Anticorrosion Performance of PVDF Membranes Modified by Blending PTFE Nanoemulsion and Prepared through Usual Non-Solvent-Induced Phase Inversion Method
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of PVDF/PTFE Composite Membrane
2.3. Chemical Stability Test Conducted on the Prepared Membranes
2.4. Characterization of Membranes
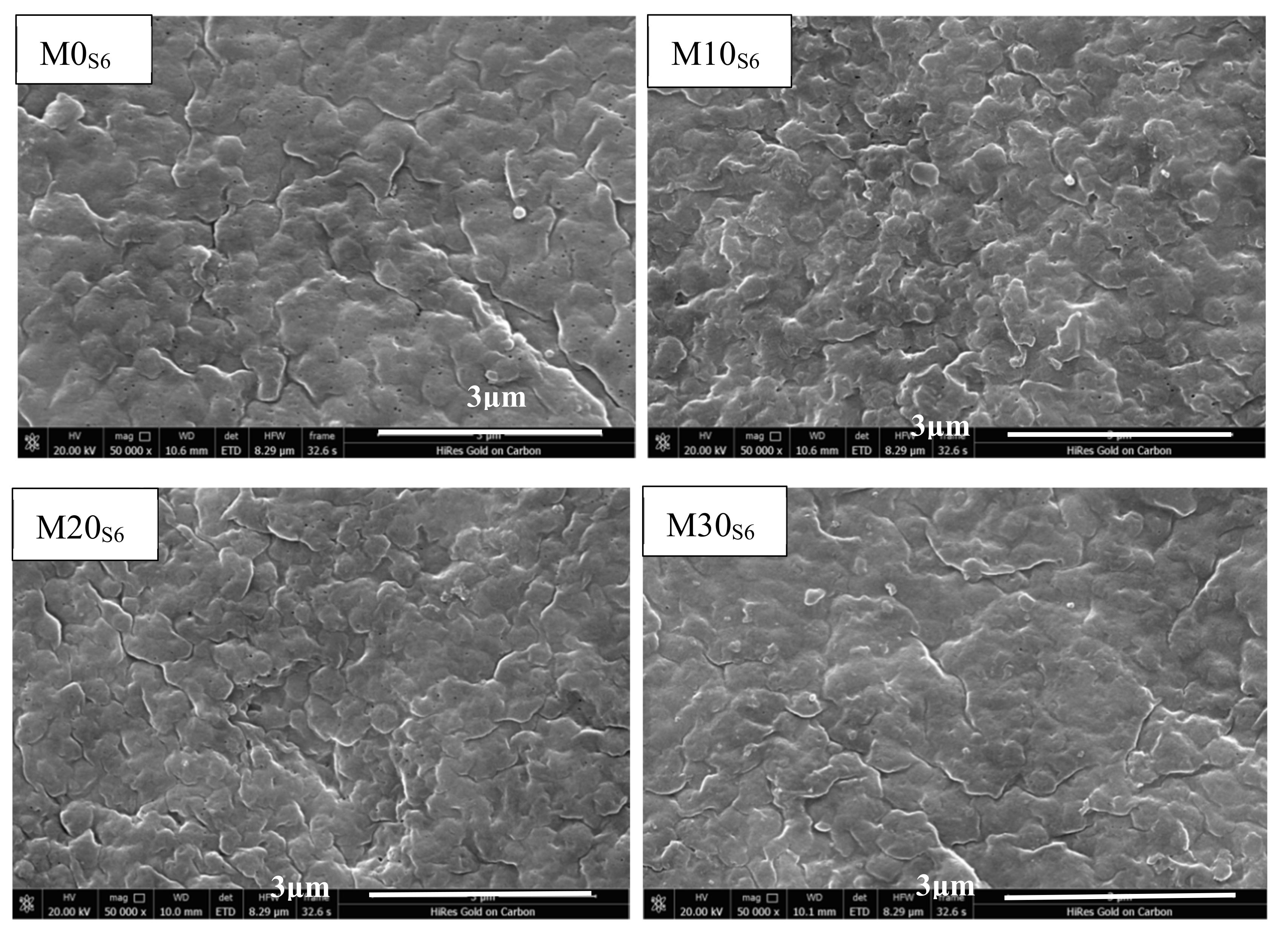
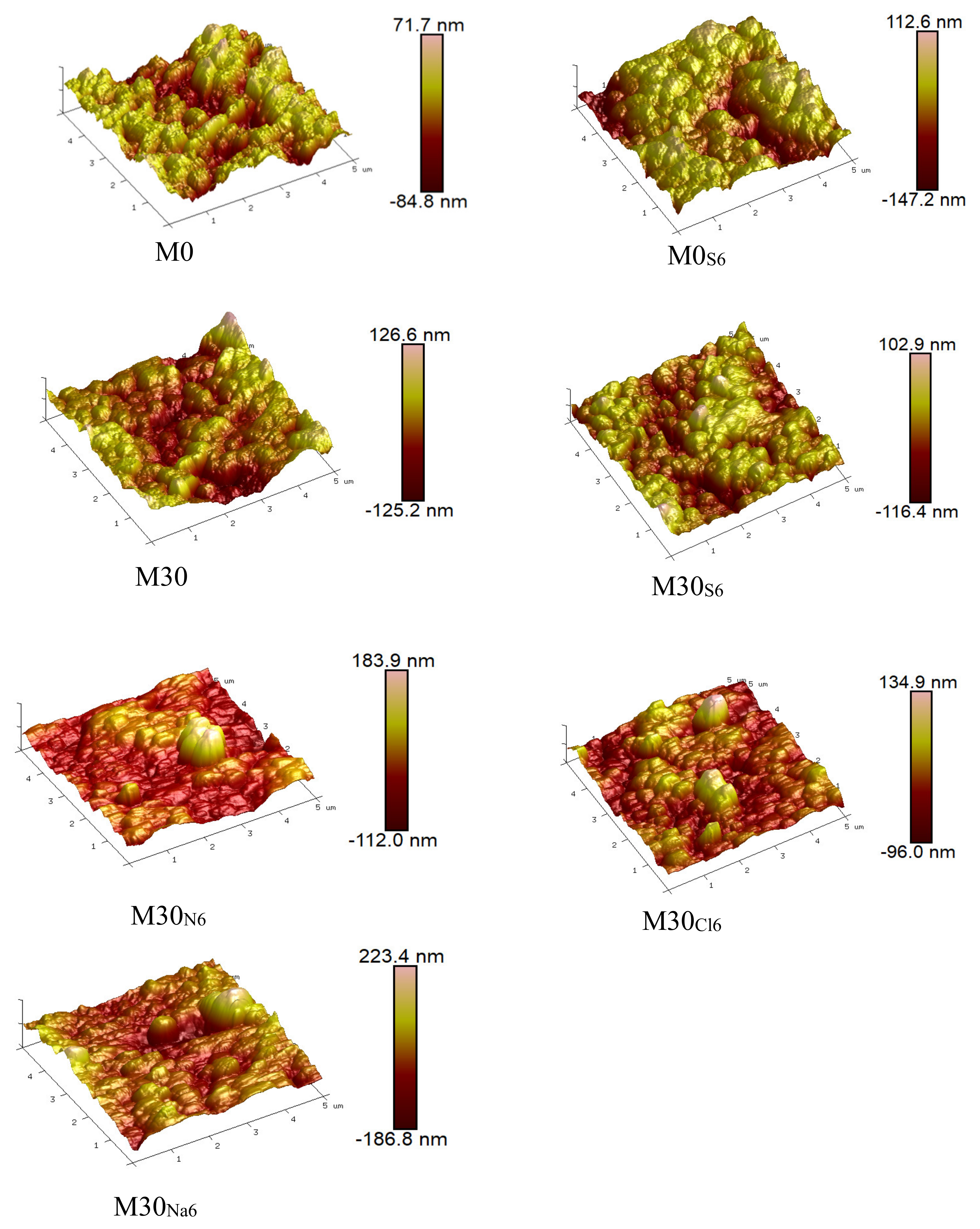
2.4.1. Microimage Analysis
2.4.2. Mechanical Properties
2.4.3. Water Contact Angle Measurements
2.4.4. Membrane Porosity
2.4.5. Membrane Permeation Test
3. Results and Discussion
3.1. Membrane Morphologies and PTFE Dispersion
3.2. Membrane Strength and Surface Properties
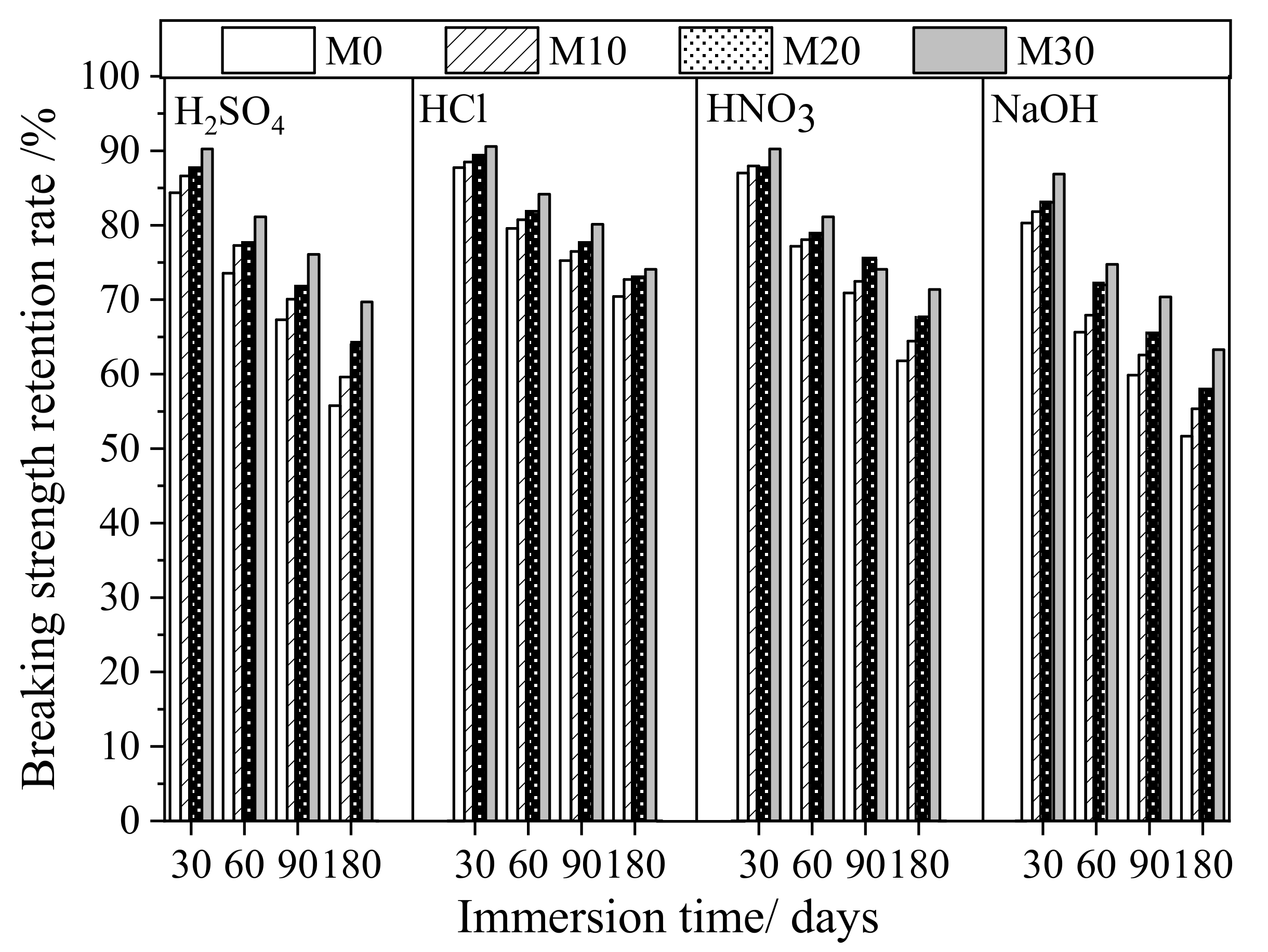
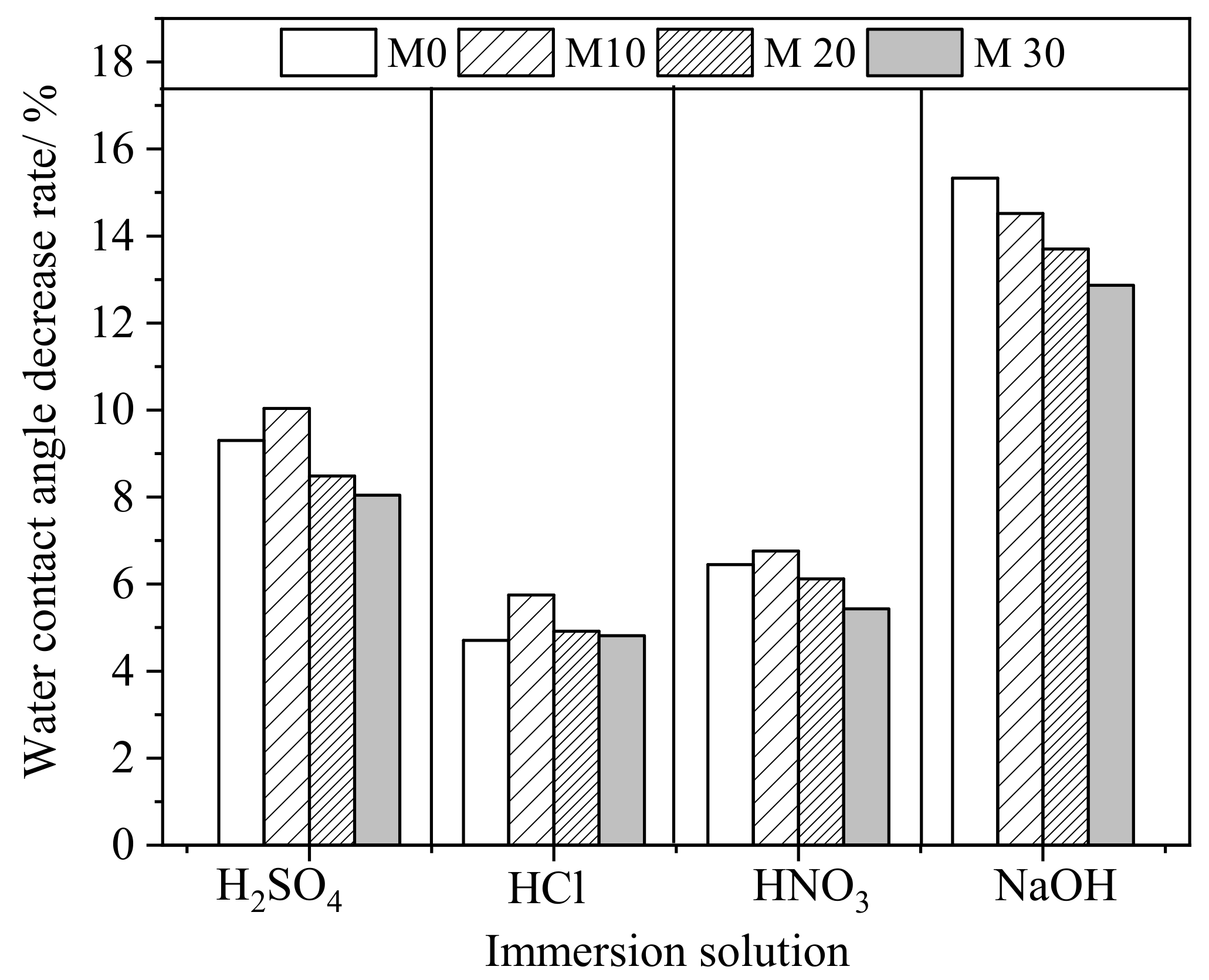
3.3. Anticorrosion Stability of PVDF/PTFE Composite Membranes
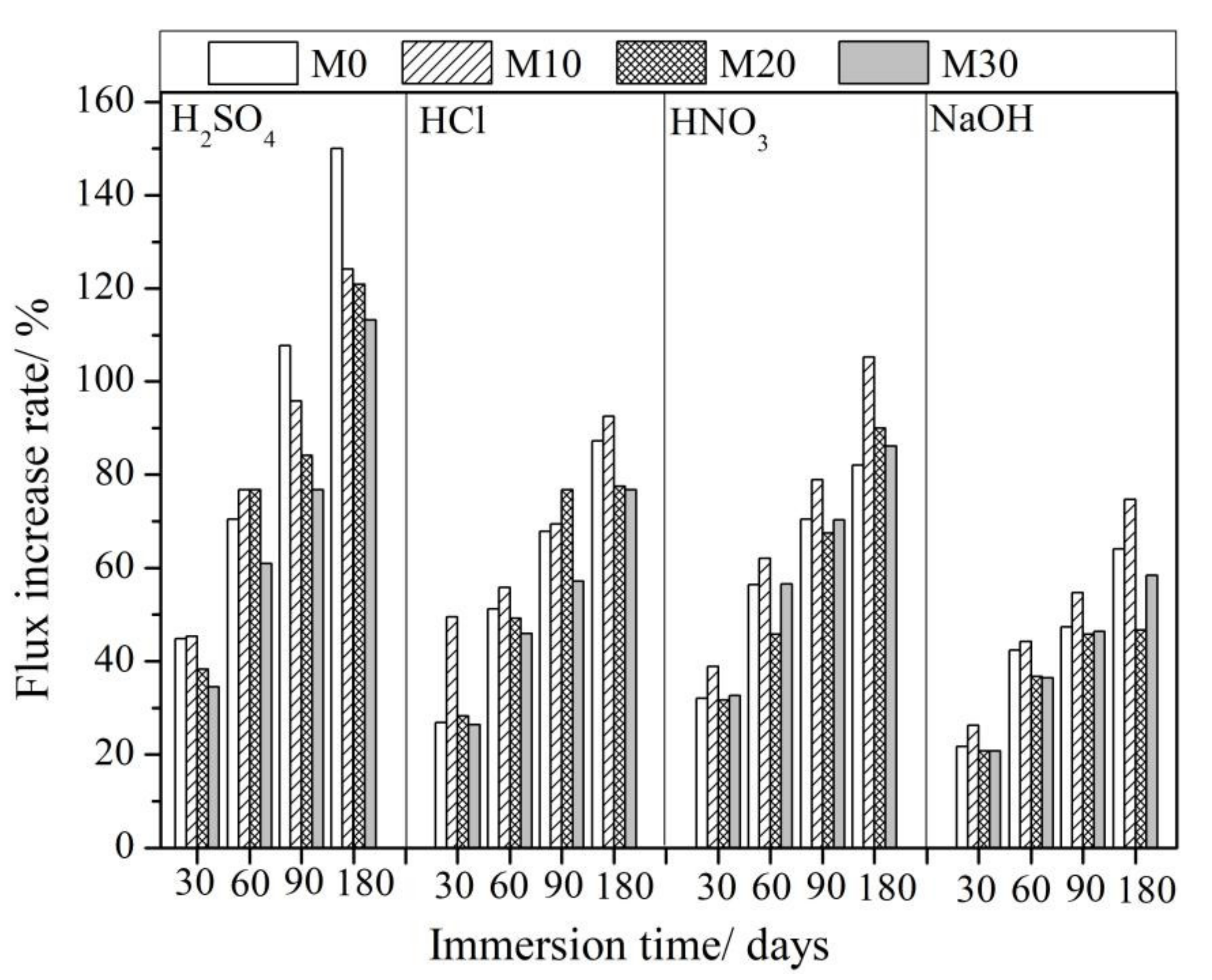
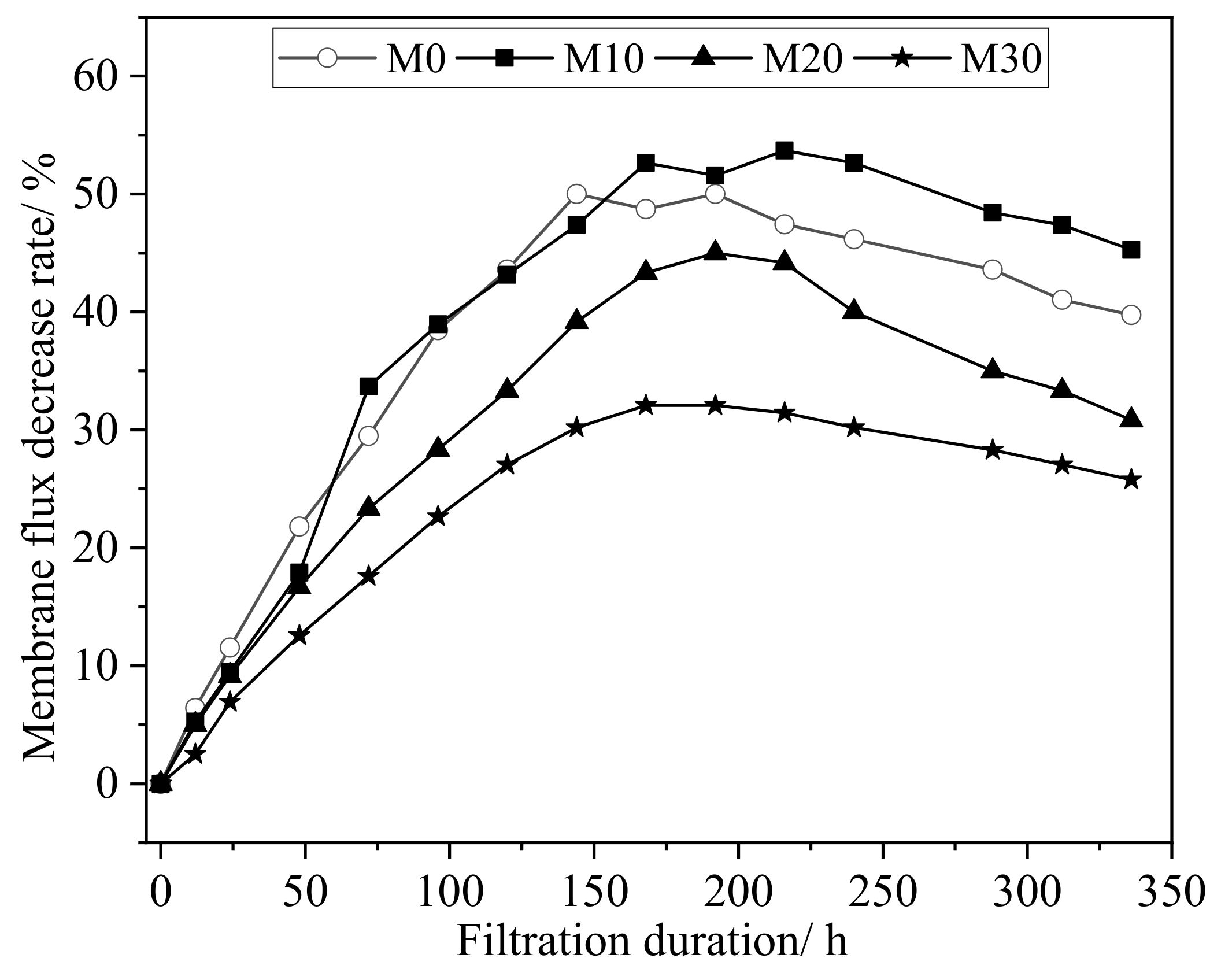
3.4. Dynamic Filtration Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chindaprasirt, P.; Rattanasak, U. Synthesis of porous alkali-activated materials for high-acidic wastewater treatment. J. Water Process Eng. 2020, 33, 101118. [Google Scholar] [CrossRef]
- Vélez-Pérez, L.S.; Ramirez-Nava, J.; Hernández-Flores, G.; Talavera-Mendoza, O.; Escamilla-Alvarado, C.; Poggi-Varaldo, H.M.; Solorza-Feria, O.; López-Díaz, J.A. Industrial acid mine drainage and municipal wastewater co-treatment by dual-chamber microbial fuel cells. Int. J. Hydrog. Energy 2020, 45, 13757–13766. [Google Scholar] [CrossRef]
- Wang, C.; Lin, G.; Zhao, J.; Wang, S.; Zhang, L.; Xi, Y.; Li, X.; Ying, Y. Highly selective recovery of Au(III) from wastewater by thioctic acid modified Zr-MOF: Experiment and DFT calculation. Chem. Eng. J. 2020, 380, 122511. [Google Scholar] [CrossRef]
