Multidrug Resistance Like Protein 1 Activity in Malpighian Tubules Regulates Lipid Homeostasis in Drosophila
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Fly Strains and Maintenance
2.2. Knockdown of MRP
2.3. Starvation Assay
2.4. Carbohydrate Assay
2.5. Triacylglyceride (TAG) Assay
2.6. Fly Proboscis and Activity Detector (FlyPAD)
2.7. ROS Detection
2.8. Paraquat Resistance
2.9. Quantitative Real-Time PCR
- Rp49-F: 5′-CACACCAAATCTTACAAAATGTGTGA-3′;
- Rp49-R: 5′-AATCCGGCCTTGCACATG-3′;
- Hr96-F: 5′-GATATGTTCCTCCAGGCCCTA-3′;
- Hr96-R: 5′-TGTGCGTGGCAAAGAAGACT-3′;
- Cnc-F: 5′-CTGCATCGTCATGTCTTCCAGT-3′;
- Cnc-R: 5′-AGCAAGTAGACGGAGCCAT-3′;
- Keap1-F: 5′-AGGCCAATGTGTTTATTGAGCG-3′;
- Keap1-R: 5′-GCAATCAACTGATATGCCGAAAG-3′;
- ss-F: 5′-GATATGTTCCTCCAGGCCCTA-3′;
- ss-R: 5′-TGTGCGTGGCAAAGAAGACT-3′;
- MRP-R: 5′-GAATCTGGGTCTGCTGGTAATC;
- MRP-R: 5′-AAACATCCAGGTCGTAGAGCG.
3. Data Analysis
4. Results
4.1. Successful Knockdown of MRP
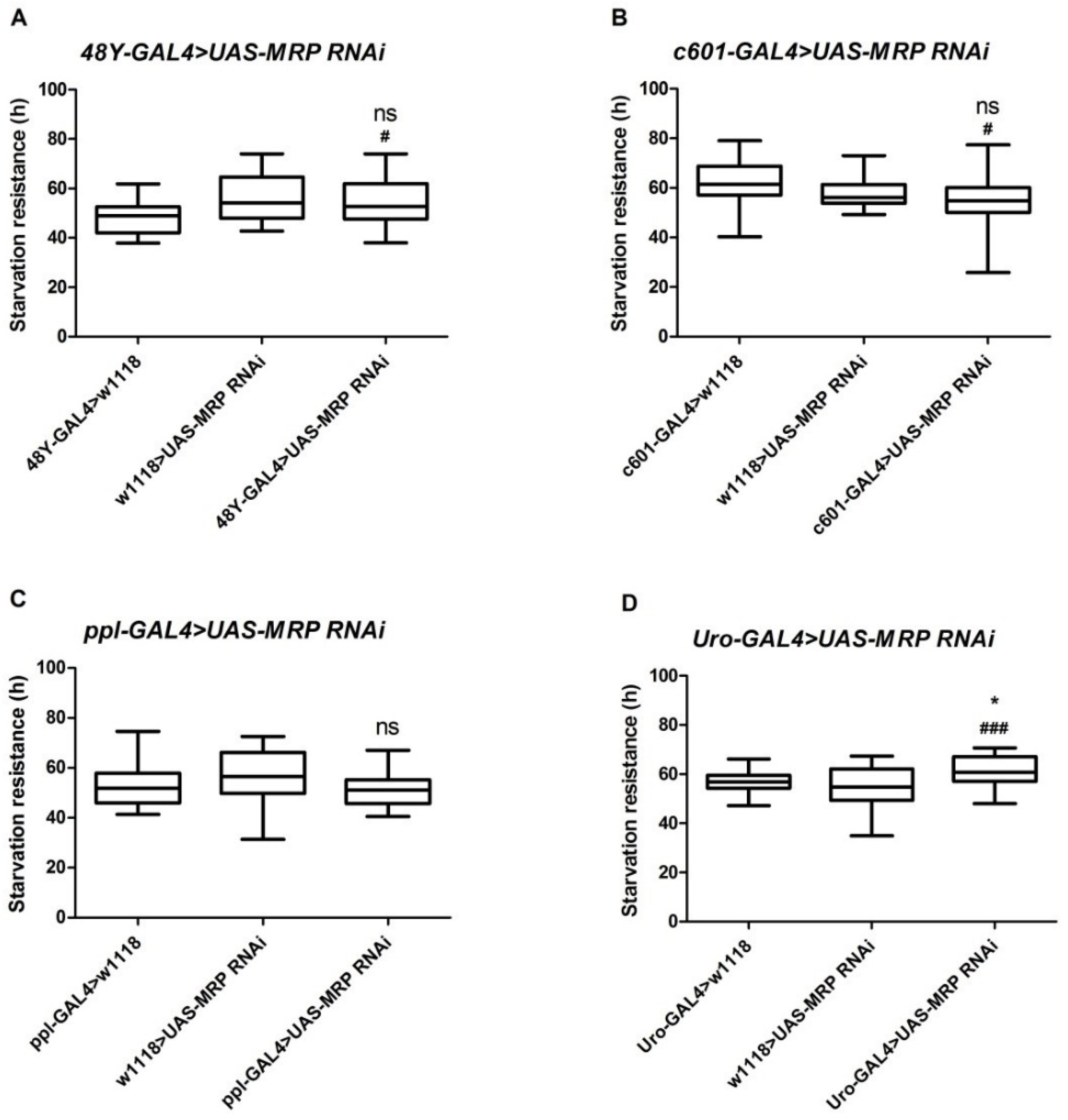
4.2. Loss of MRP in Malpighian Tubules Increases Starvation Resistance
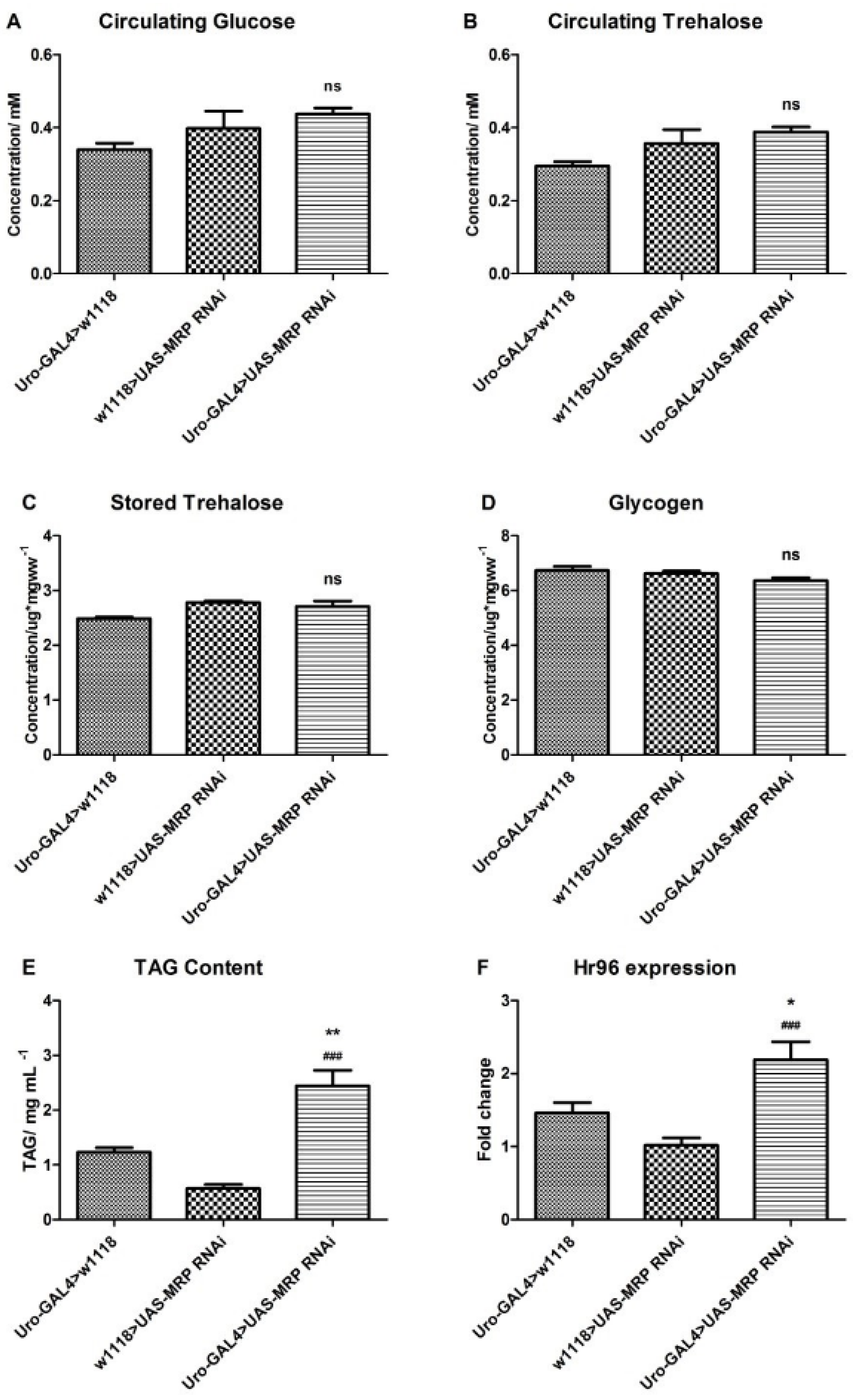
4.3. Loss of MRP in Malpighian Tubules Influences Triacylglyceride Levels
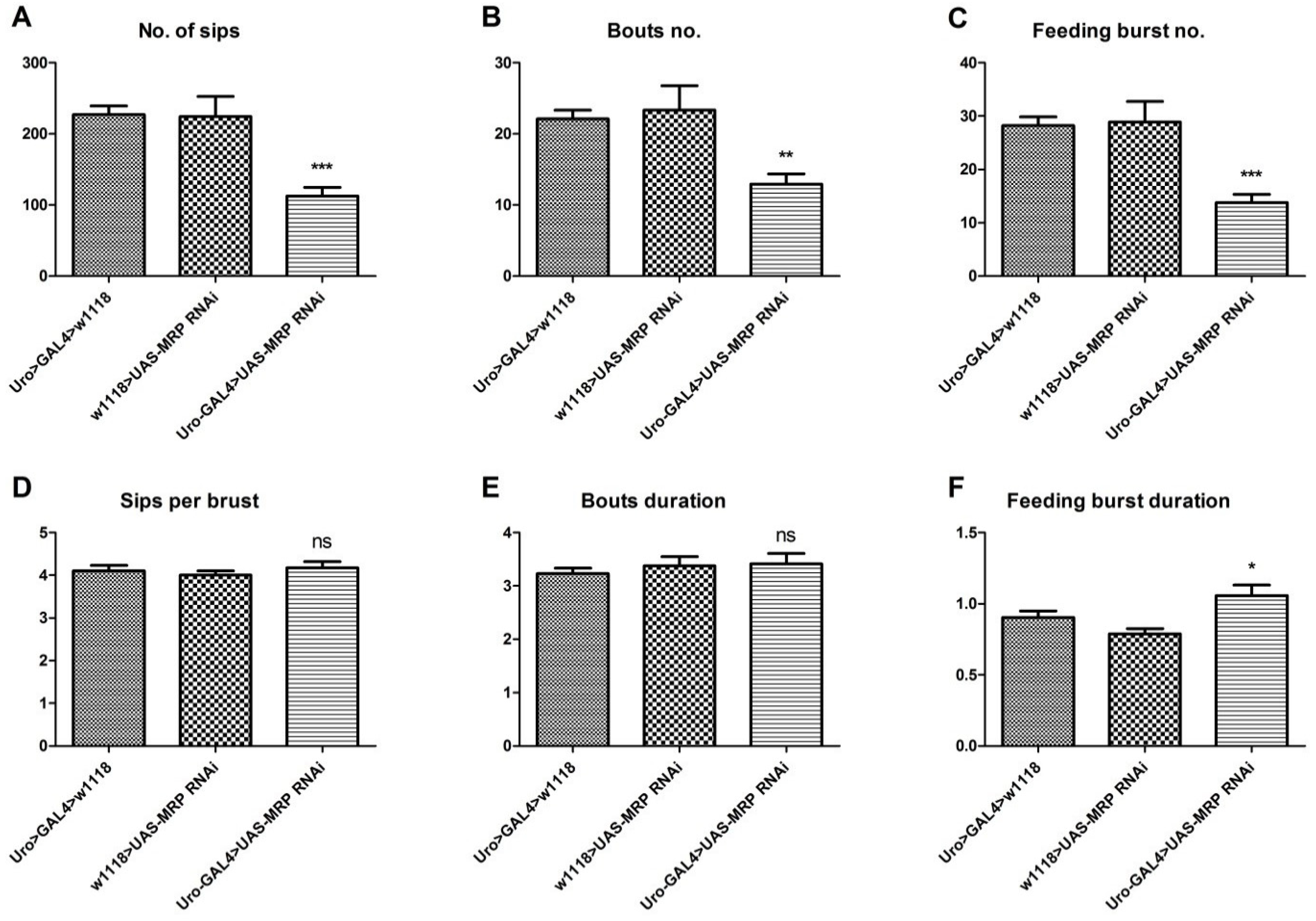
4.4. Loss of MRP in the Malpighian Tubules Influences Feeding Behavior
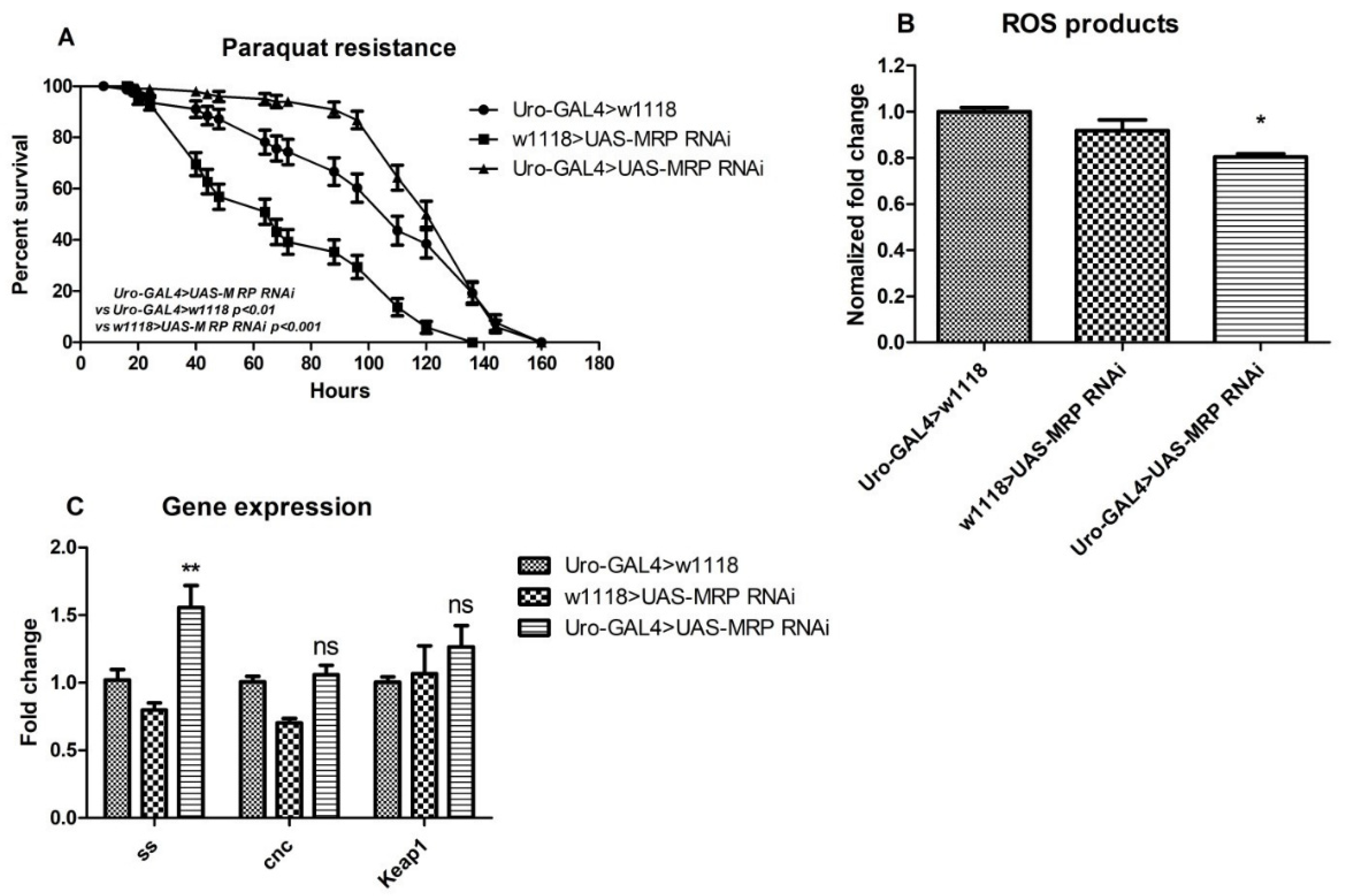
4.5. MRP Knockdown in Malpighian Tubules Confers Oxidative Resistance to Drosophila melanogaster
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, X.; Manautou, J.E. Regulation of hepatic ABCC transporters by xenobiotics and in disease states. Drug Metab. Rev. 2010, 42, 482–538. [Google Scholar] [CrossRef]
- Keppler, D. Multidrug resistance proteins (MRPs, ABCCs): Importance for pathophysiology and drug therapy. Handb. Exp. Pharmacol. 2011, 299–323. [Google Scholar] [CrossRef]
