Lanolin-Based Synthetic Membranes for Transdermal Permeation and Penetration Drug Delivery Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pig Skin Membrane
2.3. Artificial Lanolin Membrane Preparation
2.4. Water Permeability by Trans-Epidermal Water Loss (TEWL)
2.5. Finite-Dose Penetration Test using Vertical Franz Diffusion Cells
2.6. Infinite-Dose Kinetic Permeation Assay using Vertical Franz Diffusion Cells
2.7. High-Pressure Liquid Chromatography-Diode Array Detector (HPLC-DAD) Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Lanolin-Based Membranes
3.2. Physico-Chemical Properties of the Active Compounds
3.3. Transdermal Drug Delivery Assays
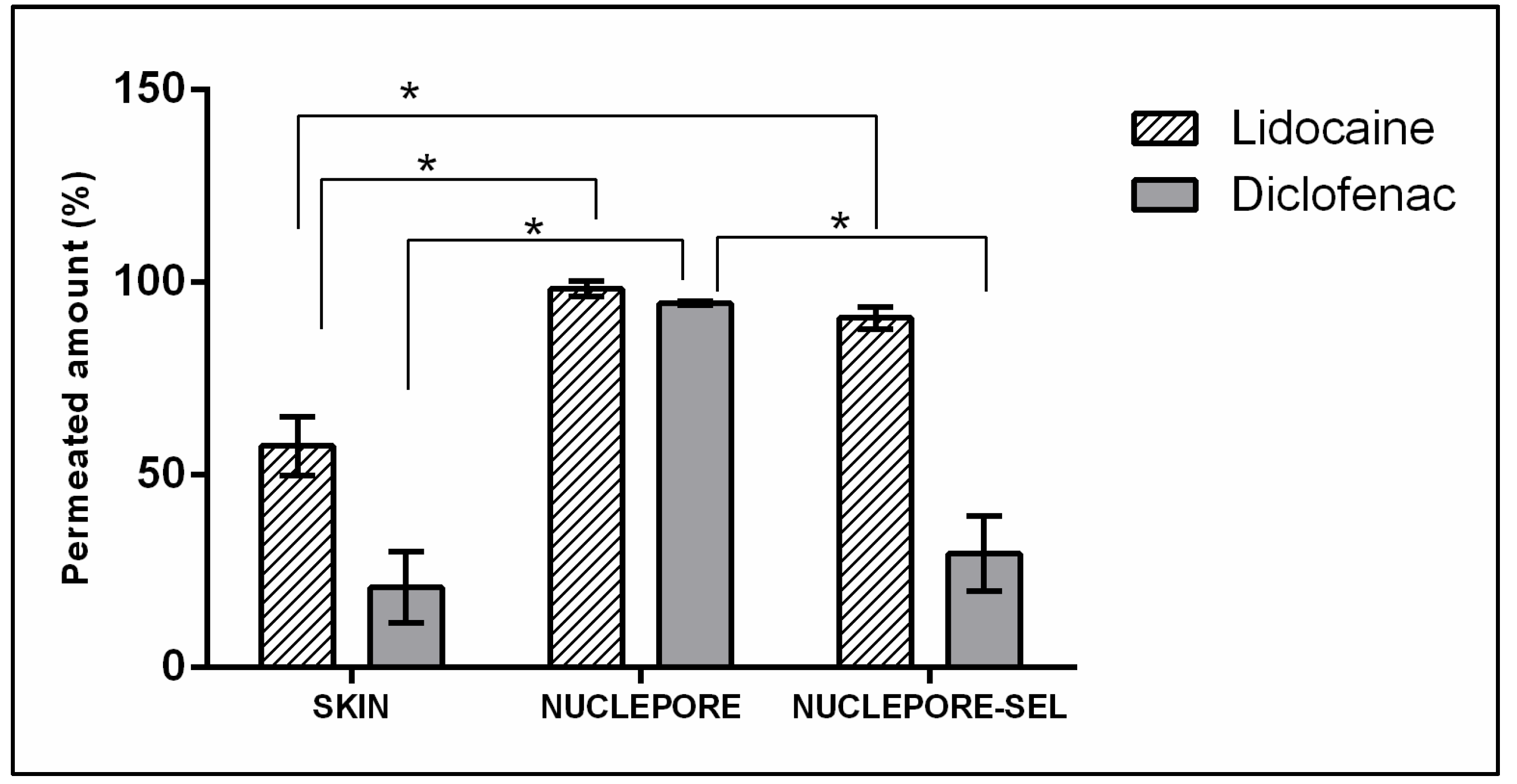
3.3.1. Finite Dose Drug Delivery Assay
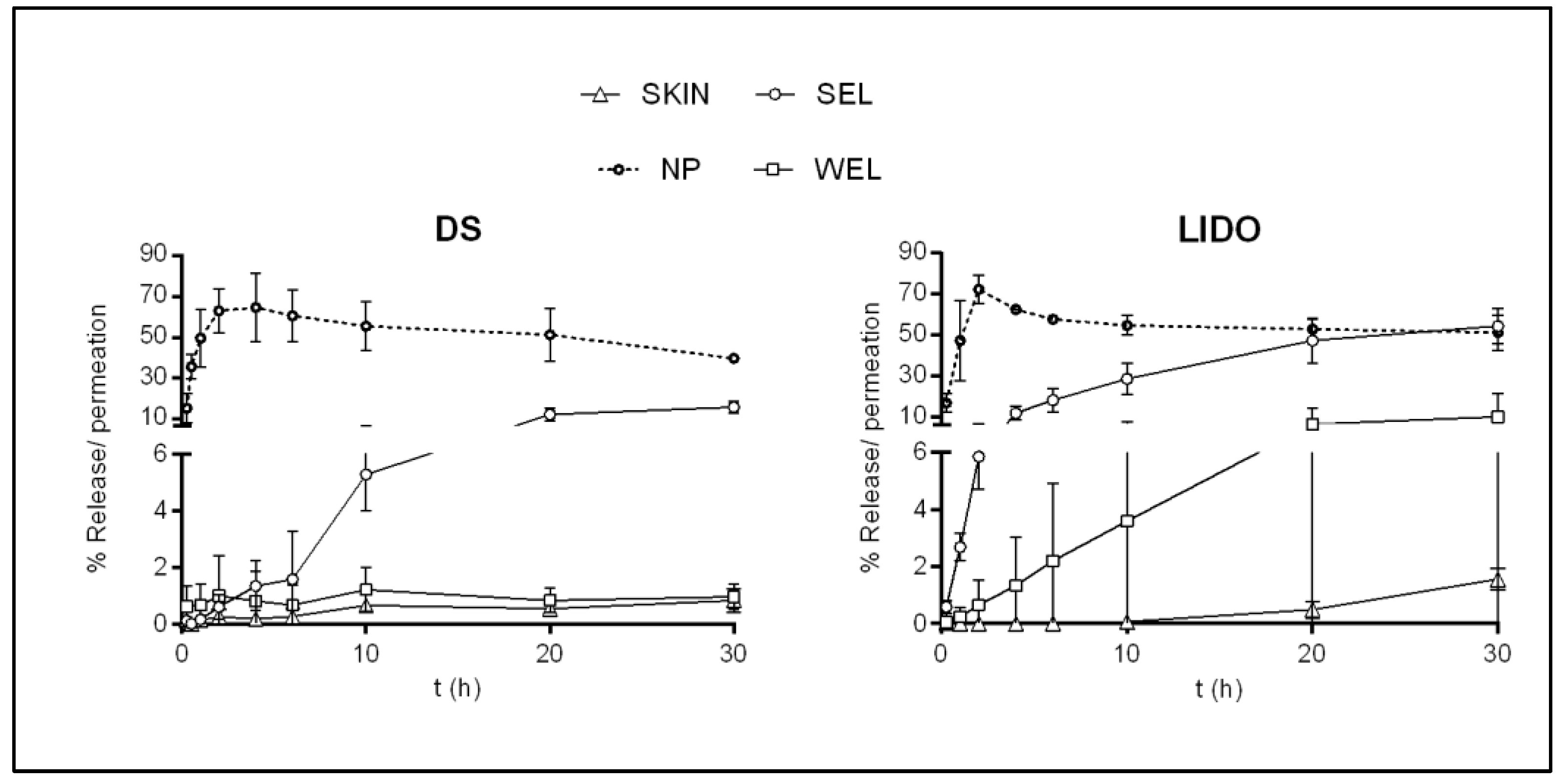
3.3.2. Infinite Dose Drug Delivery Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartosova, L.; Bajgar, J. Transdermal drug delivery in vitro using diffusion cells. Curr. Med. Chem. 2012, 19, 4671–4677. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Honeywell-Nguyen, P.L.; Gooris, G.S.; Ponec, M. Structure of the skin barrier and its modulation by vesicular formulations. Prog. Lipid Res. 2003, 42, 1–36. [Google Scholar] [CrossRef]
