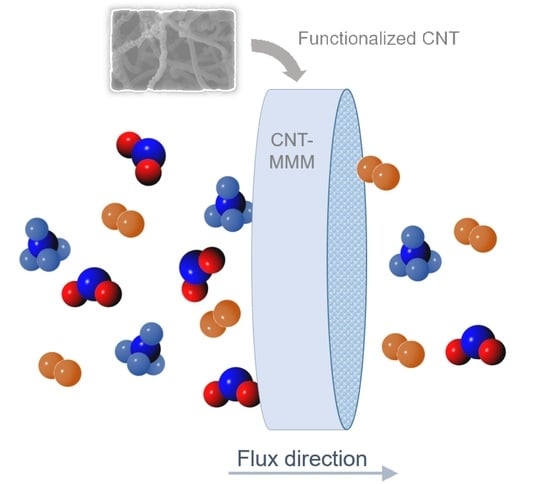
Review: Mixed-Matrix Membranes with CNT for CO2 Separation Processes
Abstract
:1. Introduction
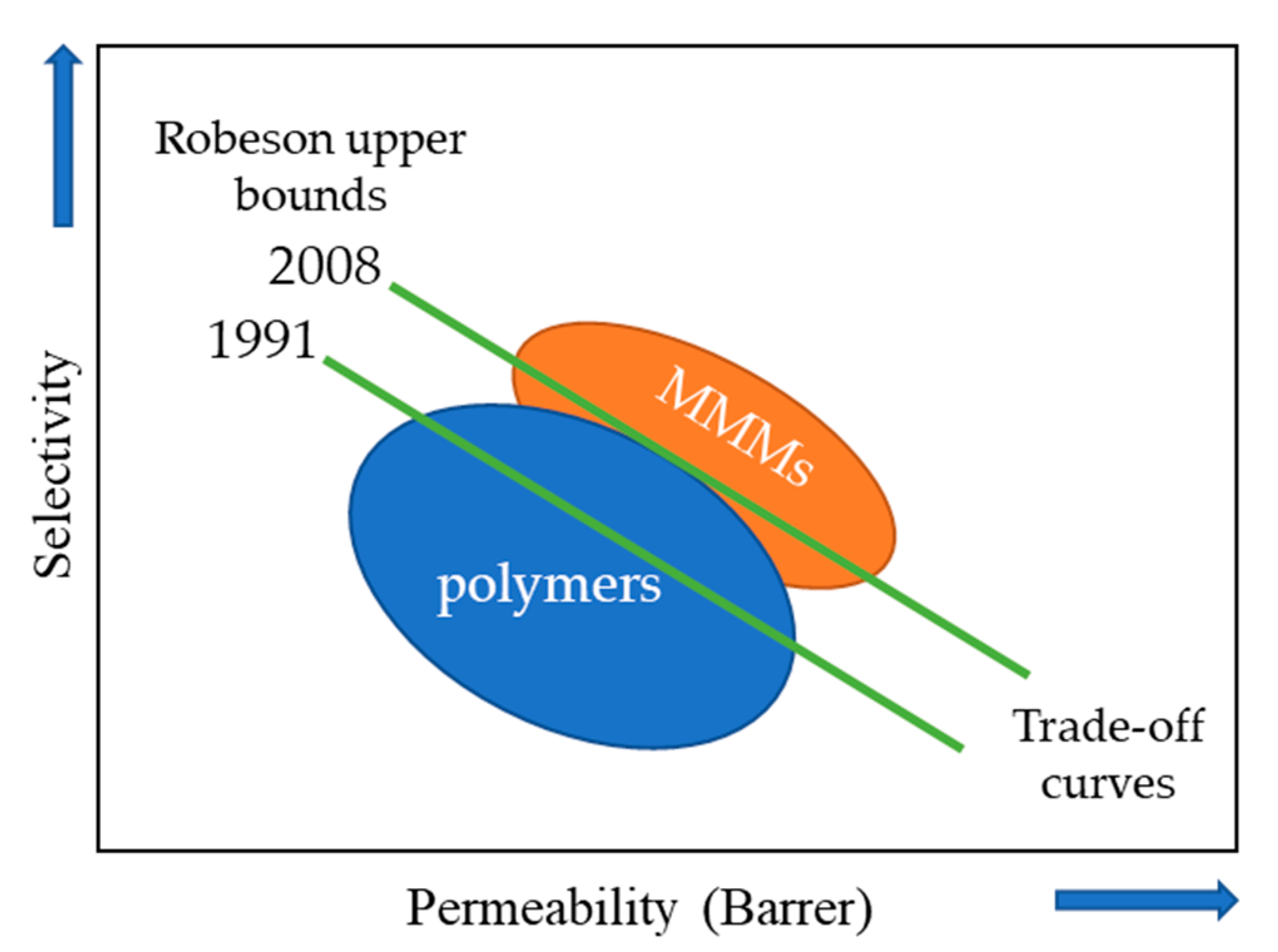
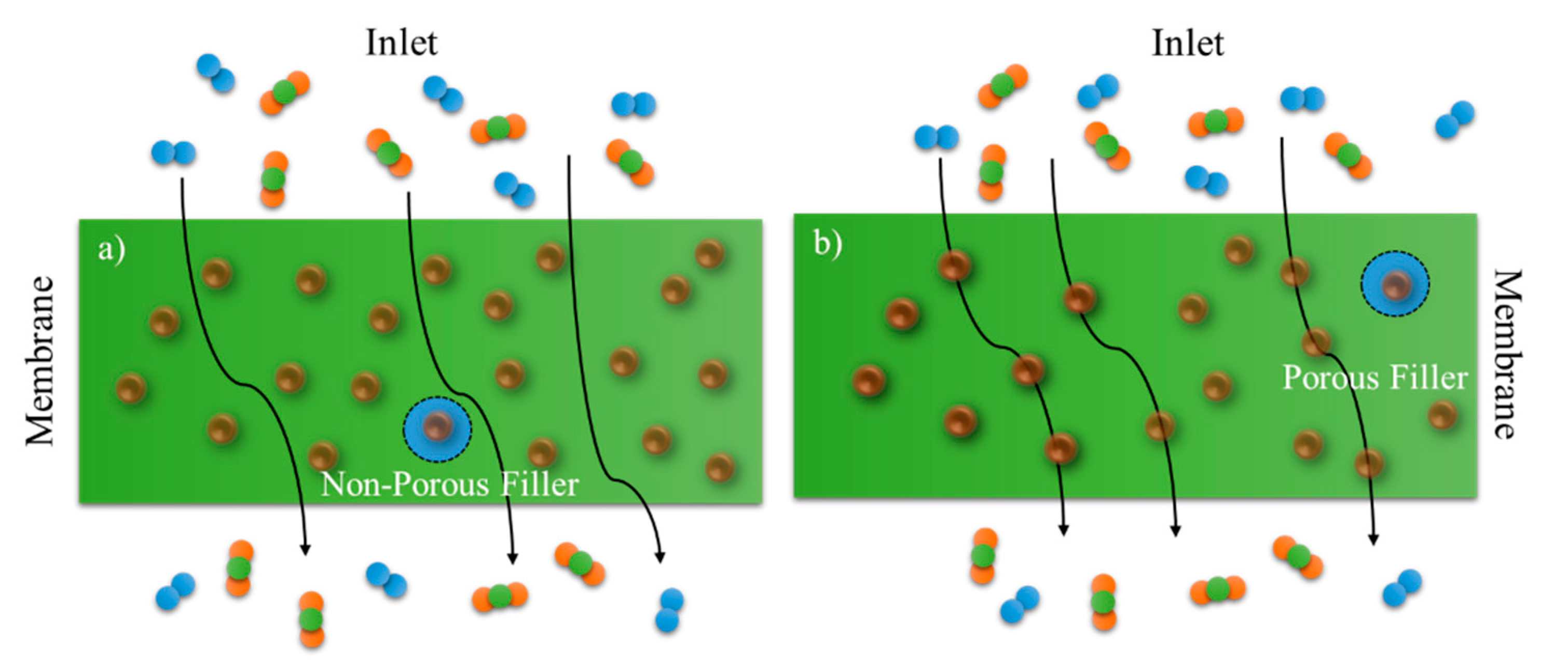
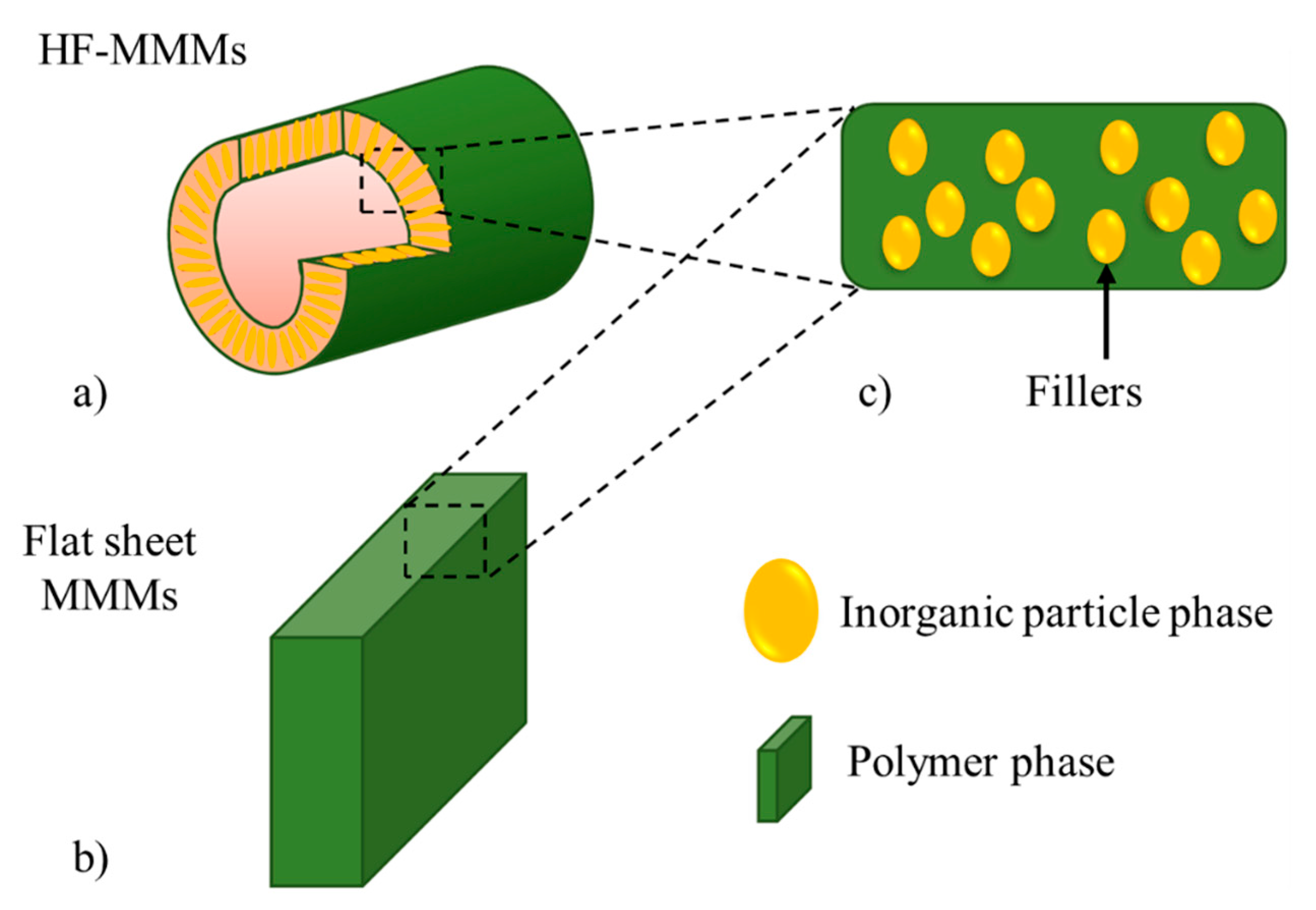
2. Mixed-Matrix Membranes with CNT as Fillers
3. Application of CNT–MMM in CO2 Separation
3.1. Influence of CNT as Fillers in MMM
3.2. Influence of Functionalized CNT as Fillers in MMM
3.3. Influence of CNT and Other Compounds Added to MMM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, C.; Li, Q.; Hua, Y.; Zhou, B.; Duan, J.; Jingui, D. Mechanical Synthesis of COF Nanosheet Cluster and Its Mixed Matrix Membrane for Efficient CO2 Removal. ACS Appl. Mater. Interfaces 2017, 9, 29093–29100. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Liu, J.; Hou, J.; Zhang, Y. Enzyme-embedded metal-organic framework membranes on polymeric substrates for efficient CO2 capture. J. Mater. Chem. A 2017, 5, 19954–19962. [Google Scholar] [CrossRef]
- World Meteorological Organization (WMO). WMO Greenhouse Gas Bulletin: The State of Greenhouse Gases in the Atmosphere Based on Global Observations Through; World Meteorological Organization: Geneva, Switzerland, 2015; p. 12. Available online: https://reliefweb.int/report/world/wmo-greenhouse-gasbulletin-state-greenhouse-gases-atmosphere-based-global-observations (accessed on 8 May 2018).
