Production Strategies of TiNx Coatings via Reactive High Power Impulse Magnetron Sputtering for Selective H2 Separation
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
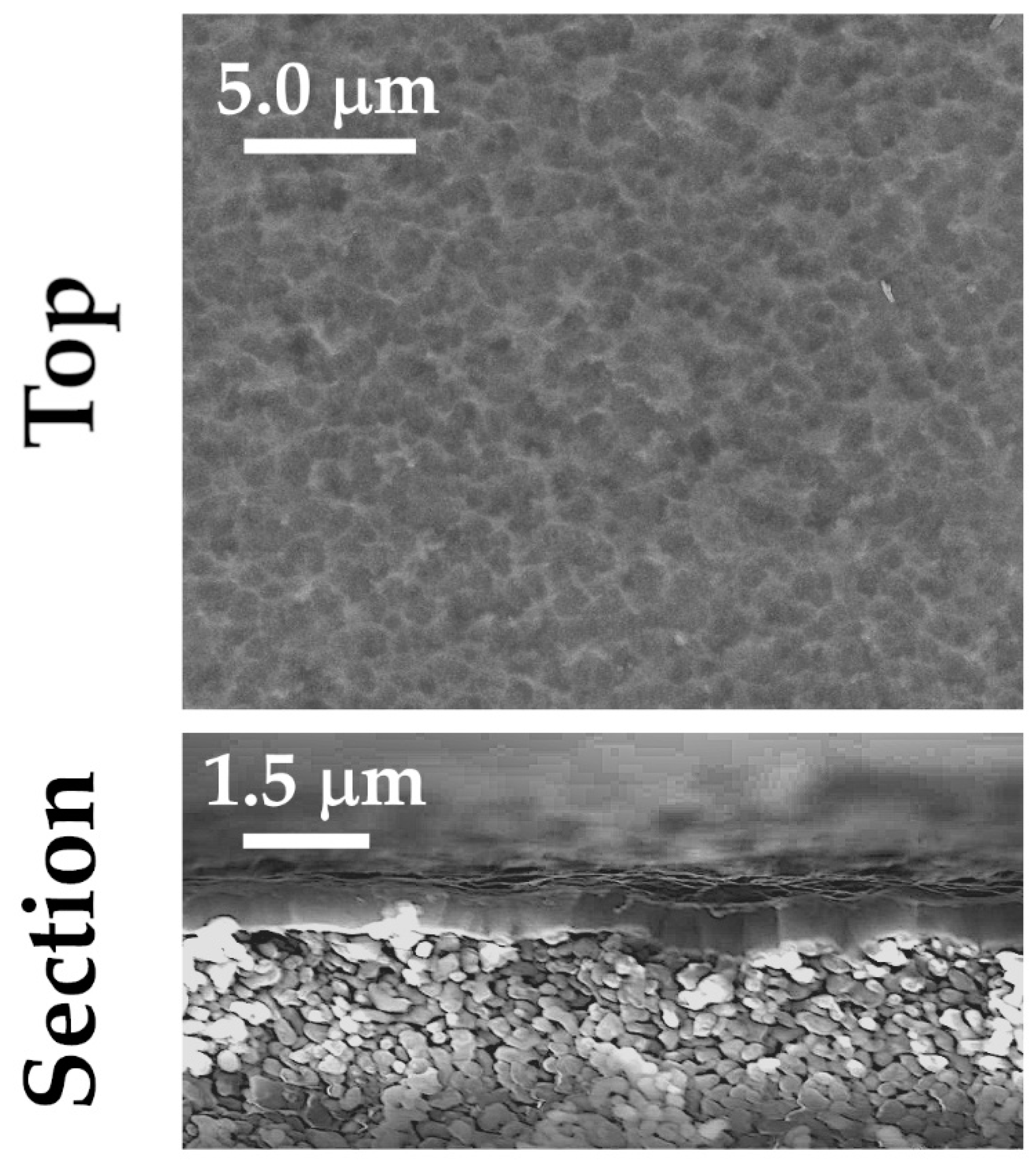
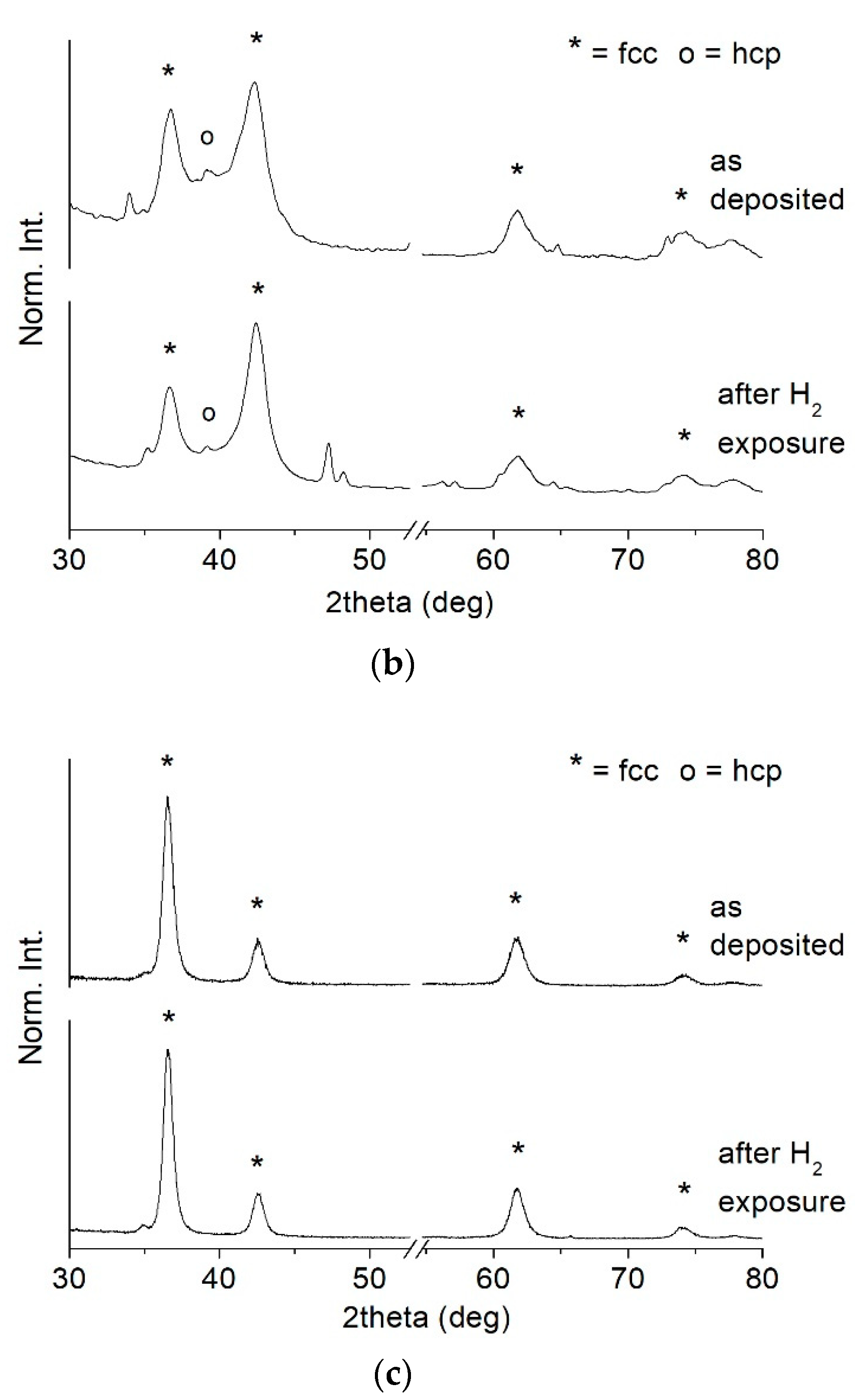
3.1. As Deposited Coatings
3.2. Stability under H2-Containing Atmosphere
4. Conclusions
- With the same process parameters, the substrate temperature change from 140 °C to 300 °C promotes a N/Ti ratio increase (enhanced chemisorption of atomic N plus dissociative chemisorption of N2) and a reduction in thickness (i.e., >density).
