Study of Tissue-Specific Reactive Oxygen Species Formation by Cell Membrane Microarrays for the Characterization of Bioactive Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Samples and Reagents
2.1.1. Drugs and Reagents
2.1.2. Samples
2.2. Cell Membrane Microarray Fabrication
2.3. Determination of Superoxide Formation Mediated by NADH-Ubiquinone Oxidoreductase in CMMA
2.4. Determination of Superoxide Formation Mediated by Antimalarials in CMMA
2.5. Data Analysis and Normalization
2.6. Plant Material and Cultivation
2.7. Essential Oil Extraction
2.8. Gas Chromatography–Mass Spectrometry Analysis
2.9. Ferriprotoporphyrin (FP) IX Biocrystallization Inhibition Test (FBIT)
3. Results
3.1. Protocol Optimization to Detect Superoxide Formation in Cell Membrane Microarrays
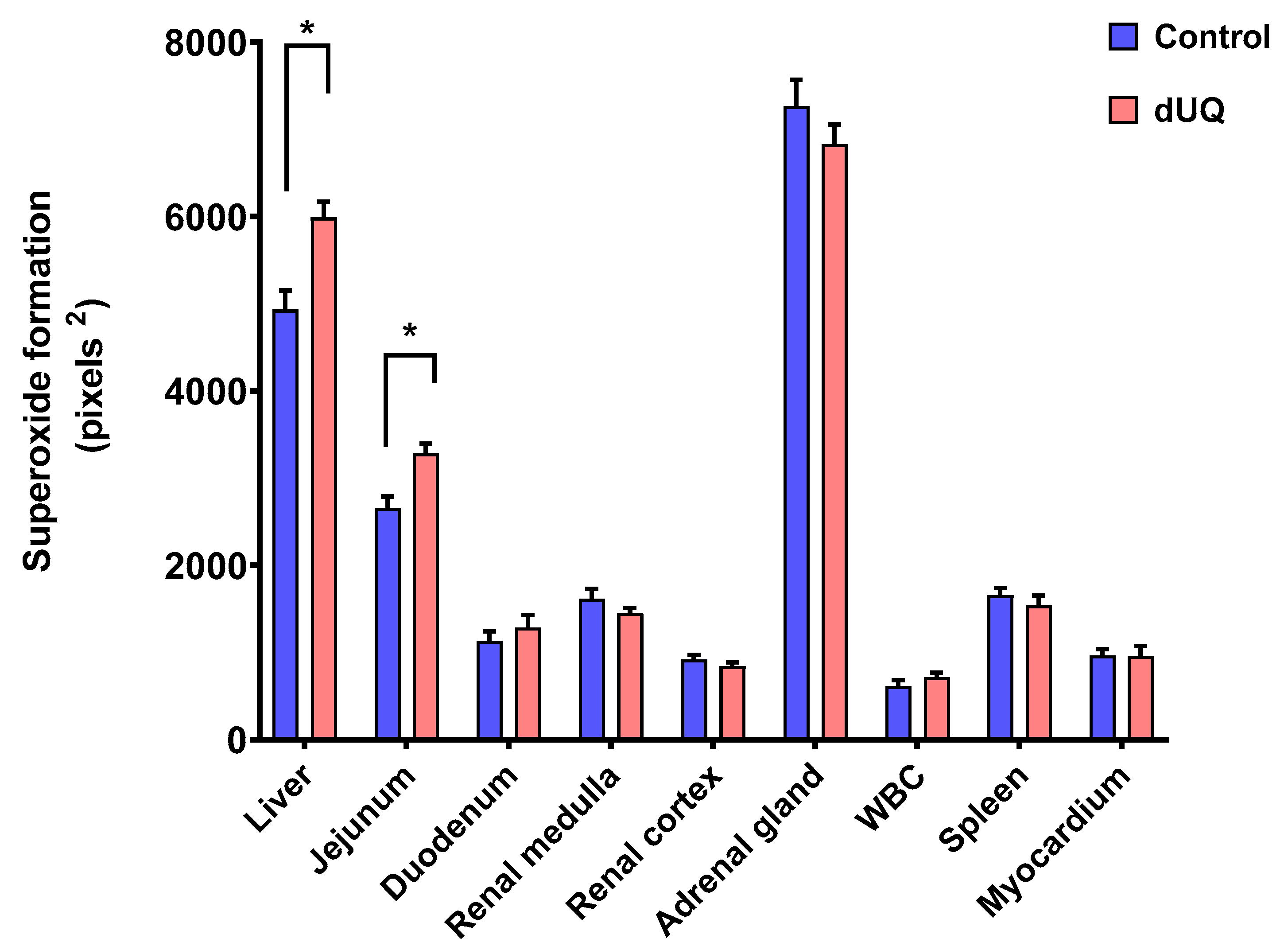
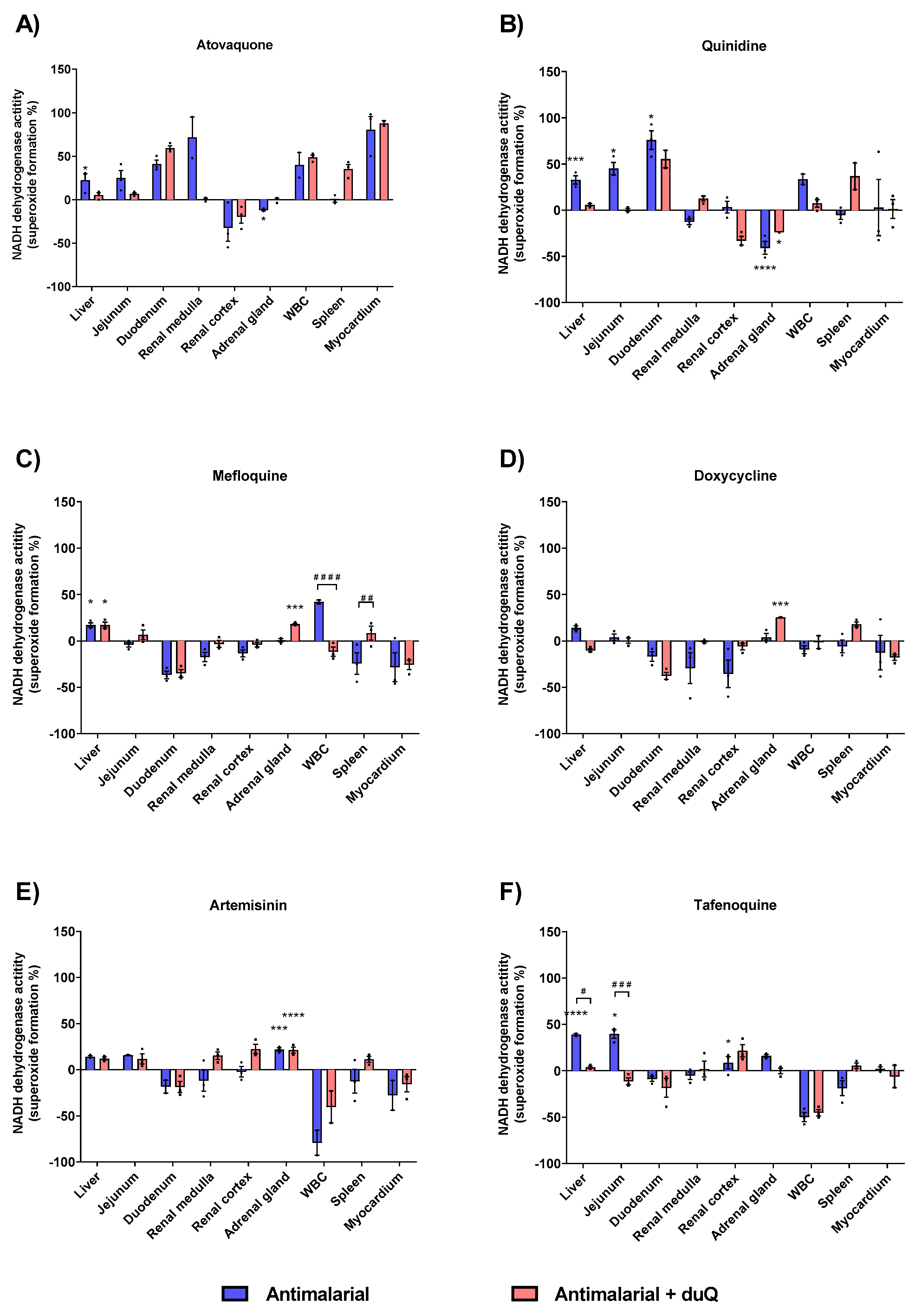
3.2. Effect of Antimalarials on Superoxide Formation in Human Tissues
3.2.1. Basal Superoxide Formation in Human Tissues
3.2.2. Superoxide Formation with Antimalarials in Human Tissues
3.3. Composition, Antimalarial Activity and Effect on Superoxide Formation of Essential Oils
3.3.1. Antimalarial Activity of EOs
3.3.2. Chemical Composition of EOs
3.3.3. Effect on Superoxide Formation of EOs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Youdim, M.B.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Kaludercic, N.; Mialet-Perez, J.; Paolocci, N.; Parini, A.; di Lisa, F. Monoamine oxidases as sources of oxidants in the heart. J. Mol. Cell. Cardiol. 2014, 73, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Di Lisa, F.; Kaludercic, N.; Carpi, A.; Menabò, R.; Giorgio, M. Mitochondrial pathways for ROS formation and myocardial injury: The relevance of p66(Shc) and monoamine oxidase. Basic Res. Cardiol. 2009, 104, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Wu, S.B.; Wei, Y.H. Metabolic reprogramming of human cells in response to oxidative stress: Implications in the pathophysiology and therapy of mitochondrial diseases. Curr. Pharm. Des. 2014, 20, 5510–5526. [Google Scholar] [CrossRef] [PubMed]
- Dan Dunn, J.; Alvarez, L.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Fišar, Z.; Hroudová, J. Common aspects of neuroplasticity, stress, mood disorders and mitochondrial functions. Citeseer 2010, 52, 52010–52011. [Google Scholar]
- Hroudová, J.; Fišar, Z. In vitro inhibition of mitochondrial respiratory rate by antidepressants. Toxicol. Lett. 2012, 213, 345–352. [Google Scholar] [CrossRef]