- Zhang, Z.; Chen, F.; Rezakazemi, M.; Zhang, W.; Lu, C.; Chang, H.; Quan, X. Modeling of a CO2-piperazine-membrane absorption system. Chem. Eng. Res. Des. 2018, 131, 375–384. [Google Scholar] [CrossRef]
- De Vos, R.M. High-Selectivity, High-Flux Silica Membranes for Gas Separation. Science 1998, 279, 1710–1711. [Google Scholar] [CrossRef]
- Shiflett, M.B. Ultrasonic Deposition of High-Selectivity Nanoporous Carbon Membranes. Science 1999, 285, 1902–1905. [Google Scholar] [CrossRef] [PubMed]
- Merkel, T.C.; Freeman, B.D.; Spontak, R.J.; He, Z.; Pinnau, I.; Meakin, P.; Hill, A.J. Ultrapermeable, Reverse-Selective Nanocomposite Membranes. Science 2002, 296, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Sazali, N.; Salleh, W.N.W.; Ismail, A.F.; Wong, K.C.; Iwamoto, Y. Exploiting pyrolysis protocols on BTDA-TDI/MDI (P84) polyimide/nanocrystalline cellulose carbon membrane for gas separations. J. Appl. Polym. Sci. 2018, 136, 136. [Google Scholar] [CrossRef]
- Basile, A.; Gallucci, F. Membranes for Membrane Reactors: Preparation, Optimization and Selection; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Nandi, B.; Uppaluri, R.; Purkait, M. Preparation and characterization of low cost ceramic membranes for micro-filtration applications. Appl. Clay Sci. 2008, 42, 102–110. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D. Perry’s Chemical Engineers’ Handbook, 8th ed.; McGraw-Hill Education: New York, NY, USA, 2007. [Google Scholar]
- Gandia, L.; Arzamedi, G.; Dieguez, P. Renewable Hydrogen Technologies: Production, Purification, Storage, Applications and Safety; Elsevier Science: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Venna, S.R.; Lartey, M.; Li, T.; Spore, A.; Kumar, S.; Nulwala, H.B.; Luebke, D.R.; Rosi, N.L.; Albenze, E. Fabrication of MMMs with improved gas separation properties using externally-functionalized MOF particles. J. Mater. Chem. A 2015, 3, 5014–5022. [Google Scholar] [CrossRef]
- Pal, R. Permeation models for mixed matrix membranes. J. Colloid Interface Sci. 2008, 317, 191–198. [Google Scholar] [CrossRef]
- Widjojo, N.; Chung, N.T.-S.; Kulprathipanja, S. The fabrication of hollow fiber membranes with double-layer mixed-matrix materials for gas separation. J. Membr. Sci. 2008, 325, 326–335. [Google Scholar] [CrossRef]
- Amooghin, A.E.; Mashhadikhan, S.; Sanaeepur, H.; Moghadassi, A.; Matsuura, T.; Ramakrishna, S. Substantial breakthroughs on function-led design of advanced materials used in mixed matrix membranes (MMMs): A new horizon for efficient CO2 separation. Prog. Mater. Sci. 2019, 102, 222–295. [Google Scholar] [CrossRef]
- Japip, S.; Xiao, Y.; Chung, T.-S. Particle-Size Effects on Gas Transport Properties of 6FDA-Durene/ZIF-71 Mixed Matrix Membranes. Ind. Eng. Chem. Res. 2016, 55, 9507–9517. [Google Scholar] [CrossRef]
- Basu, S.; Odena, A.; Vankelecom, I.F. MOF-containing mixed-matrix membranes for CO2/CH4 and CO2/N2 binary gas mixture separations. Sep. Purif. Technol. 2011, 81, 31–40. [Google Scholar] [CrossRef]
- Abetz, V.; Brinkmann, T.; Dijkstra, M.; Ebert, K.; Fritsch, D.; Ohlrogge, K.; Paul, D.; Peinemann, K.-V.; Pereira-Nunes, S.; Scharnagl, N.; et al. Developments in Membrane Research: From Material via Process Design to Industrial Application. Adv. Eng. Mater. 2006, 8, 328–358. [Google Scholar] [CrossRef]
- Soltani, B.; Asghari, M. Effects of ZnO Nanoparticle on the Gas Separation Performance of Polyurethane Mixed Matrix Membrane. Membranes 2017, 7, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiq, S.; Deng, L.; Hägg, M.-B. Role of Facilitated Transport Membranes and Composite Membranes for Efficient CO2 Capture—A Review. ChemBioEng Rev. 2016, 3, 68–85. [Google Scholar] [CrossRef]
- Wee, S.-L.; Tye, C.-T.; Bhatia, S. Membrane separation process—Pervaporation through zeolite membrane. Sep. Purif. Technol. 2008, 63, 500–516. [Google Scholar] [CrossRef]
- Lozano, L.; Godínez, C.; Ríos, A.D.L.; Hernández-Fernández, F.; Sánchez-Segado, S.; Alguacil, F.J. Recent advances in supported ionic liquid membrane technology. J. Membr. Sci. 2011, 376, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Korelskiy, D.; Ye, P.; Fouladvand, S.; Karimi, S.; Sjöberg, E.; Hedlund, J. Efficient ceramic zeolite membranes for CO2/H2 separation. J. Mater. Chem. A 2015, 3, 12500–12506. [Google Scholar] [CrossRef] [Green Version]