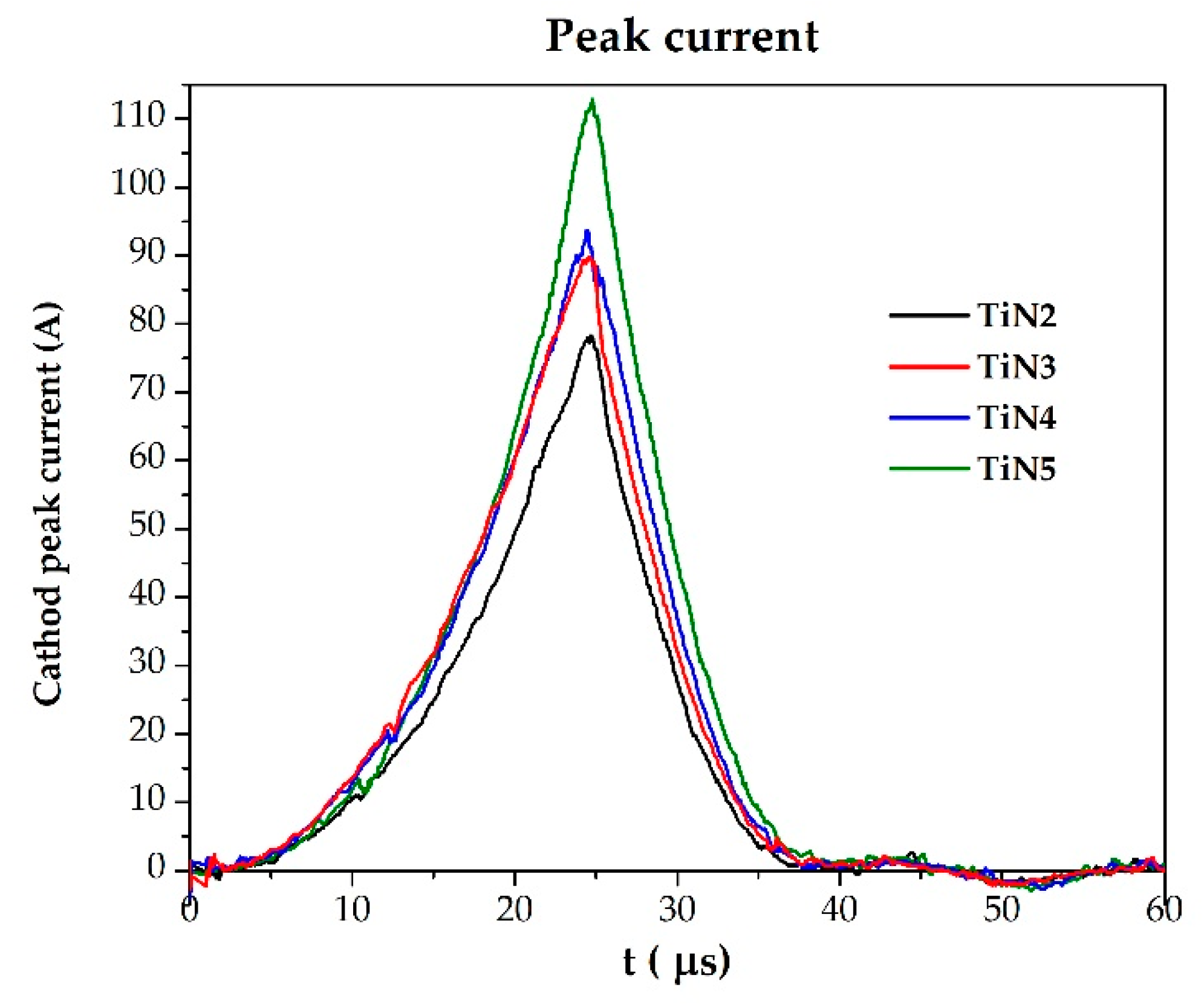
- While the process N2/Ar ratio increases, TiNx film stoichiometry increases (SEM-EDS), but deposition rate decreases (Calotest).