- Goyal, A.; Srivastava, V.C. Treatment of highly acidic wastewater containing high energetic compounds using dimensionally stable anode. Chem. Eng. J. 2017, 325, 289–299. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Agboola, O.; Onyango, M.S.; Popoola, P.; Bobape, M.F. Chapter 6—Current Methods for the Remediation of Acid Mine Drainage Including Continuous Removal of Metals From Wastewater and Mine Dump. In Bio-Geotechnologies for Mine Site Rehabilitation; Prasad, M.N.V., Favas, P.J.d.C., Maiti, S.K., Eds.; Elsevier: Cambridge, UK, 2018; pp. 103–114. [Google Scholar]
- You, S.; Lu, J.; Tang, C.Y.; Wang, X. Rejection of heavy metals in acidic wastewater by a novel thin-film inorganic forward osmosis membrane. Chem. Eng. J. 2017, 320, 532–538. [Google Scholar] [CrossRef]
- Dhir, B. Chapter 4—Biotechnological Tools for Remediation of Acid Mine Drainage (Removal of Metals from Wastewater and Leachate); Elsevier: Cambridge, UK, 2018; pp. 67–82. [Google Scholar]
- López, J.; Reig, M.; Gibert, O.; Cortina, J.L. Integration of nanofiltration membranes in recovery options of rare earth elements from acidic mine waters. J. Clean. Prod. 2019, 210, 1249–1260. [Google Scholar] [CrossRef]
- Choi, J.; Im, S.J.; Jang, A. Application of volume retarded osmosis-Low pressure membrane hybrid process for recovery of heavy metals in acid mine drainage. Chemosphere 2019, 232, 264–272. [Google Scholar] [CrossRef]
- López, J.; Reig, M.; Gibert, O.; Cortina, J.L. Increasing sustainability on the metallurgical industry by integration of membrane nanofiltration processes: Acid recovery. Sep. Purif. Technol. 2019, 226, 267–277. [Google Scholar] [CrossRef]
- Amaya-Vías, D.; Tataru, L.; Herce-Sesa, B.; López-López, J.A.; López-Ramírez, J.A. Metals removal from acid mine drainage (Tinto River, SW Spain) by water gap and air gap membrane distillation. J. Membr. Sci. 2019, 582, 20–29. [Google Scholar] [CrossRef]