- Kumbhare, M.B.; Sapkal, V.S.; Sapkal, R.S. A Review: Membrane for CO2 Separation from Syngas and Hydrogen. Int. J. Basic Appl. Res. 2018, 8, 651–677. [Google Scholar]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Swaidan, R.; Ma, X.; Litwiller, E.; Pinnau, I. High pressure pure- and mixed-gas separation of CO2/CH4 by thermally-rearranged and carbon molecular sieve membranes derived from a polyimide of intrinsic microporosity. J. Membr. Sci. 2013, 447, 387–394. [Google Scholar] [CrossRef]
- Shantarovich, V.P.; Kevdina, I.B.; Yampolskii, Y.P.; Alentiev, A.Y. Positron Annihilation Lifetime Study of High and Low Free Volume Glassy Polymers: Effects of Free Volume Sizes on the Permeability and Permselectivity. Macromolecules 2000, 33, 7453–7466. [Google Scholar] [CrossRef]
- Raj, K.R.; Sunarti, A.R. Preliminary Fractional Factorial Design (FFD) study using incorporation of Graphene Oxide in PVC in mixed matrix membrane to enhance CO2/CH4 separation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 702, 012041. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Lua, A.C. Preparation and characterization of mixed matrix membranes based on PVDF and three inorganic fillers (fumed nonporous silica, zeolite 4A and mesoporous MCM-41) for gas separation. Chem. Eng. J. 2012, 192, 201–210. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Hunger, K.; Schmeling, N.; Jeazet, H.B.T.; Janiak, C.; Staudt, C.; Kleinermanns, K. Investigation of Cross-Linked and Additive Containing Polymer Materials for Membranes with Improved Performance in Pervaporation and Gas Separation. Membranes 2012, 2, 727–763. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.T.; Lee, J.S.; Bae, T.-H.; Ward, J.K.; Johnson, J.; Jones, C.W.; Nair, S.; Koros, W.J. CO2–CH4 permeation in high zeolite 4A loading mixed matrix membranes. J. Membr. Sci. 2011, 367, 197–203. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Challenges and opportunities for mixed-matrix membranes for gas separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Li, N.; Liao, J.; Zhao, S.; Wang, J.; Wang, S. Relationship between polymer–filler interfaces in separation layers and gas transport properties of mixed matrix composite membranes. J. Membr. Sci. 2015, 495, 252–268. [Google Scholar] [CrossRef]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-Matrix Membranes. Angew. Chem. Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef]
- Barankova, E.; Pradeep, N.; Peinemann, K.-V. Zeolite-imidazolate framework (ZIF-8) membrane synthesis on a mixed-matrix substrate. Chem. Commun. 2013, 49, 9419–9421. [Google Scholar] [CrossRef]
- Arjmandi, M.; Pakizeh, M. Mixed matrix membranes incorporated with cubic-MOF-5 for improved polyetherimide gas separation membranes: Theory and experiment. J. Ind. Eng. Chem. 2014, 20, 3857–3868. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed matrix membranes using carbon molecular sieves: I. Preparation and experimental results. J. Membr. Sci. 2003, 211, 311–334. [Google Scholar] [CrossRef]
- Koresh, J.E.; Soffer, A. The Carbon Molecular Sieve Membranes. General Properties and the Permeability of CH4/H2 Mixture. Sep. Sci. Technol. 1987, 22, 973–982. [Google Scholar] [CrossRef]
- Zornoza, B.; Téllez, C.; Coronas, J. Mixed matrix membranes comprising glassy polymers and dispersed mesoporous silica spheres for gas separation. J. Membr. Sci. 2011, 368, 100–109. [Google Scholar] [CrossRef]
- Zhao, Y.; Jung, B.T.; Ansaloni, L.; Ho, W.W. Multiwalled carbon nanotube mixed matrix membranes containing amines for high pressure CO2/H2 separation. J. Membr. Sci. 2014, 459, 233–243. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Teow, Y.H.; Mohammad, A.W.; Ba-Abbad, M.M. The Effect of Graphene Oxide (GO) Loading for the Enhancement of Nylon 6,6-GO Mixed-matrix. Membr. Perform. 2020, 24, 2. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, Y.; Wang, Z.; Zhang, J.; Guo, R.; Li, X. Incorporating the magnetic alignment of GO composites into Pebax matrix for gas separation. J. Energy Chem. 2019, 31, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cheng, Y.; Zhang, H.; Wang, S.; Jiang, Z.; Guo, R.; Wu, H. Efficient CO2 Capture by Functionalized Graphene Oxide Nanosheets as Fillers To Fabricate Multi-Permselective Mixed Matrix Membranes. ACS Appl. Mater. Interfaces 2015, 7, 5528–5537. [Google Scholar] [CrossRef] [PubMed]
