigation and modeling efforts. Water Res. 2021, 188, 116522. [Google Scholar] [CrossRef] [PubMed]
- Tae Hwan, C.; Ho Bum, P. Membrane and virus filter trends in the processes of biopharmaceutical production. Membr. J. 2020, 30, 9–20. [Google Scholar]
- Syedain, Z.; Bohonak, D.; Zydney, A. Protein Fouling of Virus Filtration Membranes: Effects of Membrane Orientation and Operating Conditions. Biotechnol. Prog. 2006, 22, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.; Blackbeard, J.; Leslie, G. Removal Efficiency and Integrity Monitoring Techniques for Virus Removal by Membrane Processes. Crit. Rev. Environ. Sci. Technol. 2012, 42, 891–933. [Google Scholar] [CrossRef]
- Chua, M.H.; Cheng, W.; Goh, S.S.; Kong, J.; Li, B.; Lim, J.Y.C.; Mao, L.; Wang, S.; Xue, K.; Yang, L.; et al. Face Masks in the New COVID-19 Normal: Materials, Testing, and Perspectives. Research 2020, 2020, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-Y.; Hsieh, G.-W. Electrostatic polyester air filter composed of conductive nanowires and photocatalytic nanoparticles for particulate matter removal and formaldehyde decomposition. Environ. Sci. Nano 2020, 7, 3746–3758. [Google Scholar] [CrossRef]
- Choi, D.Y.; Heo, K.J.; Kang, J.; An, E.J.; Jung, S.-H.; Lee, B.U.; Lee, H.M.; Jung, J.H. Washable antimicrobial polyester/aluminum air filter with a high capture efficiency and low pressure drop. J. Hazard. Mater. 2018, 351, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Bulejko, P. Numerical Comparison of Prediction Models for Aerosol Filtration Efficiency Applied on a Hollow-Fiber Membrane Pore Structure. Nanomaterials 2018, 8, 447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durthi, C.P.; Rajulapati, S.B.; Palliparambi, A.A.; Kola, A.K.; Sonawane, S.H. Studies on removal of arsenic using cellulose acetate–zinc oxide nanoparticle mixed matrix membrane. Int. Nano Lett. 2018, 8, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Kim, J.; Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.H.; Terrien, A.; Figueiredo, A.S.; Gonçalves, M.C.; De Pinho, M.N. The role of silver nanoparticles on mixed matrix Ag/cellulose acetate asymmetric membranes. Polym. Compos. 2015, 38, 32–39. [Google Scholar] [CrossRef]
- Essa, W.; Yasin, S.; Saeed, I.; Ali, G. Nanofiber-Based Face Masks and Respirators as COVID-19 Protection: A Review. Membranes 2021, 11, 250. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, D.; He, H.; Ramakrishna, S. Electrospun ultrafine fibers for advanced face masks. Mater. Sci. Eng. R Rep. 2021, 143, 100594. [Google Scholar] [CrossRef] [PubMed]
- Fahimirad, S.; Fahimirad, Z.; Sillanpää, M. Efficient removal of water bacteria and viruses using electrospun nanofibers. Sci. Total Environ. 2021, 751, 141673. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, K.; Nair, K.M.; Forouzandeh, P.; Mathew, S.; Grant, J.; Moran, R.; Bartlett, J.; Bird, J.; Pillai, S.C. Face Masks and Respirators in the Fight against the COVID-19 Pandemic: A Review of Current Materials, Advances and Future Perspectives. Materials 2020, 13, 3363. [Google Scholar] [CrossRef]
- Yang, J.; Monnot, M.; Ercolei, L.; Moulin, P. Membrane-Based Processes Used in Municipal Wastewater Treatment for Water Reuse: State-of-the-Art and Performance Analysis. Membranes 2020, 10, 131. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K.; Rajca, M. Membranes in water and wastewater disinfection—Review. Arch. Environ. Prot. 2019, 45, 3–18. [Google Scholar] [CrossRef]
- Li, L.; Visvanathan, C. Membrane technology for surface water treatment: Advancement from microfiltration to membrane bioreactor. Rev. Environ. Sci. BioTechnol. 2017, 16, 737–760. [Google Scholar] [CrossRef]