- Leslie, E.M.; Deeley, R.G.; Cole, S.P. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005, 204, 216–237. [Google Scholar] [CrossRef] [PubMed]
- Glavinas, H.; Krajcsi, P.; Cserepes, J.; Sarkadi, B. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr. Drug Deliv. 2004, 1, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Deeley, R.G.; Cole, S.P. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS Lett. 2006, 580, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Nixon, M.; Mackenzie, S.D.; Taylor, A.I.; Homer, N.Z.; Livingstone, D.E.; Mouras, R.; Morgan, R.A.; Mole, D.J.; Stimson, R.H.; Reynolds, R.M.; et al. ABCC1 confers tissue-specific sensitivity to cortisol versus corticosterone: A rationale for safer glucocorticoid replacement therapy. Sci. Transl. Med. 2016, 8, 352ra109. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kuo, M.T.; Furuta, K.; Suzuki, M. A new aspect on glutathione-associated biological function of MRP/GS-X pump and its gene expression. Cytotechnology 1998, 27, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Hipfner, D.R.; Deeley, R.G.; Cole, S.P. Structural, mechanistic and clinical aspects of MRP1. Biochim. Biophys. Acta 1999, 1461, 359–376. [Google Scholar] [CrossRef]
- Wijnholds, J.; Evers, R.; van Leusden, M.R.; Mol, C.A.; Zaman, G.J.; Mayer, U.; Beijnen, J.H.; van der Valk, M.; Krimpenfort, P.; Borst, P. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat. Med. 1997, 3, 1275–1279. [Google Scholar] [CrossRef]
- Neuser, J.; Fraccarollo, D.; Wick, M.; Bauersachs, J.; Widder, J.D. Multidrug resistance associated protein-1 (MRP1) deficiency attenuates endothelial dysfunction in diabetes. J. Diabetes Complicat. 2016, 30, 623–627. [Google Scholar] [CrossRef]
- Vander Borght, S.; Komuta, M.; Libbrecht, L.; Katoonizadeh, A.; Aerts, R.; Dymarkowski, S.; Verslype, C.; Nevens, F.; Roskams, T. Expression of multidrug resistance-associated protein 1 in hepatocellular carcinoma is associated with a more aggressive tumour phenotype and may reflect a progenitor cell origin. Liver Int. 2008, 28, 1370–1380. [Google Scholar] [CrossRef]