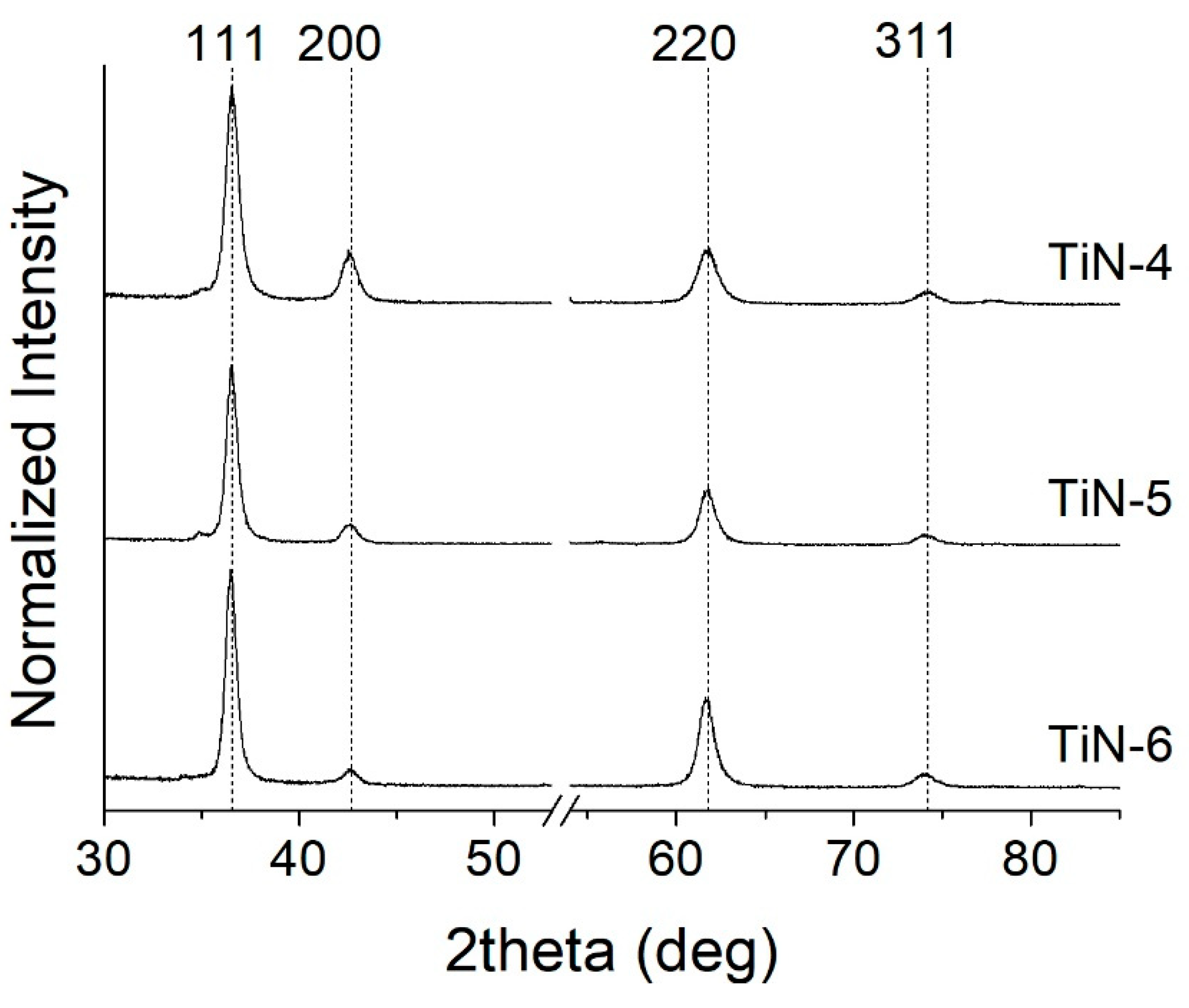
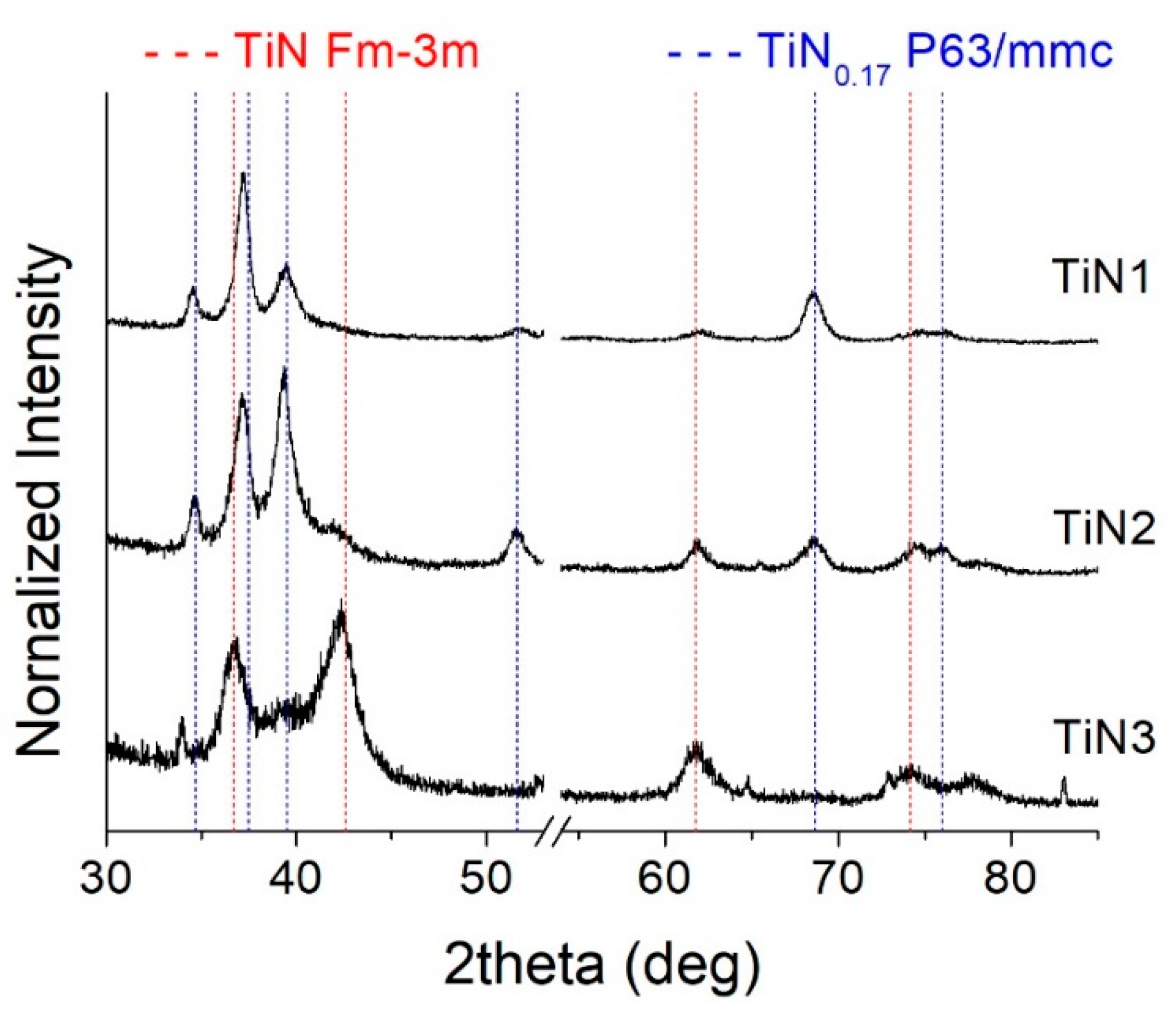
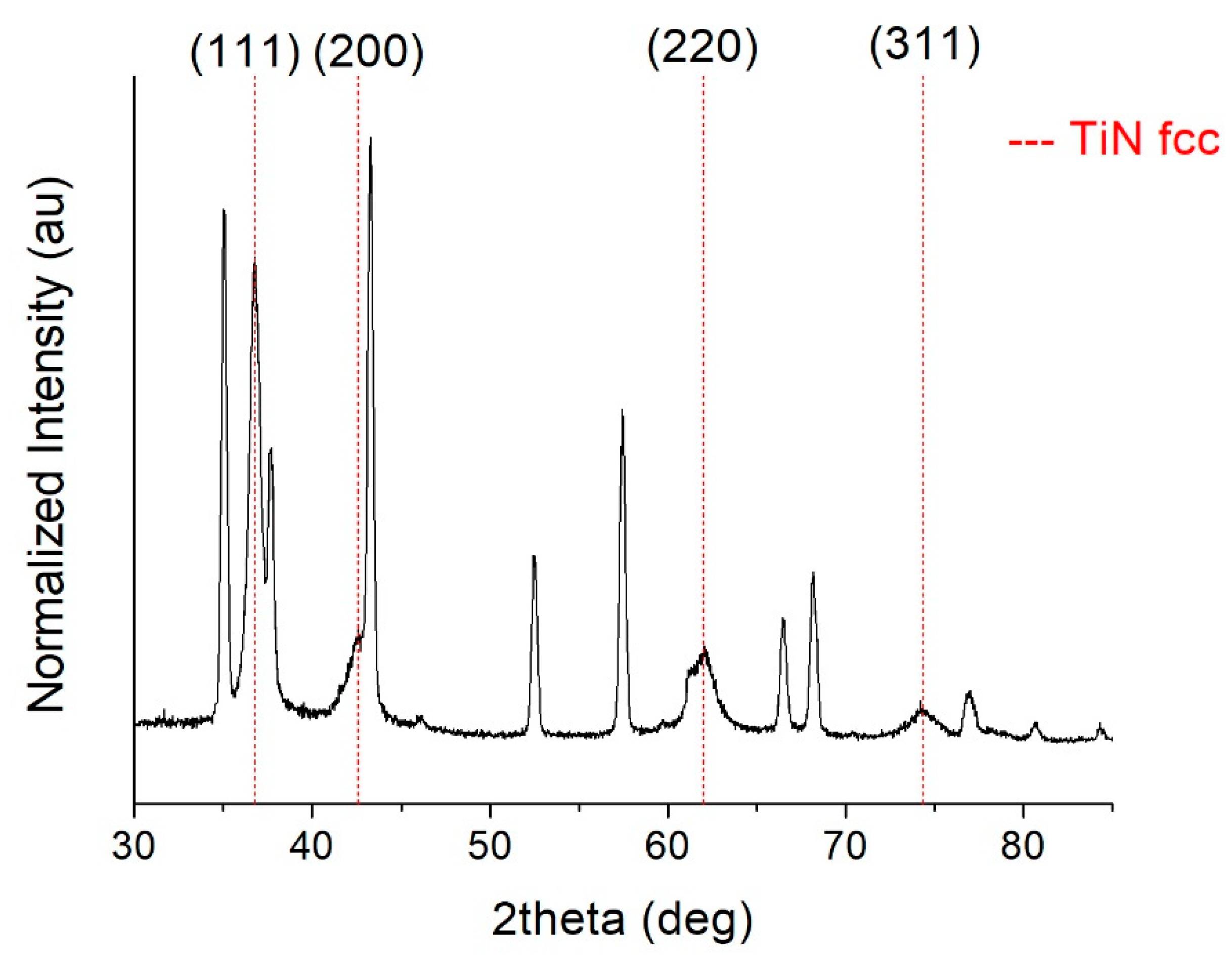
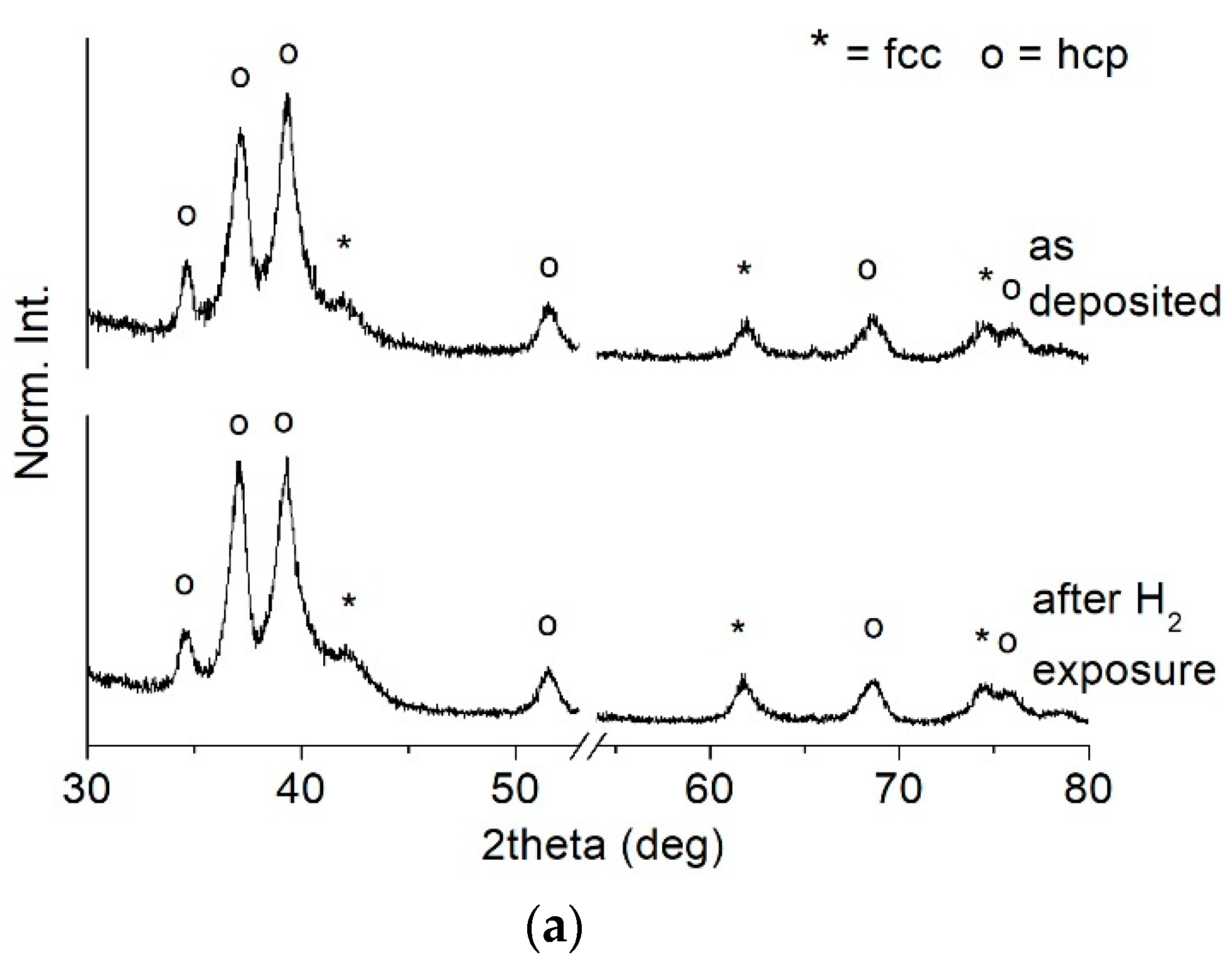
- At high nitrogen flows (>1.2 sccm), the preponderant and stable phase that forms in the film is a face-centered cubic structure (ICSD #604220 TiN, Fm-3m space group) with a (111) preferential orientation. On the