- Zhang, C.-M.; Xu, L.-M.; Xu, P.-C.; Wang, X.C. Elimination of viruses from domestic wastewater: Requirements and technologies. World J. Microbiol. Biotechnol. 2016, 32, 1–9. [Google Scholar] [CrossRef]
- Altintas, Z.; Gittens, M.; Pocock, J.; Tothill, I.E. Biosensors for waterborne viruses: Detection and removal. Biochimie 2015, 115, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, V.; Lozovski, V.; Lokshyn, M.; Gomeniuk, Y.V.; Dorovskih, A.; Rusinchuk, N.; Pankivska, Y.; Povnitsa, O.; Zagorodnya, S.; Tertykh, V.; et al. Nanoparticles as antiviral agents against adenoviruses. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025021. [Google Scholar] [CrossRef]
- Balasubramaniam, B.; Prateek; Ranjan, S.; Saraf, M.; Kar, P.; Singh, S.P.; Thakur, V.K.; Singh, A.; Gupta, R.K. Antibacterial and antiviral functional materials: Chemistry and biological activity toward tackling COVID-19-like pandemics. ACS Pharmacol. Transl. Sci. 2021, 4, 8–54. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.-H.; Song, H. Antiviral Potential of Nanoparticles—Can Nanoparticles Fight Against Coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef]
- El-Mohamady, R.S.; Ghattas, T.; Zawrah, M.; El-Hafeiz, Y.A. Inhibitory effect of silver nanoparticles on bovine herpesvirus-1. Int. J. Vet. Sci. Med. 2018, 6, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speshock, J.L.; Murdock, R.C.; Braydich-Stolle, L.K.; Schrand, A.M.; Hussain, S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Sun, R.W.-Y.; Chen, R.; Hui, C.-K.; Ho, C.-M.; Luk, J.M.; Lau, G.K.K.; Che, C.-M. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008, 13, 253. [Google Scholar]
- Du, T.; Liang, J.; Dong, N.; Lu, J.; Fu, Y.; Fang, L.; Xiao, S.; Han, H. Glutathione-Capped Ag2S Nanoclusters Inhibit Coronavirus Proliferation through Blockage of Viral RNA Synthesis and Budding. ACS Appl. Mater. Interfaces 2018, 10, 4369–4378. [Google Scholar] [CrossRef]
- Meléndez-Villanueva, M.A.; Morán-Santibañez, K.; Martínez-Sanmiguel, J.J.; Rangel-López, R.; Garza-Navarro, M.A.; Rodríguez-Padilla, C.; Zarate-Triviño, D.G.; Trejo-Ávila, L.M. Virucidal Activity of Gold Nanoparticles Synthesized by Green Chemistry Using Garlic Extract. Viruses 2019, 11, 1111. [Google Scholar] [CrossRef] [Green Version]
- Bawage, S.; Tiwari, P.M.; Singh, A.; Dixit, S.; Pillai, S.R.; Dennis, V.A.; Singh, S.R. Gold nanorods inhibit respiratory syncytial virus by stimulating the innate immune response. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2299–2310. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, A.; Hashemzadeh, M.S. Inhibition of herpes simplex virus type 1 by copper oxide nanoparticles. J. Virol. Methods 2020, 275, 113688. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral activity of cuprous oxide nanoparticles against Hepatitis C Virus in vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef]
- Tavakoli, A.; Ataei-Pirkooh, A.; Sadeghi, G.M.; Bokharaei-Salim, F.; Sahrapour, P.; Kiani, S.J.; Moghoofei, M.; Farahmand, M.; Javanmard, D.; Monavari, S.H. Polyethylene glycol-coated zinc oxide nanoparticle: An efficient nanoweapon to fight against herpes simplex virus type 1. Nanomedicine 2018, 13, 2675–2690. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, S.; Shahzad, K.; Mushtaq, S.; Ali, I.; Rafe, M.H.; Fazal-Ul-Karim, S.M. Antibacterial and antiviral potential of colloidal Titanium dioxide (TiO2) nanoparticles suitable for biological applications. Mater. Res. Express 2019, 6, 105409. [Google Scholar] [CrossRef]
- Kim, J.; Cho, I.; Kim, I.; Kim, C.; Heo, N.H.; Suh, S. Manufacturing of antiviral inorganic materials from colloidal silver and titanium oxide. Rev. Roum. Chim. 2006, 51, 1121. [Google Scholar]