- Nowicki, M.T.; Aleksunes, L.M.; Sawant, S.P.; Dnyanmote, A.V.; Mehendale, H.M.; Manautou, J.E. Renal and hepatic transporter expression in type 2 diabetic rats. Drug Metab. Lett. 2008, 2, 11–17. [Google Scholar] [CrossRef]
- van de Water, F.M.; Masereeuw, R.; Russel, F.G. Function and regulation of multidrug resistance proteins (MRPs) in the renal elimination of organic anions. Drug Metab. Rev. 2005, 37, 443–471. [Google Scholar] [CrossRef]
- Hirrlinger, J.; Konig, J.; Keppler, D.; Lindenau, J.; Schulz, J.B.; Dringen, R. The multidrug resistance protein MRP1 mediates the release of glutathione disulfide from rat astrocytes during oxidative stress. J. Neurochem. 2001, 76, 627–636. [Google Scholar] [CrossRef]
- Cole, S.P. Multidrug resistance protein 1 (MRP1, ABCC1), a “multitasking” ATP-binding cassette (ABC) transporter. J. Biol. Chem. 2014, 289, 30880–30888. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Wang, Y.J.; Gupta, P.; Chen, Z.S. Multidrug Resistance Proteins (MRPs) and Cancer Therapy. AAPS J. 2015, 17, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Ditzel, E.J.; Li, H.; Foy, C.E.; Perrera, A.B.; Parker, P.; Renquist, B.J.; Cherrington, N.J.; Camenisch, T.D. Altered Hepatic Transport by Fetal Arsenite Exposure in Diet-Induced Fatty Liver Disease. J. Biochem. Mol. Toxicol. 2016, 30, 321–330. [Google Scholar] [CrossRef]
- Ghose, R.; Omoluabi, O.; Gandhi, A.; Shah, P.; Strohacker, K.; Carpenter, K.C.; McFarlin, B.; Guo, T. Role of high-fat diet in regulation of gene expression of drug metabolizing enzymes and transporters. Life Sci. 2011, 89, 57–64. [Google Scholar] [CrossRef]