other hand, at low nitrogen flows (≤1.2 sccm), the structure evolves towards a hexagonal symmetry and space group P63/mmc (ICSD #644765). This phase is rich in Ti and contains a small amount of nitrogen. It becomes predominant at 1 sccm.
- The sample grown on alumina (TiN4A) is quasi-stoichiometric, and the XRD spectrum shows the presence of a well-crystallized fcc Fm-3m TiN phase. Moreover, a very predominant (111) preferential orientation is exhibited.
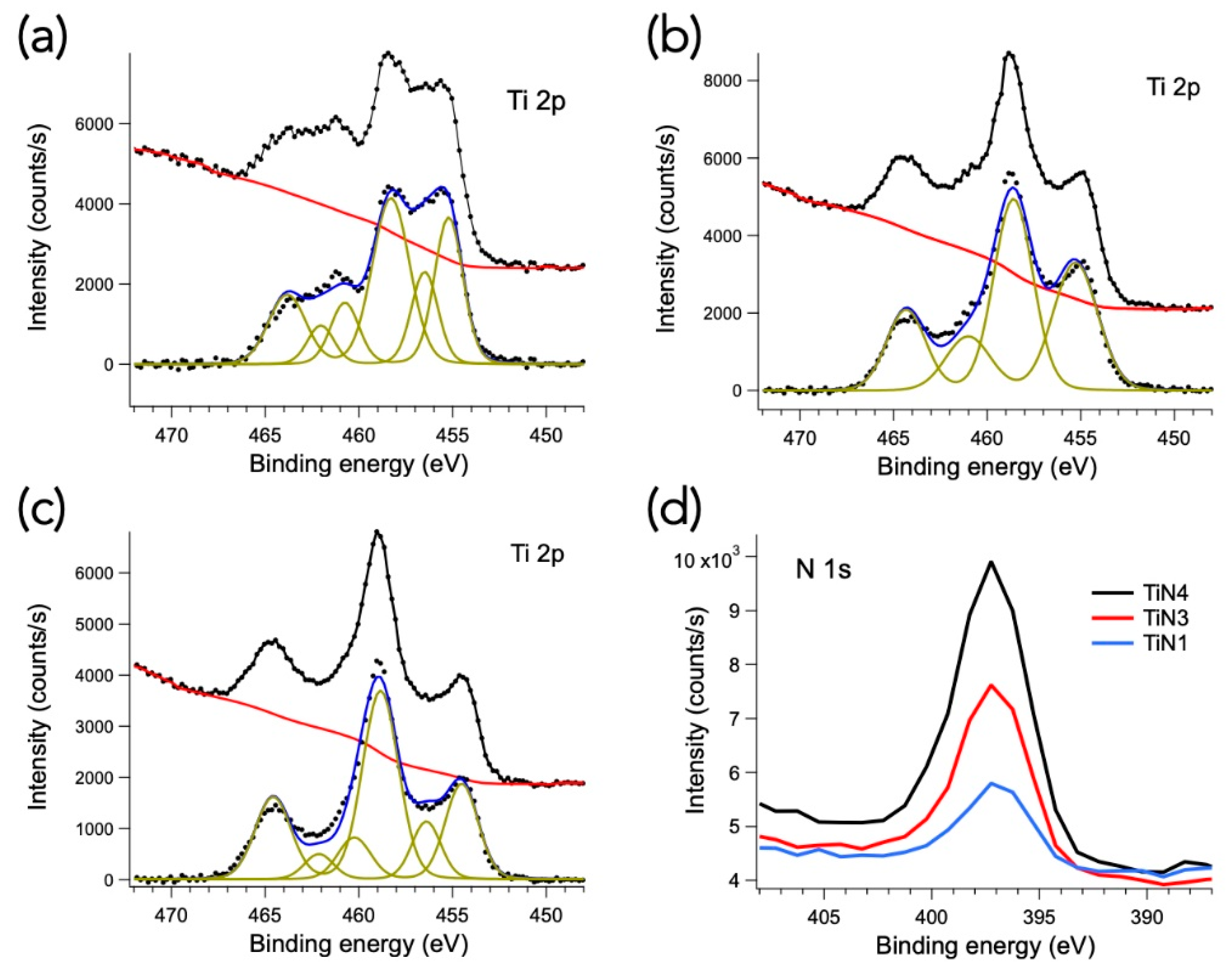
- XPS analyses confirmed that TiNx membranes might be susceptible to oxidation that could hinder hydrogen permeation. The addition of protective films (i.e., Pd or Pd-alloy) to form a multilayer Pd-TiNx-Pd architecture on alumina could be extremely useful.