- Martinez, Z.S.; Castro, E.; Seong, C.-S.; Cerón, M.R.; Echegoyen, L.; Llano, M. Fullerene Derivatives Strongly Inhibit HIV-1 Replication by Affecting Virus Maturation without Impairing Protease Activity. Antimicrob. Agents Chemother. 2016, 60, 5731–5741. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Liu, G.-L.; Ling, F.; Wang, G.-X. Carbon nanotube-based nanocarrier loaded with ribavirin against grass carp reovirus. Antivir. Res. 2015, 118, 29–38. [Google Scholar] [CrossRef]
- Botta, G.; Bizzarri, B.M.; Garozzo, A.; Timpanaro, R.; Bisignano, B.; Amatore, D.; Palamara, A.T.; Nencioni, L.; Saladino, R. Carbon nanotubes supported tyrosinase in the synthesis of lipophilic hydroxytyrosol and dihydrocaffeoyl catechols with antiviral activity against DNA and RNA viruses. Bioorg. Med. Chem. 2015, 23, 5345–5351. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Shao, K.; Li, Z.; Guo, N.; Zuo, Y.; Li, Q.; Lu, Z.; Chen, L.; He, Q.; Han, H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces 2015, 7, 21571–21579. [Google Scholar] [CrossRef]
- Rajak, B.L.; Kumar, R.; Gogoi, M.; Patra, S. Antimicrobial Activity of Nanomaterials. In Nanosensors for Environment, Food and Agriculture Vol. 1; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; Volume 1, pp. 147–185. [Google Scholar]
- Osminkina, L.A.; Timoshenko, V.; Shilovsky, I.P.; Kornilaeva, G.V.; Shevchenko, S.; Gongalsky, M.; Tamarov, K.P.; Abramchuk, S.S.; Nikiforov, V.; Khaitov, M.R.; et al. Porous silicon nanoparticles as scavengers of hazardous viruses. J. Nanopart. Res. 2014, 16, 2430. [Google Scholar] [CrossRef]
- Silva, J.M.D.S.E.; Hanchuk, T.D.M.; Santos, M.I.; Kobarg, J.; Bajgelman, M.C.; Cardoso, M.B. Viral Inhibition Mechanism Mediated by Surface-Modified Silica Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 16564–16572. [Google Scholar] [CrossRef]
- Trigilio, J.; Antoine, T.E.; Paulowicz, I.; Mishra, Y.; Adelung, R.; Shukla, D. Tin Oxide Nanowires Suppress Herpes Simplex Virus-1 Entry and Cell-to-Cell Membrane Fusion. PLoS ONE 2012, 7, e48147. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [Green Version]
- De Gusseme, B.; Hennebel, T.; Christiaens, E.; Saveyn, H.; Verbeken, K.; Fitts, J.P.; Boon, N.; Verstraete, W. Virus disinfection in water by biogenic silver immobilized in polyvinylidene fluoride membranes. Water Res. 2011, 45, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Han, T.; Yin, J.; Li, Q.; Chen, Z.; Wei, Z.; Zhang, Y.; Dong, L. Bumpy structured nanofibrous membrane as a highly efficient air filter with antibacterial and antiviral property. Sci. Total Environ. 2021, 777, 145768. [Google Scholar] [CrossRef]
- Rafiei, S.; Rezatofighi, S.E.; Ardakani, M.R.; Rastegarzadeh, S. Gold Nanoparticles Impair Foot-and-Mouth Disease Virus Replication. IEEE Trans. NanoBiosci. 2015, 15, 34–40. [Google Scholar] [CrossRef]
- Papp, I.; Sieben, C.; Ludwig, K.; Roskamp, M.; Böttcher, C.; Schlecht, S.; Herrmann, A.; Haag, R. Inhibition of Influenza Virus Infection by Multivalent Sialic-Acid-Functionalized Gold Nanoparticles. Small 2010, 6, 2900–2906. [Google Scholar] [CrossRef]
- Alayande, A.B.; Obaid, M.; Kim, I.S. Antimicrobial mechanism of reduced graphene oxide-copper oxide (rGO-CuO) nanocomposite films: The case of Pseudomonas aeruginosa PAO1. Mater. Sci. Eng. C 2020, 109, 110596. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J. Copper, an ancient remedy returning to fight microbial, fungal and viral infections. Curr. Chem. Biol. 2009, 3, 272–278. [Google Scholar]