- Flynn, G.L. Percutaneous Absorption: Mechanisms—Methodology—Drug Delivery; Bronough, R., Maibach, H.I., Eds.; Marcel Dekker: New York, NY, USA, 1985; pp. 17–42. [Google Scholar]
- Madison, K.C.; Swartzendruber, D.C.; Wertz, P.W.; Downing, D.T. Presence of Intact Intercellular Lipid Lamellae in the Upper Layers of the Stratum Corneum. J. Investig. Dermatol. 1987, 88, 714–718. [Google Scholar] [CrossRef]
- Swartzendruber, D.C.; Wertz, P.W.; Kitko, D.J.; Madison, K.C.; Downing, D.T. Molecular models of the Intercellular Lipid Lamellae in Mammalian Stratum Corneum. J. Investig. Dermatol. 1989, 92, 251–257. [Google Scholar] [CrossRef]
- Kaur, L.; Singh, K.; Paul, S.; Singh, S.; Singh, S.; Jain, S.K. A mechanistic study to determine the structural similarities between artificial membrane Strat-MTM and biological membranes and its application to carry out skin permeation study of Amphotericin B nanoformulations. AAPS Pharm. Sci. Tech. 2018, 19, 1606–1624. [Google Scholar] [CrossRef]
- Kessner, D.; Ruettinger, A.; Kiselev, M.A.; Wartewig, S.; Neubert, R.H.H. Properties of ceramides and their impact on the stratum corneum structure: A review—Part 2: Stratum corneum lipid model systems. Skin Pharmacol. Physiol. 2008, 21, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Najib, O.N.; Martin, G.P.; Kirton, S.B.; Botha, M.J.; Sallam, A.S.; Murnane, D. The Influence of Oily Composition and Vehicle-Membrane Interactions on the Diffusion of Model Permeants across barrier Membranes. Membranes 2021, 11, 57. [Google Scholar] [CrossRef]
- Flynn, T.C.; Petros, J.; Clark, R.E.; Viehman, G.E. Dry skin and moisturizers. Clin. Dermatol. 2001, 19, 387–392. [Google Scholar] [CrossRef]
- White, S.H.; Mirejovsky, D.; King, G.I. Structure of Lamellar Lipid Domains and Corneocyte Envelopes of Murine Stratum Corneum. An X-ray Diffraction Study. Biochemistry 1988, 27, 3725–3732. [Google Scholar] [CrossRef]
- Wooldryscouring (WDS)—Eco-Efficient Dry Wool Scouring with Total by-Products Recovery. Available online: http://ec.europa.eu/environment/life/project/Projects/index.cfm?fuseaction=search.dspPage&n_proj_id=4254#RM (accessed on 15 February 2019).