- López, J.; Reig, M.; Gibert, O.; Cortina, J.L. Comparison of acid-resistant ceramic and polymeric nanofiltration membranes for acid mine waters treatment. Chem. Eng. J. 2020, 382, 122786. [Google Scholar] [CrossRef]
- Samaei, S.M.; Trinidad, S.G.; Altaee, A. The application of pressure-driven ceramic membrane technology for the treatment of industrial wastewaters—A review. Sep. Purif. Technol. 2018, 200, 198–220. [Google Scholar] [CrossRef]
- Radulovic, L.L.; Wojcinski, Z.W. PTFE (Polytetrafluoroethylene; Teflon®). In Encyclopedia of Toxicology; Academic Press: Oxford, UK, 2014; pp. 1133–1136. [Google Scholar]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and perspectives in PTFE membrane: Preparation, modification, and applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Adnan, S.; Hoang, M.; Wang, H.; Xie, Z. Commercial PTFE membranes for membrane distillation application: Effect of microstructure and support material. Desalination 2012, 284, 297–308. [Google Scholar] [CrossRef]
- Zhu, H.L.; Wang, H.J.; Wang, F.; Guo, Y.H.; Zhang, H.P.; Chen, J.Y. Preparation and properties of PTFE hollow fiber membranes for desalination through vacuum membrane distillation. J. Membr. Sci. 2013, 446, 145–153. [Google Scholar] [CrossRef]
- Xue, S.; Li, C.C.; Li, J.M.; Zhu, H.L.; Guo, Y.H. A catechol-based biomimetic strategy combined with surface mineralization to enhance hydrophilicity and anti-fouling property of PTFE flat membrane. J. Membr. Sci. 2017, 524, 409–418. [Google Scholar] [CrossRef]
- Huang, Q.-L.; Xiao, C.-F.; Hu, X.-Y.; Li, X.-F. Study on the effects and properties of hydrophobic poly(tetrafluoroethylene) membrane. Desalination 2011, 277, 187–192. [Google Scholar] [CrossRef]