- Mazurkow, J.M.; Yüzbasi, N.S.; Domagala, K.W.; Pfeiffer, S.; Kata, D.; Graule, T. Nano-Sized Copper (Oxide) on Alumina Granules for Water Filtration: Effect of Copper Oxidation State on Virus Removal Performance. Environ. Sci. Technol. 2019, 54, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A Novel Anti-Influenza Copper Oxide Containing Respiratory Face Mask. PLoS ONE 2010, 5, e11295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalpana, V.N.; Rajeswari, V.D. A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Subhapriya, S.; Gomathipriya, P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 2018, 116, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Innocenzi, P.; Stagi, L. Carbon-based antiviral nanomaterials: Graphene, C-dots, and fullerenes. A perspective. Chem. Sci. 2020, 11, 6606–6622. [Google Scholar] [CrossRef]
- Matsushita, T.; Suzuki, H.; Shirasaki, N.; Matsui, Y.; Ohno, K. Adsorptive virus removal with super-powdered activated carbon. Sep. Purif. Technol. 2013, 107, 79–84. [Google Scholar] [CrossRef]
- Du, T.; Liang, J.; Dong, N.; Liu, L.; Fang, L.; Xiao, S.; Han, H. Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon 2016, 110, 278–285. [Google Scholar] [CrossRef]
- Park, K.-T.; Hwang, J. Filtration and inactivation of aerosolized bacteriophage MS2 by a CNT air filter fabricated using electro-aerodynamic deposition. Carbon 2014, 75, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharmin, S.; Rahaman, M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as antimicrobial and antiviral agents: A literature-based perspective study. Heliyon 2021, 7, e06456. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cagno, V.; Andreozzi, P.; D’Alicarnasso, M.; Silva, P.J.; Mueller, M.; Galloux, M.; Le Goffic, R.; Jones, S.T.; Vallino, M.; Hodek, J.; et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018, 17, 195–203. [Google Scholar] [CrossRef]
- Zhan, S.; Yang, Y.; Shen, Z.; Shan, J.; Li, Y.; Yang, S.; Zhu, D. Efficient removal of pathogenic bacteria and viruses by multifunctional amine-modified magnetic nanoparticles. J. Hazard. Mater. 2014, 274, 115–123. [Google Scholar] [CrossRef]
- Illescas, B.M.; Rojo, J.; Delgado, R.; Martín, N. Multivalent Glycosylated Nanostructures To Inhibit Ebola Virus Infection. J. Am. Chem. Soc. 2017, 139, 6018–6025. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Mu, L.; Wen, J.; Zhou, Q. Covalently synthesized graphene oxide-aptamer nanosheets for efficient visible-light photocatalysis of nucleic acids and proteins of viruses. Carbon 2012, 50, 2772–2781. [Google Scholar] [CrossRef]
- Singh, L.; Kruger, H.G.; Maguire, G.; Govender, T.; Parboosing, R. The role of nanotechnology in the treatment of viral infections. Ther. Adv. Infect. Dis. 2017, 4, 105–131. [Google Scholar] [CrossRef]
- Velthuis, A.J.W.T.; Worm, S.H.E.V.D.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; Van Hemert, M.J. Zn2+ Inhibits Coronavirus and Arterivirus RNA Polymerase Activity In Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Obregón, S.; Patrón-Soberano, O.A.; Terashima, C.; Fujishima, A. An approach to the photocatalytic mechanism in the TiO2-nanomaterials microorganism interface for the control of infectious processes. Appl. Catal. B Environ. 2020, 270, 118853. [Google Scholar] [CrossRef] [PubMed]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Hernandez, B.V.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Ali, I.; Raza, M.A.; Mehmood, R.; Islam, A.; Sabir, A.; Gull, N.; Haider, B.; Park, S.H.; Khan, R.U. Novel Maleic Acid, Crosslinked, Nanofibrous Chitosan/Poly (Vinylpyrrolidone) Membranes for Reverse Osmosis Desalination. Int. J. Mol. Sci. 2020, 21, 7338. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.; Setiawan, L.; Wei, L.; Si, R.; Fane, A.G.; Wang, R.; Chen, Y. Graphene oxide as effective selective barriers on a hollow fiber membrane for water treatment process. J. Membr. Sci. 2015, 474, 244–253. [Google Scholar] [CrossRef]