- Barba, C.; Carrer, V.; Marti, M.; Iglesias, J.; Iglesias, J.; Coderch, L. Solvent-extracted wool wax: Thermotropic properties and skin efficacy. Ski. Pharmacol. Press. 2018, 31, 198–205. [Google Scholar]
- Dick, I.P.; Scott, R.C. Pig Ear Skin as an In-vitro Model for Human Skin Permeability. J. Pharm. Pharmacol. 1992, 44, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Barbero, A.M.; Frasch, H.F. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol. Vitr. 2009, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Babita, K.; Kumar, V.; Rana, V.; Jain, S.; Tiwary, A.K. Thermotropic and spectroscopic behavior of skin: Relationship with percutaneous permeation enhancement. Curr. Drug Deliv. 2006, 3, 95–113. [Google Scholar] [CrossRef]
- Arseneault, M.; Lafleur, M. Cholesterol sulfate and Ca2+ modulate the mixing properties of lipids in stratum corneum model mixtures. Biophys. J. 2007, 92, 99–114. [Google Scholar] [CrossRef]
- Wagner, H.; Kostka, K.H.; Lehr, C.M.; Schaefer, U.F. Interrelation of permeation and penetration parameters obtained from in vitro experiments with human skin and skin equivalents. J. Control. Release 2001, 75, 283–295. [Google Scholar] [CrossRef]
- Selzer, D.; Abdel-Mottaleb, M.M.A.; Hahn, T.; Schaefer, U.F.; Neumann, D. Finite and infinite dosing: Difficulties in measurements, evaluations and predictions. Adv. Drug Deliv. Rev. 2013, 65, 278–294. [Google Scholar] [CrossRef]
- Moss, G.P.; Dearden, J.C.; Patel, H.; Cronin, M.T.D. Quantitative structure-permeability relationships (QSPRs) for percutaneous absorption. Toxicol. Vitr. 2002, 16, 299–317. [Google Scholar] [CrossRef]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef]
- Carrer, V.; Guzmán, B.; Martí, M.; Alonso, C.; Coderch, L. Lanolin-based synthetic membranes as percutaneous absorption models for transdermal drug delivery. Pharmaceutics 2018, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Collini, I.; Carrer, V.; Barba, C.; Marti, M.; Coderch, L. Permeation kinetics of active drugs through lanolin-based artificial membranes. Colloids Surf. B Biointerfaces 2020, 192, 111024. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals; National Research Council. Guide for the Care and Use of Laboratory Animals; The National Academic Press: Washington, DC, USA, 2010; pp. 115–118. Available online: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf (accessed on 15 February 2019).
- Pullmannová, P.; Pavlíková, L.; Kováčik, A.; Sochorová, M.; Školová, B.; Slepička, P.; Maixner, J.; Zbytovská, J.; Vávrová, K. Permeability and microstructure of model stratum corneum lipid membranes containing ceramides with long (C16) and very long (C24) acyl chains. Biophys. Chem. 2017, 224, 20–31. [Google Scholar] [CrossRef] [PubMed]
- OECD. Skin Absorption: In Vitro Method; Test, no. 428.; OECD: Paris, France, 2004; pp. 1–8. [Google Scholar]
- Carrer, V.; Alonso, C.; Pont, M.; Zanuy, M.; Córdoba, M.; Espinosa, S.; Barba, B.; Oliver, M.A.; Martí, M.; Coderch, L. Effect of propylene glycol on the skin penetration of drugs. Arch. Dermatol. Res. 2020, 312, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Ich. ICH Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology. Int. Conf. Harmon. 1994, 17. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf (accessed on 21 February 2019).