- Quezada, C.; Alarcon, S.; Carcamo, J.G.; Yanez, A.; Casanello, P.; Sobrevia, L.; San Martin, R. Increased expression of the multidrug resistance-associated protein 1 (MRP1) in kidney glomeruli of streptozotocin-induced diabetic rats. Biol. Chem. 2011, 392, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.J.; Wiemerslage, L.; Gohel, P.; Kheder, S.; Kothegala, L.V.; Schioth, H.B. Dibutyl Phthalate Exposure Disrupts Evolutionarily Conserved Insulin and Glucagon-Like Signaling in Drosophila Males. Endocrinology 2016, 157, 2309–2321. [Google Scholar] [CrossRef] [PubMed]
- Denholm, B.; Sudarsan, V.; Pasalodos-Sanchez, S.; Artero, R.; Lawrence, P.; Maddrell, S.; Baylies, M.; Skaer, H. Dual origin of the renal tubules in Drosophila: Mesodermal cells integrate and polarize to establish secretory function. Curr. Biol. 2003, 13, 1052–1057. [Google Scholar] [CrossRef]
- Robinson, S.W.; Herzyk, P.; Dow, J.A.; Leader, D.P. FlyAtlas: Database of gene expression in the tissues of Drosophila melanogaster. Nucleic Acids Res. 2013, 41, D744–D750. [Google Scholar] [CrossRef]
- Pfeiffenberger, C.; Lear, B.C.; Keegan, K.P.; Allada, R. Locomotor activity level monitoring using the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5518. [Google Scholar] [CrossRef] [PubMed]
- Itskov, P.M.; Moreira, J.M.; Vinnik, E.; Lopes, G.; Safarik, S.; Dickinson, M.H.; Ribeiro, C. Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat. Commun. 2014, 5, 4560. [Google Scholar] [CrossRef] [PubMed]
- Chintapalli, V.R.; Wang, J.; Dow, J.A. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 2007, 39, 715–720. [Google Scholar] [CrossRef]
- Sieber, M.H.; Thummel, C.S. The DHR96 nuclear receptor controls triacylglycerol homeostasis in Drosophila. Cell Metab. 2009, 10, 481–490. [Google Scholar] [CrossRef]
- Wang, D.Q. New concepts of mechanisms of intestinal cholesterol absorption. Ann. Hepatol. 2003, 2, 113–121. [Google Scholar] [CrossRef]