- Dencher, N.; Frenzel, M.; Reifschneider, N.; Sugawa, W.; Krause, F. Proteome alterations in rat mitochondria caused by aging. Ann. N. Y. Acad. Sci. 2007, 1100, 291–298. [Google Scholar] [CrossRef]
- Helbig, A.; de Groot, M.; van Gestel, R.; Mohammed, S.; de Hulster, E.; Luttik, M.; Daran-Lapujade, P.; Pronk, J.; Heck, A.; Slijper, M. A three-way proteomics strategy allows differential analysis of yeast mitochondrial membrane protein complexes under anaerobic and aerobic conditions. Proteomics 2009, 9, 4787–4798. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Aguilar, S.; Keuthe, M.; Rocha, M.; Fedyaev, V.; Kramp, K.; Gupta, K.; Rasmusson, A.; Schulze, W.; van Dongen, J. The composition of plant mitochondrial supercomplexes changes with oxygen availability. J. Biol. Chem. 2011, 286, 43045–43053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogliati, S.; Frezza, C.; Soriano, M.; Varanita, T.; Quintana-Cabrera, R.; Corrado, M.; Cipolat, S.; Costa, V.; Casarin, A.; Gomes, L.; et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 2013, 155, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Rosca, M.; Vazquez, E.; Kerner, J.; Parland, W.; Chandler, M.; Stanley, W.; Sabbah, H.; Hoppel, C. Cardiac mitochondria in heart failure: Decrease in respirasomes and oxidative phosphorylation. Cardiovasc. Res. 2008, 80, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Böttinger, L.; Horvath, S.; Kleinschroth, T.; Hunte, C.; Daum, G.; Pfanner, N.; Becker, T. Phosphatidylethanolamine and cardiolipin differentially affect the stability of mitochondrial respiratory chain supercomplexes. J. Mol. Biol. 2012, 423, 677–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasseva, G.; Bai, H.; Davidescu, M.; Haromy, A.; Michelakis, E.; Vance, J. Phosphatidylethanolamine deficiency in Mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology. J. Biol. Chem. 2013, 288, 4158–4173. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, E.; Dencher, N.; Vonck, J.; Parcej, D. Three-dimensional structure of the respiratory chain supercomplex I1III2IV1 from bovine heart mitochondria. Biochemistry 2007, 46, 12579–12585. [Google Scholar] [CrossRef]
- Dudkina, N.V.; Kouril, R.; Peters, K.; Braun, H.P.; Boekema, E.J. Structure and function of mitochondrial supercomplexes. Biochim. Biophys. Acta 2010, 1797, 664–670. [Google Scholar] [CrossRef] [Green Version]