- Lim, S.; Park, K.H.; Tran, V.H.; Akther, N.; Phuntsho, S.; Choi, J.Y.; Shon, H.K. Size-controlled graphene oxide for highly permeable and fouling-resistant outer-selective hollow fiber thin-film composite membranes for forward osmosis. J. Membr. Sci. 2020, 609, 118171. [Google Scholar] [CrossRef]
- Chou, W.-L.; Yu, D.-G.; Yang, M.-C. The preparation and characterization of silver-loading cellulose acetate hollow fiber membrane for water treatment. Polym. Adv. Technol. 2005, 16, 600–607. [Google Scholar] [CrossRef]
- Botes, M.; Cloete, T. The potential of nanofibers and nanobiocides in water purification. Crit. Rev. Microbiol. 2010, 36, 68–81. [Google Scholar] [CrossRef]
- Yao, L.; Haas, T.W.; Guiseppi-Elie, A.; Bowlin, G.L.; Simpson, D.G.; Wnek, G.E. Electrospinning and Stabilization of Fully Hydrolyzed Poly(Vinyl Alcohol) Fibers. Chem. Mater. 2003, 15, 1860–1864. [Google Scholar] [CrossRef]
- Ramakrishna, S. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005. [Google Scholar]
- Ding, B.; Kimura, E.; Sato, T.; Fujita, S.; Shiratori, S. Fabrication of blend biodegradable nanofibrous nonwoven mats via multi-jet electrospinning. Polymer 2004, 45, 1895–1902. [Google Scholar] [CrossRef]
- Um, C.; Fang, D.; Hsiao, B.S.; Okamoto, A.A.; Chu, B. Electro-Spinning and Electro-Blowing of Hyaluronic Acid. Biomacromolecules 2004, 5, 1428–1436. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Hammond, P.T. Form and Function in Multilayer Assembly: New Applications at the Nanoscale. Adv. Mater. 2004, 16, 1271–1293. [Google Scholar] [CrossRef]
- Shi, Y.; Wan, D.; Huang, J.; Liu, Y.; Li, J. Stable LBL self-assembly coating porous membrane with 3D heterostructure for enhanced water treatment under visible light irradiation. Chemosphere 2020, 252, 126581. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Mi, B. Layer-by-layer assembly of graphene oxide membranes via electrostatic interaction. J. Membr. Sci. 2014, 469, 80–87. [Google Scholar] [CrossRef]
- Sinclair, T.; Patil, A.; Raza, B.; Reurink, D.; Hengel, S.V.D.; Rutjes, S.; Husman, A.D.R.; Roesink, H.; de Vos, W. Cationically modified membranes using covalent layer-by-layer assembly for antiviral applications in drinking water. J. Membr. Sci. 2019, 570, 494–503. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Yin, W.; Chong, T.H.; Wang, R. Design and development of layer-by-layer based low-pressure antifouling nanofiltration membrane used for water reclamation. J. Membr. Sci. 2019, 584, 309–323. [Google Scholar] [CrossRef]
- He, M.; Wang, Q.; Zhao, W.; Zhao, C.-S. A substrate-independent ultrathin hydrogel film as an antifouling and antibacterial layer for a microfiltration membrane anchored via a layer-by-layer thiol-ene click reaction. J. Mater. Chem. B 2018, 6, 3904–3913. [Google Scholar] [CrossRef]
- Akther, N.; Ali, S.M.; Phuntsho, S.; Shon, H. Surface modification of thin-film composite forward osmosis membranes with polyvinyl alcohol–graphene oxide composite hydrogels for antifouling properties. Desalination 2020, 491, 114591. [Google Scholar] [CrossRef]
- Hernandez, S.; Papp, J.K.; Bhattacharyya, D. Iron-Based Redox Polymerization of Acrylic Acid for Direct Synthesis of Hydrogel/Membranes and Metal Nanoparticles for Water Treatment. Ind. Eng. Chem. Res. 2014, 53, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Peeva, P.D.; Million, N.; Ulbricht, M. Factors affecting the sieving behavior of anti-fouling thin-layer cross-linked hydrogel polyethersulfone composite ultrafiltration membranes. J. Membr. Sci. 2012, 390-391, 99–112. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, X.; Wei, J.; Li, J.; Zhou, X.; Liu, D.; Liu, Z.; Li, J. Calcium alginate hydrogel filtration membrane with excellent anti-fouling property and controlled separation performance. J. Membr. Sci. 2015, 492, 536–546. [Google Scholar] [CrossRef]