- Whyte-Allman, S.K.; Hoque, M.T.; Jenabian, M.A.; Routy, J.P.; Bendayan, R. Xenobiotic Nuclear Receptors Pregnane X Receptor and Constitutive Androstane Receptor Regulate Antiretroviral Drug Efflux Transporters at the Blood-Testis Barrier. J. Pharmacol. Exp. Ther. 2017, 363, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Lucia, M.B.; Savarino, A.; Straface, E.; Golotta, C.; Rastrelli, E.; Matarrese, P.; Rutella, S.; Malorni, W.; Cauda, R. Role of lymphocyte multidrug resistance protein 1 in HIV infection: Expression, function, and consequences of inhibition. JAIDS J. Acquir. Immune Defic. Syndr. 2005, 40, 257–266. [Google Scholar] [CrossRef]
- Bonilla, E.; Medina-Leendertz, S.; Villalobos, V.; Molero, L.; Bohorquez, A. Paraquat-induced oxidative stress in drosophila melanogaster: Effects of melatonin, glutathione, serotonin, minocycline, lipoic acid and ascorbic acid. Neurochem. Res. 2006, 31, 1425–1432. [Google Scholar] [CrossRef]
- Huang, H.; Lu-Bo, Y.; Haddad, G.G. A Drosophila ABC transporter regulates lifespan. PLoS Genet. 2014, 10, e1004844. [Google Scholar] [CrossRef]
- Kuzin, B.A.; Nikitina, E.A.; Cherezov, R.O.; Vorontsova, J.E.; Slezinger, M.S.; Zatsepina, O.G.; Simonova, O.B.; Enikolopov, G.N.; Savvateeva-Popova, E.V. Combination of hypomorphic mutations of the Drosophila homologues of aryl hydrocarbon receptor and nucleosome assembly protein family genes disrupts morphogenesis, memory and detoxification. PLoS ONE 2014, 9, e94975. [Google Scholar] [CrossRef]
- Pitoniak, A.; Bohmann, D. Mechanisms and functions of Nrf2 signaling in Drosophila. Free Radic Biol. Med. 2015, 88, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Spiers, J.G.; Breda, C.; Robinson, S.; Giorgini, F.; Steinert, J.R. Drosophila Nrf2/Keap1 Mediated Redox Signaling Supports Synaptic Function and Longevity and Impacts on Circadian Activity. Front. Mol. Neurosci. 