- Quan, S.; Li, S.W.; Xiao, Y.C.; Shao, L. CO 2 -selective mixed matrix membranes (MMMs) containing graphene oxide (GO) for enhancing sustainable CO 2 capture. Int. J. Greenh. Gas Control. 2017, 56, 22–29. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A.; Sanip, S.; Ng, B.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Wijenayake, S.N.; Panapitiya, N.P.; Versteeg, S.H.; Nguyen, C.N.; Goel, S.; Balkus, J.K.J.; Musselman, I.H.; Ferraris, J.P. Surface Cross-Linking of ZIF-8/Polyimide Mixed Matrix Membranes (MMMs) for Gas Separation. Ind. Eng. Chem. Res. 2013, 52, 6991–7001. [Google Scholar] [CrossRef]
- Ismail, A.; Rahim, N.; Mustafa, A.; Matsuura, T.; Ng, B.; Abdullah, S.; Hashemifard, S.A. Gas separation performance of polyethersulfone/multi-walled carbon nanotubes mixed matrix membranes. Sep. Purif. Technol. 2011, 80, 20–31. [Google Scholar] [CrossRef]
- Hamid, M.R.A.; Jeong, H.-K. Recent advances on mixed-matrix membranes for gas separation: Opportunities and engineering challenges. Korean J. Chem. Eng. 2018, 35, 1577–1600. [Google Scholar] [CrossRef]
- Ismail, A.; Goh, P.; Sanip, S.; Aziz, M. Transport and separation properties of carbon nanotube-mixed matrix membrane. Sep. Purif. Technol. 2009, 70, 12–26. [Google Scholar] [CrossRef]
- Krishna, R.; van Baten, J.M. Maxwell–Stefan modeling of slowing-down effects in mixed gas permeation across porous membranes. J. Membr. Sci. 2011, 383, 289–300. [Google Scholar] [CrossRef]
- Saito, Y.; Hirai, K.; Emori, H.; Murata, S.; Uetani, Y.; Kii, K. Carrier Diffusivity in Porous Membranes. J. Phys. Chem. B 2004, 108, 1137–1142. [Google Scholar] [CrossRef]
- Nuhnen, A.; Dietrich, D.; Millan, S.; Janiak, C. Role of Filler Porosity and Filler/Polymer Interface Volume in Metal–Organic Framework/Polymer Mixed-Matrix Membranes for Gas Separation. ACS Appl. Mater. Interfaces 2018, 10, 33589–33600. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Mikami, H.; Tanaka, M.; Isaji, T.; Odaka, K.; Yamato, M.; Kawakami, H. Mixed matrix membranes comprising a polymer of intrinsic microporosity loaded with surface-modified non-porous pearl-necklace nanoparticles. J. Membr. Sci. 2020, 597, 117627. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Amooghin, A.E.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Noble, R.D. Perspectives on mixed matrix membranes. J. Membr. Sci. 2011, 378, 393–397. [Google Scholar] [CrossRef]
- Kiadehi, A.D.; Rahimpour, A.; Jahanshahi, M.; Ghoreyshi, A.A. Novel carbon nano-fibers (CNF)/polysulfone (PSf) mixed matrix membranes for gas separation. J. Ind. Eng. Chem. 2015, 22, 199–207. [Google Scholar] [CrossRef]
- Nour, M.; Berean, K.; Balendhran, S.; Ou, J.Z.; Du Plessis, J.; McSweeney, C.; Bhaskaran, M.; Sriram, S.; Kalantar-Zadeh, K. CNT/PDMS composite membranes for H2 and CH4 gas separation. Int. J. Hydrog. Energy 2013, 38, 10494–10501. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.Y.; Chung, T.; Kulprathipanja, S.; Chung, N.T.-S. An investigation to revitalize the separation performance of hollow fibers with a thin mixed matrix composite skin for gas separation. J. Membr. Sci. 2006, 276, 113–125. [Google Scholar] [CrossRef]