- Park, G.-C.; Kim, D. Porous PTFE reinforced SPEEK proton exchange membranes for enhanced mechanical, dimensional, and electrochemical stability. Polymer 2021, 218, 123506. [Google Scholar] [CrossRef]
- Khumalo, N.P.; Nthunya, L.N.; De Canck, E.; Derese, S.; Verliefde, A.R.; Kuvarega, A.T.; Mamba, B.B.; Mhlanga, S.D.; Dlamini, D.S. Congo red dye removal by direct membrane distillation using PVDF/PTFE membrane. Sep. Purif. Technol. 2019, 211, 578–586. [Google Scholar] [CrossRef]
- Khumalo, N.; Nthunya, L.; Derese, S.; Motsa, M.; Verliefde, A.; Kuvarega, A.; Mamba, B.B.; Mhlanga, S.; Dlamini, D.S. Water recovery from hydrolysed human urine samples via direct contact membrane distillation using PVDF/PTFE membrane. Sep. Purif. Technol. 2019, 211, 610–617. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, L.; Loh, C.H.; Wang, R. Preparation of PVDF/PTFE hollow fiber membranes for direct contact membrane distillation via thermally induced phase separation method. Desalination 2018, 430, 86–97. [Google Scholar] [CrossRef]
- Dong, Z.Q.; Ma, X.H.; Xu, Z.L.; You, W.T.; Li, F.B. Superhydrophobic PVDF–PTFE electrospun nanofibrous membranes for desalination by vacuum membrane distillation. Desalination 2014, 347, 175–183. [Google Scholar] [CrossRef]
- Teoh, M.M.; Chung, T.S.; Yeo, Y.S. Dual-layer PVDF/PTFE composite hollow fibers with a thin macrovoid-free selective layer for water production via membrane distillation. Chem. Eng. J. 2011, 171, 684–691. [Google Scholar] [CrossRef]
- Pourhashem, S.; Saba, F.; Duan, J.; Rashidi, A.; Guan, F.; Nezhad, E.G.; Hou, B. Polymer/Inorganic nanocomposite coatings with superior corrosion protection performance: A review. J. Ind. Eng. Chem. 2020, 88, 29–57. [Google Scholar] [CrossRef]