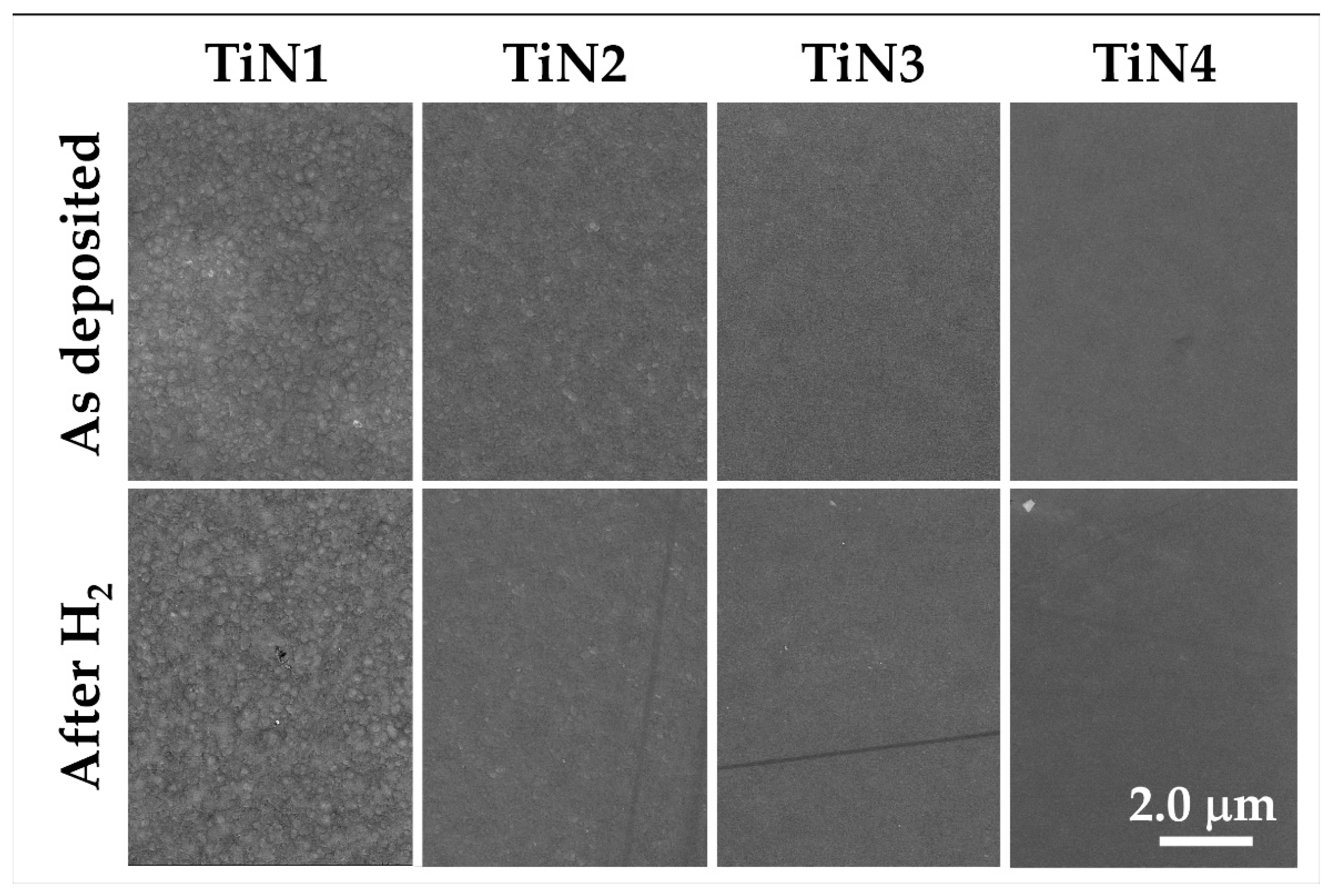
- Chemical robustness was confirmed after the thermal treatment at 500 °C for 20 h under a hydrogen-containing atmosphere: composition and microstructure were maintained by all the samples, thus highlighting their stability under reducing conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallucci, F.; Fernandez, E.; Corengia, P.; Annaland, M. Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Al-Mufachi, N.A.; Rees, N.V.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sustain. Energ. Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Barison, S.; Fasolin, S.; Boldrini, S.; Ferrario, A.; Romano, M.; Montagner, F.; Deambrosis, S.M.; Fabrizio, M.; Armelao, L. PdAg/alumina membranes prepared by high power impulse magnetron sputtering for hydrogen separation. Int. J. Hydrogen Energy 2018, 43, 7982–7989. [Google Scholar] [CrossRef]
- Available online: https://www.kitco.com/charts/livepalladium.html (accessed on 27 April 2021).