- Chen, K.; Gou, W.; Wang, X.; Zeng, C.; Ge, F.; Dong, Z.; Wang, C. UV-Cured Fluoride-Free Polyurethane Functionalized Textile with pH-Induced Switchable Superhydrophobicity and Underwater Superoleophobicity for Controllable Oil/Water Separation. ACS Sustain. Chem. Eng. 2018, 6, 16616–16628. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, J.; Ge, F.; Zhao, R.; Wang, C. Smart UV-curable fabric coatings with self-healing ability for durable self-cleaning and intelligent oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 86–96. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, W.; Ziemann, E.; Be’Er, A.; Lu, X.; Elimelech, M.; Bernstein, R. Functionalization of ultrafiltration membrane with polyampholyte hydrogel and graphene oxide to achieve dual antifouling and antibacterial properties. J. Membr. Sci. 2018, 565, 293–302. [Google Scholar] [CrossRef]
- Chaudhary, J.P.; Vadodariya, N.; Nataraj, S.K.; Meena, R. Chitosan-Based Aerogel Membrane for Robust Oil-in-Water Emulsion Separation. ACS Appl. Mater. Interfaces 2015, 7, 24957–24962. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Ultra-hydrophobic and mesoporous silica aerogel membranes for efficient separation of surfactant-stabilized water-in-oil emulsion separation. Sep. Purif. Technol. 2019, 212, 597–604. [Google Scholar] [CrossRef]
- He, M.; Zhang, R.; Zhang, K.; Liu, Y.; Su, Y.; Jiang, Z. Reduced graphene oxide aerogel membranes fabricated through hydrogen bond mediation for highly efficient oil/water separation. J. Mater. Chem. A 2019, 7, 11468–11477. [Google Scholar] [CrossRef]
- Esmaielzadeh, S.; Ahmadizadegan, H. Preparation and characterization of novel polyimide/functionalized ZnO bionanocomposite for gas separation and study of their antibacterial activity. Solid State Sci. 2018, 78, 46–57. [Google Scholar] [CrossRef]
- Ji, S.-M.; Tiwari, A.P.; Oh, H.J.; Kim, H.-Y. ZnO/Ag nanoparticles incorporated multifunctional parallel side by side nanofibers for air filtration with enhanced removing organic contaminants and antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126564. [Google Scholar] [CrossRef]
- Muhammad, S.; Siddiq, M.; Niazi, J.H.; Qureshi, A. Role of quaternary ammonium compound immobilized metallic graphene oxide in PMMA/PEG membrane for antibacterial, antifouling and selective gas permeability properties. Polym. Bull. 2018, 75, 5695–5712. [Google Scholar] [CrossRef]
- Ahmadizadegan, H.; Esmaielzadeh, S.; Ranjbar, M.; Marzban, Z.; Ghavas, F. Synthesis and characterization of polyester bionanocomposite membrane with ultrasonic irradiation process for gas permeation and antibacterial activity. Ultrason. Sonochem. 2018, 41, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shi, M.; Zhao, S.; Wang, Z.; Wang, J.; Wang, S. Preparation and characterization of a polyethersulfone/polyaniline nanocomposite membrane for ultrafiltration and as a substrate for a gas separation membrane. RSC Adv. 2015, 5, 27211–27223. [Google Scholar] [CrossRef]
- Choi, J.; Yang, B.J.; Bae, G.-N.; Jung, J.H. Herbal Extract Incorporated Nanofiber Fabricated by an Electrospinning Technique and its Application to Antimicrobial Air Filtration. ACS Appl. Mater. Interfaces 2015, 7, 25313–25320. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, W.-N.; Liu, D.; Nie, Y.; Li, W.; Wu, J.; Zhang, F.; Biswas, P.; Fortner, J.D. Engineered Crumpled Graphene Oxide Nanocomposite Membrane Assemblies for Advanced Water Treatment Processes. Environ. Sci. Technol. 2015, 49, 6846–6854. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lee, M.W.; An, S.; Sinha-Ray, S.; Khansari, S.; Joshi, B.; Hong, S.; Hong, J.-H.; Kim, J.-J.; Pourdeyhimi, B.; et al. Antibacterial activity of photocatalytic electrospun titania nanofiber mats and solution-blown soy protein nanofiber mats decorated with silver nanoparticles. Catal. Commun. 2013, 34, 35–40. [Google Scholar] [CrossRef]