2019, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Pohl, A.; Devaux, P.F.; Herrmann, A. Function of prokaryotic and eukaryotic ABC proteins in lipid transport. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2005, 1733, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Soderberg, J.A.; Birse, R.T.; Nassel, D.R. Insulin production and signaling in renal tubules of Drosophila is under control of tachykinin-related peptide and regulates stress resistance. PLoS ONE 2011, 6, e19866. [Google Scholar] [CrossRef]
- Sun, J.; Usune, S.; Zhao, Y.; Migita, K.; Katsuragi, T. Multidrug resistance protein transporter and Ins(1,4,5)P(3)-sensitive Ca(2)+-signaling involved in adenosine triphosphate export via Gq protein-coupled NK(2)-receptor stimulation with neurokinin A. J. Pharmacol. Sci. 2010, 114, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Raggers, R.J.; van Helvoort, A.; Evers, R.; van Meer, G. The human multidrug resistance protein MRP1 translocates sphingolipid analogs across the plasma membrane. J. Cell Sci. 1999, 112 Pt 3, 415–422. [Google Scholar] [CrossRef]
- Kamp, D.; Haest, C.W. Evidence for a role of the multidrug resistance protein (MRP) in the outward translocation of NBD-phospholipids in the erythrocyte membrane. Biochim. Biophys. Acta Biomembr. 1998, 1372, 91–101. [Google Scholar] [CrossRef][Green Version]
- Cheng, Z.; Guo, S.; Copps, K.; Dong, X.; Kollipara, R.; Rodgers, J.T.; Depinho, R.A.; Puigserver, P.; White, M.F. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat. Med. 2009, 15, 1307–1311. [Google Scholar] [CrossRef]
- Sengupta, N.; Reardon, D.C.; Gerard, P.D.; Baldwin, W.S. Exchange of polar lipids from adults to neonates in Daphnia magna: Perturbations in sphingomyelin allocation by dietary lipids and environmental toxicants. PLoS ONE 2017, 12, e0178131. [Google Scholar] [CrossRef] [PubMed]