- Du, J.; Li, N.; Tian, Y.; Zhang, J.; Zuo, W. Preparation of PVDF membrane blended with graphene oxide-zinc sulfide (GO-ZnS) nanocomposite for improving the anti-fouling property. J. Photochem. Photobiol. A Chem. 2020, 400, 112694. [Google Scholar] [CrossRef]
- Qing, W.H.; Shi, X.N.; Zhang, W.D.; Wang, J.Q.; Wu, Y.F.; Wang, P.Y.; Tang, C.Y. Solvent-thermal induced roughening: A novel and versatile method to prepare superhydrophobic membranes. J. Membr. Sci. 2018, 564, 465–472. [Google Scholar] [CrossRef]
- Kima, C.U.; Lee, J.M.; Ihm, S.K. Emulsion polymerization of tetrafluoroethylene: Effects of reaction conditions on particle formation. J. Fluor. Chem. 1999, 96, 11–21. [Google Scholar] [CrossRef]
- Zuo, J.H.; Wei, C.; Cheng, P.; Yan, X.; Chen, Y.; Lang, W.Z. Breakthrough the upperbond of permeability vs. tensile strength of TIPS-prepared PVDF membranes. J. Membr. Sci. 2020, 604, 118089. [Google Scholar] [CrossRef]
- Yang, Y.J.; Li, Y.Q.; Cao, L.X.P.; Wang, Y.J.; Li, L.; Li, W.L. Electrospun PVDF-SiO2 nanofibrous membranes with enhanced surface roughness for oil-water coalescence separation. Sep. Purif. Technol. 2021, 269, 118726. [Google Scholar] [CrossRef]
- Platt, S.; Nystrom, M.; Bottino, A. Capannelli; Stability of NF membranes under extreme acidic conditions. J. Membr. Sci. 2004, 239, 91–103. [Google Scholar] [CrossRef]
- Frick, A. Book Review: Compositional and Failure Analysis of Polymers: A Practical Approach. By John Scheirs. Macromol. Mater. Eng. 2002, 287, 634. [Google Scholar] [CrossRef]
- Yang, L.X.; Cao, X.L.; Wu, Y.T.; Chen, S.; Xie, X.C.; Zhu, Q.L.; Wang, J.X.; Qu, J.E.; Chen, S.; Zheng, P.H. Improvement of corrosion resistance and mechanism analysis for self-assembled vinyltriethoxysilane (VS) films on low carbon steel using a novel chemical etching method. Corros. Sci. 2020, 177, 109002. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Zhang, D.; Wang, Y.; Yu, Y.; Gao, L.; Huang, M. Preparation of triazole compounds via click chemistry reaction and formation of the protective self-assembled membrane against copper corrosion. Colloids Surf. A Physicochem. Eng. Asp. 2018, 550, 145–154. [Google Scholar] [CrossRef]
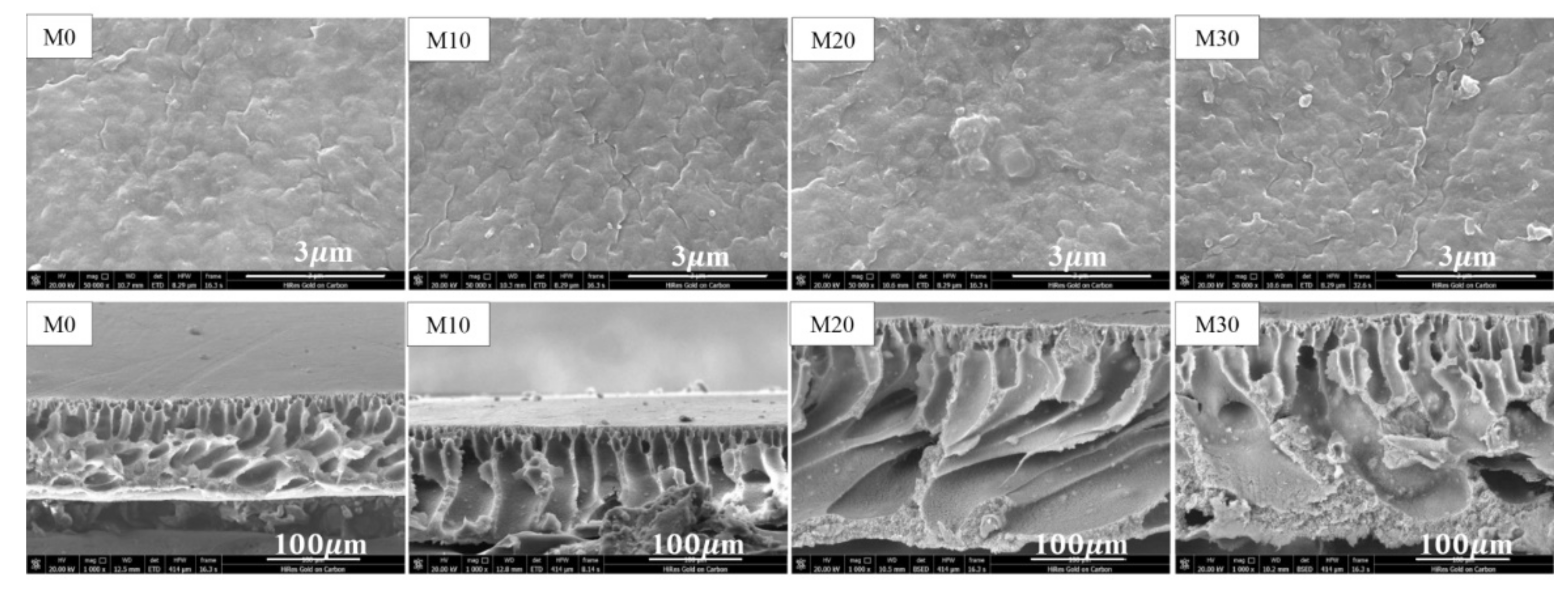
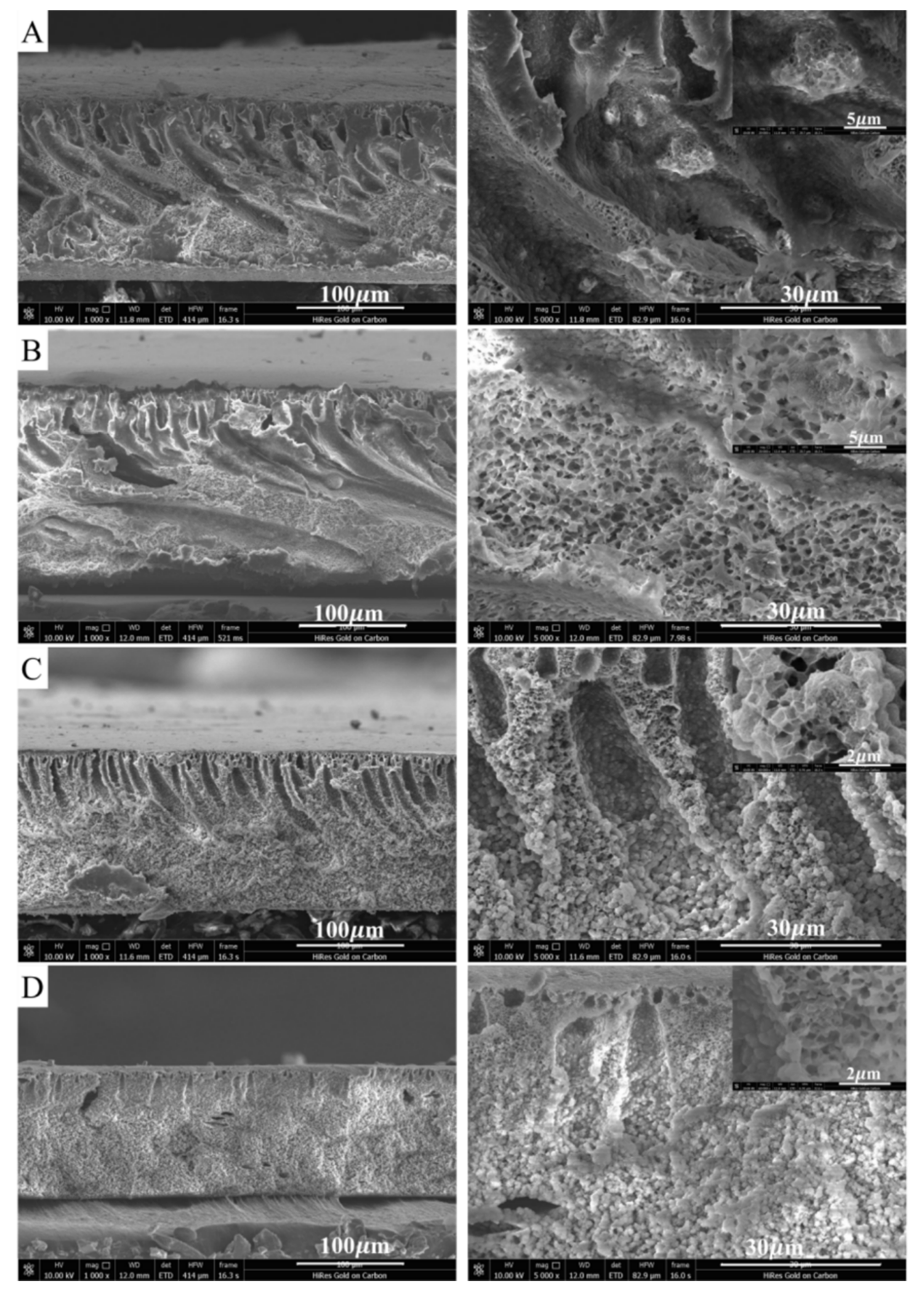
| Composition or Physical Feature | Membrane Type | |||
|---|---|---|---|---|
| M0 | M10 | M20 | M30 | |
| Weight of NMP/g | 84 | 84 | 84 | 84 |
| Weight of PVDF/g | 16 | 16 | 16 | 16 |
| Weight of 62% PTFE nanoemulsion/g | 0 | 2.87 | 6.45 | 11.06 |
| PTFE content relative to PVDF and PTFE/wt% | 0 | 10 | 20 | 30 |
| Total solid content in the casting solution/% | 16 | 17.3 | 18.8 | 20.6 |
| Breaking strength/MPa | 4.16 | 3.74 | 3.25 | 2.97 |
| Breaking elongation/% | 246.8 | 217.2 | 175.2 | 131.0 |
| WCA/° | 78.6 | 81.2 | 83.8 | 88.5 |
| Oil contact angle in water/° | 100.3 | 106.1 | 114.7 | 125.0 |
| Porosity/% | 73.9 | 78.2 | 78.9 | 80.3 |
| Water flux/L·m−2·h−1 | 7.8 | 9.5 | 12.0 | 15.9 |
| Breaking Strength/MPa | Breaking Strength Retention Rate/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Immersion Solution | 0 | 30 | 60 | 90 | 180 | 30 | 60 | 90 | 180 | |
| H2SO4 | M0 | 4.16 | 3.51 | 3.06 | 2.80 | 2.32 | 84.3 | 73.6 | 67.3 | 55.8 |
| M10 | 3.74 | 3.24 | 2.89 | 2.62 | 2.23 | 86.6 | 77.3 | 70.1 | 59.6 | |
| M20 | 3.25 | 2.85 | 2.52 | 2.33 | 2.08 | 87.7 | 77.5 | 71.7 | 64.0 | |
| M30 | 2.97 | 2.68 | 2.41 | 2.26 | 2.07 | 90.2 | 81.1 | 76.1 | 69.7 | |
| HCl | M0 | 4.16 | 3.65 | 3.31 | 3.13 | 2.93 | 87.7 | 79.6 | 75.2 | 70.4 |
| M10 | 3.74 | 3.31 | 3.02 | 2.86 | 2.72 | 88.5 | 80.7 | 76.5 | 72.7 | |
| M20 | 3.25 | 2.9 | 2.66 | 2.52 | 2.37 | 89.2 | 81.8 | 77.5 | 72.9 | |
| M30 | 2.97 | 2.69 | 2.50 | 2.38 | 2.20 | 90.6 | 84.2 | 80.1 | 74.1 | |