- Cado, G.; Aslam, R.; Séon, L.; Garnier, T.; Fabre, R.; Parat, A.; Chassepot, A.; Voegel, J.-C.; Senger, B.; Schneider, F.; et al. Self-Defensive Biomaterial Coating Against Bacteria and Yeasts: Polysaccharide Multilayer Film with Embedded Antimicrobial Peptide. Adv. Funct. Mater. 2013, 23, 4801–4809. [Google Scholar] [CrossRef]
- Weigel, T.; Solomaier, T.; Wehmeyer, S.; Peuker, A.; Wolff, M.; Reichl, U. A membrane-based purification process for cell culture-derived influenza A virus. J. Biotechnol. 2016, 220, 12–20. [Google Scholar] [CrossRef]
- Carvalho, S.B.; Silva, R.J.S.; Moleirinho, M.; Cunha, B.; Moreira, A.S.; Xenopoulos, A.; Alves, P.; Carrondo, M.; Peixoto, C. Membrane-Based Approach for the Downstream Processing of Influenza Virus-Like Particles. Biotechnol. J. 2019, 14, e1800570. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.B.; Fortuna, A.R.; Wolff, M.W.; Peixoto, C.; Alves, P.; Reichl, U.; Carrondo, M. Purification of influenza virus-like particles using sulfated cellulose membrane adsorbers. J. Chem. Technol. Biotechnol. 2017, 93, 1988–1996. [Google Scholar] [CrossRef]
- Komaladewi, A.; Khoiruddin, K.; Surata, I.; Subagia, I.A.; Wenten, I. Recent advances in antimicrobial air filter. E3S Web Conf. 2018, 67, 03016. [Google Scholar] [CrossRef]
- Wang, Y.; El Deen, A.G.; Li, P.; Oh, B.H.; Guo, Z.; Khin, M.M.; Vikhe, Y.S.; Wang, J.; Hu, R.G.; Boom, R.M.; et al. High-Performance Capacitive Deionization Disinfection of Water with Graphene Oxide-graft-Quaternized Chitosan Nanohybrid Electrode Coating. ACS Nano 2015, 9, 10142–10157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A Chem. 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Xu, H.; Fang, Z.; Tian, W.; Wang, Y.; Ye, Q.; Zhang, L.; Cai, J. Green Fabrication of Amphiphilic Quaternized β-Chitin Derivatives with Excellent Biocompatibility and Antibacterial Activities for Wound Healing. Adv. Mater. 2018, 30, e1801100. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wen, Y.; Li, Y.; Liu, P.; Li, Z.; Shi, Y.; Lan, J.; Guo, R.; Tan, L. Facile Fabrication of Sandwich Structural Membrane with a Hydrogel Nanofibrous Mat as Inner Layer for Wound Dressing Application. Front. Chem. 2018, 6, 490. [Google Scholar] [CrossRef]
- Tan, L.; Hu, J.; Huang, H.; Han, J.; Hu, H. Study of multi-functional electrospun composite nanofibrous mats for smart wound healing. Int. J. Biol. Macromol. 2015, 79, 469–476. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, J.; Xu, J.; Tian, M.; Li, L.; Tan, L.; Li, Z. Fast-acting and highly rechargeable antibacterial composite nanofibrous membrane for protective applications. Compos. Sci. Technol. 2021, 202, 108574. [Google Scholar] [CrossRef]
- Kang, J.; Han, J.; Gao, Y.; Gao, T.; Lan, S.; Xiao, L.; Zhang, Y.; Gao, G.; Chokto, H.; Dong, A. Unexpected Enhancement in Antibacterial Activity of N-Halamine Polymers from Spheres to Fibers. ACS Appl. Mater. Interfaces 2015, 7, 17516–17526. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Xiang, P.; Lu, J.; Yuan, J.; Shen, J. Electrospun polyurethane/keratin/AgNP biocomposite mats for biocompatible and antibacterial wound dressings. J. Mater. Chem. B 2016, 4, 635–648. [Google Scholar] [CrossRef]
- Irwansyah, I.; Li, Y.-Q.; Shi, W.; Qi, D.; Leow, W.R.; Tang, M.B.Y.; Li, S.; Chen, X. Gram-Positive Antimicrobial Activity of Amino Acid-Based Hydrogels. Adv. Mater. 2015, 27, 648–654. [Google Scholar] [CrossRef]