- Elcombe, C.R.; Elcombe, B.M.; Foster, J.R.; Chang, S.C.; Ehresman, D.J.; Butenhoff, J.L. Hepatocellular hypertrophy and cell proliferation in Sprague-Dawley rats from dietary exposure to potassium perfluorooctanesulfonate results from increased expression of xenosensor nuclear receptors PPARalpha and CAR/PXR. Toxicology 2012, 293, 16–29. [Google Scholar] [CrossRef]
- Dong, B.; Saha, P.K.; Huang, W.; Chen, W.; Abu-Elheiga, L.A.; Wakil, S.J.; Stevens, R.D.; Ilkayeva, O.; Newgard, C.B.; Chan, L.; et al. Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proc. Natl. Acad. Sci. USA 2009, 106, 18831–18836. [Google Scholar] [CrossRef] [PubMed]
- Kast, H.R.; Goodwin, B.; Tarr, P.T.; Jones, S.A.; Anisfeld, A.M.; Stoltz, C.M.; Tontonoz, P.; Kliewer, S.; Willson, T.M.; Edwards, P.A. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J. Biol. Chem. 2002, 277, 2908–2915. [Google Scholar] [CrossRef]
- Takahashi, K.; Shibata, T.; Oba, T.; Ishikawa, T.; Yoshikawa, M.; Tatsunami, R.; Takahashi, K.; Tampo, Y. Multidrug-resistance-associated protein plays a protective role in menadione-induced oxidative stress in endothelial cells. Life Sci. 2009, 84, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tatsunami, R.; Sato, K.; Tampo, Y. Multidrug resistance associated protein 1 together with glutathione plays a protective role against 4-hydroxy-2-nonenal-induced oxidative stress in bovine aortic endothelial cells. Biol. Pharm. Bull. 2012, 35, 1269–1274. [Google Scholar] [CrossRef]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Bohmann, D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 2008, 14, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Moore, J.N.; Newsted, J.L.; Hecker, M.; Zwiernik, M.J.; Jones, P.D.; Bursian, S.J.; Giesy, J.P. Sequencing and characterization of mixed function monooxygenase genes CYP1A1 and CYP1A2 of Mink (Mustela vison) to facilitate study of dioxin-like compounds. Toxicol. Appl. Pharmacol. 