- Lapuente-Brun, E.; Moreno-Loshuertos, R.; Acín-Pérez, R.; Latorre-Pellicer, A.; Colás, C.; Balsa, E.; Perales-Clemente, E.; Quirós, P.; Calvo, E.; Rodríguez-Hernández, M.; et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 2013, 340, 1567–1570. [Google Scholar] [CrossRef]
- Maranzana, E.; Barbero, G.; Falasca, A.I.; Lenaz, G.; Genova, M.L. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex i. Antioxid. Redox Signal. 2013, 19, 1469–1480. [Google Scholar] [CrossRef] [Green Version]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Rich, P.; Maréchal, A. The mitochondrial respiratory chain. Essays Biochem. 2010, 47, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hekimi, S. Understanding Ubiquinone. Trends Cell Biol. 2016, 26, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Lenaz, G. Mitochondria and reactive oxygen species. Which role in physiology and pathology? In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 942, pp. 93–136. ISBN 9789400728684. [Google Scholar]
- Vademecum Hidroquinidina Serecor. Available online: https://www.vademecum.es/medicamento-hidroquinidina+serecor_47498 (accessed on 9 September 2021).
- Vidal Vademecum Spain LARIAM Tablet 250 mg. Available online: https://www.vademecum.es/equivalencia-lista-lariam+tablet+250+mg-estados+unidos-p01bc02-28001325-us_1 (accessed on 28 October 2021).
- Haston, J.C. Guidance for Using Tafenoquine for Prevention and Antirelapse Therapy for Malaria—United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1062–1068. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Gu, S.; Sun, D.; Dai, H.; Chen, H.; Zhang, Z. The selectivity of artemisinin-based drugs on human lung normal and cancer cells. Environ. Toxicol. Pharmacol. 2018, 57, 86–94. [Google Scholar] [CrossRef]
- Luo, Y.; Che, M.J.; Liu, C.; Liu, H.G.; Fu, X.W.; Hou, Y.P. Toxicity and related mechanisms of dihydroartemisinin on porcine oocyte maturation in vitro. Toxicol. Appl. Pharmacol. 2018, 341, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Yang, H.; Zhao, X.; Wei, S.; Tao, Y.; Liu, M.; Bo, R.; Li, J. Antimalarial agent artesunate induces G0/G1 cell cycle arrest and apoptosis via increasing intracellular ROS levels in normal liver cells. Hum. Exp. Toxicol. 2020, 39, 1681–1689. [Google Scholar] [CrossRef]
- Bachmann, E.; Weber, E.; Zbinden, G. Biochemical mechanisms of quinidine cardiotoxicity. J. Cardiovasc. Pharmacol. 1986, 8, 826–831. [Google Scholar] [CrossRef]
- WHO. Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 28 October 2021).