- Thakker, K.D.; Chern, W.H. Development and validation of in vitro release tests for semisolid dosage forms—case study. Dissolution Technol. 2003, 10, 10–15. [Google Scholar] [CrossRef]
- Mallandrich, M.; Fernández-Campos, F.; Clares, B.; Halbaut, L.; Alonso, C.; Coderch, L.; Garduño-Ramírez, M.L.; Andrade, B.; Del Pozo, A.; Lane, M.E.; et al. Developing Transdermal Applications of Ketorolac Tromethamine Entrapped in Stimuli Sensitive Block Copolymer Hydrogels. Pharm. Res. 2017, 34, 1728–1740. [Google Scholar] [CrossRef]
- Bhagavan, N.V. Water, Acids, Bases, and Buffers. In Medical Biochemistry, 4th ed.; Harcourt Academic Press: Orlando, FL, USA, 2002; pp. 1–16. [Google Scholar]
- Soldatov, V.S. Physical meaning of parameters of generalized Henderson-Hasselbach equation. Dokl. Akad. Nauk. 1994, 336, 782–785. [Google Scholar]
- Parry, G.E.; Bunge, A.L.; Silcox, G.D.; Pershing, L.K.; Pershing, D.W. Percutaneous Absorption of Benzoic Acid Across Human Skin. I. In Vitro Experiments and Mathematical Modeling. Pharm. Res. 1990, 7, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Michaels, A.S.; Chandrasekaran, S.K.; Shaw, J.E. Drug Permeation Through Human Skin: Theory and in Vitro Experimental Measurement. AIChE J. 1975, 21, 985–996. [Google Scholar] [CrossRef]
- EMA. The European Agency for the Evaluation of Medicinal Products. Available online: https://www.ema.europa.eu/documents/scientific-guideline/note-guidance-quality-modified-releaseproducts-oral-dosage-forms-b-transdermal-dosage-forms-section_en.pdf (accessed on 15 February 2019).
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Zsikó, S.; Cutcher, K.; Kovács, A.; Budai-Szücs, M.; Gácsi, A.; Baki, G.; Csányi, E.; Berkó, S. Nanostructured Lipid Carrier Gel for the Dermal Application of Lidocaine: Comparison of Skin Penetration Testing Methods. Pharmaceutics 2019, 11, 310. [Google Scholar] [CrossRef]
- Zhang, Y.; Lane, M.E.; Hadgraft, J.; Heinrich, M.; Chen, T.; Lian, G.; Sinko, B. A comparison of the in vitro permeation of niacinamide in mammalian skin and in the Parallel Artificial Membrane Permeation Assay (PAMPA) model. Int. J. Pharm. 2019, 556, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Carrer, V.; Espinosa, S.; Zanuy, M.; Córdoba, M.; Vidal, B.; Domínguez, M.; Godessart, N.; Coderch, L.; Pont, M. 656 Prediction of the skin permeability of topical drugs using in silico and in vitro models. Eur. J. Pharm. Sci. 2019, 136, 104945. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Wendtner, M.H.; Korting, H.C. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Briggaman, R.A.; Wheeler, C.E. The Epidermal-Dermal Junction. J. Investig. Dermatol. 1975, 65, 71–84. [Google Scholar] [CrossRef]
| Parameters | Diclofenac Sodium | Lidocaine |
|---|---|---|
| Extractor solvent | CH3CN (+CF3COOH 0.5%) | CH3OH |