2009, 234, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Aleksunes, L.M.; Klaassen, C.D. Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARalpha-, and Nrf2-null mice. Drug Metab. Dispos. 2012, 40, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.S.; Klaassen, C.D. Importance of hepatic induction of constitutive androstane receptor and other transcription factors that regulate xenobiotic metabolism and transport. Drug Metab. Dispos. 2007, 35, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Maher, J.M.; Cheng, X.; Slitt, A.L.; Dieter, M.Z.; Klaassen, C.D. Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab. Dispos. 2005, 33, 956–962. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Cao, H.; Kimari, M.; Maronitis, G.; Williams, M.J.; Schiöth, H.B. Multidrug Resistance Like Protein 1 Activity in Malpighian Tubules Regulates Lipid Homeostasis in Drosophila. Membranes 2021, 11, 432. https://doi.org/10.3390/membranes11060432
Liu W, Cao H, Kimari M, Maronitis G, Williams MJ, Schiöth HB. Multidrug Resistance Like Protein 1 Activity in Malpighian Tubules Regulates Lipid Homeostasis in Drosophila. Membranes. 2021; 11(6):432. https://doi.org/10.3390/membranes11060432
Chicago/Turabian StyleLiu, Wen, Hao Cao, Moses Kimari, Georgios Maronitis, Michael J. Williams, and Helgi B Schiöth. 2021. "Multidrug Resistance Like Protein 1 Activity in Malpighian Tubules Regulates Lipid Homeostasis in Drosophila" Membranes 11, no. 6: 432. https://doi.org/10.3390/membranes11060432
APA StyleLiu, W., Cao, H., Kimari, M., Maronitis, G., Williams, M. J., & Schiöth, H. B. (2021). Multidrug Resistance Like Protein 1 Activity in Malpighian Tubules Regulates Lipid Homeostasis in Drosophila. Membranes, 11(6), 432. https://doi.org/10.3390/membranes11060432