- Aronson, J.K. Meyler’s Side Effects of Drugs: The International Ecyclopedia of Adverse Drug Reactions and Interactions, 16th ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Meshnick, S.R. Artemisinin: Mechanisms of action, resistance and toxicity. Int. J. Parasitol. 2002, 32, 1655–1660. [Google Scholar] [CrossRef]
- Jones, R.A.; Panda, S.S.; Hall, C.D. Quinine conjugates and quinine analogues as potential antimalarial agents. Eur. J. Med. Chem. 2015, 97, 335–355. [Google Scholar] [CrossRef]
- Beaufay, C.; Bero, J.; Quetin-Leclerq, J. Antimalarial terpenic coumponds isolated from plants used in traditional medicine. Nat. Antimicrob. Agents 2018, 19, 247–268. [Google Scholar]
- Menard, D.; Dondorp, A. Antimalarial drug resistance: A threat to malaria elimination. Cold Spring Harb. Perspect. Med. 2017, 7, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monzote, L.; Alarcón, O.; Setzer, W. Antiprotozoal activity of essential oils. Agric. Conspec. Sci. 2012, 77, 167–175. [Google Scholar]
- Sülsen, V.; Cazorla, S.; Frank, F.; di Leo Lira, P.; Anesini, C.; Gutierrez-Yapu, D.; Giménez-Turba, A.; Bandoni, A.; Malchiodi, E.; Muschietti, L. In vitro Antiprotozoal Activity and Chemical Composition of Ambrosia tenuifolia and A. scabra Essential Oils. Nat. Prod. Commun. 2008, 3, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Ortet, R.; Thomas, O.P.; Regalado, E.L.; Pino, J.A.; Filippi, J.J.; Fernández, M.D. Composition and biological properties of the volatile oil of Artemisia gorgonum Webb. Chem. Biodivers. 2010, 7, 1325–1332. [Google Scholar] [CrossRef]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef]
- Baricevic, D.; Bartol, T. The biological/pharmacological activity of the Origanum genus. In Oregano, the Genera Origanum and Lippia; Kintzios, S.E., Ed.; Taylor & Francis Inc.: Abingdon, UK, 2002. [Google Scholar]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, M.; González-Coloma, A.; Fe Andrés, M.; Navarro-Rocha, J.; Martínez-Díaz, R.A. Biological Evaluation of Essential Oils from Selected Medicinal Plants and Their Main Components against Phytomonas davidi (Kinetoplastea: Trypanosomatidae). Chem. Biodivers. 2020, 17, e2000521. [Google Scholar] [CrossRef]
- Milhau, G.; Valentin, A.; Benoit, F.; Mallié, M.; Bastide, J.M.; Pélissier, Y.; Bessière, J.M. In Vitro Antimalarial Activity of Eight Essential Oils. J. Essent. Oil Res. 1997, 9, 329–333. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Burillo, J.; García-Vallejo, M.C. Investigación y Experimentación de Plantas Aromáticas y Medicinales en Aragón. Cultivo, Transformación y Analítica; Gobierno de Aragón: Zaragoza, Spain, 2003. [Google Scholar]
- Burillo, J. Insecticidas y Repelentes de Insectos de Origen Natural; Burillo, J., González-Coloma, A., Eds.; Centro de Investigación y Tecnología Agroalimentaria: Zaragoza, Spain, 2009. [Google Scholar]
- European Pharmacopeia. Available online: http://www.edqm.eu/en/Homepage-628.html (accessed on 28 October 2021).