- Sanip, S.; Ismail, A.; Goh, P.; Soga, T.; Tanemura, M.; Yasuhiko, H. Gas separation properties of functionalized carbon nanotubes mixed matrix membranes. Sep. Purif. Technol. 2011, 78, 208–213. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas sep-aration applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Shan, M.; Seoane, B.; Andres-Garcia, E.; Kapteijn, F.; Gascon, J. Mixed-matrix membranes containing an azine-linked covalent organic framework: Influence of the polymeric matrix on post-combustion CO2-capture. J. Membr. Sci. 2018, 549, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Hernandez, B.V.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Janakiram, S.; Ahmadi, M.; Dai, Z.; Ansaloni, L.; Deng, L. Performance of Nanocomposite Membranes Containing 0D to 2D Nanofillers for CO2 Separation: A Review. J. Membr. Sci. 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.M.; Filiz, V.; Bengtson, G.; Shishatskiy, S.; Rahman, M.; Lillepaerg, J.; Abetz, V. Enhanced gas permeability by fabricating mixed matrix membranes of functionalized multiwalled carbon nanotubes and polymers of intrinsic microporosity (PIM). J. Membr. Sci. 2013, 436, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Sokhan, V.P.; Nicholson, D.; Quirke, N. Transport properties of nitrogen in single walled carbon nanotubes. J. Chem. Phys. 2004, 120, 3855–3863. [Google Scholar] [CrossRef]
- Matranga, C.; Bockrath, B.; Chopra, N.; Hinds, B.J.; Andrews, R. Raman Spectroscopic Investigation of Gas Interactions with an Aligned Multiwalled Carbon Nanotube Membrane. Langmuir 2006, 22, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhu, Z.; Li, F.; Liu, S.; Wang, L.; Tang, X.; Rudolph, V. Investigation of Gas Permeability in Carbon Nanotube (CNT)−Polymer Matrix Membranes via Modifying CNTs with Functional Groups/Metals and Controlling Modification Location. J. Phys. Chem. C 2011, 115, 6661–6670. [Google Scholar] [CrossRef]
- Lee, R.; Jawad, Z.; Ahmad, A.; Chua, H. Incorporation of functionalized multi-walled carbon nanotubes (MWCNTs) into cellulose acetate butyrate (CAB) polymeric matrix to improve the CO2/N2 separation. Process. Saf. Environ. Prot. 2018, 117, 159–167. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, S.; Tripathi, B.; Singh, M.; Vijay, Y. Aligned CNT/Polymer nanocomposite membranes for hydrogen separation. Int. J. Hydrog. Energy 2009, 34, 3977–3982. [Google Scholar] [CrossRef]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Lin, R.; Ge, L.; Diao, H.; Rudolph, V.; Zhu, Z. Propylene/propane selective mixed matrix membranes with grape-branched MOF/CNT filler. J. Mater. Chem. A 2016, 4, 6084–6090. [Google Scholar] [CrossRef]
- Ranjbaran, F.; Omidkhah, M.R.; Amooghin, A.E. The novel Elvaloy4170/functionalized multi-walled carbon nanotubes mixed matrix membranes: Fabrication, characterization and gas separation study. J. Taiwan Inst. Chem. Eng. 2015, 49, 220–228. [Google Scholar] [CrossRef]
- Swain, S.S.; Unnikrishnan, L.; Mohanty, S.; Nayak, S.K. Carbon nanotubes as potential candidate for separation of H2CO2 gas pairs. Int. J. Hydrog. Energy 2017, 42, 29283–29299. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.; Montazer-Rahmati, M.; Matsuura, T. Effect of chitosan as a functionalization agent on the performance and separation properties of polyimide/multi-walled carbon nanotubes mixed matrix flat sheet membranes. J. Membr. Sci. 2010, 364, 309–317. [Google Scholar] [CrossRef]
- Eitan, A.; Jiang, K.; Dukes, D.; Andrews, A.R.; Schadler, L.S. Surface Modification of Multiwalled Carbon Nanotubes: Toward the Tailoring of the Interface in Polymer Composites. Chem. Mater. 2003, 15, 3198–3201. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Raimondo, M.; Naddeo, C.; Vertuccio, L.; Bonnaud, L.; Dubois, P.; Binder, W.H.; Sorrentino, A.; Guadagno, L. Multifunctionality of structural nanohybrids: The crucial role of carbon nanotube covalent and non-covalent functionalization in enabling high thermal, mechanical and self-healing performance. Nanotechnology 2020, 31, 225708. [Google Scholar] [CrossRef]
- Merum, S.; Veluru, J.B.; Seeram, R. Functionalized carbon nanotubes in bio-world: Applications, limitations and future directions. Mater. Sci. Eng. B 2017, 223, 43–63. [Google Scholar] [CrossRef]
- Bilalis, P.; Katsigiannopoulos, D.; Avgeropoulos, A.; Sakellariou, G. Non-covalent functionalization of carbon nanotubes with polymers. RSC Adv. 2014, 4, 2911–2934. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Cong, H.; Zhang, J.; Radosz, M.; Shen, Y. Carbon nanotube composite membranes of brominated poly(2,6-diphenyl-1,4-phenylene oxide) for gas separation. J. Membr. Sci. 2007, 294, 178–185. [Google Scholar] [CrossRef]