- Alayande, A.B.; Goh, K.; Son, M.; Kim, C.-M.; Chae, K.-J.; Kang, Y.; Jang, J.; Kim, I.S.; Yang, E. Recent Progress in One- and Two-Dimensional Nanomaterial-Based Electro-Responsive Membranes: Versatile and Smart Applications from Fouling Mitigation to Tuning Mass Transport. Membranes 2020, 11, 5. [Google Scholar] [CrossRef]

| Nanomaterials (NMs) | Target Virus | Mode of Action | References |
|---|---|---|---|
| AgNMs | Bovine herpesvirus-1 (BoHV-1), MS2 bacteriophage, Tacaribe virus (TCRV), Hepatitis B virus (HBV), | ROS production, viral replication inhibition, direct viral inactivation, viral binding, and interaction with double-stranded DNA | [35,36,37,38] |
| Ag2S NCs | Porcine epidemic diarrhea virus (PEDV) | Blockage of viral DNA and budding | [39] |
| AuNMs | Measles virus (MeV), Respiratory syncytial virus (RSV) | Direct virus blocking and viral replication inhibition | [40,41] |
| CuNMs | Herpes simplex virus−1 (HSV−1), hepatitis C virus (HCV) | Viral proteins and genome oxidation (ROS) and degradation, viral protein denaturation | [42,43] |
| ZnO NMs | Human influenza A virus (H1N1), HSV−1 | Polymerase function inhibition, contact killing | [44,45,46] |
| TiO2 NMs | Newcastle disease virus (NDV) | Viral lipid envelope destruction | [47,48] |
| Carbon-based NMs (fullerene, SWCNTs, MWCNTs, graphene, graphene oxide) | Human immunodeficiency virus (HIV−1), HSV−1,2, coxsackievirus (Cox B3), cytomegalovirus, grass carp reovirus (GCRV) | Viral maturation and replication inhibition interact with viral enzyme, RNA polymerase inhibition, viral membrane destruction | [49,50,51,52,53] |
| Silica-based NMs | RSV, HIV | Viral inactivation, disruption of DNA replication | [54,55] |
| SnO2 NMs | HSV−1 | Inhibition of cell entry and cell-to-cell spread | [56] |
| Application | Material | Membrane Structure | Ref. |
|---|---|---|---|
| Air purification | ZnO | Nanocomposite | [113] |
| Air purification | ZnO/Ag | SBS nanofibers | [114] |
| Air purification | CTAB@MGO | Nanocomposite | [115] |
| Air purification | Cellulose/silica BNCs | Nanocomposite | [116] |
| Air purification | PANI nanorods | Nanocomposite | [117] |
| Air purification | Herbal extract | HEI nanofiber | [118] |
| Sanitation in ultrafiltration | Ag@TiO2/crumpled GO | Stacked composite | [119] |
| Sanitation in water treatment | TiO2-decorated Nylon nanofiber | Nanofiber membrane | [120] |
| Sanitation in medical tool | Peptide-functionalized sodium hyaluronate and chitosan | Layered coating | [121] |
| Virus purification | Sulfated cellulose | Film on support | [122] |
| Virus purification | Polyethersulfone | Thin-film composite | [123] |
| Virus-like particle purification | Sulfated cellulose | Film on support | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alayande, A.B.; Kang, Y.; Jang, J.; Jee, H.; Lee, Y.-G.; Kim, I.S.; Yang, E. Antiviral Nanomaterials for Designing Mixed Matrix Membranes. Membranes 2021, 11, 458. https://doi.org/10.3390/membranes11070458
Alayande AB, Kang Y, Jang J, Jee H, Lee Y-G, Kim IS, Yang E. Antiviral Nanomaterials for Designing Mixed Matrix Membranes. Membranes. 2021; 11(7):458. https://doi.org/10.3390/membranes11070458
Chicago/Turabian StyleAlayande, Abayomi Babatunde, Yesol Kang, Jaewon Jang, Hobin Jee, Yong-Gu Lee, In S. Kim, and Euntae Yang. 2021. "Antiviral Nanomaterials for Designing Mixed Matrix Membranes" Membranes 11, no. 7: 458. https://doi.org/10.3390/membranes11070458
APA StyleAlayande, A. B., Kang, Y., Jang, J., Jee, H., Lee, Y.-G., Kim, I. S., & Yang, E. (2021). Antiviral Nanomaterials for Designing Mixed Matrix Membranes. Membranes, 11(7), 458. https://doi.org/10.3390/membranes11070458