- Sainz, P.; Andrés, M.F.; Martínez-Díaz, R.A.; Bailén, M.; Navarro-Rocha, J.; Díaz, C.E.; González-Coloma, A. Chemical composition and biological activities of artemisia pedemontana subsp. Assoana essential oils and hydrolate. Biomolecules 2019, 9, 558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, R.; Garate, J.; Tolentino-Cortez, T.; Herraiz, A.; Lombardero, L.; Ducrocq, F.; Rodríguez-Puertas, R.; Trifilieff, P.; Astigarraga, E.; Barreda-Gómez, G.; et al. Microarray and Mass Spectrometry-Based Methodology for Lipid Profiling of Tissues and Cell Cultures. Anal. Chem. 2019, 91, 15967–15973. [Google Scholar] [CrossRef]
- Rienda, B.; Elexpe, A.; Tolentino-Cortez, T.; Gulak, M.; Bruzos-Cidón, C.; Torrecilla, M.; Astigarraga, E.; Barreda-Gómez, G. Analysis of Acetylcholinesterase Activity in Cell Membrane Microarrays of Brain Areas as a Screening Tool to Identify Tissue Specific Inhibitors. Analytica 2021, 2, 3. [Google Scholar] [CrossRef]
- Hebert-Chatelain, E.; Desprez, T.; Serrat, R.; Bellocchio, L.; Soria-Gomez, E.; Busquets-Garcia, A.; Pagano Zottola, A.C.; Delamarre, A.; Cannich, A.; Vincent, P.; et al. A cannabinoid link between mitochondria and memory. Nature 2016, 539, 555–559. [Google Scholar] [CrossRef]
- Manuel, I.; Barreda-Gómez, G.; González de San Román, E.; Veloso, A.; Fernández, J.A.; Giralt, M.T.; Rodríguez-Puertas, R. Neurotransmitter receptor localization: From autoradiography to imaging mass spectrometry. ACS Chem. Neurosci. 2015, 6, 362–373. [Google Scholar] [CrossRef]
- Sánchez-Magraner, L.; de la Fuente, M.; Evans, C.; Miles, J.; Elexpe, A.; Rodriguez-Astigarraga, M.; Astigarraga, E.; Barreda-Gómez, G. Quantification of PD-1/PD-L1 Interaction between Membranes from PBMCs and Melanoma Samples Using Cell Membrane Microarray and Time-Resolved Förster Resonance Energy Transfer. Analytica 2021, 2, 15. [Google Scholar] [CrossRef]
- Pollard, A.K.; Craig, E.L.; Chakrabarti, L. Mitochondrial complex 1 activity measured by spectrophotometry is reduced across all brain regions in ageing and more specifically in neurodegeneration. PLoS ONE 2016, 11, e0150545. [Google Scholar] [CrossRef] [Green Version]
- Talpade, D.J.; Greene, J.G.; Higgins, D.S.; Greenamyre, J.T. In Vivo Labeling of Mitochondrial Complex I (NADH: Ubiquinone Oxidoreductase) in Rat Brain Using [3H] Dihydrorotenone. J. Neurochem. 2000, 75, 2611–2621. [Google Scholar] [CrossRef] [Green Version]
- Stroh, A.; Kadenbach, B. Tissue-specific and species-specific distribution of -SH groups in cytochrome c oxidase subunits. Eur. J. Biochem. 1986, 156, 199–204. [Google Scholar] [CrossRef]