- Sapalidis, A.A.; Karantzis, P.I.; Vairis, A.; Nitodas, S.F.; Barbe, S.; Favvas, E.P. A Study of the Reinforcement Effect of MWCNTs onto Polyimide Flat Sheet Membranes. Polymers 2020, 12, 1381. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-H.; Kumar, I.A.; Weng, T.-H.; Lu, C.-Y.; Wey, M.-Y. Preparation and characterization of carbon molecular sieve membranes for gas separation—the effect of incorporated multi-wall carbon nanotubes. Desalination 2009, 240, 40–45. [Google Scholar] [CrossRef]
- Yu, B.; Cong, H.; Li, Z.; Tang, J.; Zhao, X.S. Pebax-1657 nanocomposite membranes incorporated with nanoparticles/colloids/carbon nanotubes for CO2/N2 and CO2/H2 separation. J. Appl. Polym. Sci. 2013, 130, 2867–2876. [Google Scholar] [CrossRef]
- Moghadassi, A.R.; Rajabi, Z.; Hosseini, S.M.; Mohammadi, M. Preparation and Characterization of Polycarbonate-Blend-Raw/Functionalized Multi-Walled Carbon Nano Tubes Mixed Matrix Membrane for CO2 Separation. Sep. Sci. Technol. 2013, 48, 1261–1271. [Google Scholar] [CrossRef]
- Habibiannejad, S.A.; Aroujalian, A.; Raisi, A. Pebax-1657 mixed matrix membrane containing surface modified multi-walled carbon nanotubes for gas separation. RSC Adv. 2016, 6, 79563–79577. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Li, H.; Li, X.; Deng, M. Gas separation properties of poly(amide-6-b-ethylene oxide)/amino modified multi-walled carbon nanotubes mixed matrix membranes. J. Membr. Sci. 2014, 467, 41–47. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Wang, Y.; Qiu, Y.; Li, H.; Hua, K.; Li, X.; Ji, J.; Deng, M. High CO2 separation performance of Pebax®/CNTs/GTA mixed matrix membranes. J. Membr. Sci. 2017, 521, 104–113. [Google Scholar] [CrossRef]
- Sun, H.; Wang, T.; Xu, Y.; Gao, W.; Li, P.; Niu, Q.J. Fabrication of polyimide and functionalized multi-walled carbon nanotubes mixed matrix membranes by in-situ polymerization for CO2 separation. Sep. Purif. Technol. 2017, 177, 327–336. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Wang, C.; Chang, H.-C.; Guo, R. Carbon nanotube-based mixed-matrix membranes with supramolecularly engineered interface for enhanced gas separation performance. J. Membr. Sci. 2020, 598, 117794. [Google Scholar] [CrossRef]
- Iranagh, F.R.; Asghari, M.; Parnian, M.J. Dispersion engineering of MWCNT to control structural and gas transport properties of PU mixed matrix membranes. J. Environ. Chem. Eng. 2020, 8, 104493. [Google Scholar] [CrossRef]
- Amirkhani, F.; Mosadegh, M.; Asghari, M.; Parnian, M.J. The beneficial impacts of functional groups of CNT on structure and gas separation properties of PEBA mixed matrix membranes. Polym. Test. 2020, 82, 106285. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, Y.; Yao, D.; Yang, Z.; Xin, Y.; Wang, F.; Wang, Y.; Ning, H.; Wu, H.; Wang, H. Liquid-like CNT/SiO2 nanoparticle organic hybrid materials as fillers in mixed matrix composite membranes for enhanced CO2-selective separation. New J. Chem. 2019, 43, 11949–11958. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.S.; Taniguchi, T.; Ismail, A.F.; Zahri, K. The role of geometrically different carbon-based fillers on the formation and gas separation performance of nanocomposite membranes. Carbon 2019, 149, 33–44. [Google Scholar] [CrossRef]
- Lia, X.; Yua, S.; Lia, K.; Mab, C.; Zhanga, J.; Lia, H.; Changa, X.; Zhua, L.; Xuea, Q. Enhanced gas separation performance of Pebax mixed matrix membranes by incorporating ZIF-8 in situ inserted by multiwalled carbon nanotubes. Sep. Purif. Technol. 2020, 248, 117080. [Google Scholar] [CrossRef]
- Sun, H.; Gao, W.; Zhang, Y.; Cao, X.; Bao, S.; Li, P.; Kang, Z.; Niu, Q.J. Bis(phenyl)fluorene-based polymer of intrinsic microporosity/functionalized multi-walled carbon nanotubes mixed matrix membranes for enhanced CO2 separation performance. React. Funct. Polym. 2020, 147, 104465. [Google Scholar] [CrossRef]
- Li, X.; Ma, L.; Zhang, H.; Wang, S.; Jiang, Z.; Guo, R.; Wu, H.; Cao, X.; Yang, J.; Wang, B. Synergistic effect of combining carbon nanotubes and graphene oxide in mixed matrix membranes for efficient CO2 separation. J. Membr. Sci. 2015, 479, 1–10. [Google Scholar] [CrossRef]