- Meulenberg, W.A.; Schulze-Küppers, F.; Deibert, W.; Van Gestel, T.; Baumann, S. Ceramic Membranes: Materials—Components—Potential Applications. ChemBioEng Rev. 2019, 6, 198–208. [Google Scholar] [CrossRef]
- Mortalò, C.; Rebollo, E.; Escolastico, S.; Deambrosis, S.; Haas-Santo, K.; Rancan, M.; Dittmeyer, R.; Armelao, L.; Fabrizio, M. Enhanced sulfur tolerance of BaCe0.65Zr0.20Y0.15O3−δ–Ce0.85Gd0.15O2−δ composite for hydrogen separation membranes. J. Membr. Sci. 2018, 564, 123–132. [Google Scholar] [CrossRef]
- Montaleone, D.; Mercadelli, E.; Escolàstico, S.; Gondolini, A.; Serra, J.M.; Sanson, A. All-ceramic asymmetric membranes with superior hydrogen permeation. J. Mater. Chem. A 2018, 6, 15718–15727. [Google Scholar] [CrossRef]
- Mortalò, C.; Santoru, A.; Pistidda, C.; Rebollo, E.; Boaro, M.; Leonelli, C.; Fabrizio, M. Structural evolution of BaCe0.65Zr0.20Y0.15O3−δ–Ce0.85Gd0.15O2−δ composite MPEC membrane by in-situ synchrotron XRD analyses. Mater. Today Energy 2019, 13, 331–341. [Google Scholar] [CrossRef]
- Cannio, M.; Mortalò, C.; Prestianni, M.; Andreola, F.; Deambrosis, S.M.; Miorin, E.; Zin, V.; Boccaccini, D.N.; Romagnoli, M. Manufacturing of BaCe0.65Zr0.20Y0.15O3−δ–Ce0.85Gd0.15O2−δ structures by micro-extrusion 3D-printing. Mater. Lett. 2021, 284, 128970. [Google Scholar] [CrossRef]
- Van de Walle, C.G.; Neugebauer, J. Universal alignment of hydrogen levels in semiconductors, insulators and solutions. Nature 2003, 423, 626–628. [Google Scholar] [CrossRef]
- Chevallier, J. Hydrogen and doping issues in wide band gap semiconductors. Mater. Sci. Eng. 2000, 71, 62–68. [Google Scholar] [CrossRef]
- Kura, C.; Fujimoto, S.; Kunisada, Y.; Kiwalski, D.; Tsuji, E.; Zhu, C.; Hazabaki, H.; Aoki, Y. Enhanced hydrogen permeability of hafnium nitride nanocrystalline membranes by interfacial hydride conduction. J. Mater. Chem. A 2018, 6, 2730–2741. [Google Scholar] [CrossRef]
- Kim, K.-I.; Hong, T.-W. Hydrogen permeation of TiN–graphene membrane by hot press sintering (HPS) process. Int. J. Hydrogen Energy 2010, 35, 12981–12985. [Google Scholar] [CrossRef]
- Kura, C.; Kunisada, Y.; Tsuji, E.; Zhu, C.; Habazaki, H.; Nagata, S.; Müller, M.P.; De Souza, R.A.; Aoki, Y. Hydrogen separation by nanocrystalline titanium nitride membranes with high hydride ion conductivity. Nat. Energy 2017, 2, 786–794. [Google Scholar] [CrossRef]
- Goncharov, A.; Guglya, A.; Kalchenko, A.; Solopikhina, E.; Vlasov, V.; Lyubchenko, E. Nanocrystalline Porous Hydrogen Storage Based on Vanadium and Titanium Nitrides. J. Nanotechnol. 2017, 4106067. [Google Scholar] [CrossRef]
- Krella, A. Resistance of PVD Coatings to Erosive and Wear Processes: A Review. Coatings 2020, 10, 921. [Google Scholar] [CrossRef]
- D’Avico, L.; Beltrami, R.; Lecis, N.; Trasatti, S.P. Corrosion Behavior and Surface Properties of PVD Coatings for Mold Technology Applications. Coatings 2019, 9, 7. [Google Scholar] [CrossRef]
- Barna, P.B.; Adamik, M. Fundamental structurebforming phenomena of polycrystalline films an the structure zone models. Thin Solid Film. 1998, 317, 27–33. [Google Scholar] [CrossRef]
- Lundin, D.; Tiberiu Minea, T.; Gudmundsson, J.T. High Power Impulse Magnetron Sputtering Fundamentals, Technologies, Challenges and Applications; Imprint Elsevier, 2020; ISBN 978-0-12-812454-3. [Google Scholar] [CrossRef]
- Anders, A. Tutorial: Reactive high power impulse magnetron sputtering (R-HiPIMS). J. Appl. Phys. 2017, 121, 171101. [Google Scholar] [CrossRef]
- Lutterotti, L.; Scardi, P. Simultaneous structure and size–strain refinement by the Rietveld method. J. Appl. Cryst. 1990, 23, 246–252. [Google Scholar] [CrossRef]
- Lutterotti, L.; Chateigner, D.; Ferrari, S.; Ricote, J. Texture, residual stress and structural analysis of thin films using a combined X-ray analysis. Thin Solid Film. 2004, 450, 34–41. [Google Scholar] [CrossRef]
- Paufler, P. The Rietveld Method, International Union of Crystallography; Young, R.A., Ed.; Oxford University Press: Oxford, UK, 1993; ISBN 0-19-855577-6. [Google Scholar]
- Magnus, F.; Ingason, A.S.; Sveinsson, O.B.; Olafsson, S.; Gudmundsson, J.T. Morphology of TiN thin films grown on SiO2 by reactive high power impulse magnetron sputtering. Thin Solid Film. 2011, 520, 1621–1624. [Google Scholar] [CrossRef]
- Vasu, K.; Ghanashyam Krishna, M.; Padmanabhan, K.A. Substrate-temperature dependent structure and composition variations in RF magnetron sputtered titanium nitride thin films. Appl. Surf. Sci. 2011, 257, 3069–3074. [Google Scholar] [CrossRef]
- Petrov, I.; Hultman, L.; Sundgren, J.E.; Greene, J.E. Polycrystalline TiN films deposited by reactive bias magnetron sputtering: Effects of ion bombardment on resputtering rates, film composition and microstructure. J. Vac. Sci. Technol. A 1992, 10, 265. [Google Scholar] [CrossRef]
- Anders, A. A structure zone diagram including plasma-based deposition and ion etching. Thin Solid Film. 2010, 518, 4087–4090. [Google Scholar] [CrossRef]
- Wang, D.; He, Y.; Cui, W. Secondary electron emission characteristics of TiN coatings produced by RF magnetron sputtering. J. Appl. Phys. 2018, 124, 053301. [Google Scholar]