- Casademont, J.; Rodriguez-Santiago, B.; Miró, Ò.; Beato, A.; López, S.; Nunes, V.; Cardellach, F. Mitochondrial respiratory chain in brain homogenates: Activities in different brain areas in patients with Alzheimer’s disease. Aging Clin. Exp. Res. 2005, 17, 1–7. [Google Scholar] [CrossRef]
- Fecher, C.; Trovò, L.; Müller, S.A.; Snaidero, N.; Wettmarshausen, J.; Heink, S.; Ortiz, O.; Wagner, I.; Kühn, R.; Hartmann, J.; et al. Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat. Neurosci. 2019, 22, 1731–1742. [Google Scholar] [CrossRef]
- Adav, S.S.; Park, J.E.; Sze, S.K. Quantitative profiling brain proteomes revealed mitochondrial dysfunction in Alzheimer’s disease. Mol. Brain 2019, 12, 8. [Google Scholar] [CrossRef]
- Villa, R.F.; Ferrari, F.; Bagini, L.; Gorini, A.; Brunello, N.; Tascedda, F. Mitochondrial energy metabolism of rat hippocampus after treatment with the antidepressants desipramine and fluoxetine. Neuropharmacology 2017, 121, 30–38. [Google Scholar] [CrossRef]
- Villa, R.F.; Gorini, A.; Ferrari, F.; Hoyer, S. Energy metabolism of cerebral mitochondria during aging, ischemia and post-ischemic recovery assessed by functional proteomics of enzymes. Neurochem. Int. 2013, 63, 765–781. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Camb. Companion Philos. Biol. 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Song, S.; Jiang, L.; Oyarzabal, E.A.; Wilson, B.; Li, Z.; Shih, I.; Wang, Q.; Hong, J.; Sciences, H.; Carolina, N.; et al. Loss of brain norepinephrine elicits neuroinflammation-mediated oxidative injury and selective caudo-rostral neurodegeneration. Mol. Neurobiol. 2019, 56, 2653–2669. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Oyarzabal, E.A.; Song, S.; Wilson, B.; Santos, J.H.; Hong, J.S. Locus coeruleus neurons are most sensitive to chronic neuroinflammation-induced neurodegeneration. Brain. Behav. Immun. 2020, 87, 359–368. [Google Scholar] [CrossRef]
- Braak, H.; del Tredici, K. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. J. Parkinsons Dis. 2017, 7, S73–S87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maria Michel, T.; Pulschen, D.; Thome, J. The role of oxidative stress in depressive disorders. Curr. Pharm. Des. 2012, 18, 5890–5899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manji, H.; Kato, T.; di Prospero, N.A.; Ness, S.; Beal, M.F.; Krams, M.; Chen, G. Impaired mitochondrial function in psychiatric disorders. Nat. Rev. Neurosci. 2012, 13, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H.; Pfeiffer, K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000, 19, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, E.; Seelert, H.; Reifschneider, N.; Krause, F.; Dencher, N.; Vonck, J. Architecture of active mammalian respiratory chain supercomplexes. J. Biol. Chem. 2006, 281, 15370–15375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, D.; Greenamyre, J. [3H]dihydrorotenone binding to NADH: Ubiquinone reductase (complex I) of the electron transport chain: An autoradiographic study. J. Neurosci. 1996, 16, 3807–3816. [Google Scholar] [CrossRef] [Green Version]