| HNO3 | M0 | 4.16 | 3.62 | 3.21 | 2.95 | 2.57 | 87.0 | 77.2 | 70.9 | 61.8 |
| M10 | 3.74 | 3.29 | 2.92 | 2.71 | 2.41 | 88.0 | 78.1 | 72.5 | 64.4 | |
| M20 | 3.25 | 2.85 | 2.56 | 2.45 | 2.20 | 87.7 | 78.8 | 75.4 | 67.7 | |
| M30 | 2.97 | 2.68 | 2.41 | 2.20 | 2.12 | 90.2 | 81.1 | 74.1 | 71.4 | |
| NaOH | M0 | 4.16 | 3.34 | 2.73 | 2.49 | 2.15 | 80.3 | 65.6 | 59.9 | 51.7 |
| M10 | 3.74 | 3.06 | 2.54 | 2.34 | 2.07 | 81.8 | 67.9 | 62.6 | 55.3 | |
| M20 | 3.25 | 2.70 | 2.34 | 2.12 | 1.88 | 83.1 | 72.0 | 65.2 | 57.8 | |
| M30 | 2.97 | 2.58 | 2.22 | 2.09 | 1.88 | 86.9 | 74.7 | 70.4 | 63.3 | |
| Flux/L/m2h | Increase in Flux/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Immersion Immersion Time/Days | 0 | 30 | 60 | 90 | 180 | 30 | 60 | 90 | 180 | |
| H2SO4 | M0 | 7.8 | 11.3 | 13.3 | 16.2 | 19.5 | 44.9 | 70.59 | 107.7 | 150.0 |
| M10 | 9.5 | 13.8 | 16.8 | 18.6 | 21.3 | 45.3 | 76.8 | 95.8 | 124.2 | |
| M20 | 12.0 | 16.6 | 21.2 | 22.1 | 26.5 | 38.3 | 76.7 | 84.2 | 120.8 | |
| M30 | 15.9 | 21.4 | 25.6 | 28.1 | 33.9 | 34.6 | 61.0 | 76.7 | 113.2 | |
| HCl | M0 | 7.8 | 9.9 | 11.8 | 13.1 | 14.6 | 26.9 | 51.3 | 67.9 | 87.2 |
| M10 | 9.5 | 14.2 | 14.8 | 16.1 | 18.3 | 49.5 | 55.8 | 69.5 | 92.65 | |
| M20 | 12.0 | 15.4 | 17.9 | 21.2 | 21.3 | 28.3 | 49.2 | 76.7 | 77.5 | |
| M30 | 15.9 | 20.1 | 23.2 | 25.0 | 28.1 | 26.4 | 45.9 | 57.2 | 76.7 | |
| HNO3 | M0 | 7.8 | 10.3 | 12.2 | 13.3 | 14.2 | 32.1 | 56.4 | 70.5 | 82.1 |
| M10 | 9.5 | 13.2 | 15.4 | 17.0 | 19.5 | 38.9 | 62.1 | 78.9 | 105.3 | |
| M20 | 12.0 | 15.8 | 17.5 | 20.1 | 22.8 | 31.7 | 45.8 | 67.5 | 90.0 | |
| M30 | 15.9 | 21.1 | 24.9 | 27.1 | 29.6 | 32.7 | 56.6 | 70.4 | 86.2 | |
| NaOH | M0 | 7.8 | 9.5 | 11.1 | 11.5 | 12.8 | 21.8 | 42.3 | 47.4 | 64.1 |
| M10 | 9.5 | 12.0 | 13.7 | 14.7 | 16.6 | 26.3 | 44.2 | 54.7 | 74.7 | |