| Column | LiChrocart® 250-4 Lichrosphere® 100RP-18, 5 µm | LiChrocart® 125-4 Lichrosphere® 100RP-18, 5 µm |
| Wavelength (nm) | 254 | 205 |
| Injection volume (µL) | 20 | 20 |
| Mobil phase (flux) | 66% CH3OH 34% H3PO4 0.7% (1 mL/min) | 70% NaH2PO4, 0.05 M pH 7.4 30% CH3CN (1 mL/min) |
| Linear regression Equation (R2) | A = 80,050(DS) − 2484 (0.9997) | A = 414,046(LIDO) − 68,532 (0.9999) |
| LoD/LoQ (µg/mL) | 0.07/0.22 | 0.12/0.35 |
| Precision (%CV) Intra day | 2.05 ± 0.71 | 5.21 ± 2.86 |
| Inter day | 6.02 ± 1.98 | 5.30 ± 3.03 |
| Membranes | Lanolin Amount (mg) | pH | TEWL (g/hm2) |
|---|---|---|---|
| Skin | - | 5.7 ± 0.3 | 5.9 ± 2.3 |
| NP | - | 6.5 ± 0.2 | 52.5 ± 9.0 * |
| NP-SEL 5% | 14.3 ± 1.8 | 6.6 ± 0.3 | 14.9 ± 3.6 |
| NP-WEL 5% | 13.3 ± 3.8 | 5.3 ± 0.2 | 15.2 ± 1.2 |
| Compound | pKa | LogD at pH 5.5 | LogD at pH 7.4 | MW | LogKp (Potts & Guy) |
|---|---|---|---|---|---|
| Diclofenac Sodium | 4.15 [23] | 2.75 | 1.10 | 318.1 | −7.42 |
| Lidocaine | 7.70 [24] | 0.61 | 2.33 | 234.3 | −5.99 |
| Membrane | API | Kinetics of Release | J(µg/cm2/t) a | Kp (cm/t) | C max (µg/mL) | AUC (a.u.) |
|---|---|---|---|---|---|---|
| SKIN | DS | Ord 0 | 3.94 | 0.0001 | 38.10 | 167.55 |
| LIDO | Ord 0 | 2.29 | 0.000125 | 49.95 | 109.30 | |
| NP | DS | Higuchi | 2307.12 * | 0.0642 * | 3056.54 * | 14,821.56 * |
| LIDO | Higuchi | 1360.27 * | 0.0843 * | 2021.17 * | 13,197.15 * | |
| SEL | DS | Ord 0 | 30.40 * | 0.00121 * | 615.29 * | 1977.11 * |
| LIDO | Higuchi | 343.05 * | 0.0213 * | 1540.43 * | 7341.61 * | |
| WEL | DS | Ord 0 | 6.18 | 0.000208 | 48.43 | 317.06 |
| LIDO | Ord 0 | 4.85 | 0.000269 | 144.78 | 509.19 |
| Compound | pKa | Log Kp | Log Kp | ||
|---|---|---|---|---|---|
| In Silico | Permeation Infinite Dosage | ||||
| (Potts & Guy) | Skin | SEL | WEL | ||
| Diclofenac Sodium | 4.15 | −7.42 | −3.98 | −2.92 | −3.68 |
| Lidocaine | 7.70 | −5.99 | −3.90 | −1.67 | −3.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso, C.; Collini, I.; Martí, M.; Barba, C.; Coderch, L. Lanolin-Based Synthetic Membranes for Transdermal Permeation and Penetration Drug Delivery Assays. Membranes 2021, 11, 444. https://doi.org/10.3390/membranes11060444
Alonso C, Collini I, Martí M, Barba C, Coderch L. Lanolin-Based Synthetic Membranes for Transdermal Permeation and Penetration Drug Delivery Assays. Membranes. 2021; 11(6):444. https://doi.org/10.3390/membranes11060444
Chicago/Turabian StyleAlonso, Cristina, Ilaria Collini, Meritxell Martí, Clara Barba, and Luisa Coderch. 2021. "Lanolin-Based Synthetic Membranes for Transdermal Permeation and Penetration Drug Delivery Assays" Membranes 11, no. 6: 444. https://doi.org/10.3390/membranes11060444
APA StyleAlonso, C., Collini, I., Martí, M., Barba, C., & Coderch, L. (2021). Lanolin-Based Synthetic Membranes for Transdermal Permeation and Penetration Drug Delivery Assays. Membranes, 11(6), 444. https://doi.org/10.3390/membranes11060444