- Deambrosis, S.M.; Montagner, F.; Zin, V.; Fabrizio, M.; Badini, C.; Padovano, E.; Sebastiani, M.; Bemporad, E.; Brunelli, K.; Miorin, E. Ti1−xAlxN coatings by Reactive High Power Impulse Magnetron Sputtering: Film/substrate interface effect on residual stress and high temperature oxidation. Surf. Coat. Technol. 2018, 354, 56–65. [Google Scholar] [CrossRef]
- Daniel, R.; Martinschitz, K.J.; Keckes, J.; Mitterer, C. The origin of stresses in magnetron-sputtered thin films with zone T structures. Acta Mater. 2010, 58, 2621–2633. [Google Scholar] [CrossRef]
- Machunze, R.; Ehiasarian, A.P.; Tichelaar, F.D.; Janssen, G.C.A.M. Stress and texture in HIPIMS TiN thin films. Thin Solid Film. 2009, 518, 1561–1565. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Thirumurugesan, R.; Mohandas, E.; Sastikumar, D.; Kuppusami, P.; Song, J.I. Phase Transition and Thermal Expansion Studies of Alumina Thin Films Prepared by Reactive Pulsed Laser Deposition. J. Nanosci. Nanotechnol. 2014, 14, 7728–7733. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, L.; Yang, H.; Gao, K.; Volinsky, A.A. Residual Stress and Surface Energy of Sputtered TiN Films. J. Mater. Eng. Perform. 2015, 24, 1185–1191. [Google Scholar] [CrossRef]
- Jiang, C.-C.; Goto, T.; Hirai, T. Non-stoichiometry of titanium nitride plates prepared by chemical vapour deposition. J. Alloys Compd. 1993, 190, 197–200. [Google Scholar] [CrossRef]
- Patsalas, P.; Gravalidis, C.; Logothetidis, S. Surface kinetics and subplantation phenomena affecting the texture, morphology, stress, and growth evolution of titanium nitride films. J. Appl. Phys. 2004, 96, 6234–6246. [Google Scholar] [CrossRef]
- Hultman, L.; Sundgren, J.-E.; Greene, J.E.; Bergstrom, D.B.; Petrov, I. High-flux low energy (∼20 eV) N+2 ion irradiation during TiN deposition by reactive magnetron sputtering: Effects on microstructure and preferred orientation. J. Appl. Phys. 1995, 78, 5395–5403. [Google Scholar] [CrossRef]
- Holmberg, B. Structural Studies on the Titanium-Nitrogen System. Acta Chem. Scand. 1962, 16, 1255–1261. [Google Scholar] [CrossRef][Green Version]
- Oktay, S.; Kahraman, Z.; Urgen, M.; Kazmanli, K. XPS investigations of tribolayers formed on TiN and (Ti, Re) N coatings. Appl. Surf. Sci. 2015, 328, 255–261. [Google Scholar] [CrossRef]
- Fasolin, S.; Barison, S.; Boldrini, S.; Ferrario, A.; Romano, M.; Montagner, F.; Miorin, E.; Fabrizio, M.; Armelao, L. Hydrogen separation by thin vanadium-based multi-layered membranes. Int. J. Hydrogen Energy 2018, 43, 3235–3243. [Google Scholar] [CrossRef]

| Sample | Substate | Dep. T (°C) | Duration (min) | N2 Partial p (×10−3 Pa) | N2/Ar % | Thickness (nm) | Dep. Rate (nm/min) |
|---|---|---|---|---|---|---|---|
| TiN1 | Si | 140 | 120 | 2.8 (1 sccm) | 0.6 | 1207 | 10.1 |
| TiN2 | Si | 300 | 120 | 2.8 (1 sccm) | 0.6 | 1160 | 9.7 |
| TiN3 | Si | 300 | 120 | 3.5 (1.2 sccm) | 0.7 | 1080 | 9.0 |
| TiN4 | Si | 300 | 120 | 4.4 (1.5 sccm) | 0.9 | 1090 | 9.1 |
| TiN5 | Si | 300 | 120 | 6.2 (2 sccm) | 1.2 | 643 | 5.4 |
| TiN6 | Si | 300 | 120 | 9.5 (3 sccm) | 1.8 | 430 | 3.6 |
| TiN4A | Al2O3 | 300 | 65 | 4.4 (1.5 sccm) | 0.9 | 590 | 9.1 |
| Sample | As Deposited | After H2 |
|---|---|---|
| TiN1 | 0.43 | 0.44 |
| TiN2 | 0.66 | 0.62 |
| TiN3 | 0.85 | 0.87 |
| TiN4 | 1.1 | 1.09 |
| TiN5 | 1.25 | not treated |
| TiN6 | 1.75 | not treated |
| TiN4A | 1.01 | not treated |
| Sample | Structure | Phase% | Lattice Parameters | Grain Size | Texturing | ||
|---|---|---|---|---|---|---|---|
| %wt | a | c | (nm) | Fm-3m | P63/mmc | ||
| (Å) | (Å) | I111/220 | I002/101 | ||||
| TiN (ref) ICSD #604220 | Fm-3m | - | 4.24 | - | - | 1.4 | - |
| Ti0.83N0.17 (ref) ICSD #644765 | P63/mmc | - | 2.969 | 4.777 | - | - | 0.27 |
| TiN1 | P63/mmc | 100 | 2.989 | 4.835 | 16 | - | 2.1 |
| TiN2 | Fm-3m | 5 | 4.277 | - | - | - | |
| P63/mmc | 95 | 2.989 | 4.833 | 13 | - | 0.87 | |
| TiN3 | Fm-3m | 95 | 4.237 | - | 12 | 2.4 | - |
| P63/mmc | 5 | - | - | - | - | - | |
| TiN4 | Fm-3m | 4.24 | - | 26 | 3.85 | - | |
| TiN5 | Fm-3m | 4.238 | - | 30 | 3.24 | - | |
| TiN6 | Fm-3m | 4.238 | - | 34 | 2.5 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortalò, C.; Deambrosis, S.M.; Montagner, F.; Zin, V.; Fabrizio, M.; Pasquali, L.; Capelli, R.; Montecchi, M.; Miorin, E. Production Strategies of TiNx Coatings via Reactive High Power Impulse Magnetron Sputtering for Selective H2 Separation. Membranes 2021, 11, 360. https://doi.org/10.3390/membranes11050360
Mortalò C, Deambrosis SM, Montagner F, Zin V, Fabrizio M, Pasquali L, Capelli R, Montecchi M, Miorin E. Production Strategies of TiNx Coatings via Reactive High Power Impulse Magnetron Sputtering for Selective H2 Separation. Membranes. 2021; 11(5):360. https://doi.org/10.3390/membranes11050360
Chicago/Turabian StyleMortalò, Cecilia, Silvia Maria Deambrosis, Francesco Montagner, Valentina Zin, Monica Fabrizio, Luca Pasquali, Raffaella Capelli, Monica Montecchi, and Enrico Miorin. 2021. "Production Strategies of TiNx Coatings via Reactive High Power Impulse Magnetron Sputtering for Selective H2 Separation" Membranes 11, no. 5: 360. https://doi.org/10.3390/membranes11050360
APA StyleMortalò, C., Deambrosis, S. M., Montagner, F., Zin, V., Fabrizio, M., Pasquali, L., Capelli, R., Montecchi, M., & Miorin, E. (2021). Production Strategies of TiNx Coatings via Reactive High Power Impulse Magnetron Sputtering for Selective H2 Separation. Membranes, 11(5), 360. https://doi.org/10.3390/membranes11050360