- Althoff, T.; Mills, D.; Popot, J.; Kühlbrandt, W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. EMBO J. 2011, 30, 4652–4664. [Google Scholar] [CrossRef] [Green Version]
- Letts, J.; Fiedorczuk, K.; Sazanov, L. The architecture of respiratory supercomplexes. Nature 2016, 537, 644–648. [Google Scholar] [CrossRef]
- Gu, J.; Wu, M.; Guo, R.; Yan, K.; Lei, J.; Gao, N.; Yang, M. The architecture of the mammalian respirasome. Nature 2016, 537, 639–643. [Google Scholar] [CrossRef]
- Milenkovic, D.; Blaza, J.; Larsson, N.; Hirst, J. The Enigma of the Respiratory Chain Supercomplex. Cell Metab. 2017, 25, 765–776. [Google Scholar] [CrossRef] [Green Version]
- Sun, N.; Wu, Y.; Nanba, K.; Sbiera, S.; Kircher, S.; Kunzke, T.; Aichler, M.; Berezowska, S.; Reibetanz, J.; Rainey, W.E.; et al. High-Resolution Tissue Mass Spectrometry Imaging Reveals a Refined Functional Anatomy of the Human Adult Adrenal Gland. Endocrinology 2018, 159, 1511–1524. [Google Scholar] [CrossRef]
- Tsukada, H.; Nishiyama, S.; Fukumoto, D.; Kanazawa, M.; Harada, N. Novel PET probes 18F-BCPP-EF and 18F-BCPP-BF for mitochondrial complex I: A PET study in comparison with 18F-BMS-747158-02 in rat brain. J. Nucl. Med. 2014, 55, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Nixon, G.; Pidathala, C.; Shone, A.; Antoine, T.; Fisher, N.; O’Neill, P.; Ward, S.; Biagini, G. Targeting the mitochondrial electron transport chain of Plasmodium falciparum: New strategies towards the development of improved antimalarials for the elimination era. Future Med. Chem. 2013, 5, 1573–1591. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sisodia, J. Quinidine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Li, H.; Jiao, S.; Li, X.; Banu, H.; Hamal, S.; Wang, X. Therapeutic effects of antibiotic drug mefloquine against cervical cancer through impairing mitochondrial function and inhibiting mtor pathway. Can. J. Physiol. Pharmacol. 2017, 95, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Aemps Ficha Tecnica Doxiciclina Normon 100 MG Comprimidos Recubiertos. Available online: https://cima.aemps.es/cima/dochtml/ft/47077/FT_47077.html (accessed on 28 October 2021).
- Chu, C.; Hwang, J. Tafenoquine: A toxicity overview. Expert Opin. Drug Saf. 2021, 20, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Okun, G.; Lu, P.; Brandt, U. Three Classes of Inhibitors Share a Common Binding Domain in Mitochondrial Complex I (NADH: Ubiquinone Oxidoreductase). J. Biol. Chem. 1999, 274, 2625–2630. [Google Scholar] [CrossRef] [PubMed] [Green Version]