| M20 | 12.0 | 14.5 | 16.4 | 17.5 | 17.6 | 20.8 | 36.7 | 45.8 | 46.7 | |
| M30 | 15.9 | 19.2 | 21.7 | 23.3 | 25.2 | 20.8 | 36.5 | 46.5 | 58.5 | |
| M0 | M0S6 | M30 | M30S6 | M30N6 | M30Cl6 | M30Na6 | |
|---|---|---|---|---|---|---|---|
| Ra (nm) | 15.7 | 22.5 | 17.6 | 21.0 | 21.2 | 18.5 | 22.0 |
| Rq (nm) | 19.8 | 28.0 | 22.9 | 26.9 | 30.3 | 25.9 | 30.1 |
| Initial WCA/° | WCA after Immersion in Different Corrosive Solutions for 180 Days/° | ||||
|---|---|---|---|---|---|
| H2SO4 | HCl | HNO3 | NaOH | ||
| M0 | 78.6 | 71.3 | 74.9 | 73.5 | 66.6 |
| M10 | 81.2 | 73.1 | 76.5 | 75.7 | 69.4 |
| M20 | 83.8 | 76.7 | 79.7 | 78.7 | 72.3 |
| M30 | 88.5 | 81.4 | 84.2 | 83.7 | 77.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Zhou, X.; Sun, Y.; Bai, R. Anticorrosion Performance of PVDF Membranes Modified by Blending PTFE Nanoemulsion and Prepared through Usual Non-Solvent-Induced Phase Inversion Method. Membranes 2021, 11, 420. https://doi.org/10.3390/membranes11060420
Liu T, Zhou X, Sun Y, Bai R. Anticorrosion Performance of PVDF Membranes Modified by Blending PTFE Nanoemulsion and Prepared through Usual Non-Solvent-Induced Phase Inversion Method. Membranes. 2021; 11(6):420. https://doi.org/10.3390/membranes11060420
Chicago/Turabian StyleLiu, Tianshu, Xiaoji Zhou, Yizhuo Sun, and Renbi Bai. 2021. "Anticorrosion Performance of PVDF Membranes Modified by Blending PTFE Nanoemulsion and Prepared through Usual Non-Solvent-Induced Phase Inversion Method" Membranes 11, no. 6: 420. https://doi.org/10.3390/membranes11060420
APA StyleLiu, T., Zhou, X., Sun, Y., & Bai, R. (2021). Anticorrosion Performance of PVDF Membranes Modified by Blending PTFE Nanoemulsion and Prepared through Usual Non-Solvent-Induced Phase Inversion Method. Membranes, 11(6), 420. https://doi.org/10.3390/membranes11060420