- Sarfraz, M.; Ba-Shammakh, M. Harmonious interaction of incorporating CNTs and zeolitic imidazole frameworks into polysulfone to prepare high performance MMMs for CO2 separation from humidified post combustion gases. Braz. J. Chem. Eng. 2018, 35, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Guo, R.; Hou, J.; Wei, Z.; Li, X. Mixed-Matrix Membranes Containing Carbon Nanotubes Composite with Hydrogel for Efficient CO2 Separation. ACS Appl. Mater. Interfaces 2016, 8, 29044–29051. [Google Scholar] [CrossRef] [PubMed]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.C.; Sposito, E.; Fuoco, A.; Jansen, J.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ul-trapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef] [Green Version]
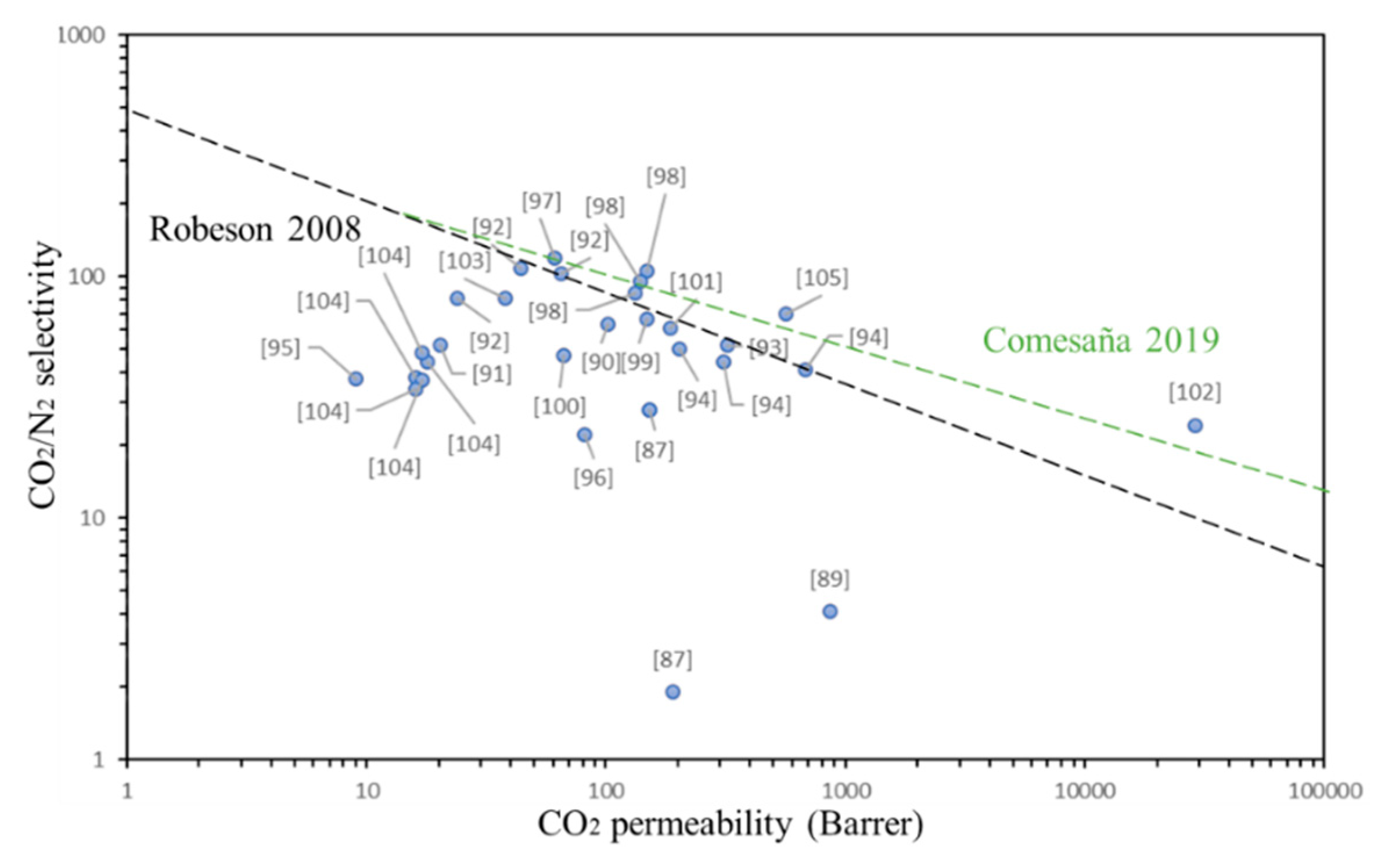

| Fillers | Polymer Matrix | CNT wt% | Pressure (Bar) | Temperature (°C) | Permeability (Barrer) CO2 | Selectivity (CO2/N2) | Selectivity (CO2/CH4) | Reference |
|---|---|---|---|---|---|---|---|---|
| CNTs | Brominated poly(2,6-diphenyl-1,4-phenylene oxide) | 5 | 0.689 | 25 | 153 | 28 | - | [87] |
| CNTs | Polyimide | 15 | 1 | 25 | 866.6 | 4.1 | - | [89] |
| C-MWCNTs | PC/PEG | 10 | 2 | 25 | 20.32 | 52.10 | 36.64 | [91] |
| SWNT | PEBA MMMs | 5 | 2.3 | 21 | 102 | 63 | - | [90] |
| MWNTs–NH2 | Pebax 1657 | 23 | 7 | 34.85 | 320 | 52 | 14 | [93] |
| CNTs-GO | Matrimids | 5 | 4 × 10−5 | 25 | 38.07 | 81 | 84.6 | [103] |
| CNTs | Pebax | 2 | 25 | 567 | 70 | 35 | [105] | |
| MWCNTs-COOH-4 | Pebax | 4 | 3 | 30 | 24 | 81 | - | [92] |
| MWCNTs-X100 | Pebax | 4 | 3 | 30 | 65 | 103 | - | [92] |
| MWCNTs-NH2 | Pebax | 4 | 3 | 30 | 44 | 108 | - | [92] |
| MWNT-COOH-OH | polyimide (PI) | 3 | 1 | 15 | 9.06 | 37.74 | 24 | [95] |
| MWNT | Pebax | 5 | 7 | 34.85 | 202 | 50 | - | [94] |
| MWNT | Pebax | 10 | 7 | 34.85 | 310 | 44 | - | [94] |
| MWNT | Pebax | 15 | 7 | 34.85 | 680 | 41 | - | [94] |
| CNTs | Brominated poly(2,6-diphenyl-1,4-phenylene oxide) | 5 | 0.689 | 25 | 153 | 28 | - | [87] |
| CNT/ZIF-301(6) | PSF | 10 | 2 | 25 | 16 | 38 | - | [104] |
| CNTs/ZIF-301(12) | PSF | 8 | 2 | 25 | 17 | 37 | - | [104] |
| CNTs/ZIF-301(18) | PSF | 6 | 2 | 25 | 18 | 44 | - | [104] |
| CNTs/ZIF-301(24) | PSF | 4 | 2 | 25 | 17 | 48 | - | [104] |
| CNTs/ZIF-301(30) | PSF | 2 | 2 | 25 | 16 | 34 | - | [104] |
| CNTs–GO | TFN | Ratio CNT and GO 1:1 | 4 | 70 | 66.3 | 47.1 | - | [100] |
| MWCNT–COOH | PEBA | 0.75 | 10 | 25 | 132.30 | 85.32 | 24.18 | [98] |
| MWCNT–NCO | PEBA | 0.3 | 10 | 25 | 148.86 | 104.92 | 28.95 | [98] |
| MWCNT–NH2 | PEBA | 0.5 | 10 | 25 | 139.53 | 95.62 | 26.28 | [98] |
| CNT/SiO2 (NOHM) | Pebax-1657 | 10 | 20 | 66.5 | 148.3 | 66.5 | - | [99] |
| MWCNTs | BTDA–TDI/MDI (P84) co-polyimide | 2 | 1 | 25 | 190.5 | 1.9 | - | [87] |
| MWCNTs ZIF-8–8 | Pebax1657 | 8 | 5 | 35 | 186.3 | 61.3 | - | [101] |
| MWCNT–NCO | polyurethane (PU) | 0.3 | 10 | 30 | 61.36 | 119.51 | 40.87 | [97] |
| f-MWCNTs | bis(phenyl) fluorene-based PIMs (Cardo-PIM-1) | 7.5 | 1 | 25 | 2.9 × 104 | 24.2 | - | [102] |
| AP-SWNTs | 6FDA-TP polyimide | 2 | 16.4 | 35 | 81 | 22 | 36 | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, M.J.; Vences, L.J.; Moreno, H.; Pacheco, J.O.; Valdivia, R.; Hernández, C. Review: Mixed-Matrix Membranes with CNT for CO2 Separation Processes. Membranes 2021, 11, 457. https://doi.org/10.3390/membranes11060457
Pacheco MJ, Vences LJ, Moreno H, Pacheco JO, Valdivia R, Hernández C. Review: Mixed-Matrix Membranes with CNT for CO2 Separation Processes. Membranes. 2021; 11(6):457. https://doi.org/10.3390/membranes11060457
Chicago/Turabian StylePacheco, Marquidia J., Luis J. Vences, Hilda Moreno, Joel O. Pacheco, Ricardo Valdivia, and Celso Hernández. 2021. "Review: Mixed-Matrix Membranes with CNT for CO2 Separation Processes" Membranes 11, no. 6: 457. https://doi.org/10.3390/membranes11060457
APA StylePacheco, M. J., Vences, L. J., Moreno, H., Pacheco, J. O., Valdivia, R., & Hernández, C. (2021). Review: Mixed-Matrix Membranes with CNT for CO2 Separation Processes. Membranes, 11(6), 457. https://doi.org/10.3390/membranes11060457