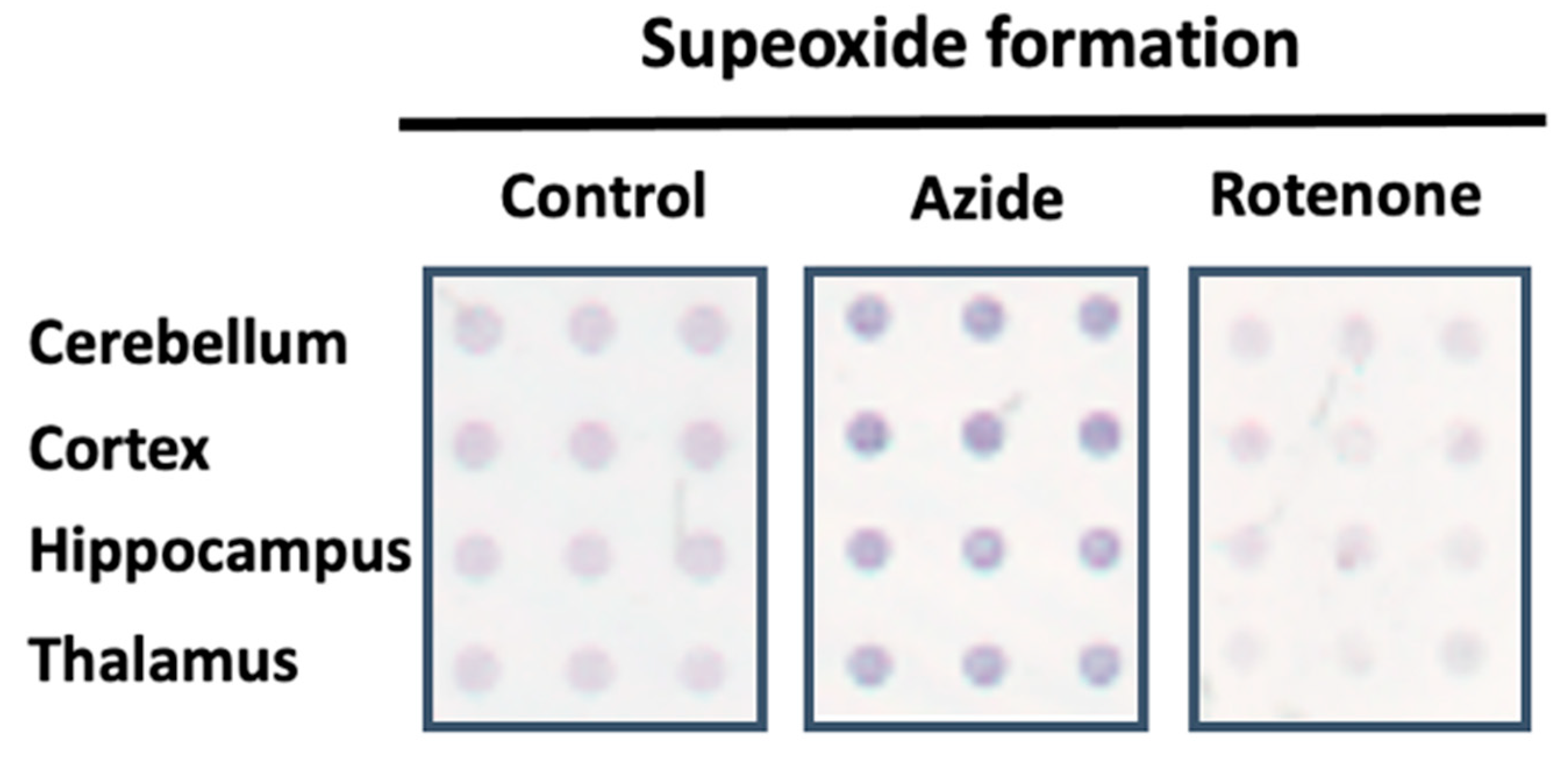
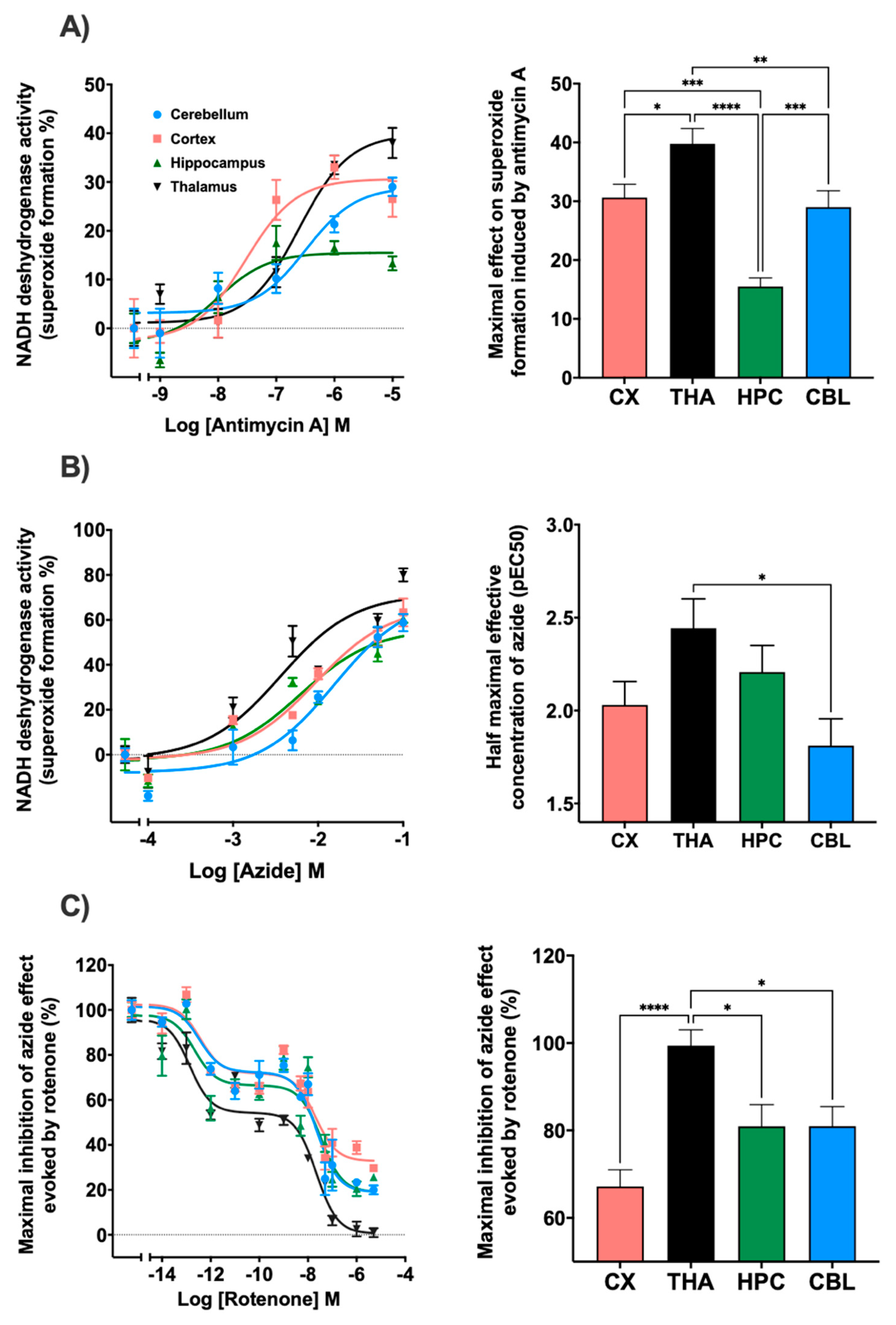


| Cerebellum | Cortex | Hippocampus | Thalamus | ||
|---|---|---|---|---|---|
| pIC50 Hi | −12.4 ± 0.4 | −12.4 ± 0.3 | −12.6 ± 0.3 | −12.8 ± 0.2 | |
| Rotenone | pIC50 Low | −7.6 ± 0.1 | −7.6 ± 0.2 | −7.4 ± 0.2 | −7.6 ± 0.1 |
| Imax | 81.0 ± 4.5 | 67.2 ± 3.8 | 81.0 ± 4.9 | 99.4 ± 3.6 | |
| Antimycin A | pEC50 | −6.4 ± 0.2 | −7.5 ± 0.2 | −8.0 ± 0.2 | −6.6 ± 0.1 |
| Emax | 29.0 ± 2.7 | 30.6 ± 2,7 | 15.5 ± 1.4 | 39.7 ± 2.5 | |
| Azide | pEC50 | −1.8 ± 0.1 | −2.0 ± 0.1 | −2.2 ± 0.1 | −2.4 ± 0.1 |
| Emax | 78.0 ± 6.6 | 68.2 ± 5.0 | 58.6 ± 5.1 | 73.3 ± 6.8 |
| EO | Main Components |
|---|---|
| Rosmarinus officinalis | 1,8-cineol (26%), α-pinene (21%), camphor (13%), camphene (9%), β-pinene (5%), |
| Origanum majoricum | 4-terpineol (30%), γ-terpinene (11%), α-terpinene (6%), sabinene (6%), p-cymene (5%), trans-β-ocimene (5%), caryophyllene (5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elexpe, A.; Nieto, N.; Fernández-Cuétara, C.; Domínguez-Fernández, C.; Morera-Herreras, T.; Torrecilla, M.; Miguélez, C.; Laso, A.; Ochoa, E.; Bailen, M.; et al. Study of Tissue-Specific Reactive Oxygen Species Formation by Cell Membrane Microarrays for the Characterization of Bioactive Compounds. Membranes 2021, 11, 943. https://doi.org/10.3390/membranes11120943
Elexpe A, Nieto N, Fernández-Cuétara C, Domínguez-Fernández C, Morera-Herreras T, Torrecilla M, Miguélez C, Laso A, Ochoa E, Bailen M, et al. Study of Tissue-Specific Reactive Oxygen Species Formation by Cell Membrane Microarrays for the Characterization of Bioactive Compounds. Membranes. 2021; 11(12):943. https://doi.org/10.3390/membranes11120943
Chicago/Turabian StyleElexpe, Ane, Nerea Nieto, Claudia Fernández-Cuétara, Celtia Domínguez-Fernández, Teresa Morera-Herreras, María Torrecilla, Cristina Miguélez, Antonio Laso, Eneko Ochoa, María Bailen, and et al. 2021. "Study of Tissue-Specific Reactive Oxygen Species Formation by Cell Membrane Microarrays for the Characterization of Bioactive Compounds" Membranes 11, no. 12: 943. https://doi.org/10.3390/membranes11120943
APA StyleElexpe, A., Nieto, N., Fernández-Cuétara, C., Domínguez-Fernández, C., Morera-Herreras, T., Torrecilla, M., Miguélez, C., Laso, A., Ochoa, E., Bailen, M., González-Coloma, A., Angulo-Barturen, I., Astigarraga, E., & Barreda-Gómez, G. (2021). Study of Tissue-Specific Reactive Oxygen Species Formation by Cell Membrane Microarrays for the Characterization of Bioactive Compounds. Membranes, 11(12), 943. https://doi.org/10.3390/membranes11120943








